The Effect of Intermittent Fasting on Appetite: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
- Participants: Humans of any age and any BMI.
- Intervention: Intermittent fasting interventions of any type (e.g., alternate day fasting, time-restricted eating, 5:2 diet) and any duration.
- Control/comparator: Continuous energy restriction intervention.
- Outcomes: To be included in the review, the RCT must have measured the primary outcome of appetite, e.g., visual-analogue scales of hunger, fullness, desire to eat, and prospective food consumption (PFC). Where measured, secondary outcomes were also included in the review: body weight (kg), energy intake (kcal/day), eating behavior questionnaire scores (e.g., Three-Factor Eating Questionnaire), physical activity, adherence to interventions (%), and dropout.
3. Results
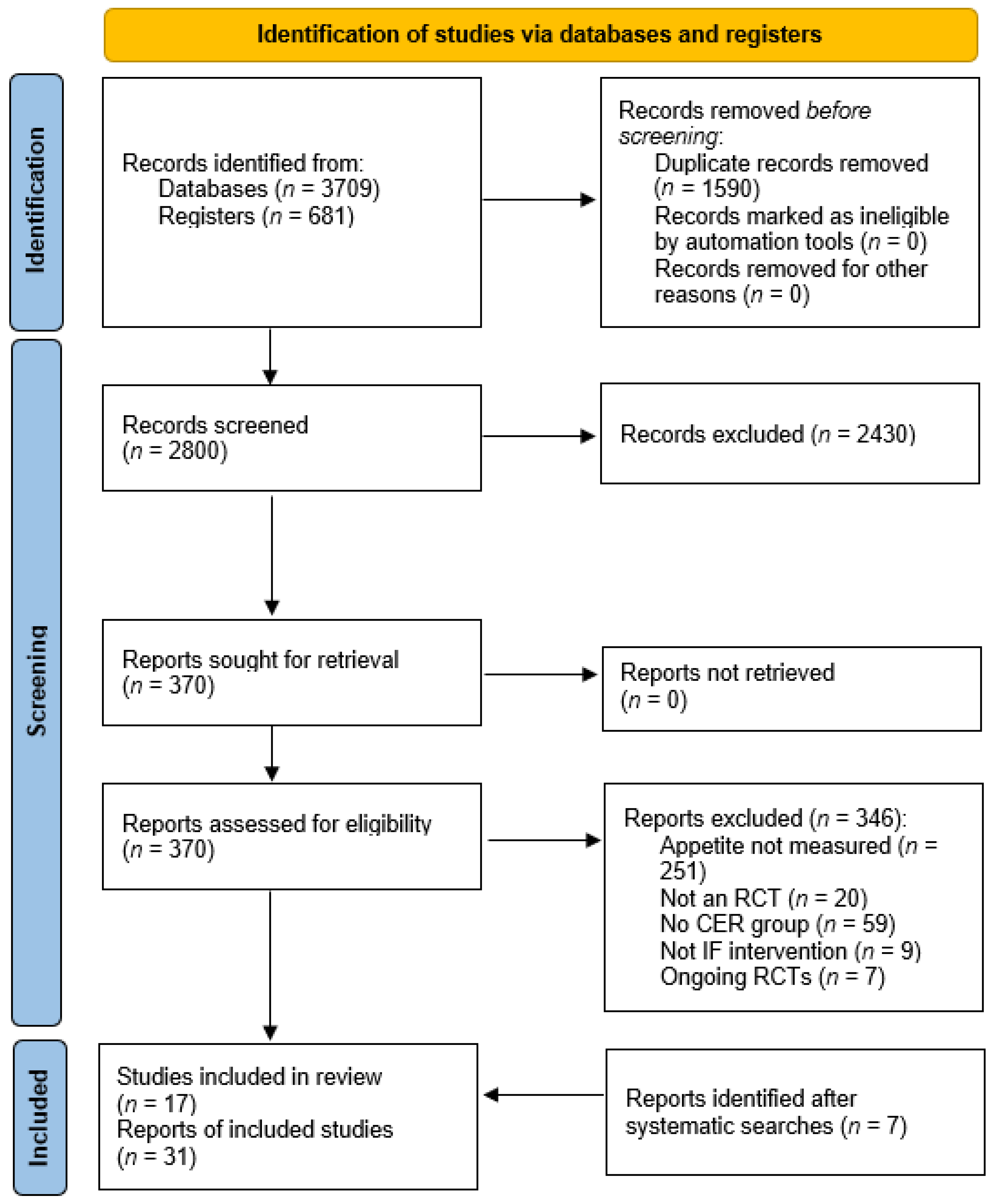
3.1. Study Selection
3.2. Study Characteristics
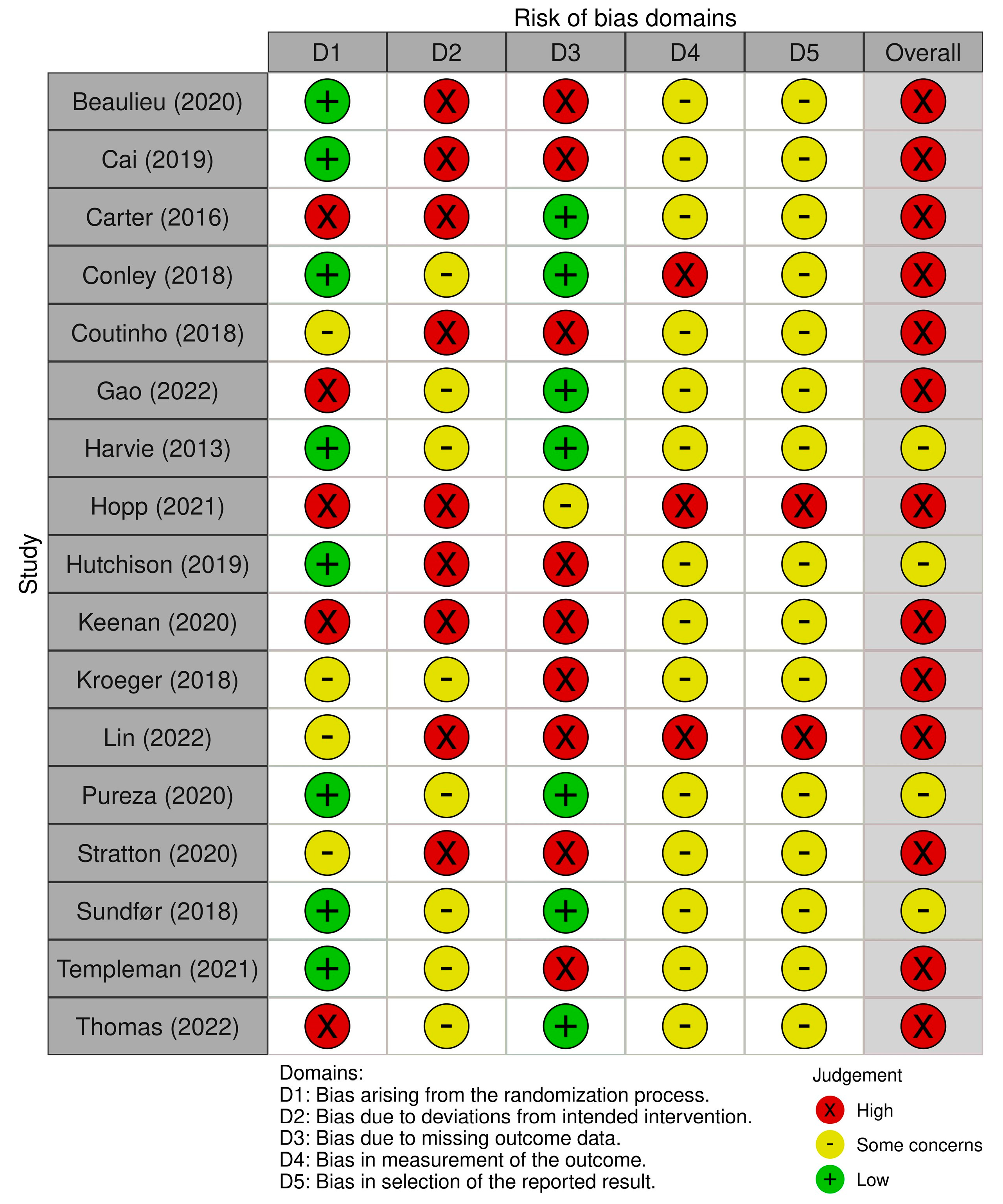
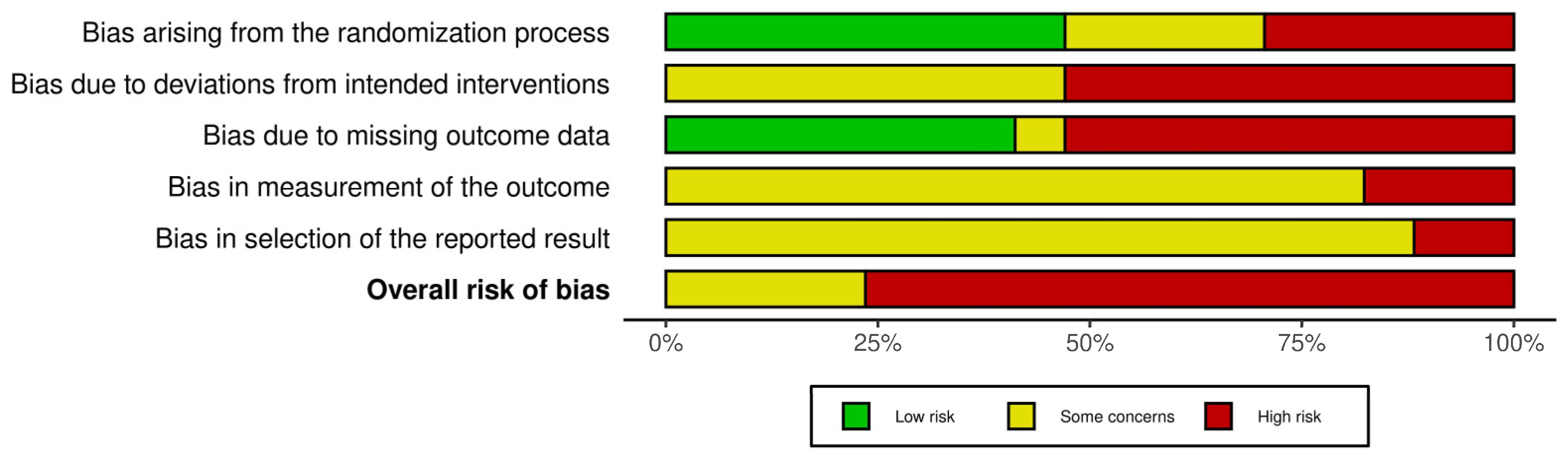
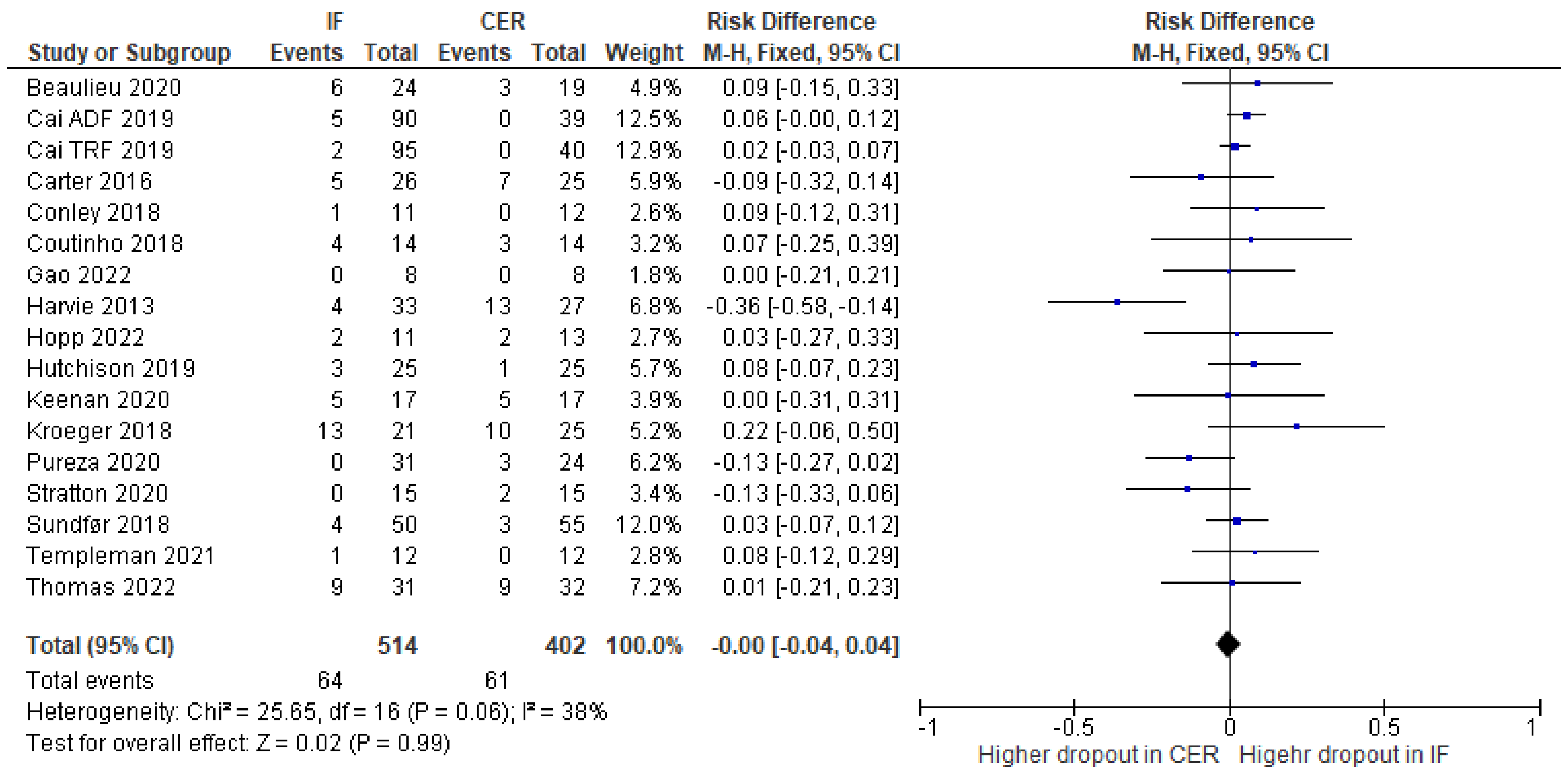
3.3. Risk of Bias
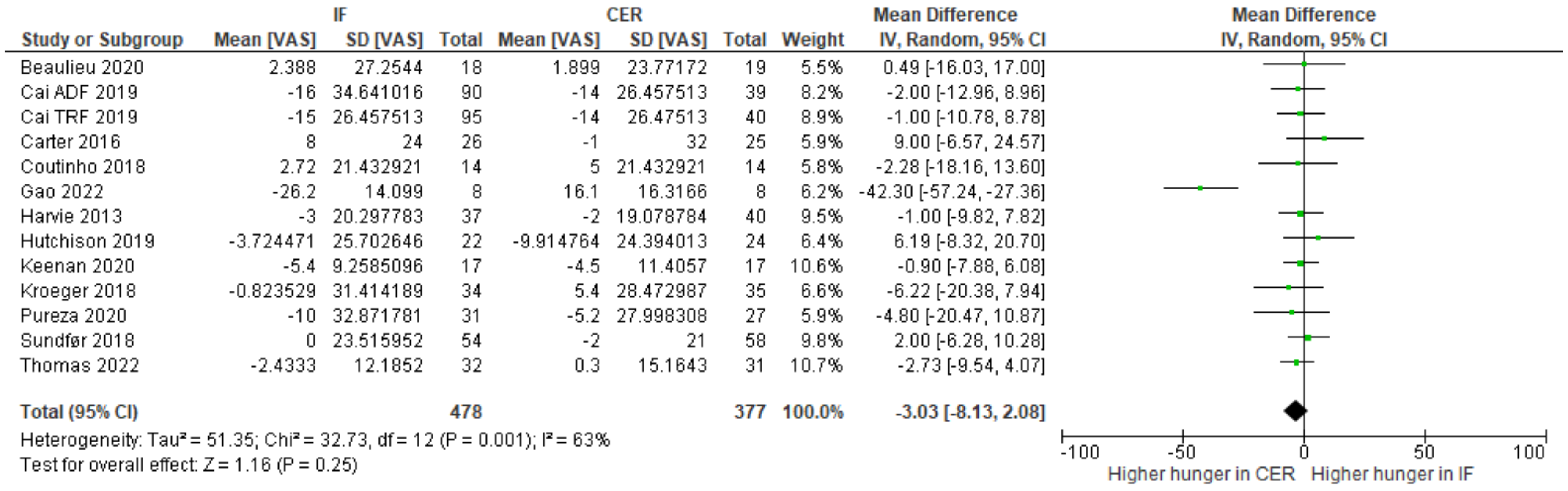
3.4. Primary Outcomes
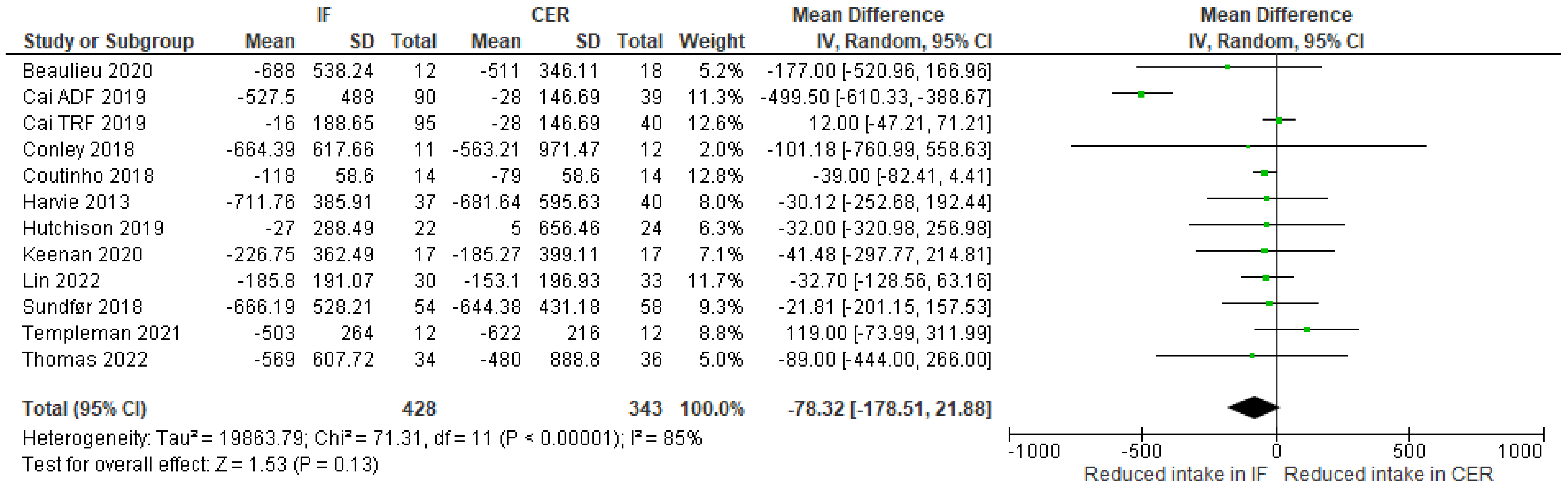
3.5. Secondary Outcomes
3.6. Certainty of the Evidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Topic | No. | Item | Location Where Item Is Reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | 1–3 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist | |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | 51–72 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | 71–78 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | 90–104 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | 105–117 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | 117–120, Appendix B |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | 121–127 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | 128–137 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | 98–104 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | 128–137 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | 138–143 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | 151–153 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item 5)). | 144–147 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | 159–166 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | 157–158 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | 147–158 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | 167–168 | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | 168–171 | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | 171–172 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | 140–143 |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | 174–186 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | 184 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | 187–221 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | 222–232 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | 233–341 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | 222–341 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | 233–341 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | 262–265 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | 338–342 | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | 319–320 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | 316–320 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | 321–330 |
| 23b | Discuss any limitations of the evidence included in the review. | 338–350 | |
| 23c | Discuss any limitations of the review processes used. | 350–354 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | 355–371 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | 80–81 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | 81–84 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | 84–86 | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | 386–391 |
| Competing interests | 26 | Declare any competing interests of review authors. | 397–399 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | Appendix C |
Appendix B. Search Strategy in MEDLINE (via OvidSP)
Appendix C
| Study details | Study number | |
| Title | ||
| Authors | ||
| Year | ||
| Form | ||
| Country | ||
| Status | ||
| Funding | ||
| Conflicts of interest | ||
| RCT design | ||
| Trial registration link | ||
