Low Dose Monacolin K Combined with Coenzyme Q10, Grape Seed, and Olive Leaf Extracts Lowers LDL Cholesterol in Patients with Mild Dyslipidemia: A Multicenter, Randomized Controlled Trial
Abstract
1. Introduction
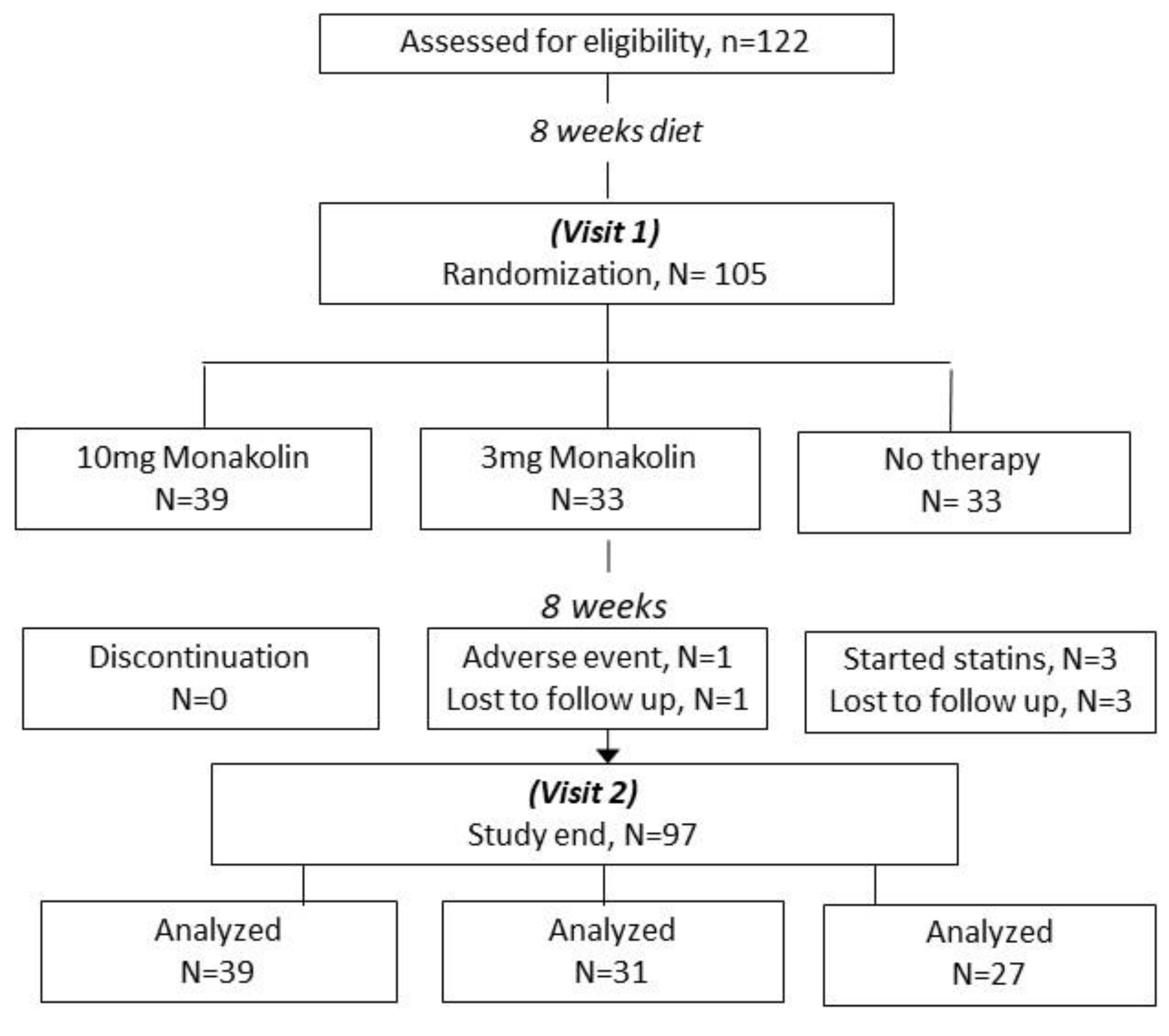
2. Materials and Methods
2.1. Study Protocol
2.2. Measurements
3. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASCVD | Atherosclerotic cardiovascular disease |
| BMI | Body mass index |
| Co Q10 | Coenzyme Q10 |
| CPK | creatine phosphokinase |
| γ-gt | gamma-glutamyl transferase |
| GSE | grape seed extract |
| HDL-C | High-density lipoprotein cholesterol |
| HMG-CoA | hydroxymethylglutaryl-coenzyme A |
| LDL-C | Low-density lipoprotein cholesterol |
| MDRD GFR | Modification of Diet in Renal Disease calculated glomerular filtration rate |
| OLE | Olive leaf extract |
| RYR | Red yeast rice extract |
| SAMS | Statin-associated muscle symptoms |
| SGOT | aspartate aminotransferase |
| SGPT | Alanine aminotransferase |
| TC | Total cholesterol |
| TG | Triglycerides |
References
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the Evidence for the Efficacy and Safety of Statin Therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J. 2018 American Heart Association/American College of Cardiology Multisociety Guideline on the Management of Blood Cholesterol. JAMA Cardiol. 2019, 4, 488–489. [Google Scholar] [CrossRef]
- Catapano, A.L.; Graham, I.; De Backer, G.; Wiklund, O.; Chapman, M.J.; Drexel, H.; Hoes, A.W.; Jennings, C.S.; Landmesser, U.; Pedersen, T.R.; et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Atherosclerosis 2016, 253, 281–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, Z.; Chi, J.; Wang, W.; Su, M.; Kou, W.; Yu, P.; Yu, L.; Chen, L.; Zhu, J.