Gut Microbiota Perturbation in Early Life Could Influence Pediatric Blood Pressure Regulation in a Sex-Dependent Manner in Juvenile Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Breeding
2.2. Tail-Cuff Pressure Measurement
2.3. Feces Collection, DNA Extraction, and Analysis of Microbiota Composition
2.4. Euthanasia of Rats and Organ Collection
2.5. Total Tissue RNA Extraction, Reverse Transcription, and Real-Time Quantitative PCR
2.6. Detection of Cytokines in the Colon and Prefrontal Cortex
2.7. Fecal SCFA Analysis
2.8. Analysis of Serum TMAO and ITS precursors
2.9. Statistical Analysis
3. Results
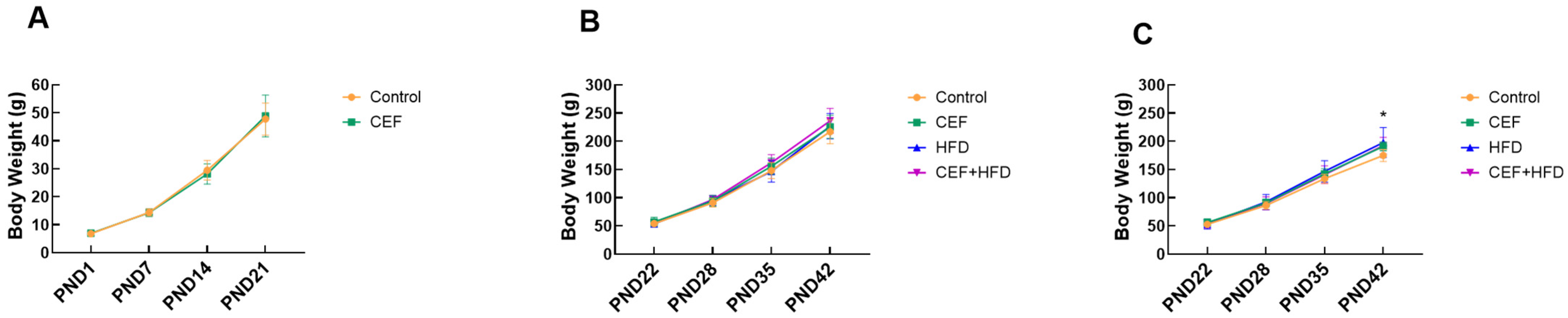
3.1. Effects of Early Life CEF and HFD Treatment on BW Increment
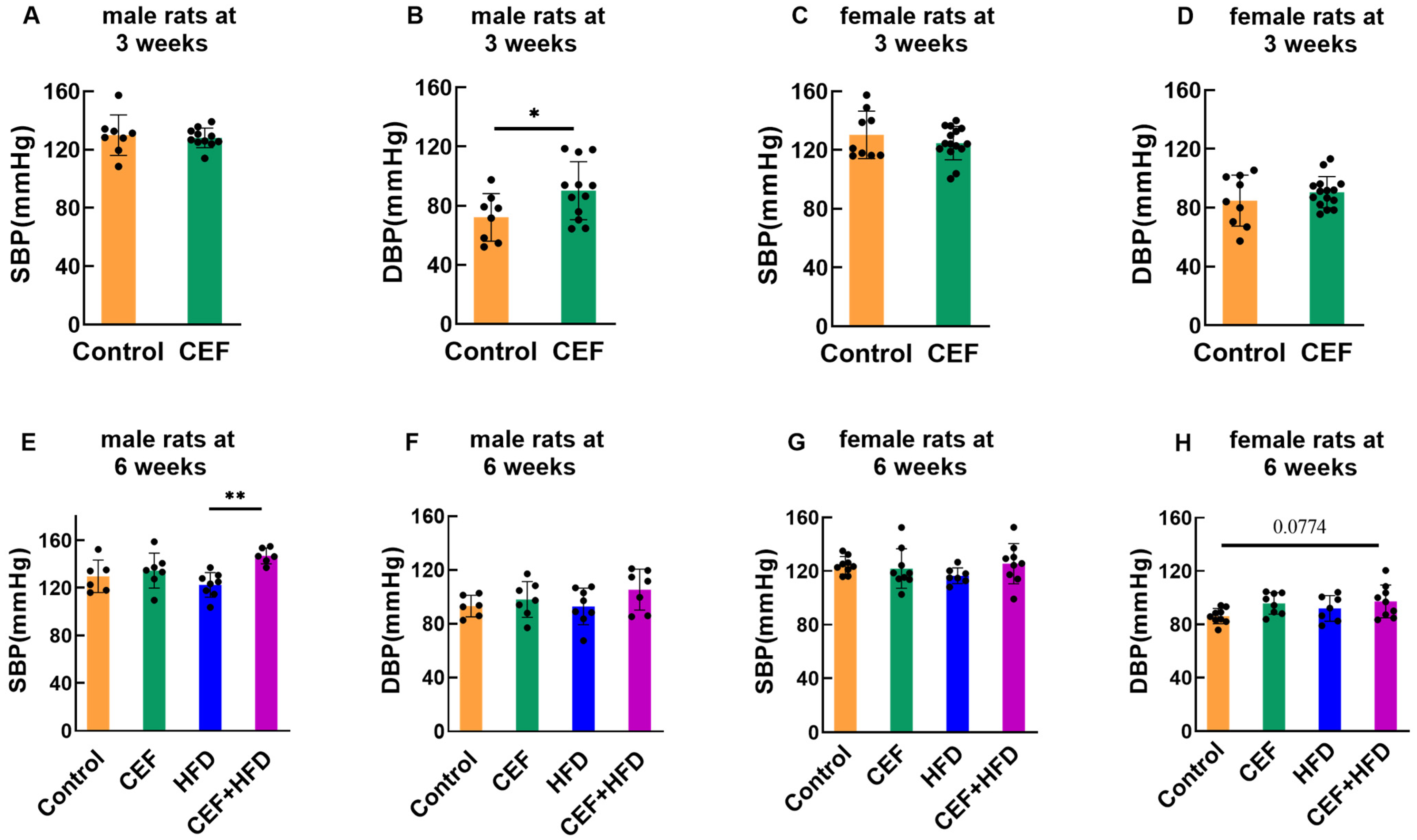
3.2. Effects of Early-Life CEF and HFD Treatment on SBP and DBP
3.3. Modulation of the RAS in Different Tissues after Eraly Life CEF and HFD Treatment
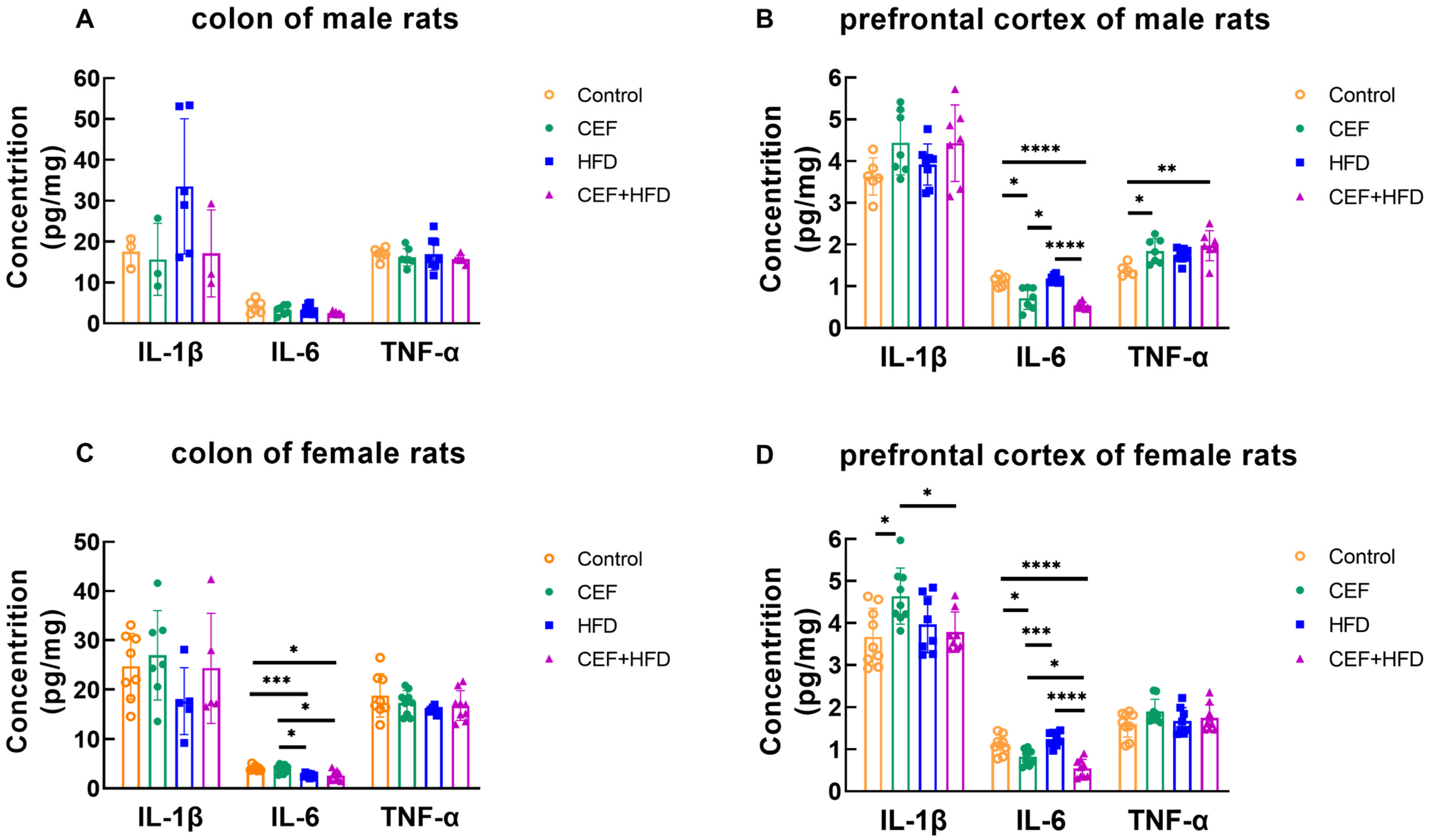
3.4. Changes in the Concentrations of Cytokines in the Colon and Prefrontal Cortex
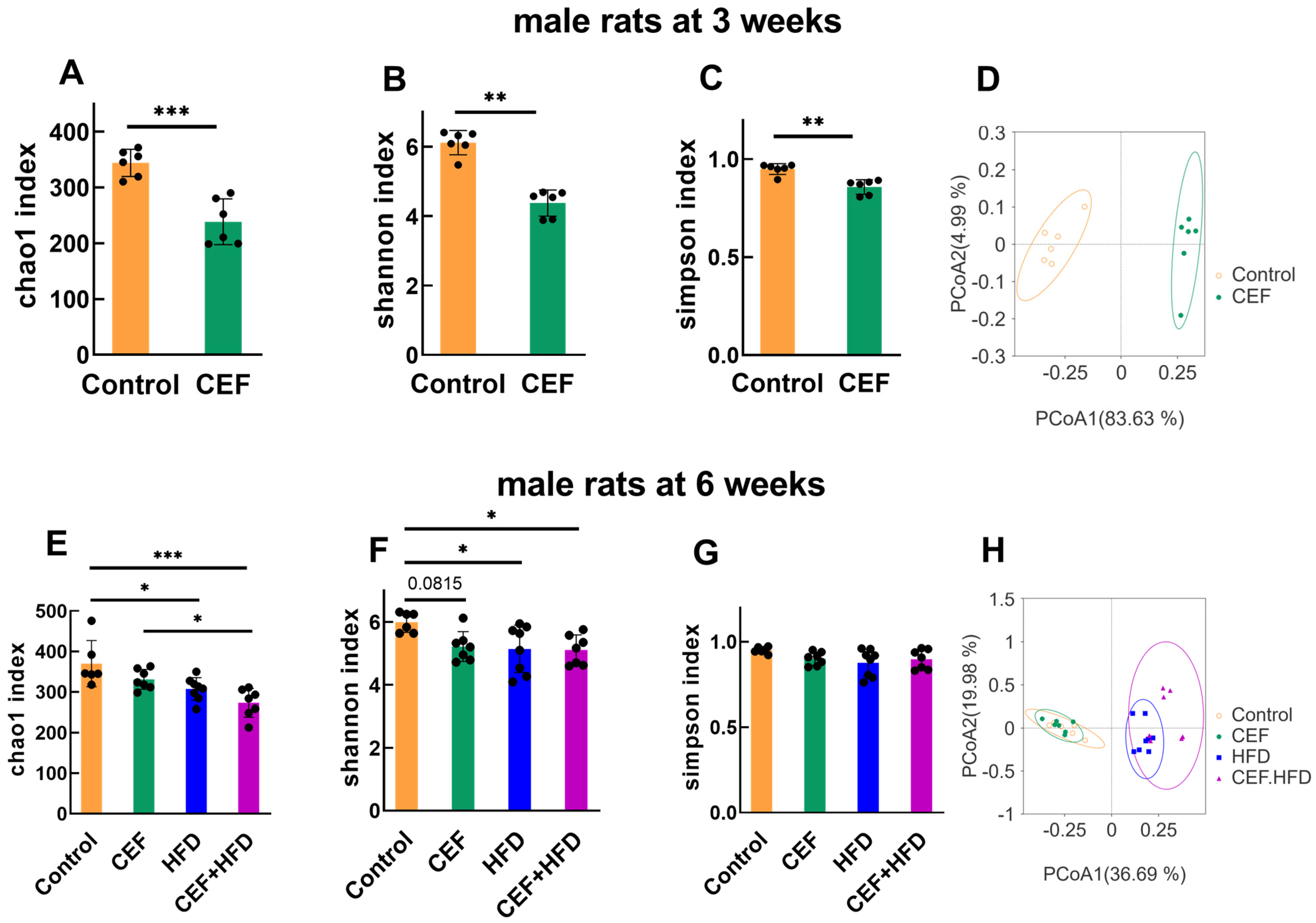
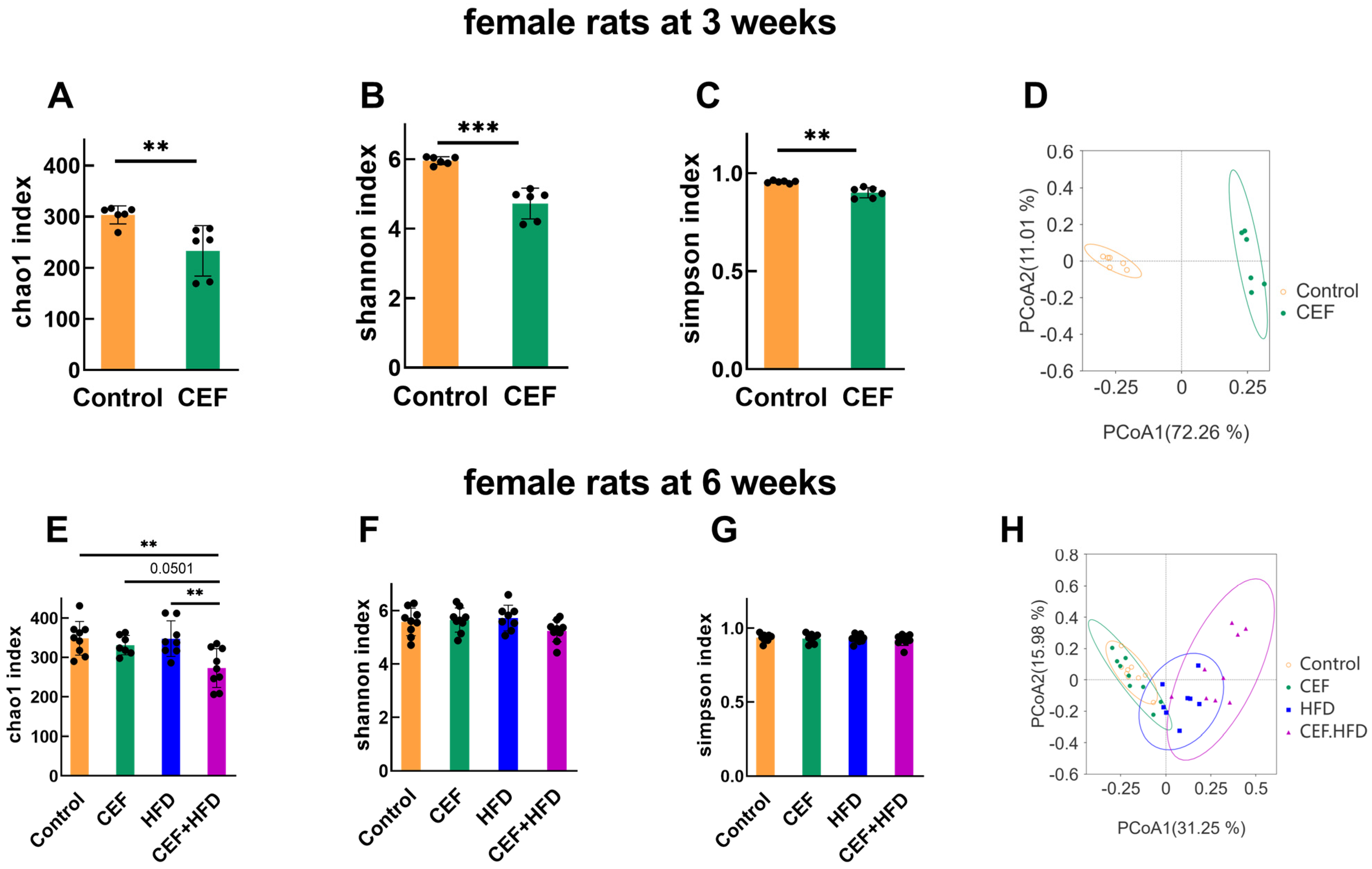
3.5. Alterations in Gut Microbiota Diversity after Early-Life CEF and HFD Treatment
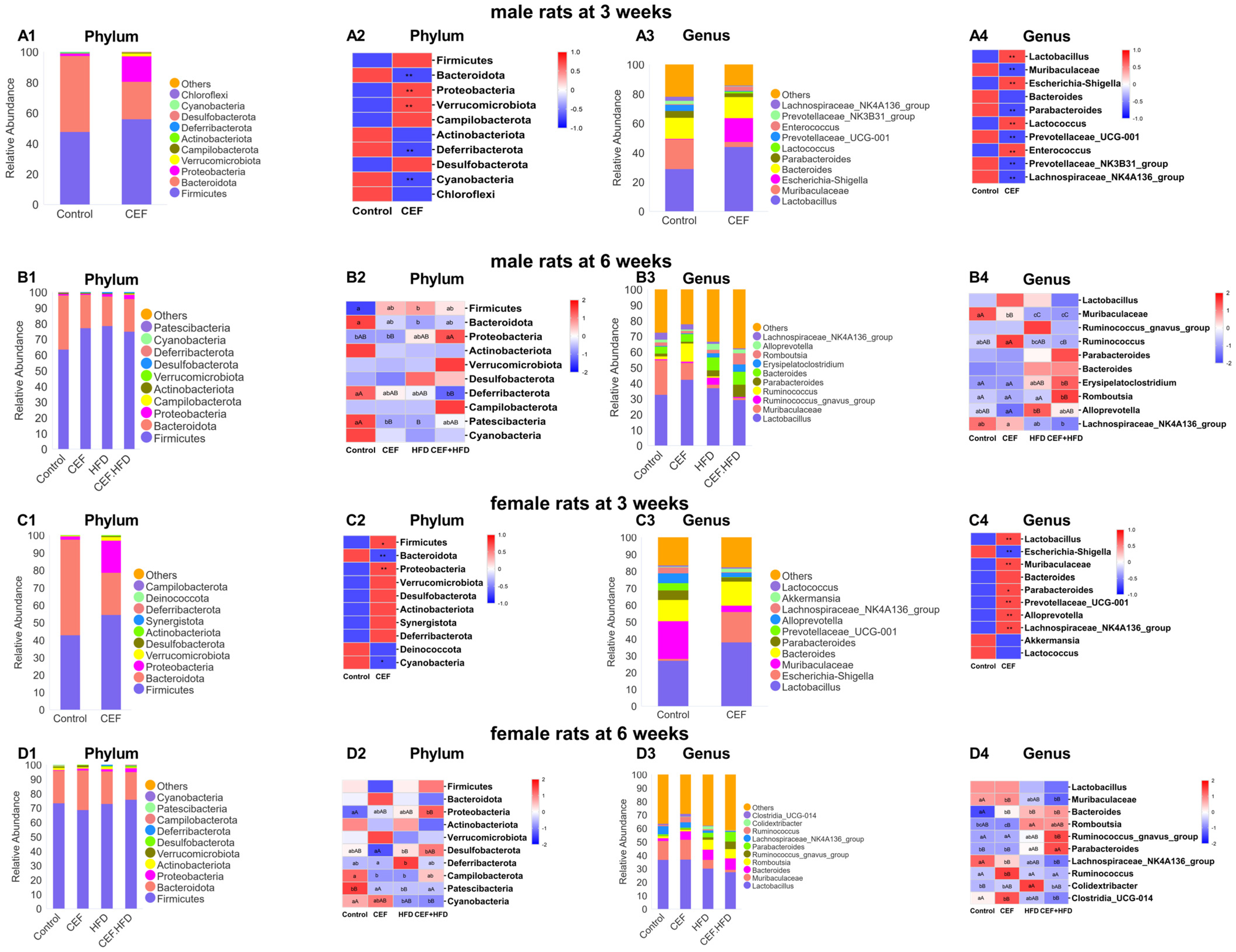
3.6. Alterations in the Relative Abundance of GUT Microbiota after Early-Life CEF and HFD Treatment
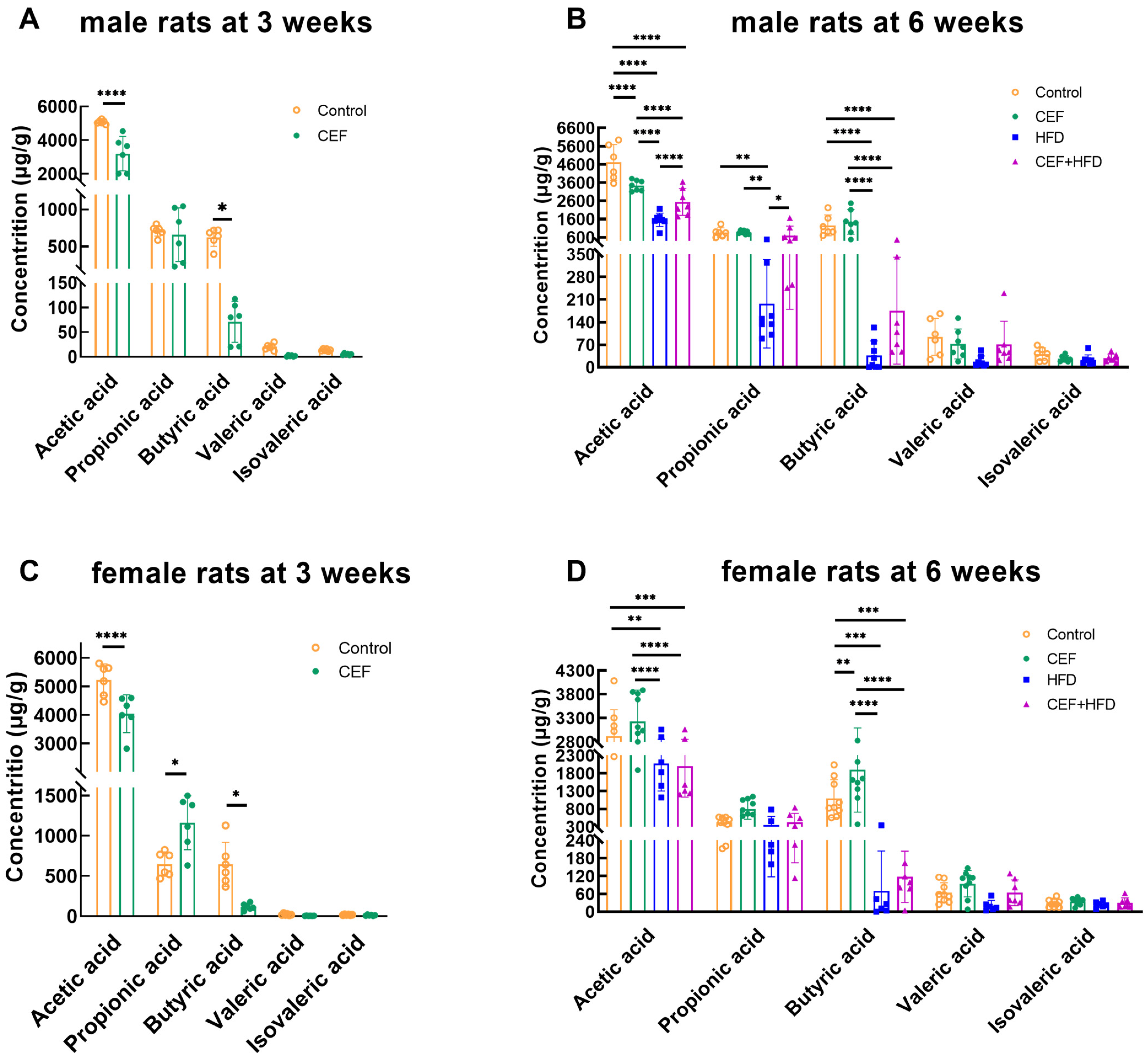
3.7. Changes in the Level of Fecal SCFAs after Early-Life CEF and HFD Treatment
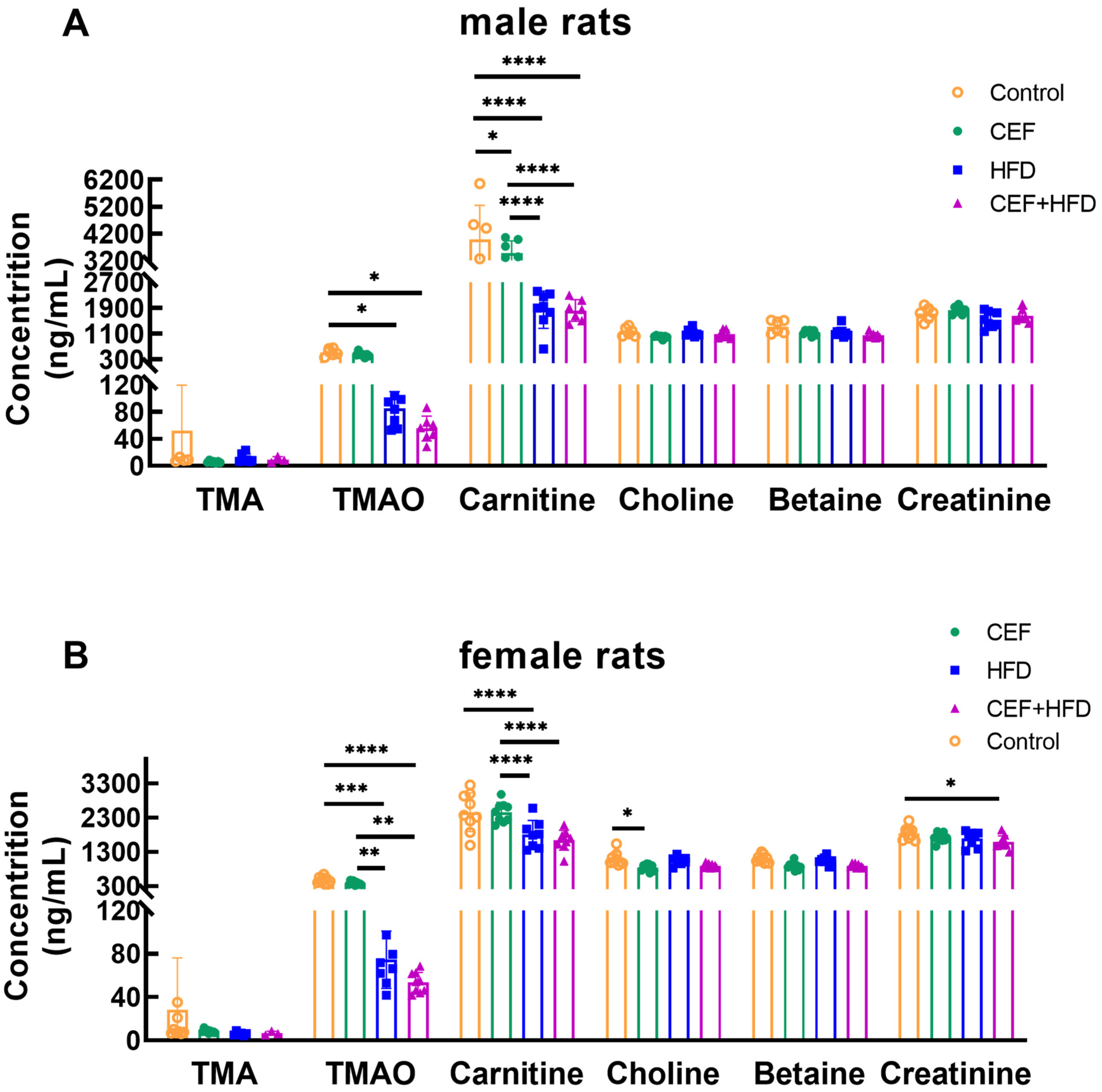
3.8. Changes in the Levels of Serum TMAO and Its Precursors after Early-Life CEF and HFD Treatment
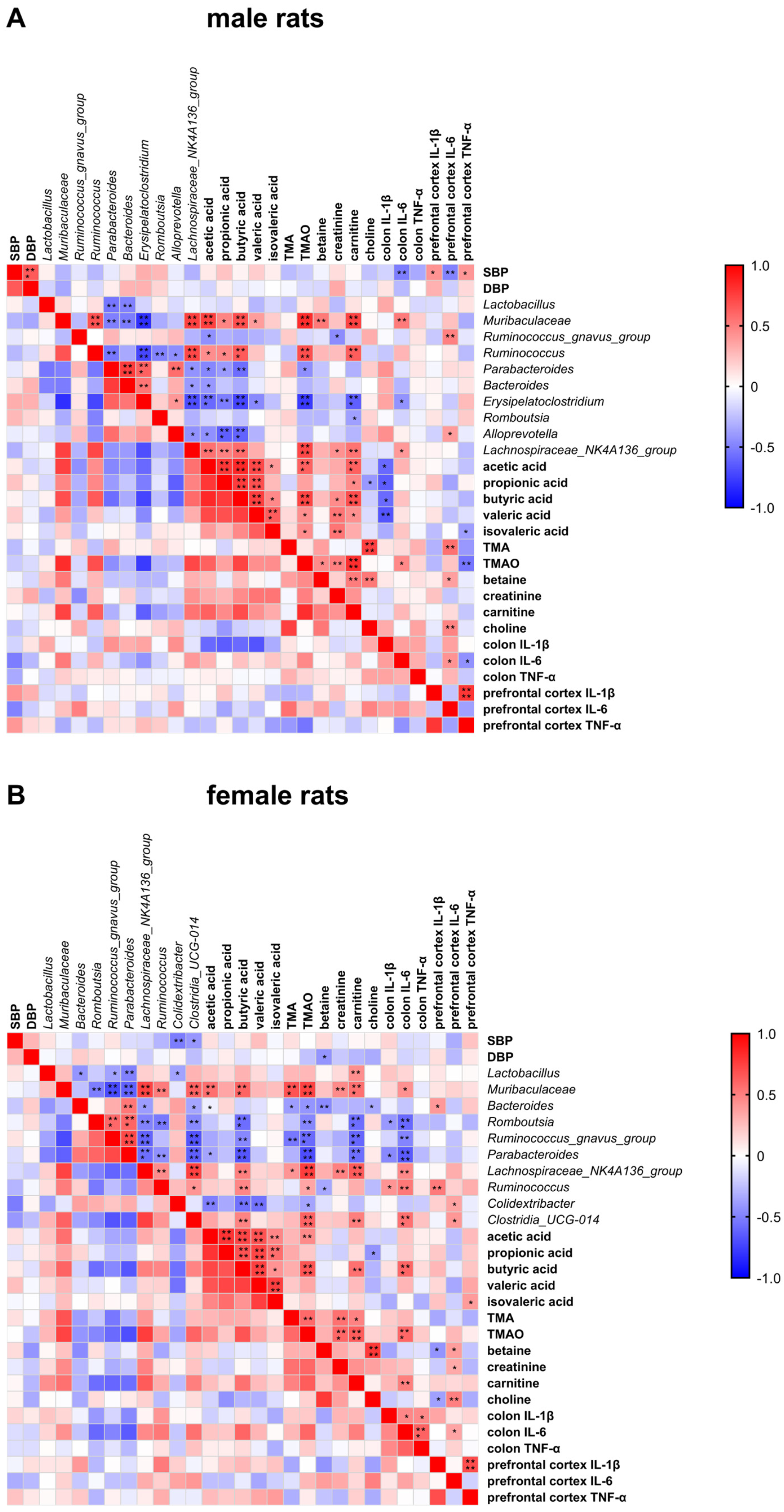
3.9. Spearman’s Rank Correlation Analysis between SBP/DBP and Biochemical Indicators
4. Discussion
4.1. Sexual Differences in the Changes in Pediatric Blood Pressure
4.2. RAS Shows More Activation in Males than in Females during Pediatric Blood Pressure Regulation
4.3. Sex-Specific Changes in Proinflammatory Cytokine Levels Are Related to Pediatric Blood Pressure Regulation
4.4. Early-Life Gut Microbiota of Males Is More Susceptible than That of Females
4.5. Gut Microbiota Related to Pediatric Blood Pressure Regulation Were Found Only in Female Rats
4.6. Sex-Specific Differences Are Reflected in the Levels of SCFAs Rather than in TMAO in Pediatric Blood Pressure Regulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzman-Limon, M.; Samuels, J. Pediatric Hypertension: Diagnosis, Evaluation, and Treatment. Pediatr. Clin. N. Am. 2019, 66, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Niiranen, T.J. Gut Microbiome over a Lifetime and the Association with Hypertension. Curr. Hypertens. Rep. 2021, 23, 15. [Google Scholar] [CrossRef]
