Association between Iron Status and Survival in Patients on Chronic Hemodialysis
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Study’s Variables
2.3. Statistical Analyses
3. Results
3.1. Clinical Characteristics
3.2. Survival Analyses
3.3. Erythropoiesis-Related Indicators according to Iron Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levey, A.S.; Eknoyan, G. Cardiovascular disease in chronic renal disease. Nephrol. Dial. Transplant. 1994, 14, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, F.; Zhang, X.; Jin, Y.; Li, Q.; Shen, H.; Fu, H.; Mao, J. Benefits and risks of essential trace elements in chronic kidney disease: A narrative review. Ann. Transl. Med. 2022, 10, 1400. [Google Scholar] [CrossRef] [PubMed]
- KDIGO Anemia Work Group. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. Suppl. 2012, 2, 279–335. [Google Scholar]
- Walther, C.P.; Triozzi, J.L.; Deswal, A. Iron deficiency and iron therapy in heart failure and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2020, 29, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Regidor, D.L.; McAllister, C.J.; Michael, B.; Warnock, D.G. Time-dependent associations between iron and mortality in hemodialysis patients. J. Am. Soc. Nephrol. 2005, 16, 3070–3080. [Google Scholar] [CrossRef]
- Pollak, V.E.; Lorch, J.A.; Shukla, R.; Satwah, S. The importance of iron in long-term survival of maintenance hemodialysis patients treated with epoetin-alfa and intravenous iron: Analysis of 9.5 years of prospectively collected data. BMC Nephrol. 2009, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.M.; Kim, C.H.; Doh, F.M.; Lee, M.J.; Kim, E.J.; Han, J.S.; Oh, H.J.; Park, J.T.; Han, S.H.; Yoo, T.H.; et al. The relationship of initial transferrin saturation to cardiovascular parameters and outcomes in patients initiating dialysis. PLoS ONE 2014, 9, e87231. [Google Scholar] [CrossRef]
- Sato, M.; Hanafusa, N.; Tsuchiya, K.; Kawaguchi, H.; Nitta, K. Impact of Transferrin Saturation on All-Cause Mortality in Patients on Maintenance Hemodialysis. Blood Purif. 2019, 48, 158–166. [Google Scholar] [CrossRef]
- Yeh, S.C.; Lin, Y.C.; Hong, Y.C.; Hsu, C.C.; Lin, Y.C.; Wu, M.S. Different Effects of Iron Indices on Mortality in Patients With Autosomal Dominant Polycystic Kidney Disease After Long-Term Hemodialysis: A Nationwide Population-Based Study. J. Ren. Nutr. 2019, 29, 444–453. [Google Scholar] [CrossRef]
- Kim, H.W.; Jhee, J.H.; Joo, Y.S.; Yang, K.H.; Jung, J.J.; Shin, J.H.; Han, S.H.; Yoo, T.H.; Kang, S.W.; Park, J.T. Clinical significance of hemodialysis quality of care indicators in very elderly patients with end stage kidney disease. J. Nephrol. 2022, 35, 2351–2361. [Google Scholar] [CrossRef]
- Health Insurance Review & Assessment Service. 6th Hemodialysis Quality Assessment Program. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=6619#none (accessed on 20 April 2023).