-S.; et al. Multicenter Clinical Trial of the Serum Lipid-Lowering Effects of a Monascus Purpureus (Red Yeast) Rice Preparation from Traditional Chinese Medicine. Curr. Ther. Res. 1997, 58, 964–978. [Google Scholar] [CrossRef]
- Ma, J.; Li, Y.; Ye, Q.; Li, J.; Hua, Y.; Ju, D.; Zhang, D.; Cooper, R.; Chang, M. Constituents of Red Yeast Rice, a Traditional Chinese Food and Medicine. J. Agric. Food Chem. 2000, 48, 5220–5225. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.W.; Mousa, S.A. The Effect of Red Yeast Rice (Monascus Purpureus) in Dyslipidemia and Other Disorders. Complement. Ther. Med. 2012, 20, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Yip, I.; Ashley, J.M.; Elashoff, D.A.; Elashoff, R.M.; Go, V.L.W. Cholesterol-Lowering Effects of a Proprietary Chinese Red-Yeast-Rice Dietary Supplement. Am. J. Clin. Nutr. 1999, 69, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Derosa, G.; Parini, A.; Maffioli, P.; D’Addato, S.; Reggi, A.; Giovannini, M.; Borghi, C. Red Yeast Rice Improves Lipid Pattern, High-Sensitivity C-Reactive Protein, and Vascular Remodeling Parameters in Moderately Hypercholesterolemic Italian Subjects. Nutr. Res. 2013, 33, 622–628. [Google Scholar] [CrossRef]
- Scientific Opinion on the Substantiation of Health Claims Related to Monacolin K from Red Yeast Rice and Maintenance of Normal Blood LDL Cholesterol Concentrations (ID 1648, 1700) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006|EFSA. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2304 (accessed on 3 April 2023).