| Sample | Total N allocated | |
| Analysis type | ||
| Total N analysed (intention to treat) | ||
| Total N analysed (completers analysis) | ||
| Total N analysed (per protocol analysis) | ||
| Per protocol or completers requirements | ||
| N allocated | IF | |
| CER | ||
| N analysed | IF | |
| CER | ||
| Age (mean, SD) | IF | |
| CER | ||
| Gender | IF | |
| CER | ||
| BMI | IF | |
| CER | ||
| Comorbidities | ||
| Other demographic information | ||
| Outcome measures | Test day details | |
| Hunger | Details | |
| Timepoint | ||
| Fullness | Details | |
| Timepoint | ||
| Desire to eat | Details | |
| Timepoint | ||
| Prospective food consumption | Details | |
| Timepoint | ||
| Body weight | Details | |
| Timepoint | ||
| Energy intake | Details | |
| Timepoint | ||
| Eating behaviour | Details | |
| Timepoint | ||
| Physical activity | Details | |
| Timepoint | ||
| Adherence | Details | |
| Timepoint | ||
| IF intervention | Protocol | |
| Duration | ||
| N analysed | ||
| Hunger (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| Fullness (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| Desire to eat (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| PFC (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| Body weight (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score (weight loss) | ||
| Text from paper | ||
| Energy intake (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| Eating behaviour (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| Physical activity (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| Withdrew/ lost to follow up (n) | ||
| Completed (n) | ||
| Attrition (%) | ||
| Adherence (%) | ||
| CER intervention | Protocol | |
| Duration | ||
| N analysed | ||
| Hunger (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| Fullness (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| Desire to eat (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| PFC (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| Body weight (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score (weight loss) | ||
| Text from paper | ||
| Energy intake (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| Eating behaviour (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| Physical activity (unadjusted mean, SD) | Pre | |
| Post | ||
| Change score | ||
| Text from paper | ||
| Withdrew/ lost to follow up (n) | ||
| Completed (n) | ||
| Attrition (%) | ||
| Adherence (%) | ||
| Training | ||
| Papers from references | ||
Appendix D. Ongoing RCTs
Appendix E
| First Author (Year) | Papers | Protocol Paper | Conference Abstract | Trial Register | Thesis |
|---|---|---|---|---|---|
| Beaulieu (2020) [35] | [35,57] | [58] | [59] | ||
| Cai (2019) [49] | [49] | ||||
| Carter (2016) [33] | [33] | ||||
| Conley (2018) [34] | [34] | ||||
| Coutinho (2018) [36] | [36] | [60] | |||
| Gao (2022) [44] | [44] | ||||
| Harvie (2013) [41] | [41] | ||||
| Hopp (2021) [40] | [40] | ||||
| Hutchison (2019) [37] | [37] | [61] | |||
| Keenan (2020) [42] | [45,62] * | [63] * | [64] | [65] * | |
| Kroeger (2018) [38] | [38] | [66] * | |||
| Lin (2022) [48] | [48] | ||||
| Pureza (2020) [45] | [45,67] | ||||
| Stratton (2020) [46] | [46] | ||||
| Sundfør (2018) [43] | [56,68] * | ||||
| Templeman (2021) [39] | [39] * | [69] | [70] * | ||
| Thomas (2022) [47] | [47] |
References
- Varady, K.A. Intermittent versus daily calorie restriction: Which diet regimen is more effective for weight loss?: Weight Loss by Calorie Restriction Diets. Obes. Rev. 2011, 12, e593–e601. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.S.; Clarke, R.E.; Coulter, S.N.; Rounsefell, K.N.; Walker, R.E.; Rauch, C.E.; Huggins, C.E.; Ryan, L. Intermittent energy restriction and weight loss: A systematic review. Eur. J. Clin. Nutr. 2015, 70, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Welton, S.; Fcfp, T.O.; Willms, H.; Rpn, D.P.; Fcfp, S.M. Intermittent fasting and weight loss: Systematic review. Can. Fam. Physician 2020, 66, 117–125. [Google Scholar] [PubMed]
- Harris, L.; Hamilton, S.; Azevedo, L.B.; Olajide, J.; De Brún, C.; Waller, G.; Whittaker, V.; Sharp, T.; Lean, M.; Hankey, C.; et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: A Systematic Review and Meta-Analysis. JBI Database Syst. Rev. Implement. Rep. 2018, 16, 507–547. [Google Scholar] [CrossRef]
