Decreased Expression of KLF4 Leading to Functional Deficit in Pediatric Patients with Intestinal Failure and Potential Therapeutic Strategy Using Decanoic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. 10× Genomics Single-Cell RNA Sequencing (scRNA-seq)
2.2.1. Preparation of Single-Cell Suspension
2.2.2. Chromium 10× Genomics Library and Sequencing
2.2.3. Identification of the Major Cell Types
2.2.4. Pathway Enrichment Analysis
2.3. Culture and Treatment of Patient-Derived Organoids (PDOs)
2.4. Cell Culture and Treatment
2.5. TPN Rat Model
2.6. Immunofluorescence
2.7. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.8. Western Blot
2.9. Reagents
2.10. Statistical Analysis
3. Results
3.1. Clinical Characteristics of IF Patients
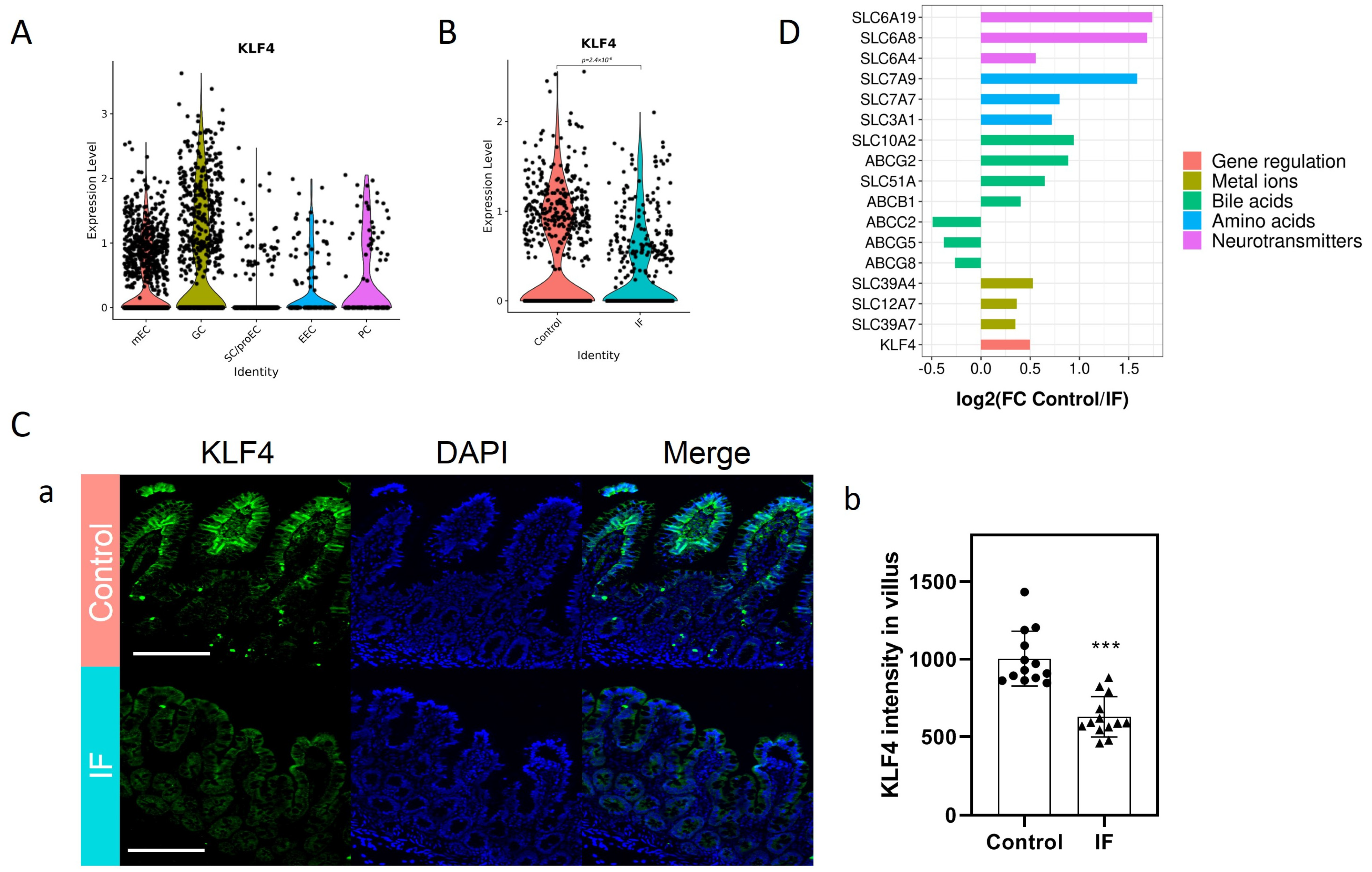
3.2. Decreased KLF4 and SLC Transporters in the Mature Enterocytes (mEC) of IF Patients
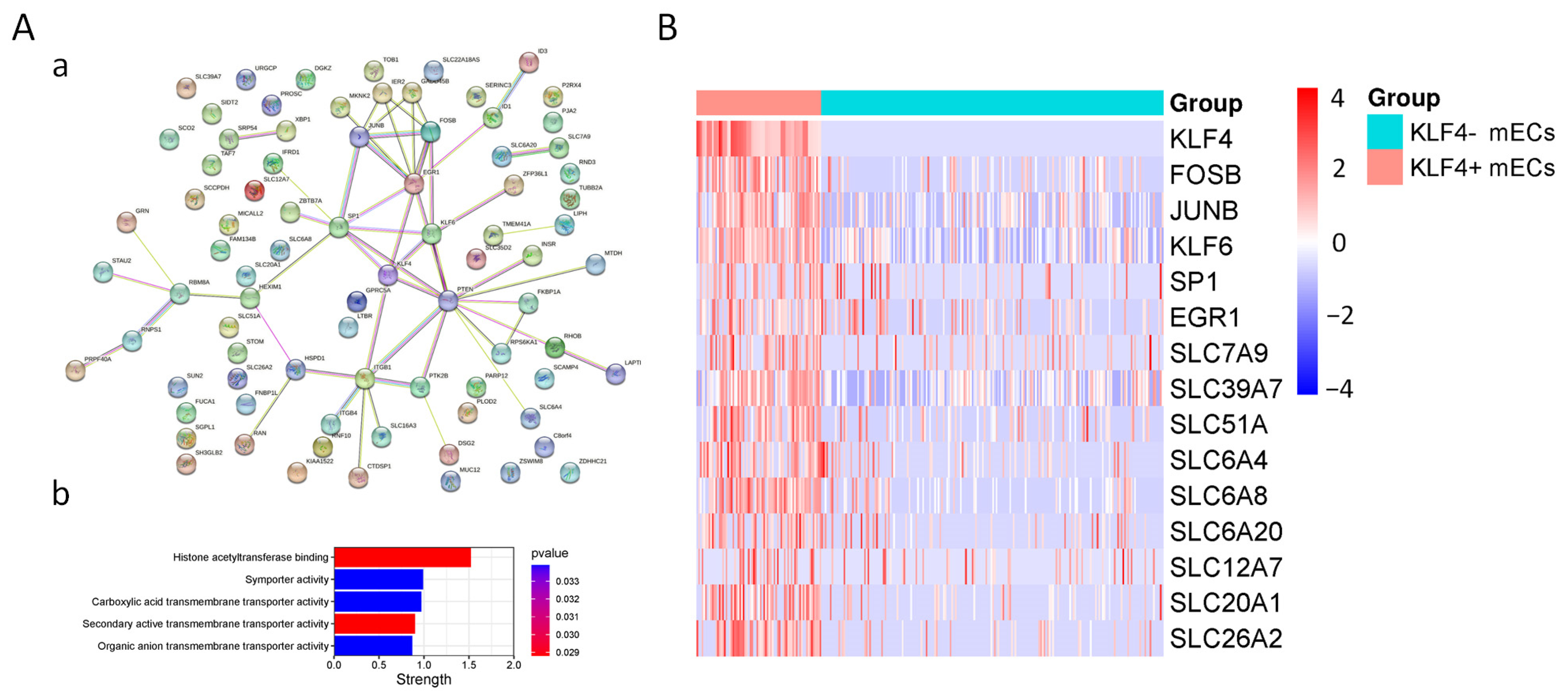
3.3. Characteristics of KLF4+ mEC
3.4. Role of Enteral Nutrition in the Regulation of Intestinal KLF4 Expression
3.5. Effect of Decanoic Acid on the Expression of KLF4 in Patient-Derived Organoids (PDOs)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pironi, L. Definitions of intestinal failure and the short bowel syndrome. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Goulet, O.; Ruemmele, F. Causes and management of intestinal failure in children. Gastroenterology 2006, 130, S16-28. [Google Scholar] [CrossRef] [PubMed]
- Alcolea Sanchez, A.; Nava Hurtado de Saracho, F.B.; Sanchez-Galan, A.M.; Gonzalez Sacristan, R. Intestinal failure in adults and children. Rev. Esp. Enferm. Dig. 2020, 112, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Tappenden, K.A. Intestinal adaptation following resection. J. Parenter. Enteral Nutr. 2014, 38, 23S–31S. [Google Scholar] [CrossRef] [PubMed]
- Grun, D.; Lyubimova, A.; Kester, L.; Wiebrands, K.; Basak, O.; Sasaki, N.; Clevers, H.; van Oudenaarden, A. Single-cell messenger RNA sequencing reveals rare intestinal cell types. Nature 2015, 525, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, W.; Wang, J.; Wang, T.; Xiong, X.; Qi, Z.; Fu, W.; Yang, X.; Chen, Y.G. Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine. J. Exp. Med. 2020, 217, e20191130. [Google Scholar] [CrossRef] [PubMed]