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, L.E.; Thomas, W.; Glen, J.; Padhi, S.; Pordes, B.A.; Wonderling, D.; Connell, R.; Stephens, S.; Mikhail, A.I.; Fogarty, D.G.; et al. Diagnosis and Management of Iron Deficiency in CKD: A Summary of the NICE Guideline Recommendations and Their Rationale. Am. J. Kidney Dis. 2016, 67, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Liao, R.; Zhou, X.; Ma, D.; Tang, J.; Zhong, H. Iron Deficiency is Associated With Platelet Count Elevation in Patients With Dialysis-dependent Chronic Kidney Disease. J. Ren. Nutr. 2022, 32, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Berns, J.S. Hemoglobin cycling in hemodialysis patients treated with recombinant human erythropoietin. Kidney Int. 2005, 68, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Liakopoulos, V.; Antoniadi, G.; Kartsios, C.; Stefanidis, I. The role of hepcidin in iron homeostasis and anemia in hemodialysis patients. Semin. Dial. 2009, 22, 70–77. [Google Scholar] [CrossRef]
- Kali, A.; Yayar, O.; Erdogan, B.; Eser, B.; Buyukbakkal, M.; Ercan, Z.; Merhametsiz, O.; Haspulat, A.; Gök Oğuz, E.; Canbakan, B.; et al. Is hepcidin-25 a predictor of atherosclerosis in hemodialysis patients? Hemodial. Int. 2016, 20, 191–197. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Hu, H.; Li, J.; Tian, T. Relationship between serum hepcidin levels and cardiovascular disease in patients with maintenance hemodialysis. Physiol. Int. 2020, 107, 491–500. [Google Scholar] [CrossRef]