- Pirro, M.; Lupattelli, G.; Del Giorno, R.; Schillaci, G.; Berisha, S.; Mannarino, M.R.; Bagaglia, F.; Melis, F.; Mannarino, E. Nutraceutical Combination (Red Yeast Rice, Berberine and Policosanols) Improves Aortic Stiffness in Low-Moderate Risk Hypercholesterolemic Patients. Pharma Nutr. 2013, 1, 73–77. [Google Scholar] [CrossRef]
- Affuso, F.; Ruvolo, A.; Micillo, F.; Saccà, L.; Fazio, S. Effects of a Nutraceutical Combination (Berberine, Red Yeast Rice and Policosanols) on Lipid Levels and Endothelial Function Randomized, Double-Blind, Placebo-Controlled Study. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 656–661. [Google Scholar] [CrossRef]
- Banach, M.; Patti, A.M.; Giglio, R.V.; Cicero, A.F.G.; Atanasov, A.G.; Bajraktari, G.; Bruckert, E.; Descamps, O.; Djuric, D.M.; Ezhov, M.; et al. The Role of Nutraceuticals in Statin Intolerant Patients. J. Am. Coll. Cardiol. 2018, 72, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Grieco, A.; Miele, L.; Pompili, M.; Biolato, M.; Vecchio, F.M.; Grattagliano, I.; Gasbarrini, G. Acute Hepatitis Caused by a Natural Lipid-Lowering Product: When “Alternative” Medicine Is No “Alternative” at All. J. Hepatol. 2009, 50, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulos, N.; Paparodis, R.D.; Androulakis, I.; Boniakos, A.; Anagnostis, P.; Tsimihodimos, V.; Livadas, S. Efficacy and Safety of Monacolin K Combined with Coenzyme Q10, Grape Seed, and Olive Leaf Extracts in Improving Lipid Profile of Patients with Mild-To-Moderate Hypercholesterolemia: A Self-Control Study. Nutraceuticals 2022, 3, 1. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific Opinion on the Safety of Monacolins in Red Yeast Rice. EFSA J. 2018, 16, e5368. [Google Scholar] [CrossRef]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.W.; Gerdes, V.E.A. Traditional Chinese Lipid-Lowering Agent Red Yeast Rice Results in Significant LDL Reduction but Safety Is Uncertain—A Systematic Review and Meta-Analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef]
- Baumgartner, S.; Bruckert, E.; Gallo, A.; Plat, J. The Position of Functional Foods and Supplements with a Serum LDL-C Lowering Effect in the Spectrum Ranging from Universal to Care-Related CVD Risk Management. Atherosclerosis 2020, 311, 116–123. [Google Scholar] [CrossRef]
- Farkouh, A.; Baumgärtel, C. Mini-Review: Medication Safety of Red Yeast Rice Products. Int. J. Gen. Med. 2019, 12, 167–171. [Google Scholar] [CrossRef]
- Heinz, T.; Schuchardt, J.P.; Möller, K.; Hadji, P.; Hahn, A. Low Daily Dose of 3 Mg Monacolin K from RYR Reduces the Concentration of LDL-C in a Randomized, Placebo-Controlled Intervention. Nutr. Res. 2016, 36, 1162–1170. [Google Scholar] [CrossRef]