- Kelly, R.K.; Thomson, R.; Smith, K.J.; Dwyer, T.; Venn, A.; Magnussen, C.G. Factors Affecting Tracking of Blood Pressure from Childhood to Adulthood: The Childhood Determinants of Adult Health Study. J. Pediatr. 2015, 167, 1422–1428. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Li, J.; Miao, Z.; Wang, Y.; Wu, S.; Wang, Y.; Wang, S.; Cheng, R.; He, F.; Shen, X. Early life administration of Bifidobacterium bifidum BD-1 alleviates long-term colitis by remodeling the gut microbiota and promoting intestinal barrier development. Front. Microbiol. 2022, 13, 916824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, H.; Wang, Y.; Cheng, R.; Pu, F.; Yang, Y.; Li, J.; Wu, S.; Shen, X.; He, F. Heat-inactivated Lacticaseibacillus paracasei N1115 alleviates the damage due to brain function caused by long-term antibiotic cocktail exposure in mice. BMC Neurosci. 2022, 23, 38. [Google Scholar] [CrossRef]
- Cheng, R.; Guo, J.; Pu, F.; Wan, C.; Shi, L.; Li, H.; Yang, Y.; Huang, C.; Li, M.; He, F. Loading ceftriaxone, vancomycin, and Bifidobacteria bifidum TMC3115 to neonatal mice could differently and consequently affect intestinal microbiota and immunity in adulthood. Sci. Rep. 2019, 9, 3254. [Google Scholar] [CrossRef]
- Hsu, C.N.; Chan, J.; Wu, K.; Yu, H.R.; Lee, W.C.; Hou, C.Y.; Tain, Y.L. Altered Gut Microbiota and Its Metabolites in Hypertension of Developmental Origins: Exploring Differences between Fructose and Antibiotics Exposure. Int. J. Mol. Sci. 2021, 22, 2674. [Google Scholar] [CrossRef]
- Hsu, C.; Hou, C.; Chang-Chien, G.; Lin, S.; Chan, J.Y.H.; Lee, C.; Tain, Y. Maternal resveratrol therapy protected adult rat offspring against hypertension programmed by combined exposures to asymmetric dimethylarginine and trimethylamine-N-oxide. J. Nutr. Biochem. 2021, 93, 108630. [Google Scholar] [CrossRef] [PubMed]
- Canale, M.P.; Noce, A.; Di Lauro, M.; Marrone, G.; Cantelmo, M.; Cardillo, C.; Federici, M.; Di Daniele, N.; Tesauro, M. Gut Dysbiosis and Western Diet in the Pathogenesis of Essential Arterial Hypertension: A Narrative Review. Nutrients 2021, 13, 1162. [Google Scholar] [CrossRef]
- Naqvi, S.; Asar, T.O.; Kumar, V.; Al-Abbasi, F.A.; Alhayyani, S.; Kamal, M.A.; Anwar, F. A cross-talk between gut microbiome, salt and hypertension. Biomed. Pharmacother. 2021, 134, 111156. [Google Scholar] [CrossRef]