- Zou, L.X.; Sun, L.; Hua, R.X.; Wu, Y. Serum Hepcidin-25 and All-Cause Mortality in Patients Undergoing Maintenance Hemodialysis. Int. J. Gen. Med. 2021, 14, 3153–3162. [Google Scholar] [CrossRef]
- Zhong, Z.; Luo, D.; Luo, N.; Li, B.; Fu, D.; Fan, L.; Li, Z.; Chen, W.; Mao, H. Serum Hepcidin-25 and Risk of Mortality in Patients on Peritoneal Dialysis. Front. Med. 2021, 8, 684548. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. Toxic effects of IV iron preparations in CKD patients. Nephrol. News Issues 2014, 28, 4–5. [Google Scholar] [PubMed]
- Luo, D.; Zhong, Z.; Qiu, Y.; Wang, Y.; Li, H.; Lin, J.; Chen, W.; Yang, X.; Mao, H. Abnormal iron status is associated with an increased risk of mortality in patients on peritoneal dialysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tian, Y.; Yuan, Z.; Zeng, Y.; Wang, S.; Fan, X.; Yang, D.; Yang, M. Iron metabolism in aging and age-related diseases. Int. J. Mol. Sci. 2022, 23, 3612. [Google Scholar] [CrossRef]
- Hatamizadeh, P.; Ravel, V.; Lukowsky, L.R.; Molnar, M.Z.; Moradi, H.; Harley, K.; Pahl, M.; Kovesdy, C.P.; Kalantar-Zadeh, K. Iron indices and survival in maintenance hemodialysis patients with and without polycystic kidney disease. Nephrol. Dial. Transplant. 2013, 28, 2889–2898. [Google Scholar] [CrossRef]
- Kim, T.; Streja, E.; Soohoo, M.; Rhee, C.M.; Eriguchi, R.; Kim, T.W.; Chang, T.I.; Obi, Y.; Kovesdy, C.P.; Kalantar-Zadeh, K. Serum Ferritin Variations and Mortality in Incident Hemodialysis Patients. Am. J. Nephrol. 2017, 46, 120–130. [Google Scholar] [CrossRef]