- Becker, D.J. Red Yeast Rice for Dyslipidemia in Statin-Intolerant Patients. Ann. Intern. Med. 2009, 150, 830. [Google Scholar] [CrossRef]
- Halbert, S.C.; French, B.; Gordon, R.Y.; Farrar, J.T.; Schmitz, K.; Morris, P.B.; Thompson, P.D.; Rader, D.J.; Becker, D.J. Tolerability of Red Yeast Rice (2,400 Mg Twice Daily) versus Pravastatin (20 Mg Twice Daily) in Patients with Previous Statin Intolerance. Am. J. Cardiol. 2010, 105, 198–204. [Google Scholar] [CrossRef]
- Flowers, N.; Hartley, L.; Todkill, D.; Stranges, S.; Rees, K. Co-Enzyme Q10 Supplementation for the Primary Prevention of Cardiovascular Disease. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- El, S.N.; Karakaya, S. Olive Tree (Olea Europaea) Leaves: Potential Beneficial Effects on Human Health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitão, F.; Coelho, A.V.; Boas, L.V. Phenolic Compounds and Antioxidant Activity of Olea Europaea L. Fruits and Leaves. Food Sci. Technol. Int. 2006, 12, 385–395. [Google Scholar] [CrossRef]
- Gariboldi, P.; Jommi, G.; Verotta, L. Secoiridoids from Olea Europaea. Phytochemistry 1986, 25, 865–869. [Google Scholar] [CrossRef]
- Hadrich, F.; Mahmoudi, A.; Bouallagui, Z.; Feki, I.; Isoda, H.; Feve, B.; Sayadi, S. Evaluation of Hypocholesterolemic Effect of Oleuropein in Cholesterol-Fed Rats. Chem.-Biol. Interact. 2016, 252, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Priore, P.; Siculella, L.; Gnoni, G.V. Extra Virgin Olive Oil Phenols Down-Regulate Lipid Synthesis in Primary-Cultured Rat-Hepatocytes. J. Nutr. Biochem. 2014, 25, 683–691. [Google Scholar] [CrossRef]
- Kennedy, C.; Köller, Y.; Surkova, E. Effect of Coenzyme Q10 on Statin-Associated Myalgia and Adherence to Statin Therapy: A Systematic Review and Meta-Analysis. Atherosclerosis 2020, 299, 1–8. [Google Scholar] [CrossRef]
- Dohadwala, M.M.; Vita, J.A. Grapes and Cardiovascular Disease. J. Nutr. 2009, 139, 1788S–1793S. [Google Scholar] [CrossRef]
- Shinagawa, F.B.; de Santana, F.C.; Torres, L.R.O.; Mancini-Filho, J. Grape Seed Oil: A Potential Functional Food? Food Sci. Technol. 2015, 35, 399–406. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol. Circulation 2018, 139, e1082–e1143. [Google Scholar] [CrossRef]
- Evans, M.; Wilson, D.; Guthrie, N. A Randomized, Double-Blind, Placebo-Controlled, Pilot Study to Evaluate the Effect of Whole Grape Extract on Antioxidant Status and Lipid Profile. J. Funct. Foods 2014, 7, 680–691. [Google Scholar] [CrossRef]