- Richards, E.M.; Pepine, C.J.; Raizada, M.K.; Kim, S. The Gut, Its Microbiome, and Hypertension. Curr. Hypertens. Rep. 2017, 19, 36. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The Good, the Bad and the Unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Leustean, A.M.; Ciocoiu, M.; Sava, A.; Costea, C.F.; Floria, M.; Tarniceriu, C.C.; Tanase, D.M. Implications of the Intestinal Microbiota in Diagnosing the Progression of Diabetes and the Presence of Cardiovascular Complications. J. Diabetes Res. 2018, 2018, 5205126. [Google Scholar] [CrossRef] [PubMed]
- Warmbrunn, M.V.; Herrema, H.; Aron-Wisnewsky, J.; Soeters, M.R.; Van Raalte, D.H.; Nieuwdorp, M. Gut microbiota: A promising target against cardiometabolic diseases. Expert Rev. Endocrinol. Metab. 2020, 15, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, A.; Schiffrin, E.L. Immune Mechanisms in Hypertension. Curr. Hypertens. Rep. 2011, 13, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Jousilahti, P.; Vartiainen, E.; Tuomilehto, J.; Puska, P. Sex, age, cardiovascular risk factors, and coronary heart disease: A prospective follow-up study of 14,786 middle-aged men and women in Finland. Circulation 1999, 99, 1165–1172. [Google Scholar] [CrossRef]
- Colafella, K.; Denton, K.M. Sex-specific differences in hypertension and associated cardiovascular disease. Nat. Rev. Nephrol. 2018, 14, 185–201. [Google Scholar] [CrossRef]
- Mushtaq, N.; Hussain, S.; Yuan, L.; Zhang, S.; Li, H.; Ullah, S.; Xu, J. Gender Based Compositional Fluctuations in Gut Microbiota in Hypertension. Cardiovasc. Pharmacol. Open Access 2018, 7, 248. [Google Scholar] [CrossRef]
- Mikolajczyk, T.P.; Guzik, T.J. Adaptive Immunity in Hypertension. Curr. Hypertens. Rep. 2019, 21, 68. [Google Scholar] [CrossRef]
- Nickenig, G.; Strehlow, K.; Wassmann, S.; Baumer, A.T.; Albory, K.; Sauer, H.; Bohm, M. Differential effects of estrogen and progesterone on AT(1) receptor gene expression in vascular smooth muscle cells. Circulation 2000, 102, 1828–1833. [Google Scholar] [CrossRef]
- Vaughan, G.M.; Becker, R.A.; Allen, J.P.; Vaughan, M.K. Elevated blood pressure after pinealectomy in the rat. J. Endocrinol. Investig. 1979, 2, 281–284. [Google Scholar] [CrossRef]
- Potje, S.R.; Munhoz, F.C.; Perassa, L.A.; Graton, M.E.; Pereira, A.A.; Nakamune, A.C.; Da, S.R.; Bendhack, L.M.; Sumida, D.H.; Antoniali, C. Mechanisms underlying the hypotensive and vasodilator effects of [Ru(terpy)(bdq)NO](3+), a nitric oxide donor, differ between normotensive and spontaneously hypertensive rats. Eur. J. Pharmacol. 2014, 741, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Lauder, L.; Mahfoud, F.; Azizi, M.; Bhatt, D.L.; Ewen, S.; Kario, K.; Parati, G.; Rossignol, P.; Schlaich, M.P.; Teo, K.K.; et al. Hypertension management in patients with cardiovascular comorbidities. Eur. Heart J. 2022; online ahead of print. [Google Scholar]