- De Cabo, R.; Mattson, M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Cioffi, I.; Evangelista, A.; Ponzo, V.; Ciccone, G.; Soldati, L.; Santarpia, L.; Contaldo, F.; Pasanisi, F.; Ghigo, E.; Bo, S. Intermittent versus continuous energy restriction on weight loss and cardiometabolic outcomes: A systematic review and meta-analysis of randomized controlled trials. J. Transl. Med. 2018, 16, 371. [Google Scholar] [CrossRef]
- Malinowski, B.; Zalewska, K.; Węsierska, A.; Sokołowska, M.M.; Socha, M.; Liczner, G.; Pawlak-Osińska, K.; Wiciński, M. Intermittent Fasting in Cardiovascular Disorders—An Overview. Nutrients 2019, 11, 673. [Google Scholar] [CrossRef]
- Cho, Y.; Hong, N.; Kim, K.; Cho, S.; Lee, M.; Lee, Y.; Lee, Y.; Kang, E.; Cha, B.-S.; Lee, B.-W. The Effectiveness of Intermittent Fasting to Reduce Body Mass Index and Glucose Metabolism: A Systematic Review and Meta-Analysis. JCM 2019, 8, 1645. [Google Scholar] [CrossRef]
- Liu, K.; Liu, B.; Heilbronn, L.K. Intermittent fasting: What questions should we be asking? Physiol. Behav. 2020, 218, 112827. [Google Scholar] [CrossRef]
- Tinsley, G.M.; La Bounty, P.M. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr. Rev. 2015, 73, 661–674. [Google Scholar] [CrossRef]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Clinical Application of Intermittent Fasting for Weight Loss: Progress and Future Directions. Nat. Rev. Endocrinol. 2022, 18, 309–321. [Google Scholar] [CrossRef]
- Varady, K. The Every-Other-Day Diet: The Diet That Lets You Eat All You Want (Half the Time) and Keep the Weight Off; Hachette Books: New York, NY, USA, 2013; ISBN 978-1-4013-0595-6. [Google Scholar]
- Mosley, D.M.; Spencer, M. The FastDiet-Revised & Updated: Lose Weight, Stay Healthy, and Live Longer with the Simple Secret of Intermittent Fasting; Simon and Schuster: New York, NY, USA, 2013; ISBN 978-1-4767-3496-5. [Google Scholar]
- Chen, W.; Liu, X.; Bao, L.; Yang, P.; Zhou, H. Health effects of the time-restricted eating in adults with obesity: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1079250. [Google Scholar] [CrossRef]
- Zinczenko, D.; Moore, P. The 8-Hour Diet: Watch the Pounds Disappear without Watching What You Eat! Harmony/Rodale: New York, NY, USA, 2013; ISBN 978-1-60961-591-8. [Google Scholar]
- Rogers, P.J.; Brunstrom, J.M. Appetite and energy balancing. Physiol. Behav. 2016, 164, 465–471. [Google Scholar] [CrossRef]
- Potter, C.; Griggs, R.L.; Brunstrom, J.M.; Rogers, P.J. Breaking the fast: Meal patterns and beliefs about healthy eating style are associated with adherence to intermittent fasting diets. Appetite 2019, 133, 32–39. [Google Scholar] [CrossRef]
- Seimon, R.V.; Roekenes, J.A.; Zibellini, J.; Zhu, B.; Gibson, A.A.; Hills, A.P.; Wood, R.E.; King, N.A.; Byrne, N.M.; Sainsbury, A. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol. Cell. Endocrinol. 2015, 418, 153–172. [Google Scholar] [CrossRef]
- Hoddy, K.K.; Marlatt, K.L.; Cetinkaya, H.; Ravussin, E. Intermittent Fasting and Metabolic Health: From Religious Fast to Time-Restricted Feeding. Obesity 2020, 28, S29–S37. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-Term Persistence of Hormonal Adaptations to Weight Loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Lefebvre, C.; Manheimer, E.; Glanville, J. Searching for Studies. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd.: New York, NY, USA, 2008; pp. 95–150. ISBN 978-0-470-71218-4. [Google Scholar]
- Hupe, M. EndNote X9. J. Electron. Resour. Med. Libr. 2019, 16, 117–119. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Review Manager (RevMan) [Computer Program], Version 5.4; The Cochrane Collaboration: London, UK, 2020.
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions; Wiley-Blackwell: New York, NY, USA, 2019; ISBN 978-1-119-53662-8. [Google Scholar]
- Balk, E.M.; Earley, A.; Patel, K.; Trikalinos, T.A.; Dahabreh, I.J. Empirical Assessment of Within-Arm Correlation Imputation in Trials of Continuous Outcomes; AHRQ Methods for Effective Health Care; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2012. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Carter, S.; Clifton, P.; Keogh, J. The effects of intermittent compared to continuous energy restriction on glycaemic control in type 2 diabetes; a pragmatic pilot trial. Diabetes Res. Clin. Pract. 2016, 122, 106–112. [Google Scholar] [CrossRef]