- Ghaffar, S.; Naqvi, M.A.; Fayyaz, A.; Abid, M.K.; Khayitov, K.N.; Jalil, A.T.; Alsaikhan, F.; Hammid, A.T.; Al-Gazally, M.E.; Mohammadparast, V.; et al. What Is the Influence of Grape Products on Liver Enzymes? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2022, 69, 102845. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects OfVitis Vinifera(Grape) and Its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Gollucke, A.P.B.; Ribeiro, D.A. Use of Grape Polyphenols for Promoting Human Health: A Review of Patents. Recent Pat. Food Nutr. Agric. 2012, 4, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Koushki, M.; Dashatan, N.A.; Meshkani, R. Effect of Resveratrol Supplementation on Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Ther. 2018, 40, 1180–1192.e5. [Google Scholar] [CrossRef] [PubMed]
- Foshati, S.; Rouhani, M.H.; Amani, R. The Effect of Grape Seed Extract Supplementation on Oxidative Stress and Inflammation: A Systematic Review and Meta-Analysis of Controlled Trials. Int. J. Clin. Pract. 2021, 75, e14469. [Google Scholar] [CrossRef]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Newby, L.K.; Piña, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women—2011 Update. Circulation 2011, 123, 1243–1262. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean Diet Attenuates Inflammation and Coagulation Process in Healthy Adults. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef]
- Clark, J.S.; Dyer, K.A.; Davis, C.R.; Shivappa, N.; Hébert, J.R.; Woodman, R.; Hodgson, J.M.; Murphy, K.J. Adherence to a Mediterranean Diet for 6 Months Improves the Dietary Inflammatory Index in a Western Population: Results from the MedLey Study. Nutrients 2023, 15, 366. [Google Scholar] [CrossRef]

| Red Yeast Rice Monacolin K | 10 mg or 3 mg |
|---|---|
| CoQ10 | 2 mg |
| Vitamin B5 | 6 mg |
| Vitamin B6 | 1.4 mg |
| Vitamin B2 | 1.4 mg |
| Vitamin B1 | 1.1 mg |
| Folic acid | 200 mcg |
| Biotin | 50 mcg |
| Vitamin B12 | 2 mcg |
| Olive leaf extract | 50 mg |
| Grape seed extract | 50 mg |
| Variable | Group A n = 39 | Group B n = 31 | Control n = 27 | p |
|---|---|---|---|---|
| Age | 56.89 ± 9.12 | 55.83 ± 9.25 | 57.42 ± 12.29 | 0.744 |
| Gender female, n (%) | 34 (87.2) | 27 (87.1) | 23 (85.2) | 0.812 * |
| BMI (kg/m2) | 25.39 ± 3.23 | 24.76 ± 3.53 | 27.10 ± 3.31 | 0.052 |
| TC (mg/dL) | 259.8 ± 25.9 | 255.3 ± 25.1 | 247.0 ± 21.9 | 0.174 |
| LDL-C (mg/dL) | 167.7 ± 14.22 | 166.5 ± 14.31 | 162.2 ± 15.42 | 0.281 |
| HDL (mg/dL) | 64.12 ± 16.79 | 62.16 ± 14.69 | 56.65 ± 11.44 | 0.191 |
| TG (mg/dL) | 125.9 ± 73.5 | 132.3 ± 56.1 | 146.6 ± 51.4 | 0.062 |