- Karaboyas, A.; Morgenstern, H.; Pisoni, R.L.; Zee, J.; Vanholder, R.; Jacobson, S.H.; Inaba, M.; Loram, L.C.; Port, F.K.; Robinson, B.M. Association between serum ferritin and mortality: Findings from the USA, Japan and European Dialysis Outcomes and Practice Patterns Study. Nephrol. Dial. Transplant. 2018, 33, 2234–2244. [Google Scholar] [CrossRef]
- Kuo, K.L.; Hung, S.C.; Tseng, W.C.; Tsai, M.T.; Liu, J.S.; Lin, M.H.; Hsu, C.C.; Tarng, D.C.; Taiwan Society of Nephrology Renal Registry Data System. Association of Anemia and Iron Parameters With Mortality Among Patients Undergoing Prevalent Hemodialysis in Taiwan: The AIM—HD Study. J. Am. Heart Assoc. 2018, 7, e009206. [Google Scholar] [CrossRef]
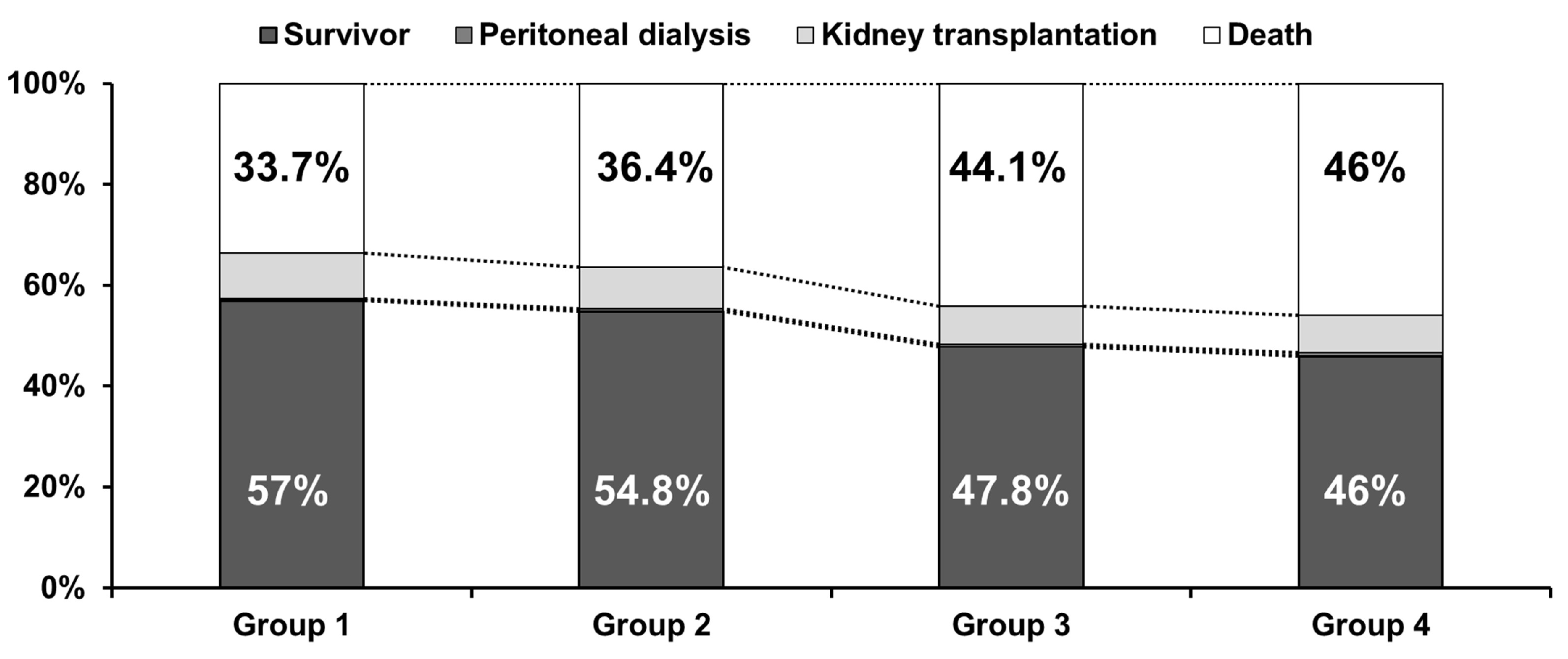
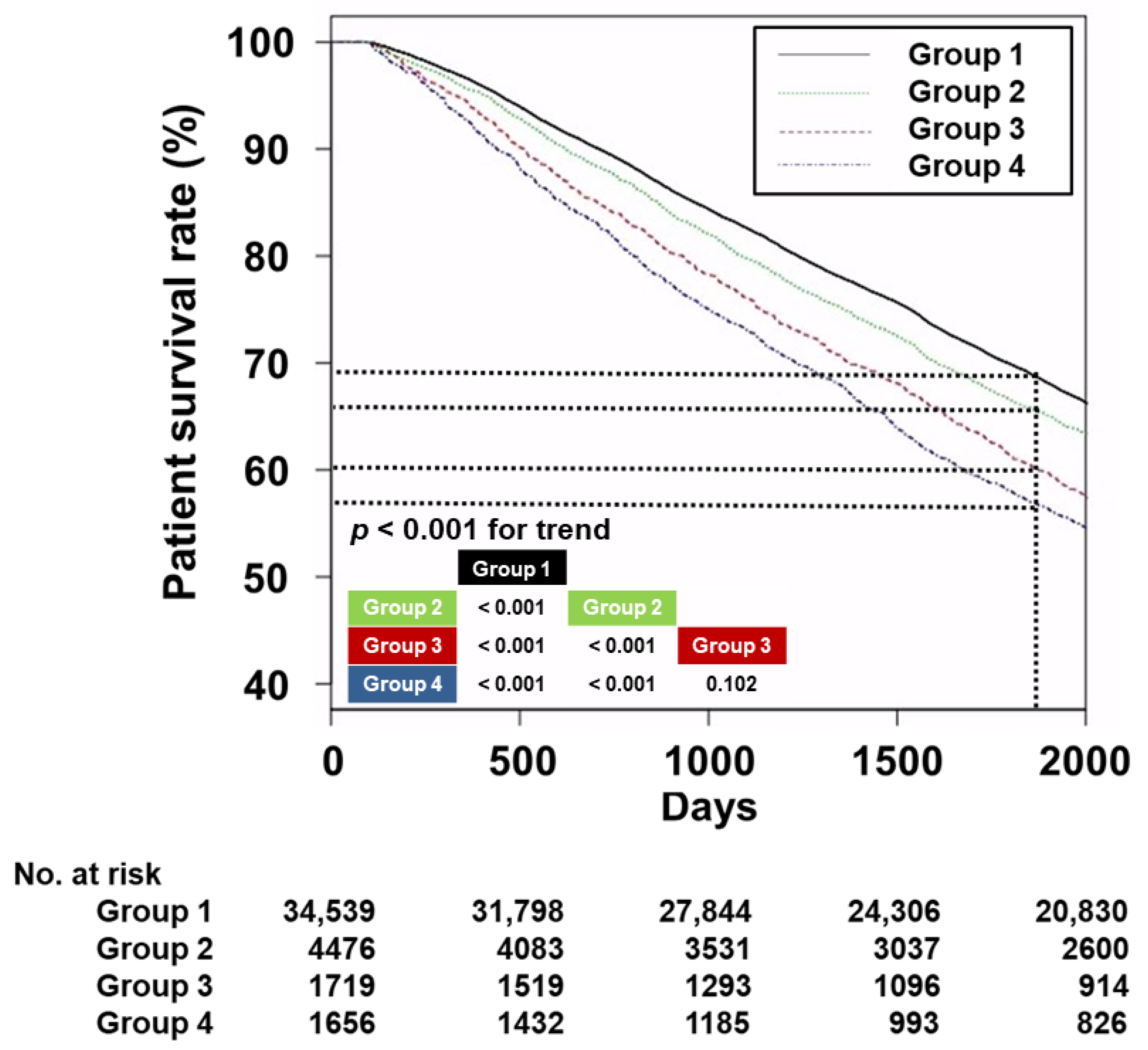
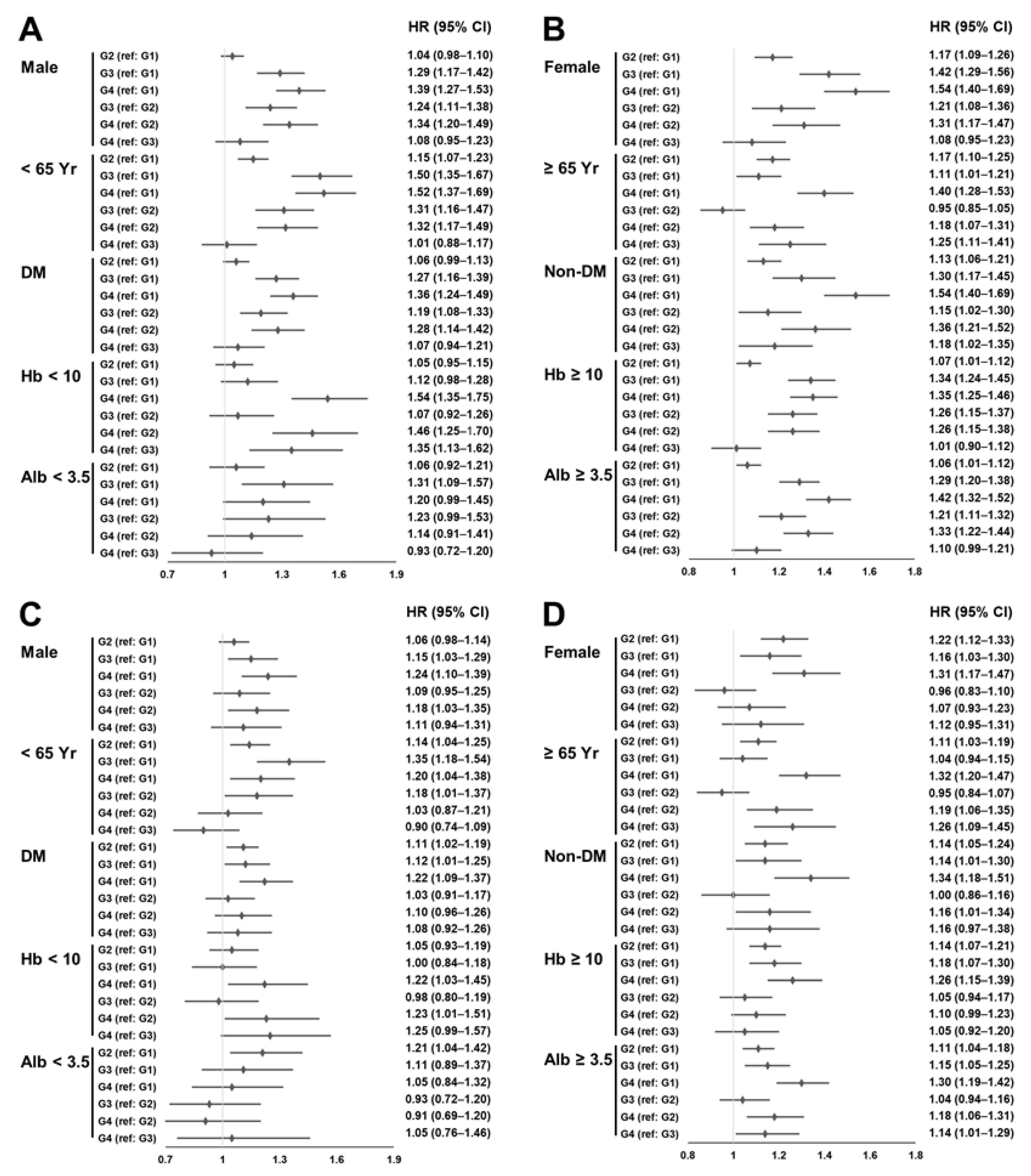
| Group 1 (n = 34,539) | Group 2 (n = 4476) | Group 3 (n = 1719) | Group 4 (n = 1656) | p | |
|---|---|---|---|---|---|
| Age (years) | 59.8 ± 12.8 | 58.3 ± 13.2 * | 60.9 ± 12.9 *# | 60.8 ± 12.7 *# | <0.001 |
| Sex (male, %) | 20,847 (60.4%) | 2511 (56.1%) | 855 (49.7%) | 816 (49.3%) | <0.001 |
| Hemodialysis vintage (months) | 40 (61) | 39 (60) | 39 (57) | 51 (71) *#+ | <0.001 |
| Underlying causes of ESRD | <0.001 | ||||
| Diabetes mellitus | 14,664 (42.5%) | 1850 (41.3%) | 835 (48.6%) | 695 (42.0%) | |
| Hypertension | 9236 (26.7%) | 1191 (26.6%) | 399 (23.2%) | 423 (25.5%) | |
| Glomerulonephritis | 3934 (11.4%) | 440 (9.8%) | 154 (9.0%) | 193 (11.7%) | |
| Others | 2818 (8.2%) | 423 (9.5%) | 154 (9.0%) | 162 (9.8%) | |
| Unknown | 3887 (11.3%) | 572 (12.8%) | 177 (10.3%) | 183 (11.1%) | |