- Conley, M.; Le Fevre, L.; Haywood, C.; Proietto, J. Is two days of intermittent energy restriction per week a feasible weight loss approach in obese males? A randomised pilot study. Nutr. Diet. 2017, 75, 65–72. [Google Scholar] [CrossRef]
- Beaulieu, K.; Casanova, N.; Oustric, P.; Turicchi, J.; Gibbons, C.; Hopkins, M.; Varady, K.; Blundell, J.; Finlayson, G. Matched Weight Loss Through Intermittent or Continuous Energy Restriction Does Not Lead To Compensatory Increases in Appetite and Eating Behavior in a Randomized Controlled Trial in Women with Overweight and Obesity. J. Nutr. 2020, 150, 623–633. [Google Scholar] [CrossRef]
- Coutinho, S.R.; Halset, E.H.; Gåsbakk, S.; Rehfeld, J.F.; Kulseng, B.; Truby, H.; Martins, C. Compensatory mechanisms activated with intermittent energy restriction: A randomized control trial. Clin. Nutr. 2018, 37, 815–823. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Liu, B.; Wood, R.E.; Vincent, A.D.; Thompson, C.H.; O’callaghan, N.J.; Wittert, G.A.; Heilbronn, L.K. Effects of Intermittent Versus Continuous Energy Intakes on Insulin Sensitivity and Metabolic Risk in Women with Overweight. Obesity 2018, 27, 50–58. [Google Scholar] [CrossRef]
- Kroeger, C.M.; Trepanowski, J.F.; Klempel, M.C.; Barnosky, A.; Bhutani, S.; Gabel, K.; Varady, K.A. Eating behavior traits of successful weight losers during 12 months of alternate-day fasting: An exploratory analysis of a randomized controlled trial. Nutr. Health 2018, 24, 5–10. [Google Scholar] [CrossRef]
- Templeman, I.; Smith, H.A.; Chowdhury, E.; Chen, Y.-C.; Carroll, H.; Johnson-Bonson, D.; Hengist, A.; Smith, R.; Creighton, J.; Clayton, D.; et al. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci. Transl. Med. 2021, 13, eabd8034. [Google Scholar] [CrossRef]
- Hopp, K.; Catenacci, V.A.; Dwivedi, N.; Kline, T.L.; Wang, W.; You, Z.; Nguyen, D.T.; Bing, K.; Poudyal, B.; Johnson, G.C.; et al. Weight loss and cystic disease progression in autosomal dominant polycystic kidney disease. iScience 2021, 25, 103697. [Google Scholar] [CrossRef]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef]
- Keenan, S.J.; Cooke, M.B.; Hassan, E.B.; Chen, W.S.; Sullivan, J.; Wu, S.X.; El-Ansary, D.; Imani, M.; Belski, R. Intermittent fasting and continuous energy restriction result in similar changes in body composition and muscle strength when combined with a 12 week resistance training program. Eur. J. Nutr. 2022, 61, 2183–2199. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Svendsen, M.; Tonstad, S. Effect of intermittent versus continuous energy restriction on weight loss, maintenance and cardiometabolic risk: A randomized 1-year trial. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 698–706. [Google Scholar] [CrossRef]
- Gao, Y.; Tsintzas, K.; Macdonald, I.A.; Cordon, S.M.; Taylor, M.A. Effects of intermittent (5:2) or continuous energy restriction on basal and postprandial metabolism: A randomised study in normal-weight, young participants. Eur. J. Clin. Nutr. 2021, 76, 65–73. [Google Scholar] [CrossRef]
- Pureza, I.R.D.O.M.; Macena, M.D.L.; Junior, A.E.D.S.; Praxedes, D.R.S.; Vasconcelos, L.G.L.; Bueno, N.B. Effect of early time-restricted feeding on the metabolic profile of adults with excess weight: A systematic review with meta-analysis. Clin. Nutr. 2020, 40, 1788–1799. [Google Scholar] [CrossRef]
- Stratton, M.T.; Tinsley, G.M.; Alesi, M.G.; Hester, G.M.; Olmos, A.A.; Serafini, P.R.; Modjeski, A.S.; Mangine, G.T.; King, K.; Savage, S.N.; et al. Four Weeks of Time-Restricted Feeding Combined with Resistance Training Does Not Differentially Influence Measures of Body Composition, Muscle Performance, Resting Energy Expenditure, and Blood Biomarkers. Nutrients 2020, 12, 1126. [Google Scholar] [CrossRef]