- Moor, A.E.; Harnik, Y.; Ben-Moshe, S.; Massasa, E.E.; Rozenberg, M.; Eilam, R.; Bahar Halpern, K.; Itzkovitz, S. Spatial Reconstruction of Single Enterocytes Uncovers Broad Zonation along the Intestinal Villus Axis. Cell 2018, 175, 1156–1167.e15. [Google Scholar] [CrossRef]
- Seiler, K.M.; Waye, S.E.; Kong, W.; Kamimoto, K.; Bajinting, A.; Goo, W.H.; Onufer, E.J.; Courtney, C.; Guo, J.; Warner, B.W.; et al. Single-Cell Analysis Reveals Regional Reprogramming during Adaptation to Massive Small Bowel Resection in Mice. Cell Mol. Gastroenterol. Hepatol. 2019, 8, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Flandez, M.; Guilmeau, S.; Blache, P.; Augenlicht, L.H. KLF4 regulation in intestinal epithelial cell maturation. Exp. Cell Res. 2008, 314, 3712–3723. [Google Scholar] [CrossRef]
- Iwasaki, M.; Tsuchiya, K.; Okamoto, R.; Zheng, X.; Kano, Y.; Okamoto, E.; Okada, E.; Araki, A.; Suzuki, S.; Sakamoto, N.; et al. Longitudinal cell formation in the entire human small intestine is correlated with the localization of Hath1 and Klf4. J. Gastroenterol. 2011, 46, 191–202. [Google Scholar] [CrossRef]
- Enman, M.A.; Wilkinson, L.T.; Meloni, K.B.; Shroyer, M.C.; Jackson, T.F.; Aban, I.; Dimmitt, R.A.; Martin, C.A.; Galloway, D.P. Key Determinants for Achieving Enteral Autonomy and Reduced Parenteral Nutrition Exposure in Pediatric Intestinal Failure. J. Parenter. Enteral Nutr. 2020, 44, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, Y.; Lu, L.; Yan, W.; Tao, Y.; Zhou, K.; Jia, J.; Cai, W. Alterations in intestinal microbiota relate to intestinal failure-associated liver disease and central line infections. J. Pediatr. Surg. 2017, 52, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feng, H.; Wang, Y.; Jiang, L.; Yan, J.; Cai, W. Impaired FXR-CPT1a signaling contributes to parenteral nutrition-induced villus atrophy in short-bowel syndrome. FASEB J. 2023, 37, e22713. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Z.; Zhou, J.; Yan, J.; Cai, W. Lin 28A/Occludin axis: An aberrantly activated pathway in intestinal epithelial cells leading to impaired barrier function under total parenteral nutrition. FASEB J. 2021, 35, e21189. [Google Scholar] [CrossRef] [PubMed]
- Struijs, M.C.; Diamond, I.R.; de Silva, N.; Wales, P.W. Establishing norms for intestinal length in children. J. Pediatr. Surg. 2009, 44, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Joosten, K.; Embleton, N.; Yan, W.; Senterre, T.; The ESPGHAN/ESPEN/ESPR/CSPEN Working Group on Pediatric Parenteral Nutrition. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition: Energy. Clin. Nutr. 2018, 37, 2309–2314. [Google Scholar] [CrossRef]