| SGOT (U/L) | 26.71 ± 6.04 | 23.89 ± 6.52 | 24.52 ± 11.46 | 0.103 |
| SGPT (U/L) | 29.71 ± 6.20 | 25.53 ± 7.96 | 27.68 ± 13.37 | 0.102 |
| γ-GT (U/L) | 20.94 ± 6.88 | 17.56 ± 9.39 | 18.50 ± 10.66 | 0.105 |
| CPK (U/L) | 79.33 ± 25.17 | 88.42 ± 40.08 | 73.69 ± 30.74 | 0.165 |
| Variable | Group A n = 39 | Group B n = 31 | Control n = 27 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Study End | p | Baseline | Study End | p | Baseline | Study End | p | p | |
| BMI (kg/m2) | 25.39 ± 3.23 | 25.28 ± 3.47 | 0.456 | 24.76 ± 3.53 | 23.57 ± 5.53 | 0.749 | 27.10 ± 3.31 | 25.95 ± 6.23 | 0.222 | 0.175 |
| TC (mg/dL) | 259.8 ± 25.9 | 211.49 ± 28.7 | <0.001 | 255.3 ± 25.1 | 198.58 ± 71.3 | <0.001 | 247.0 ± 21.9 | 238.93 ± 61.93 | 0.657 | 0.002 a |
| LDL-C (mg/dL) | 167.7 ± 14.22 | 123.38 ± 23.74 | <0.001 | 166.5 ± 14.31 | 118.97 ± 44.2 | <0.001 | 162.2 ± 15.42 | 157.41 ± 35.07 | 0.722 | <0.001 b |
| HDL (mg/dL) | 64.12 ± 16.79 | 65.08 ± 16.53 | 0.227 | 62.16 ± 14.69 | 54.23 ± 20.79 | 0.198 | 56.65 ± 11.44 | 54.19 ± 15.12 | 0.482 | 0.026 c |
| TG (mg/dL) | 125.9 ± 73.5 | 116.77 ± 55.22 | 0.004 | 132.3 ± 56.1 | 112.42 ± 68.34 | 0.071 | 146.6 ± 51.4 | 138.56 ± 51.18 | 0.424 | 0.040 d |
| SGOT (U/L) | 26.71 ± 6.04 | 28.18 ± 7.1 | 0.349 | 23.89 ± 6.52 | 22.52 ± 11.46 | 0.508 | 24.52 ± 11.46 | 24.19 ± 9.75 | 0.712 | 0.188 |
| SGPT (U/L) | 29.71 ± 6.20 | 29.05 ± 7.85 | 0.289 | 25.53 ± 7.96 | 21.81 ± 12.08 | 0.124 | 27.68 ± 13.37 | 24.81 ± 14.58 | 0.492 | 0.150 |
| γ-GT (U/L) | 20.94 ± 6.88 | 26.21 ± 7.33 | <0.001 | 17.56 ± 9.39 | 19.23 ± 11.68 | 0.170 | 18.50 ± 10.66 | 19.67 ± 13.09 | 0.644 | 0.01 |
| CPK (U/L) | 79.33 ± 25.17 | 77.92 ± 24.89 | 0.898 | 88.42 ± 40.08 | 74.29 ± 48.05 | 0.509 | 73.69 ± 30.74 | 71.59 ± 37.58 | 0.799 | 0.379 |
| Treatment | |||||
|---|---|---|---|---|---|
| Group A | Group B | Control | p | ||
| TC | Mean | −48.38 | −41.58 | 1.07 | <0.001 a |
| 95% (CI of Mean) | −56.76; −40.01 | −59.12; −24.05 | −7.96; 10.11 | ||
| SD | 26.69 | 49.81 | 23.95 | ||
| Min | −119 | −278 | −51 | ||
| Max | 2 | 19 | 52 | ||
| LDL-C | Mean | −44.38 | −30.19 | 1.19 | <0.001 b |
| SD | 21.64 | 22.29 | 21.12 | ||
| Min | −103 | −75 | −37 | ||
| Max | −7 | 2 | 45 | ||
| 95% (CI of Mean) | −51.18; −37.59 | −38.04; −22.35 | −6.78; 9.15 | ||
| HDL | Mean | 0.95 | −2.13 | −0.37 | 0.229 c |
| SD | 9.93 | 8.61 | 7.03 | ||
| Min | −23 | −25 | −12 | ||
| Max | 37 | 9 | 20 | ||
| 95% (CI of Mean) | −2.17; 4.06 | −5.16; 0.9 | −3.02; 2.28 | ||
| TG | Mean | −11.77 | −4.65 | −2.63 | 0.768 d |
| SD | 27.79 | 36.63 | 36.5 | ||
| Min | −110 | −93 | −61 | ||
| Max | 34 | 97 | 111 | ||