- Thomas, E.A.; Zaman, A.; Sloggett, K.J.; Steinke, S.; Grau, L.; Catenacci, V.A.; Cornier, M.; Rynders, C.A. Early time-restricted eating compared with daily caloric restriction: A randomized trial in adults with obesity. Obesity 2022, 30, 1027–1038. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Wang, Y.-T.; Chan, L.-C.; Chu, N.-F. Effect of time-restricted feeding on body composition and cardio-metabolic risk in middle-aged women in Taiwan. Nutrition 2022, 93, 111504. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Qin, Y.-L.; Shi, Z.-Y.; Chen, J.-H.; Zeng, M.-J.; Zhou, W.; Chen, R.-Q.; Chen, Z.-Y. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: A randomised controlled trial. BMC Gastroenterol. 2019, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- GRADEpro GDT: GRADEpro Guideline Development Tool; Available from Gradepro.Org; McMaster University and Evidence Prime: Hamilton, ON, Canada, 2022.
- Melendez-Torres, G.J.; Thomas, J.; Richardson, M.; Felix, L.; Lorenc, T.; Thomas, S.; Petticrew, M. Lessons from comparing narrative synthesis and meta-analysis in a systematic review. Lancet 2015, 386, S9. [Google Scholar] [CrossRef]
- Steger, F.L.; Jamshed, H.; Martin, C.K.; Richman, J.S.; Bryan, D.R.; Hanick, C.J.; Salvy, S.-J.; Warriner, A.H.; Peterson, C.M. Impact of early time-restricted eating on diet quality, meal frequency, appetite, and eating behaviors: A randomized trial. Obesity 2022, 31, 127–138. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Irving, P.M.; Lomer, M.C.E.; Whelan, K. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc. Nutr. Soc. 2017, 76, 203–212. [Google Scholar] [CrossRef]
- Shiffman, S.; Stone, A.A.; Hufford, M.R. Ecological Momentary Assessment. Annu. Rev. Clin. Psychol. 2008, 4, 1–32. [Google Scholar] [CrossRef]
- O’Connor, S.G.; Boyd, P.; Bailey, C.P.; Shams-White, M.M.; Agurs-Collins, T.; Hall, K.; Reedy, J.; Sauter, E.R.; Czajkowski, S.M. Perspective: Time-Restricted Eating Compared with Caloric Restriction: Potential Facilitators and Barriers of Long-Term Weight Loss Maintenance. Adv. Nutr. Int. Rev. J. 2021, 12, 325–333. [Google Scholar] [CrossRef]
- Beaulieu, K.; Casanova, N.; Oustric, P.; Hopkins, M.; Varady, K.; Finlayson, G.; Gibbons, C. An exploratory investigation of the impact of ‘fast’ and ‘feed’ days during intermittent energy restriction on free-living energy balance behaviours and subjective states in women with overweight/obesity. Eur. J. Clin. Nutr. 2020, 75, 430–437. [Google Scholar] [CrossRef]
- Proof of Concept RCT Investigating the Impact of Matched Weight Loss via Intermittent or Continuous Energy Restriction on Appetite Control|Cochrane Library. Available online: https://www.cochranelibrary.com/central/doi/10.1002/central/CN-01993808/full (accessed on 24 April 2023).
- Finlayson, D.G. The Impact of Weight Loss through Alternate Day Fasting on Homeostatic and Hedonic Appetite Control and Eating Behaviour: A Proof of Concept Randomized Controlled Trial; ClinicalTrials: Bethesda, MD, USA, 2020.
- Norwegian University of Science and Technology Effect of Intermittent Versus Continuous Energy Restriction on Compensatory Mechanisms Activated During Weight Reduction; ClinicalTrials: Bethesda, MD, USA, 2017.
- Heilbronn, A.L. Effects of Periodic Fasting Versus Daily Energy Restriction on Metabolic Health; ClinicalTrials: Bethesda, MD, USA, 2015.
- Keenan, S.; Cooke, M.B.; Chen, W.S.; Wu, S.; Belski, R. The Effects of Intermittent Fasting and Continuous Energy Restriction with Exercise on Cardiometabolic Biomarkers, Dietary Compliance, and Perceived Hunger and Mood: Secondary Outcomes of a Randomised, Controlled Trial. Nutrients 2022, 14, 3071. [Google Scholar] [CrossRef]
- Keenan, S.; Cooke, M.; Wu, S.; Hassan, E.B.; Meyer, D.; Chen, W.S.; Sullivan, J.; Duque, G.; Belski, R. The Effect Of Continuous Energy Restriction Vs Intermittent Fasting, With Resistance Training, On Lean Mass: 3082 Board #3 May 29 3:15 PM–5:15 PM. Med. Sci. Sports Exerc. 2020, 52, 846. [Google Scholar] [CrossRef]
- ANZCTR-Registration. Available online: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=380246 (accessed on 24 April 2023).
- Keenan, S. Comparing the Effects of 5:2 Intermittent Fasting and Continuous Energy Restriction When Combined with Resistance Training on Body Composition, Muscular Strength, Cardio-Metabolic Health Markers and Dietary Compliance. Available online: https://researchbank.swinburne.edu.au/items/0db549ca-232d-4621-80f6-f114d59b6ed4/1/ (accessed on 25 April 2023).
- Alternate Day Fasting Versus Calorie Restriction for Weight Maintenance-ProQuest. Available online: https://www.proquest.com/openview/6be32039192f9cddb5afd85342dd7167/1?pq-origsite=gscholar&cbl=18750 (accessed on 24 April 2023).