- Fanaroff, A.A.; Stoll, B.J.; Wright, L.L.; Carlo, W.A.; Ehrenkranz, R.A.; Stark, A.R.; Bauer, C.R.; Donovan, E.F.; Korones, S.B.; Laptook, A.R.; et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am. J. Obstet. Gynecol. 2007, 196, 147.e1–148.e8. [Google Scholar] [CrossRef]
- Stoll, B.J.; Hansen, N.I.; Bell, E.F.; Shankaran, S.; Laptook, A.R.; Walsh, M.C.; Hale, E.C.; Newman, N.S.; Schibler, K.; Carlo, W.A.; et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010, 126, 443–456. [Google Scholar] [CrossRef]
- Pironi, L.; Goulet, O.; Buchman, A.; Messing, B.; Gabe, S.; Candusso, M.; Bond, G.; Gupte, G.; Pertkiewicz, M.; Steiger, E.; et al. Outcome on home parenteral nutrition for benign intestinal failure: A review of the literature and benchmarking with the European prospective survey of ESPEN. Clin. Nutr. 2012, 31, 831–845. [Google Scholar] [CrossRef]
- Tecos, M.E.; Steinberger, A.E.; Guo, J.; Warner, B.W. Distal Small Bowel Resection Yields Enhanced Intestinal and Colonic Adaptation. J. Surg. Res. 2022, 273, 100–109. [Google Scholar] [CrossRef]
- Berlin, P.; Reiner, J.; Wobar, J.; Bannert, K.; Glass, A.; Walter, M.; Bastian, M.; Willenberg, H.S.; Vollmar, B.; Klar, E.; et al. Villus Growth, Increased Intestinal Epithelial Sodium Selectivity, and Hyperaldosteronism Are Mechanisms of Adaptation in a Murine Model of Short Bowel Syndrome. Dig. Dis. Sci. 2019, 64, 1158–1170. [Google Scholar] [CrossRef] [PubMed]
- Musch, M.W.; Bookstein, C.; Rocha, F.; Lucioni, A.; Ren, H.; Daniel, J.; Xie, Y.; McSwine, R.L.; Rao, M.C.; Alverdy, J.; et al. Region-specific adaptation of apical Na/H exchangers after extensive proximal small bowel resection. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G975–G985. [Google Scholar] [CrossRef] [PubMed]
- Sigalet, D.L.; Martin, G.R. Mechanisms underlying intestinal adaptation after massive intestinal resection in the rat. J. Pediatr. Surg. 1998, 33, 889–892. [Google Scholar] [CrossRef]
- Thompson, J.S.; Langnas, A.N.; Pinch, L.W.; Kaufman, S.; Quigley, E.M.; Vanderhoof, J.A. Surgical approach to short-bowel syndrome. Experience in a population of 160 patients. Ann. Surg. 1995, 222, 600–605; discussion 605–607. [Google Scholar] [CrossRef]