- Li, X.C.; Zhang, J.; Zhuo, J.L. The vasoprotective axes of the renin-angiotensin system: Physiological relevance and therapeutic implications in cardiovascular, hypertensive and kidney diseases. Pharmacol. Res. 2017, 125, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, S.; Kawai, T.; Scalia, R.; Rizzo, V. Understanding Angiotensin II Type 1 Receptor Signaling in Vascular Pathophysiology. Hypertension 2018, 71, 804–810. [Google Scholar] [CrossRef]
- Fraga-Silva, R.A.; Ferreira, A.J.; Dos, S.R. Opportunities for targeting the angiotensin-converting enzyme 2/angiotensin-(1-7)/mas receptor pathway in hypertension. Curr. Hypertens. Rep. 2013, 15, 31–38. [Google Scholar] [CrossRef]
- Kramer, K.M.; Plowey, E.D.; Beatty, J.A.; Little, H.R.; Waldrop, T.G. Hypothalamus, hypertension, and exercise. Brain Res. Bull. 2000, 53, 77–85. [Google Scholar] [CrossRef]
- Geraldes, V.; Laranjo, S.; Rocha, I. Hypothalamic Ion Channels in Hypertension. Curr. Hypertens. Rep. 2018, 20, 14. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Dubyak, G.R. Differing caspase-1 activation states in monocyte versus macrophage models of IL-1beta processing and release. J. Leukoc. Biol. 2004, 76, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Nemati, F.; Rahbar-Roshandel, N.; Hosseini, F.; Mahmoudian, M.; Shafiei, M. Anti-inflammatory effects of anti-hypertensive agents: Influence on interleukin-1β secretion by peripheral blood polymorphonuclear leukocytes from patients with essential hypertension. Clin. Exp. Hypertens. 2011, 33, 66–76. [Google Scholar] [CrossRef]
- White, L.R.; Juul, R.; Skaanes, K.O.; Aasly, J. Cytokine enhancement of endothelin ET(B) receptor-mediated contraction in human temporal artery. Eur. J. Pharmacol. 2000, 406, 117–122. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta BBA Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Chamarthi, B.; Williams, G.H.; Ricchiuti, V.; Srikumar, N.; Hopkins, P.N.; Luther, J.M.; Jeunemaitre, X.; Thomas, A. Inflammation and Hypertension: The Interplay of Interleukin-6, Dietary Sodium, and the Renin-Angiotensin System in Humans. Am. J. Hypertens. 2011, 24, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Jasiewicz, M.; Knapp, M.; Waszkiewicz, E.; Ptaszynska-Kopczynska, K.; Szpakowicz, A.; Sobkowicz, B.; Musial, W.J.; Kaminski, K.A. Enhanced IL-6 trans-signaling in pulmonary arterial hypertension and its potential role in disease-related systemic damage. Cytokine 2015, 76, 187–192. [Google Scholar] [CrossRef]
- Wilkins, A.T.; Reimer, R.A. Obesity, Early Life Gut Microbiota, and Antibiotics. Microorganisms 2021, 9, 413. [Google Scholar] [CrossRef] [PubMed]