| CCI score | 7.1 ± 2.8 | 7.1 ± 2.8 | 7.5 ± 2.9 *# | 7.5 ± 3.0 *# | <0.001 |
| Follow-up duration (months) | 79 (45) | 79 (42) * | 71 (46) *# | 66 (50) *# | <0.001 |
| Vascular access type | <0.001 | ||||
| Arteriovenous fistula | 29,670 (85.9%) | 3719 (83.1%) | 1459 (84.9%) | 1392 (84.1%) | |
| Arteriovenous graft | 4869 (14.1%) | 757 (16.9%) | 260 (15.1%) | 264 (15.9%) | |
| Kt/Vurea | 1.52 ± 0.27 | 1.49 ± 0.28 * | 1.54 ± 0.28 # | 1.60 ± 0.29 *#+ | <0.001 |
| Ultrafiltration volume (%/BW/session) | 3.92 ± 1.62 | 3.98 ± 1.63 * | 3.84 ± 1.61 *# | 3.95 ± 1.77 *# | <0.001 |
| Hemoglobin (g/dL) | 10.7 ± 0.8 | 10.6 ± 1.0 * | 10.4 ± 0.8 *# | 10.4 ± 0.9 *# | <0.001 |
| Serum albumin (g/dL) | 3.99 ± 0.34 | 3.95 ± 0.34 * | 3.93 ± 0.36 * | 3.91 ± 0.36 *# | <0.001 |
| Serum phosphorus (mg/dL) | 5.0 ± 1.4 | 5.2 ± 1.5 * | 4.8 ± 1.4 *# | 4.8 ± 1.5 *# | <0.001 |
| Serum calcium (mg/dL) | 8.94 ± 0.87 | 8.88 ± 0.88 * | 8.94 ± 0.84 | 8.98 ± 0.90 # | <0.001 |
| SBP (mmHg) | 141 ± 16 | 142 ± 16 * | 142 ± 16 | 140 ± 16 # | <0.001 |
| DBP (mmHg) | 78 ± 9 | 79 ± 10 * | 78 ± 10 # | 78 ± 10 # | <0.001 |
| Serum creatinine (mg/dL) | 9.7 ± 2.7 | 9.7 ± 2.9 | 9.2 ± 2.8 *# | 8.8 ± 2.5 *#+ | <0.001 |
| Use of antihypertensive drug | 23,456 (67.9%) | 2948 (65.9%) | 1175 (68.4%) | 1106 (66.8%) | 0.036 |
| Use of aspirin | 15,342 (44.4%) | 1958 (43.7%) | 787 (45.8%) | 705 (42.6%) | 0.233 |
| Use of statin | 9388 (27.2%) | 1230 (27.5%) | 561 (32.6%) | 427 (25.8%) | <0.001 |
| Transferrin saturation (%) | 33 (15) | 16 (5) * | 17 (4) *# | 41 (28) *#+ | <0.001 |