- Pureza, I.R.O.M.; Melo, I.S.V.; Macena, M.L.; Praxedes, D.R.S.; Vasconcelos, L.G.L.; Silva-Júnior, A.E.; Florêncio, T.M.M.T.; Bueno, N.B. Acute effects of time-restricted feeding in low-income women with obesity placed on hypoenergetic diets: Randomized trial. Nutrition 2020, 77, 110796. [Google Scholar] [CrossRef]
- Sundfør, T.M.; Tonstad, S.; Svendsen, M. Effects of intermittent versus continuous energy restriction for weight loss on diet quality and eating behavior. A randomized trial. Eur. J. Clin. Nutr. 2018, 73, 1006–1014. [Google Scholar] [CrossRef]
- Templeman, I.; Thompson, D.; González, J.; Walhin, J.-P.; Reeves, S.; Rogers, P.J.; Brunstrom, J.M.; Karagounis, L.G.; Tsintzas, K.; Betts, J.A. Intermittent fasting, energy balance and associated health outcomes in adults: Study protocol for a randomised controlled trial. Trials 2018, 19, 86. [Google Scholar] [CrossRef]
- Templeman, I. The Impact of Intermittent Fasting on Energy Balance and Associated Health Outcomes. Ph.D. Thesis, University of Bath, Bath, UK, 2019. [Google Scholar]

| First Author (Year) | Country | RCT Design | RCT Duration | Intermittent Fasting Protocol | Continuous Energy Restriction Protocol |
|---|---|---|---|---|---|
| Beaulieu (2020) [35] | UK, USA | 2 parallel groups | 12 weeks | ADF: 25% of daily energy requirements on fast days and ad libitum on feed days | Consume 75% of daily energy requirements each day |
| Cai (2019) [49] | China | 3 parallel groups | 12 weeks | ADF: 25% of baseline energy requirements on fast days and ad libitum on feed days. TRE: provided with a meal within an 8-h window and asked to refrain from the consumption of all food or beverages that included energy for the remaining 16 h | Consume 80% of energy needs each day |
| Carter (2016) [33] | Australia | 2 parallel groups (pilot) | 12 weeks | 5:2 diet: 1670–2500 kJ/day for two days each week and habitual eating for five days each week | 7-day continuous energy restriction diet of 5000–6500 kJ/day |
| Conley (2018) [34] | Australia | 2 parallel groups (pilot) | 6 months | 5:2 diet: daily intake restricted to 600 kcal for two non-consecutive days per week and ad libitum on the remaining five days | Daily 500 kcal reduction from the average requirement |
| Coutinho (2018) [36] | Norway, Denmark, Australia | 2 parallel groups | 12 weeks | ADF: 3 non-consecutive days of 550 kcal/day for women and 660 kcal/day for men, and a diet matching energy needs for the remaining four days (≈2118 kcal/day) | Low-calorie diet (≈1410 kcal/day) |
| Gao (2022) [44] | UK | 2 parallel groups | 2 weeks | 5:2 diet: daily calorie intake restricted to 70% of estimated energy requirements for two non-consecutive days per week, and energy intake of estimated energy requirements for the remaining five days | Daily calorie restriction of 20% from estimated energy requirements |
| Harvie (2013) [41] | UK, USA | 3 parallel groups | 12 weeks of weight loss (+4 weeks of weight maintenance) | 5:2 diet: 70% energy restriction on two consecutive days per week and meeting estimated energy requirements for the remaining 5 days | 25% energy restriction by eating an energy-restricted Mediterranean-type diet |
| Hopp (2021) [40] | UK | 2 parallel groups | 3 months of weight loss (+9 months of weight maintenance) | ADF: reduced energy intake to 20% of estimated energy requirements (eaten as a single meal) for three non-consecutive days per week and ate ad libitum for the remaining four days | Daily calorie restriction of approximately 34% of estimated energy requirements |
| Hutchison (2019) [37] | Australia | 4 parallel groups | 8 weeks | ADF: 32% of energy requirements at breakfast before a 24-h fast on three non-consecutive weekdays per week and ~100% of energy requirements on the remaining days | Consume 70% of calculated baseline energy requirements |
| Keenan (2020) [42] | Australia | 2 parallel groups | 12 weeks | 5:2 diet: consume approximately 30% of energy requirements on two non-consecutive days per week, and 100% of energy requirements on the remaining days | Consume approximately 80% of daily energy requirements |
| Kroeger (2018) [38] | USA | 2 parallel groups | 6 months (+6 months weight maintenance) | ADF: consume 25% of energy needs on the fast days and 125% of energy needs on the remainder of the days | Consume 75% of energy needs every day |
| Lin (2022) [48] | Taiwan | 2 parallel groups | 8 weeks | TRE: 1400 kcal per day consumed within an eight-hour window (10:00–18:00 or 12:00–20:00) | 1400 kcal per day with no time restriction |
| Pureza (2020) [45] | Brazil | 2 parallel groups | 3 weeks | TRE: 500 to 1000 kcal were subtracted from estimated energy requirements and only eat in a 12-h window | 500 to 1000 kcal were subtracted from participants’ estimated energy requirements |
| Stratton (2020) [46] | USA | 2 parallel groups | 4 weeks | TRE: 25% caloric deficit and only eat within an 8-h window each day | 25% caloric deficit with participants usual daily feeding schedule |
| Sundfør (2018) [43] | Norway | 2 parallel groups | 6 months (+6 months weight maintenance) | 5:2 diet: consume 400/600 kcal (female/male) on each of two non-consecutive days a week and eat as usual, the remaining five days a week | Reduce energy intake evenly each day so total weekly energy reduction is equivalent in both interventions |