| 95% (CI of Mean) | −20.49; −3.05 | −17.54; 8.25 | −16.4; 11.14 | ||
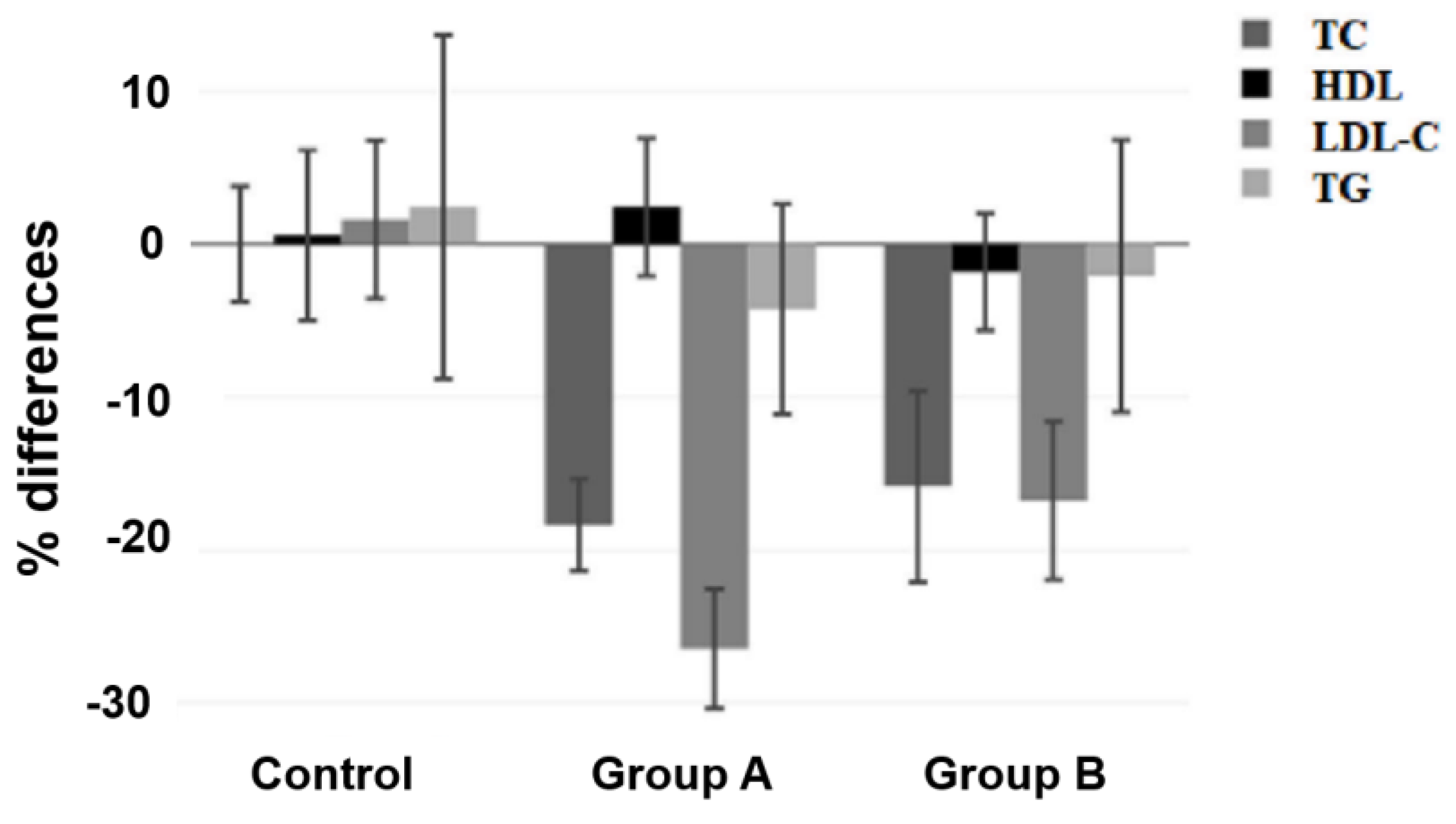
| Variable | Percentage of Difference, % | Treatment | |||
|---|---|---|---|---|---|
| Group A | Group B | Control | p | ||
| TC | Mean | −18.37 | −15.85 | 0 | <0.001 a |
| SD | 9.59 | 17.75 | 10.01 | ||
| Min | −42.31 | −100 | −23.23 | ||
| Max | 0.81 | 6.91 | 19.7 | ||
| 95% (CI of Mean) | −21.38; −15.36 | −22.1; −9.6 | −3.78; 3.78 | ||
| LDL-C | Mean | −26.46 | −16.77 | 1.61 | <0.001 b |
| SD | 12.45 | 14.68 | 13.75 | ||
| Min | −58.86 | −41.67 | −22.84 | ||
| Max | −4.12 | 20.35 | 31.03 | ||
| 95% (CI of Mean) | −30.37; −22.55 | −21.93; −11.6 | −3.58; 6.79 | ||
| HDL | Mean | 2.43 | −1.82 | 0.58 | 0.256 c |
| S | 14.32 | 10.87 | 14.78 | ||
| Min | −28.05 | −28.05 | −18.52 | ||
| Max | 55.22 | 16.07 | 54.05 | ||
| 95% (CI of Mean) | −2.07; 6.92 | −5.64; 2.01 | −4.99; 6.16 | ||
| TG | Mean | −4.25 | −2.09 | 2.42 | 0.743 d |
| SD | 21.85 | 25.29 | 29.81 | ||
| Min | −43.81 | −37.37 | −47.62 | ||
| Max | 59.65 | 56.73 | 106.73 | ||
| 95% (CI of Mean) | −11.11; 2.61 | −10.99; 6.82 | −8.82; 13.67 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelopoulos, N.; Paparodis, R.D.; Androulakis, I.; Boniakos, A.; Argyrakopoulou, G.; Livadas, S. Low Dose Monacolin K Combined with Coenzyme Q10, Grape Seed, and Olive Leaf Extracts Lowers LDL Cholesterol in Patients with Mild Dyslipidemia: A Multicenter, Randomized Controlled Trial. Nutrients 2023, 15, 2682. https://doi.org/10.3390/nu15122682
Angelopoulos N, Paparodis RD, Androulakis I, Boniakos A, Argyrakopoulou G, Livadas S. Low Dose Monacolin K Combined with Coenzyme Q10, Grape Seed, and Olive Leaf Extracts Lowers LDL Cholesterol in Patients with Mild Dyslipidemia: A Multicenter, Randomized Controlled Trial. Nutrients. 2023; 15(12):2682. https://doi.org/10.3390/nu15122682
Chicago/Turabian StyleAngelopoulos, Nicholas, Rodis D. Paparodis, Ioannis Androulakis, Anastasios Boniakos, Georgia Argyrakopoulou, and Sarantis Livadas. 2023. "Low Dose Monacolin K Combined with Coenzyme Q10, Grape Seed, and Olive Leaf Extracts Lowers LDL Cholesterol in Patients with Mild Dyslipidemia: A Multicenter, Randomized Controlled Trial" Nutrients 15, no. 12: 2682. https://doi.org/10.3390/nu15122682
APA StyleAngelopoulos, N., Paparodis, R. D., Androulakis, I., Boniakos, A., Argyrakopoulou, G., & Livadas, S. (2023). Low Dose Monacolin K Combined with Coenzyme Q10, Grape Seed, and Olive Leaf Extracts Lowers LDL Cholesterol in Patients with Mild Dyslipidemia: A Multicenter, Randomized Controlled Trial. Nutrients, 15(12), 2682. https://doi.org/10.3390/nu15122682