- Foliaki, S.; Pearce, N.; Björkstén, B.; Mallol, J.; Montefort, S.; von Mutius, E. Antibiotic use in infancy and symptoms of asthma, rhinoconjunctivitis, and eczema in children 6 and 7 years old: International Study of Asthma and Allergies in Childhood Phase III. J. Allergy Clin. Immunol. 2009, 124, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef]
- Robles-Vera, I.; de la Visitación, N.; Toral, M.; Sánchez, M.; Romero, M.; Gómez-Guzmán, M.; Vargas, F.; Duarte, J.; Jiménez, R. Changes in Gut Microbiota Induced by Doxycycline Influence in Vascular Function and Development of Hypertension in DOCA-Salt Rats. Nutrients 2021, 13, 2971. [Google Scholar] [CrossRef]
- Tsioufis, C.; Schmieder, R.E.; Mancia, G. Interventional Therapies for Secondary and Essential Hypertension; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Sun, S.; Lulla, A.; Sioda, M.; Winglee, K.; Wu, M.C.; Jacobs, D.R.; Shikany, J.M.; Lloyd-Jones, D.M.; Launer, L.J.; Fodor, A.A.; et al. Gut Microbiota Composition and Blood Pressure. Hypertension 2019, 73, 998–1006. [Google Scholar] [CrossRef]
- Chung, Y.W.; Gwak, H.; Moon, S.; Rho, M.; Ryu, J. Functional dynamics of bacterial species in the mouse gut microbiome revealed by metagenomic and metatranscriptomic analyses. PLoS ONE 2020, 15, e227886. [Google Scholar] [CrossRef]
- Koontanatechanon, A.; Wongphatcharachai, M.; Nonthabenjawan, N.; Jariyahatthakij, P.; Leksrisompong, P.; Srichana, P.; Prasopdee, S.; Roytrakul, S.; Sriyakul, K.; Thitapakorn, V.; et al. The Effects of Increasing Dietary Fat on Serum Lipid Profile and Modification of Gut Microbiome in C57BL/6N Mice. J. Oleo Sci. 2022, 71, 1039–1049. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Z.; Zhu, Y.; Wang, H.; Dai, Z.; Yang, X.; Ren, X.; Xue, Y.; Shen, Q. Cooked Adzuki Bean Reduces High-Fat Diet-Induced Body Weight Gain, Ameliorates Inflammation, and Modulates Intestinal Homeostasis in Mice. Front. Nutr. 2022, 9, 918696. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Le, Q.; Wei, Y.; Yang, L.; Cai, B.; Liu, Y.; Hong, B. Effect of piperine on the mitigation of obesity associated with gut microbiota alteration. Curr. Res. Food Sci. 2022, 5, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of intestinal Parabacteroides in human health and diseases. Fems Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Gu, Y.; Li, X.; Yang, W.; Jia, L.; Chen, C. Alterations of the Gut Microbiome in Hypertension. Front. Cell. Infect. Microbiol. 2017, 7, 381. [Google Scholar] [CrossRef]