| Templeman (2021) [39] | UK | 3 parallel groups | 4 weeks | ADF: alternate between 24-h periods of fasting and eating to 150% of habitual daily energy intake | 25% reduction in habitual daily energy intake |
| Thomas (2022) [47] | USA | 2 parallel groups | 39 weeks (outcomes measured at 12 weeks) | TRE: 35% daily calorie restriction and only eat within a ten-hour window | 35% daily calorie restriction with no instructions on the eating window |
| First Author (Year) | Specific Characteristics | N Allocated in Intermittent Fasting/Continuous Energy Restriction | N Analyzed in Intermittent Fasting/Continuous Energy Restriction | Analysis Type | Age (Intermittent Fasting/Continuous Energy Restriction) | Female (Intermittent Fasting/Continuous Energy Restriction) | BMI (Intermittent Fasting/Continuous Energy Restriction) |
|---|---|---|---|---|---|---|---|
| Beaulieu (2020) [35] | BMI between 25.0 and 34.9 kg/m2 | 24/22 | 18/19 | Completers | 36 ± 11/34 ± 9 | 18/19 | 29.1 ± 2.2/29.1 ± 2.4 |
| Cai (2019) [49] | NAFLD, BMI > 24 kg/m2 | 95 (ADF) + 97 (TRE)/79 | 90 (ADF) + 95 (TRE)/79 | Completers | 35.50 ± 4.417 (ADF), 33.56 ± 6.23 (TRE)/34.54 ± 6.96 | 60 (ADF), 66 (TRE)/56 | 26.12 ± 2.21 (ADF), 26.76 ± 1.59 (TRE)/26.34 ± 2.73 |
| Carter (2016) [33] | T2DM with BMI > 27 kg/m2 | 31/32 | 26/25 | Completers | * 61 ± 7.5/62 ± 9.1 | * 17/16 | * 35 ± 4.8/36 ± 5.2 |
| Conley (2018) [34] | War veterans with BMI ≥ 30 kg/m2 | 12/12 | 11/12 | Completers | 68 ± 2.7/ 67.1 ± 3.9 | 0/0 | 33.4 ± 1.8/36.2 ± 4.3 |
| Coutinho (2018) [36] | BMI between 30 and 40 kg/m2 | 18/17 | 14/14 | Completers | 39.4 ± 11.0/ 39.1 ± 9.0 | 10/12 | 35.6 ± 3.2/35.1 ± 4.2 |
| Gao (2022) [44] | BMIBetween 20 and 25 kg/m2, and moderately physically active | 8/10 | 8/8 | Completers | 21 ± 2.8/26 ± 5.7 | 4/4 | 21.7 ± 2.3/22.7 ± 1.7 |
| Harvie (2013) [41] | BMI between 24 and 45 kg/m2 and a family history of breast cancer | 37/40 | 37/40 | Intention to treat | 45.6 ± 8.3/ 47.9 ± 7.7 | 37/40 | 29.6 ± 4.1/32.2 ± 5.6 |
| Hopp (2021) [40] | Autosomal dominant polycystic kidney disease | 13/15 | 11/13 | Intention to treat | 46 ± 6/47 ± 12 | 7/9 | 34.8 ± 5.1/34.6 ± 5.1 |
| Hutchison (2019) [37] | BMI between 25 and 42 kg/m2 | 25/26 | 22/24 | Completers | * 49 ± 2/51 ± 2 | * 25/26 | * 32.4 ± 0.8/32.6 ± 1.0 |
| Keenan (2020) [42] | Individuals with a BMI between 22 and 35 kg/m2, and excess body fat (>18% for males or >25% for females) | 27/27 | 17/17 | Completers | 24.8 ± 4.8/23.2 ± 3.9 ** | 8/9 | 27 ± 2.7/27.1 ± 2.9 ** |
| Kroeger (2018) [38] | BMI between 25 and 40 kg/m2 | 34/35 | 34/35 | Intention to treat | 44 ± 10/ 43 ± 12 | 30/29 | 34 ± 4.1/35.6 ± 4.2 ** |
| Lin (2022) [48] | BMI ≥ 24 kg/m2 | 30/33 | 30/33 | Completers | 50.1 ± 7.5/54.2 ± 7.9 | 30/33 | 25.9 ± 3.7/25.7 ± 3.8 |
| Pureza (2020) [45] | Socially vulnerable/low-income with BMI between 30 and <45 kg/m2 | 31/27 | 31/27 | Intention to treat | 31.8 ± 6.9/31 ± 7.1 | 31/27 | 33.53 ± 4.8/33.12 ± 3.7 |
| Stratton (2020) [46] | Recreationally active | 15/17 | 13/13 | Per protocol | 22.9 ± 3.6/22.5 ± 2.2 | 0/0 | Body mass (kg) 82.0 ± 10.6 and height (cm) 178.1 ± 7.3/Body mass (kg) 83.3 ± 15.0 and height (cm) 177.5 ± 8.8 |
| Sundfør (2018) [43] | BMI between 30 and 45 kg/m2 | 54/58 | 54/58 | Intention to treat | 49.9 ± 10.1/47.5 ± 11.6 | 26/30 | 35.1 ± 3.9/35.3 ± 3.5 |
| Templeman (2021) [39] | BMI between 20.5 and 25.0 kg/m2 | 13/12 | 12/12 | Completers | 42 ± 11/45 ± 6 | 5/7 | 23.9 ± 2.4/24.0 ± 1.9 |
| Thomas (2022) [47] | BMI between 27 to 45 kg/m2 | 40/41 | 34/36 | Completers | 38.3 ± 7.9/37.8 ± 7.8 | 34/35 | 34.6 ± 5.8/33.7 ± 5.6 |
| First Author (Year) | Primary Outcomes Measured | Timepoint Measured | Appetite Measurement Protocol |
|---|---|---|---|
| Beaulieu (2020) [35] | Hunger, fullness, desire to eat, PFC * | Baseline, week 12 | Following an overnight fast, VAS (100 mm) before and after standard breakfast |
| Cai (2019) [49] | Hunger, fullness, PFC | Baseline, week 4, week 12 | VAS (100 mm) |
| Carter (2016) [33] | Hunger, fullness | Baseline, week 12 | Following the overnight fast, VAS |
| Conley (2018) [34] | Hunger | 2 weeks, 3 months, 6 months | ‘Any side effects were recorded in individual participant visit notes’ |
| Coutinho (2018) [36] | Hunger, fullness, desire to eat, PFC * | Baseline, week 13 | Following overnight fast, VAS (100 mm) before and after standard breakfast |
| Gao (2022) [44] | Hunger, fullness, desire to eat, PFC * | Baseline, day 7 | Following an overnight fast, VAS before and after and standardized liquid breakfast |
| Harvie (2013) [41] | Hunger, fullness, desire to eat, PFC | Baseline, 1 month, 3 months, 4 months | ‘How hungry have you felt over the past day?’ for 3 days, VAS |
| Hopp (2021) [40] | Hunger | Baseline, 3 months, 12 months | Reported at adverse events |
| Hutchison (2019) [37] | Hunger, fullness, desire to eat * | Baseline, week 1, week 6 | Following overnight fast, VAS (100 mm) |
| Keenan (2020) [42] | Hunger, fullness * | Daily, from week 1 until week 12 | Assessed daily on a mobile phone with a Likert scale (0–10) adapted from VAS |
| Kroeger (2018) [38] | Hunger, fullness | Baseline, 3 months, 6 months, 9 months, 12 months | VAS (100 mm) before bed for 3 days |
| Lin (2022) [48] | Hunger | No information | Reported as a side effect |