- Doldi, S.B. Intestinal adaptation following jejuno-ileal bypass. Clin. Nutr. 1991, 10, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Jacob, Y.; Sather, S.; Martin, J.R.; Ollo, R. Analysis of Kruppel control elements reveals that localized expression results from the interaction of multiple subelements. Proc. Natl. Acad. Sci. USA 1991, 88, 5912–5916. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, P.N.; Fan, L.; Sweet, D.R.; Jain, M.K. The Kruppel-Like Factors and Control of Energy Homeostasis. Endocr. Rev. 2019, 40, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Ghaleb, A.M.; McConnell, B.B.; Kaestner, K.H.; Yang, V.W. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Kruppel-like factor 4 gene. Dev. Biol. 2011, 349, 310–320. [Google Scholar] [CrossRef]
- Yu, T.; Chen, X.; Zhang, W.; Li, J.; Xu, R.; Wang, T.C.; Ai, W.; Liu, C. Kruppel-like factor 4 regulates intestinal epithelial cell morphology and polarity. PLoS ONE 2012, 7, e32492. [Google Scholar] [CrossRef]
- Reidling, J.C.; Said, H.M. Regulation of the human biotin transporter hSMVT promoter by KLF-4 and AP-2: Confirmation of promoter activity in vivo. Am. J. Physiol. Cell Physiol. 2007, 292, C1305–C1312. [Google Scholar] [CrossRef]
- Liuzzi, J.P.; Guo, L.; Chang, S.M.; Cousins, R.J. Kruppel-like factor 4 regulates adaptive expression of the zinc transporter Zip4 in mouse small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G517–G523. [Google Scholar] [CrossRef] [PubMed]
- Furumiya, M.; Inoue, K.; Ohta, K.; Hayashi, Y.; Yuasa, H. Transcriptional regulation of PCFT by KLF4, HNF4alpha, CDX2 and C/EBPalpha: Implication in its site-specific expression in the small intestine. Biochem. Biophys. Res. Commun. 2013, 431, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Grund, T.N.; Welsch, S.; Mills, D.J.; Michel, M.; Safarian, S.; Michel, H. Structural basis for amino acid exchange by a human heteromeric amino acid transporter. Proc. Natl. Acad. Sci. USA 2020, 117, 21281–21287. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N.; Christian, W.V.; Lee, J.Y.; Dawson, P.A.; Soroka, C.J.; Boyer, J.L.; Madejczyk, M.S.; Li, N. OSTalpha-OSTbeta: A major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology 2005, 42, 1270–1279. [Google Scholar] [CrossRef]