- Yan, S.; Chen, J.; Zhu, L.; Guo, T.; Qin, D.; Hu, Z.; Han, S.; Wang, J.; Matias, F.B.; Wen, L.; et al. Oryzanol alleviates high fat and cholesterol diet-induced hypercholesterolemia associated with the modulation of the gut microbiota in hamsters. Food Funct. 2022, 13, 4486–4501. [Google Scholar] [CrossRef]
- Li, S.; Wang, M.; Li, C.; Meng, Q.; Meng, Y.; Ying, J.; Bai, S.; Shen, Q.; Xue, Y. Beneficial Effects of Partly Milled Highland Barley on the Prevention of High-Fat Diet-Induced Glycometabolic Disorder and the Modulation of Gut Microbiota in Mice. Nutrients 2022, 14, 762. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Tian, S.; Jiang, S.; Tang, Y.; Han, T. DHA-enriched phosphatidylcholine from Clupea harengus roes regulates the gut–liver axis to ameliorate high-fat diet-induced non-alcoholic fatty liver disease. Food Funct. 2022, 13, 11555–11567. [Google Scholar] [CrossRef]
- Mansuri, N.M.; Mann, N.K.; Rizwan, S.; Mohamed, A.E.; Elshafey, A.E.; Khadka, A.; Mosuka, E.M.; Thilakarathne, K.N.; Mohammed, L. Role of Gut Microbiome in Cardiovascular Events: A Systematic Review. Cureus J. Med. Sci. 2022, 14, e32465. [Google Scholar] [CrossRef]
- Poll, B.G.; Cheema, M.U.; Pluznick, J.L. Gut Microbial Metabolites and Blood Pressure Regulation: Focus on SCFAs and TMAO. Physiology 2020, 35, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Fletcher, C. Trimethylamine N-oxide: Breathe new life. Br. J. Pharmacol. 2018, 175, 1344–1353. [Google Scholar] [CrossRef]
- Kaysen, G.A.; Johansen, K.L.; Chertow, G.M.; Dalrymple, L.S.; Kornak, J.; Grimes, B.; Dwyer, T.; Chassy, A.W.; Fiehn, O. Associations of Trimethylamine N-Oxide with Nutritional and Inflammatory Biomarkers and Cardiovascular Outcomes in Patients New to Dialysis. J. Ren. Nutr. 2015, 25, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Schugar, R.C.; Shih, D.M.; Warrier, M.; Helsley, R.N.; Burrows, A.; Ferguson, D.; Brown, A.L.; Gromovsky, A.D.; Heine, M.; Chatterjee, A.; et al. The TMAO-Producing Enzyme Flavin-Containing Monooxygenase 3 Regulates Obesity and the Beiging of White Adipose Tissue. Cell Rep. 2017, 19, 2451–2461. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e4947. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Q.; Ding, X.; Chen, L.; Zhang, Z. Trimethylamine N-oxide and its precursors in relation to blood pressure: A mendelian randomization study. Front. Cardiovasc. Med. 2022, 9, 922441. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Zheng, L.; Zhuang, R.; Yu, P.; Xu, Z.; Liu, G.; Xi, X.; Zhou, X.; Fan, H. The Gut Microbial Metabolite Trimethylamine N-Oxide and Hypertension Risk: A Systematic Review and Dose-Response Meta-analysis. Adv. Nutr. 2020, 11, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; de Aguiar, V.T.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef]