| Pureza (2020) [45] | Hunger | Baseline, day 21 | Following an overnight fast, VAS (0–10) |
| Stratton (2020) [46] | Hunger, fullness, desire to eat | Weekly | VAS (0–10), at arrival to a training session |
| Sundfør (2018) [43] | Hunger | 3 months, 6 months, 12 months | Following the overnight fast, VAS (1–10) |
| Templeman (2021) [39] | Hunger, fullness, desire to eat, PFC | Week 5 (after 4 weeks of monitoring), week 9 (after 4 weeks of intervention) | Following overnight fast, VAS (100 mm) |
| Thomas (2022) [47] | Hunger, fullness, desire to eat, PFC | Baseline, week 12 | Before and after each meal for three days |
| First Author (Year) | Eating Behavior Measure | Timepoints Measured | Findings |
|---|---|---|---|
| Beaulieu (2020) [35] | Three Factor Eating Questionnaire, Binge Eating Scale, Control of Eating Questionnaire, Food Reward, The Leeds Food Preference Questionnaire | Baseline and final week | Dietary restraint increased in both groups. Susceptibility to hunger decreased in both groups. Disinhibited eating decreased more in the continuous restriction than in intermittent fasting. |
| Gao (2022) [44] | Eating Attitudes Test | At screening | Not reported |
| Hopp (2021) [40] | Questionnaire on Eating and Weight Patterns-Revised | Baseline, month 3, month 12 | Not reported |
| Kroeger (2018) [38] | Three Factor Eating Questionnaire | Baseline and month 12 | There were no significant differences in restraint from baseline to 12 months |
| Templeman (2021) [39] | Two alternate forced choice tasks | Pre and post intervention | Not reported. |
| Thomas (2022) [47] | Three Factor Eating Questionnaire | Baseline, week 12, week 39 | Dietary restraint increased in both groups similarly from baseline to week 12 and week 12. Disinhibition and susceptibility to hunger did not change-from-baseline to week 12 or 39. |
| Stratton (2020) [46] | Three Factor Eating Questionnaire | Pre and post intervention | Cognitive restraint increased in the time-restricted eating group but remained the same in the continuous energy restriction group. |
| Sundfør (2018) [43] | Three Factor Eating Questionnaire | Baseline and month 3 | Disinhibited eating and emotional eating reduced in both groups following the interventions. Cognitive restraint increased in both groups, but this increase was greater in the continuous energy restriction group than in the intermittent fasting group. |
| First Author (Year) | Adherence in Intermittent Fasting Group (%) | Adherence in Continuous Energy Restriction Group (%) |
|---|---|---|
| Beaulieu (2020) [35] | 83.5 | 89.2 |
| Conley (2018) [34] | 73 | 75 |
| Coutinho (2018) [36] | 78 | 82 |
| Harvie (2013) [41] | 80 | 80 |
| Stratton (2020) [46] | 86.7 | 86.7 |
| Certainty Assessment | № of Patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| № of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | [Intervention] | [Comparison] | Relative (95% CI) | Absolute (95% CI) | ||
| Hunger (change-from-baseline) (assessed with visual analogue scale) | ||||||||||||
| 13 | randomized trials | serious | serious | not serious | serious | none | 478 | 377 | - | MD 3.03 lower (8.13 lower to 2.08 higher) | ⨁◯◯◯ Very low | |
| Fullness (change-from-baseline) (assessed with visual analogue scales) | ||||||||||||
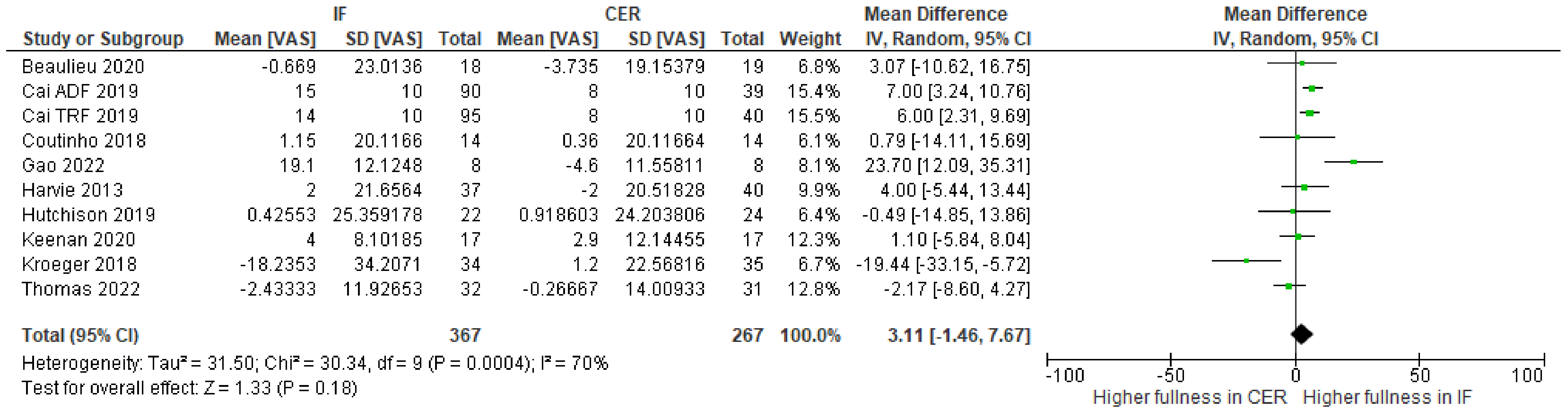
| 10 | randomized trials | serious | serious | not serious | serious | none | 367 | 267 | - | MD 3.11 higher (1.46 lower to 7.67 higher) | ⨁◯◯◯ Very low | |
| Desire to eat (change-from-baseline) (assessed with visual analogue scales) | ||||||||||||
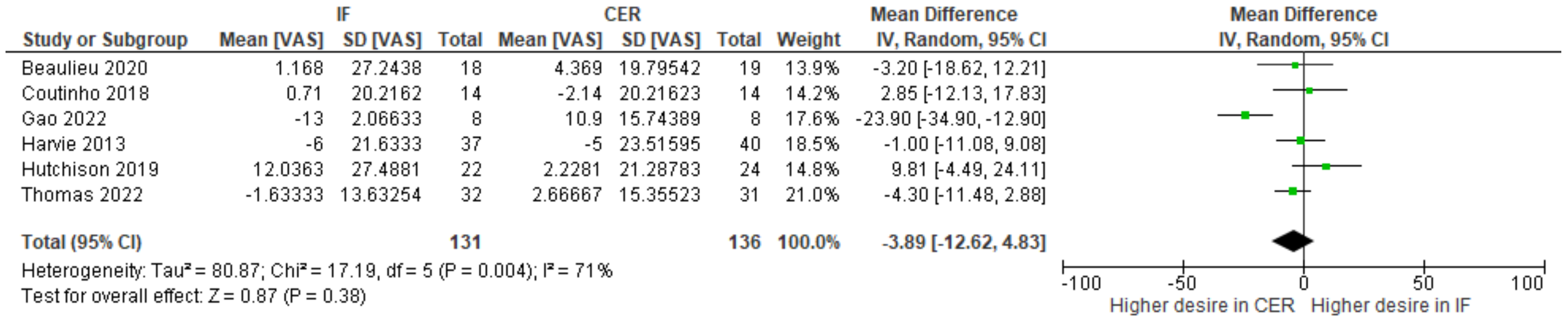
| 6 | randomized trials | serious | serious | not serious | serious | none | 131 | 136 | - | MD 3.89 lower (12.62 lower to 4.83 higher) | ⨁◯◯◯ Very low | |
| Prospective food consumption (change-from-baseline) (assessed with visual analogue scales) | ||||||||||||
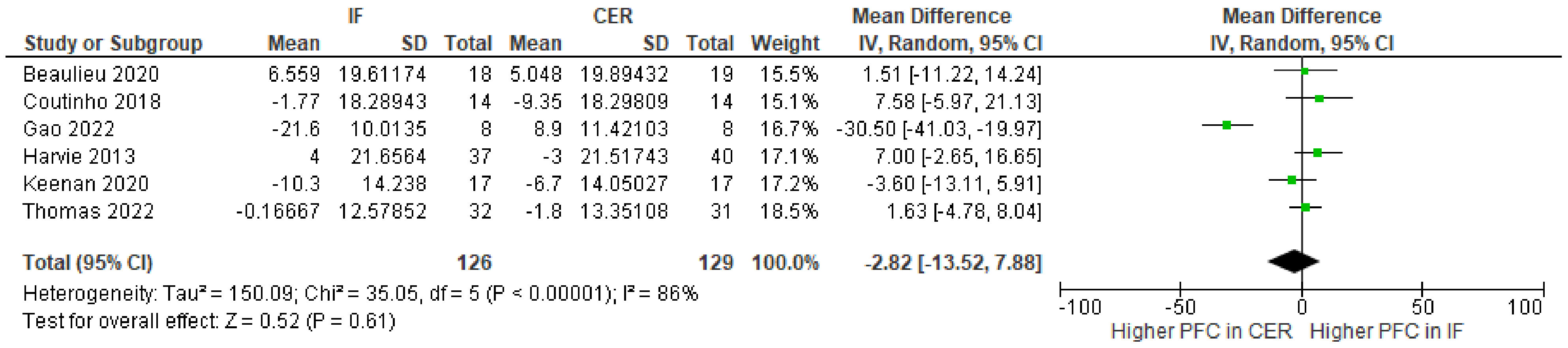
| 6 | randomized trials | serious | serious | not serious | serious | none | 126 | 129 | - | MD 2.82 lower (13.52 lower to 7.88 higher) | ⨁◯◯◯ Very low | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsworth, R.L.; Monge, A.; Perry, R.; Hinton, E.C.; Flynn, A.N.; Whitmarsh, A.; Hamilton-Shield, J.P.; Lawrence, N.S.; Brunstrom, J.M. The Effect of Intermittent Fasting on Appetite: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2604. https://doi.org/10.3390/nu15112604
Elsworth RL, Monge A, Perry R, Hinton EC, Flynn AN, Whitmarsh A, Hamilton-Shield JP, Lawrence NS, Brunstrom JM. The Effect of Intermittent Fasting on Appetite: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(11):2604. https://doi.org/10.3390/nu15112604
Chicago/Turabian StyleElsworth, Rebecca L., Angelica Monge, Rachel Perry, Elanor C. Hinton, Annika N. Flynn, Alex Whitmarsh, Julian P. Hamilton-Shield, Natalia S. Lawrence, and Jeffrey M. Brunstrom. 2023. "The Effect of Intermittent Fasting on Appetite: A Systematic Review and Meta-Analysis" Nutrients 15, no. 11: 2604. https://doi.org/10.3390/nu15112604
APA StyleElsworth, R. L., Monge, A., Perry, R., Hinton, E. C., Flynn, A. N., Whitmarsh, A., Hamilton-Shield, J. P., Lawrence, N. S., & Brunstrom, J. M. (2023). The Effect of Intermittent Fasting on Appetite: A Systematic Review and Meta-Analysis. Nutrients, 15(11), 2604. https://doi.org/10.3390/nu15112604



.png)