| Serum ferritin (ng/mL) | 197 (212) | 74 (87) * | 310 (177) *# | 1008 (392) *#+ | <0.001 |
| Use of iron | 21,676 (62.8%) | 2458 (54.9%) | 1086 (63.2%) | 902 (54.5%) | <0.001 |
| ESA dose (IU/week) | 5050 (4820) | 5470 (6120) * | 5740 (5110) *# | 5510 (5130) * | <0.001 |
| ERI (IU/kg/g/dL) | 8.0 (8.0) | 8.8 (10.6) * | 9.4 (9.0) *# | 9.5 (9.7) *# | <0.001 |
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value | |
| Group (ref: Group 1) | ||||
| Group 2 | 1.08 (1.03–1.13) | <0.001 | 1.12 (1.06–1.19) | <0.001 |
| Group 3 | 1.32 (1.24–1.41) | <0.001 | 1.14 (1.05–1.24) | 0.001 |
| Group 4 | 1.43 (1.34–1.53) | <0.001 | 1.27 (1.17–1.38) | <0.001 |
| Underlying disease of ESRD (ref: DM) | 0.80 (0.79–0.81) | <0.001 | 0.90 (0.89–0.92) | <0.001 |
| Age | 1.06 (1.06–1.07) | <0.001 | 1.06 (1.06–1.06) | <0.001 |
| Vascular access (ref: AVF) | 1.49 (1.43–1.54) | <0.001 | 1.17 (1.12–1.22) | <0.001 |
| CCI score | 1.14 (1.14–1.15) | <0.001 | 1.07 (1.06–1.08) | <0.001 |
| Sex (ref: male) | 0.86 (0.83–0.89) | <0.001 | 0.71 (0.68–0.74) | <0.001 |
| Hemodialysis vintage (ref: <1229 days) | 1.03 (0.99–1.06) | 0.079 | 1.38 (1.32–1.43) | <0.001 |
| UFV (increase per 1% of BW) | 0.97 (0.96–0.98) | <0.001 | 1.05 (1.04–1.06) | <0.001 |
| KtVurea | 0.90 (0.84–0.95) | <0.001 | 0.77 (0.71–0.83) | <0.001 |
| Serum creatinine | 0.87 (0.86–0.87) | <0.001 | 0.93 (0.92–0.94) | <0.001 |
| Serum albumin | 0.39 (0.37–0.40) | <0.001 | 0.65 (0.62–0.69) | <0.001 |
| Serum phosphorus | 0.86 (0.85–0.87) | <0.001 | 1.04 (1.02–1.05) | <0.001 |
| Serum calcium | 0.94 (0.92–0.95) | <0.001 | 1.07 (1.04–1.09) | <0.001 |