| Group | Control | CEF | HFD | CEF + HFD | |
|---|---|---|---|---|---|
| liver | AGT | 1.77 ± 1.07a | 1.91 ± 0.97a | 1.13 ± 0.80a | 1.49 ± 0.92a |
| cortex of left kidney | Ren | 3.94 ± 2.43a | 0.63 ± 0.66b | 2.21 ± 1.32ab | 0.97 ± 1.10b |
| ACE | 1.87 ± 1.02a | 1.98 ± 1.71a | 1.53 ± 0.74a | 0.64 ± 0.62a | |
| ACE2 | 1.84 + 1.38a | 1.27 ± 0.53a | 1.91 ± 1.55a | 1.44 ± 0.92a | |
| AT1R | 1.21 ± 0.35a | 1.29 ± 0.24a | 0.87 ± 0.22a | 1.28 ± 0.40a | |
| Mas1 | 1.22 ± 0.38a | 1.14 ± 0.24a | 1.01 ± 0.26a | 1.30 ± 0.24a | |
| left ventricle | ACE | 2.41 ± 1.66ab | 1.44 ± 0.21a | 1.67 ± 0.93a | 0.10 ± 0.04b |
| ACE2 | 1.62 ± 1.18a | 0.79 ± 0.55a | 1.30 ± 1.22a | 1.03 ± 0.32a | |
| AT1R | 1.60 ± 0.64a | 1.92 ± 1.04a | 2.19 ± 0.77a | 1.89 ± 0.63a | |
| Mas1 | 1.23 ± 0.42ab | 1.84 ± 0.61a | 0.85 ± 0.25b | 1.59 ± 0.51a | |
| hypothalamus | ACE | 1.09 ± 0.24b | 1.91 ± 1.28ab | 1.30 ± 0.51ab | 2.58 ± 1.26a |
| ACE2 | 0.92 ± 0.53a | 1.31 ± 0.57a | 1.82 ± 1.39a | 1.77 ± 1.02a | |
| AT1R | 0.68 ± 0.32a | 1.96 ± 1.62ab | 1.90 ± 0.80b | 1.61 ± 0.47b | |
| Mas1 | 1.77 ± 1.19a | 1.45 ± 0.80a | 1.26 ± 0.61a | 2.50 ± 1.51a | |
| aortic arch | ACE | 3.63 ± 1.81a | 1.51 ± 1.16a | 4.46 ± 4.04a | 1.89 ± 2.13a |
| ACE2 | 0.87 ± 0.45a | 0.86 ± 0.49a | 1.48 ± 1.11a | 1.29 ± 0.79a | |
| AT1R | 0.83 ± 0.46a | 0.62 ± 0.19a | 1.30 ± 0.74a | 0.67 ± 0.34a | |
| Mas1 | 1.04 ± 0.22a | 0.76 ± 0.46a | 0.98 ± 0.35a | 1.09 ± 0.55a | |
| thoracic aorta | ACE | 0.42 ± 0.34a | ND | 1.55 ± 1.30a | 12.27 ± 12.25a |
| ACE2 | 0.63 ± 0.55a | 1.05 ± 0.76a | 0.32 ± 0.19a | 1.17 ± 0.66a | |
| AT1R | 0.92 ± 0.80a | 2.32 ± 2.03a | 0.81 ± 0.63a | 2.62 ± 2.25a | |
| Mas1 | 0.97 ± 0.80ab | 2.13 ± 1.08a | 0.57 ± 0.19b | 1.71 ± 0.87ab | |
| abdominal aorta | ACE | 3.25 ± 2.38a | 1.14 ± 1.35a | 1.42 ± 1.19a | 0.52 ± 0.25a |
| ACE2 | 0.30 ± 0.23a | ND | 0.07 ± 0.04a | 0.33 ± 0.17a | |
| AT1R | 1.10 ± 0.81ab | 1.69 ± 0.84a | 0.65 ± 0.35b | 1.32 ± 0.63ab | |
| Mas1 | 0.64 ± 0.31b | 0.62 ± 0.18b | 1.06 ± 0.43ab | 1.33 ± 0.39a | |
| Group | Control | CEF | HFD | CEF + HFD | |
|---|---|---|---|---|---|
| liver | AGT | 1.02 ± 0.61a | 0.85 ± 0.33a | 1.03 ± 0.44a | 2.62 ± 1.67a |
| cortex of left kidney | Ren | 1.70 ± 1.48a | 1.58 ± 1.72a | 10.86 ± 11.05b | 0.44 ± 0.52a |
| ACE | 1.27 ± 0.96b | 0.86 ± 0.54bc | 4.10 ± 2.93a | 0.41 ± 0.39c | |
| ACE2 | 1.11 ± 0.48a | 0.96 ± 0.49a | 1.83 ± 1.3a | 1.14 ± 0.79a | |
| AT1R | 1.13 ± 0.50a | 1.02 ± 0.20a | 1.08 ± 0.33a | 0.89 ± 0.24a | |
| Mas1 | 1.08 ± 0.42a | 1.10 ± 0.43a | 1.13 ± 0.37a | 1.17 ± 0.27a | |
| left ventricle | ACE | 1.23 ± 0.75a | 1.66 ± 1.62a | 2.03 ± 1.21a | 0.14 ± 0.11b |