- Gill, R.K.; Pant, N.; Saksena, S.; Singla, A.; Nazir, T.M.; Vohwinkel, L.; Turner, J.R.; Goldstein, J.; Alrefai, W.A.; Dudeja, P.K. Function, expression, and characterization of the serotonin transporter in the native human intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G254–G262. [Google Scholar] [CrossRef]
- Yang, H.; Feng, Y.; Sun, X.; Teitelbaum, D.H. Enteral versus parenteral nutrition: Effect on intestinal barrier function. Ann. N. Y. Acad. Sci. 2009, 1165, 338–346. [Google Scholar] [CrossRef]
- Neelis, E.G.; Olieman, J.F.; Hulst, J.M.; de Koning, B.A.; Wijnen, R.M.; Rings, E.H. Promoting intestinal adaptation by nutrition and medication. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 249–261. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Mortensen, P.B. Colonic digestion and absorption of energy from carbohydrates and medium-chain fat in small bowel failure. J. Parenter. Enteral. Nutr. 1999, 23, S101–S105. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Mortensen, P.B. The influence of a preserved colon on the absorption of medium chain fat in patients with small bowel resection. Gut 1998, 43, 478–483. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, J.; Ma, X. Sodium Decanoate Improves Intestinal Epithelial Barrier and Antioxidation via Activating G Protein-Coupled Receptor-43. Nutrients 2021, 13, 2756. [Google Scholar] [CrossRef]
- Chusilp, S.; Li, B.; Lee, D.; Lee, C.; Vejchapipat, P.; Pierro, A. Intestinal organoids in infants and children. Pediatr. Surg. Int. 2020, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meran, L.; Massie, I.; Campinoti, S.; Weston, A.E.; Gaifulina, R.; Tullie, L.; Faull, P.; Orford, M.; Kucharska, A.; Baulies, A.; et al. Engineering transplantable jejunal mucosal grafts using patient-derived organoids from children with intestinal failure. Nat. Med. 2020, 26, 1593–1601. [Google Scholar] [CrossRef] [PubMed]


| Group | Control | Control | Control | IF | IF | IF |
|---|---|---|---|---|---|---|
| ID | Patient #1 | Patient #2 | Patient #3 | Patient #4 | Patient #5 | Patient #6 |
| Gender | Female | Female | Female | Male | Male | Female |
| Age (years) | 3 | 1 | 5 | 2 | 1 | 3 |
| Weight (kg) | 14 | 9.6 | 21 | 7.2 | 5.3 | 9.2 |
| Gestational age (weeks) | 38 | 37 | 37 | 41 | 29 | 38 |
| Clinical diagnosis | Ileal duplication | Abdominal cyst | Ileal duplication | Short bowel syndrome | Short bowel syndrome | Short bowel syndrome |
| Residual small bowel length (cm) | / | / | / | 149 | 108 | 117 |