| ACE2 | 0.80 ± 0.33a | 1.03 ± 0.67a | 1.14 ± 0.75a | 0.84 ± 0.22a | |
| AT1R | 1.47 ± 1.13a | 1.24 ± 0.78a | 1.89 ± 1.01a | 1.29 ± 0.54a | |
| Mas1 | 1.10 ± 0.47ab | 1.14 ± 0.57ab | 0.71 ± 0.20a | 1.35 ± 0.47b | |
| hypothalamus | ACE | 0.90 ± 0.25ab | 0.92 ± 0.50ab | 0.84 ± 0.20a | 1.36 ± 0.41b |
| ACE2 | 0.97 ± 0.50a | 1.42 ± 1.22a | 0.82 ± 0.55a | 1.10 ± 0.48a | |
| AT1R | 1.16 ± 0.64a | 1.21 ± 0.79a | 1.08 ± 0.42a | 0.74 ± 0.29a | |
| Mas1 | 1.28 ± 0.85a | 1.19 ± 0.70a | 2.62 ± 1.16a | 2.11 ± 1.49a | |
| aortic arch | ACE | 1.23 ± 0.91a | 0.90 ± 0.72a | 4.41 ± 4.61a | 2.18 ± 2.11a |
| ACE2 | 1.11 ± 0.54a | 0.91 ± 0.13a | 1.38 ± 0.98a | 1.77 ± 1.06a | |
| AT1R | 0.98 ± 0.53a | 0.79 ± 0.34a | 2.03 ± 3.08a | 1.23 ± 0.59a | |
| Mas1 | 0.98 ± 0.57a | 0.98 ± 0.64a | 1.51 ± 0.77a | 0.78 ± 0.22a | |
| thoracic aorta | ACE | 3.01 ± 3.46a | 1.23 ± 1.90a | 0.67 ± 0.38a | 5.04 ± 4.12a |
| ACE2 | 0.97 ± 0.58a | 0.91 ± 0.51a | 0.29 ± 0.08a | 0.75 ± 0.38a | |
| AT1R | 1.22 ± 0.78a | 0.83 ± 0.38a | 0.96 ± 0.47a | 0.95 ± 0.53a | |
| Mas1 | 1.24 ± 0.74a | 0.91 ± 0.44a | 0.78 ± 0.42a | 1.29 ± 0.83a | |
| abdominal aorta | ACE | 1.58 ± 1.44a | 1.08 ± 0.44a | 2.19 ± 1.56a | 1.12 ± 1.24a |
| ACE2 | 0.24 ± 0.19a | ND | 0.14 ± 0.11a | 0.43 ± 0.32a | |
| AT1R | 0.98 ± 0.32a | 1.60 ± 1.25a | 0.97 ± 0.64a | 1.27 ± 1.10a | |
| Mas1 | 1.17 ± 0.71a | 1.13 ± 0.58a | 0.90 ± 0.52a | 0.64 ± 0.22a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Li, J.; Zhou, Z.; Wu, S.; Zhao, J.; Jia, W.; Liu, M.; Shen, X.; He, F.; Cheng, R. Gut Microbiota Perturbation in Early Life Could Influence Pediatric Blood Pressure Regulation in a Sex-Dependent Manner in Juvenile Rats. Nutrients 2023, 15, 2661. https://doi.org/10.3390/nu15122661
Yang Y, Li J, Zhou Z, Wu S, Zhao J, Jia W, Liu M, Shen X, He F, Cheng R. Gut Microbiota Perturbation in Early Life Could Influence Pediatric Blood Pressure Regulation in a Sex-Dependent Manner in Juvenile Rats. Nutrients. 2023; 15(12):2661. https://doi.org/10.3390/nu15122661
Chicago/Turabian StyleYang, Yang, Jinxing Li, Zhimo Zhou, Simou Wu, Jincheng Zhao, Wen Jia, Meixun Liu, Xi Shen, Fang He, and Ruyue Cheng. 2023. "Gut Microbiota Perturbation in Early Life Could Influence Pediatric Blood Pressure Regulation in a Sex-Dependent Manner in Juvenile Rats" Nutrients 15, no. 12: 2661. https://doi.org/10.3390/nu15122661
APA StyleYang, Y., Li, J., Zhou, Z., Wu, S., Zhao, J., Jia, W., Liu, M., Shen, X., He, F., & Cheng, R. (2023). Gut Microbiota Perturbation in Early Life Could Influence Pediatric Blood Pressure Regulation in a Sex-Dependent Manner in Juvenile Rats. Nutrients, 15(12), 2661. https://doi.org/10.3390/nu15122661