| “Normal” small bowel length values (cm) * | / | / | / | 345.5 | 283.9 | 366.7 |
| Remaining bowel anatomy | / | / | / | Partial colectomy, ileocecal valve preserved | Partial colecto-my, ileocecal valve preserved | Partial colecto-my, ileocecal valve not preserved |
| Months from the 1st resection | / | / | / | 9 | 8 | 20 |
| Weight for age | <M + 1 SD | <M + 1 SD | <M + 2 SD | <M – 3 SD | <M – 3 SD | <M – 3 SD |
| Nutritional diagnosis | Normal | Normal | Normal | Severe malnutrition | Severe malnutrition | Severe malnutrition |
| On/Off PN | / | / | / | On | On | On |
| Calories provided by PN (kCal/day) | / | / | / | 492 | 470 | 639 |
| Calories provided by EN (kCal/day) | / | / | / | 241 | 160 | 200 |
| Energy requirements (kcal/day) for parenteral nutrition ** | / | / | / | 468–540 | 397.5–450.5 | 598–690 |
| Duration of PN (days) | / | / | / | 166 | 123 | 230 |
| Plasma ALT (U/L) | 11 | 10 | 36 | 85.7 | 14.8 | 45 |
| Plasma AST (U/L) | 32 | 33.4 | 29 | 102 | 36.1 | 110.4 |
| Plasma total bilirubin (μmol/L) | 5.5 | 4 | 3.6 | 25.5 | 15.3 | 7.2 |
| Plasma direct bilirubin (μmol/L) | 1.5 | 0 | <1 | 0 | 0 | 0 |
| Albumin (g/L) | 39.8 | 20.7 | 51.3 | 34 | 29.3 | 40.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, J.; Zhao, Y.; Jiang, L.; Wang, Y.; Cai, W. Decreased Expression of KLF4 Leading to Functional Deficit in Pediatric Patients with Intestinal Failure and Potential Therapeutic Strategy Using Decanoic Acid. Nutrients 2023, 15, 2660. https://doi.org/10.3390/nu15122660
Yan J, Zhao Y, Jiang L, Wang Y, Cai W. Decreased Expression of KLF4 Leading to Functional Deficit in Pediatric Patients with Intestinal Failure and Potential Therapeutic Strategy Using Decanoic Acid. Nutrients. 2023; 15(12):2660. https://doi.org/10.3390/nu15122660
Chicago/Turabian StyleYan, Junkai, Yuling Zhao, Lu Jiang, Ying Wang, and Wei Cai. 2023. "Decreased Expression of KLF4 Leading to Functional Deficit in Pediatric Patients with Intestinal Failure and Potential Therapeutic Strategy Using Decanoic Acid" Nutrients 15, no. 12: 2660. https://doi.org/10.3390/nu15122660
APA StyleYan, J., Zhao, Y., Jiang, L., Wang, Y., & Cai, W. (2023). Decreased Expression of KLF4 Leading to Functional Deficit in Pediatric Patients with Intestinal Failure and Potential Therapeutic Strategy Using Decanoic Acid. Nutrients, 15(12), 2660. https://doi.org/10.3390/nu15122660