| Hemoglobin | 0.87 (0.85–0.88) | <0.001 | 0.94 (0.91–0.96) | <0.001 |
| SBP | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.00–1.01) | <0.001 |
| DBP | 0.98 (0.98–0.99) | <0.001 | 1.01 (1.00–1.01) | 0.048 |
| Use of anti–hypertensive drug | 1.16 (1.12–1.19) | <0.001 | 1.02 (0.98–1.07) | 0.345 |
| Use of aspirin | 1.17 (1.13–1.20) | <0.001 | 1.00 (0.96–1.04) | 0.961 |
| Use of statin | 1.15 (1.12–1.19) | <0.001 | 0.99 (0.95–1.03) | 0.548 |
| ESA dose (ref: <5130 IU/week) | 1.20 (1.17–1.24) | <0.001 | 0.98 (0.94–1.03) | 0.419 |
| ERI | 1.02 (1.02–1.02) | <0.001 | 1.01 (1.01–1.01) | <0.001 |
| Use of iron | 0.92 (0.89–0.95) | <0.001 | 0.97 (0.93–1.01) | 0.110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-H.; Kim, B.-Y.; Son, E.-J.; Kim, G.-O.; Do, J.-Y. Association between Iron Status and Survival in Patients on Chronic Hemodialysis. Nutrients 2023, 15, 2577. https://doi.org/10.3390/nu15112577
Kang S-H, Kim B-Y, Son E-J, Kim G-O, Do J-Y. Association between Iron Status and Survival in Patients on Chronic Hemodialysis. Nutrients. 2023; 15(11):2577. https://doi.org/10.3390/nu15112577
Chicago/Turabian StyleKang, Seok-Hui, Bo-Yeon Kim, Eun-Jung Son, Gui-Ok Kim, and Jun-Young Do. 2023. "Association between Iron Status and Survival in Patients on Chronic Hemodialysis" Nutrients 15, no. 11: 2577. https://doi.org/10.3390/nu15112577
APA StyleKang, S.-H., Kim, B.-Y., Son, E.-J., Kim, G.-O., & Do, J.-Y. (2023). Association between Iron Status and Survival in Patients on Chronic Hemodialysis. Nutrients, 15(11), 2577. https://doi.org/10.3390/nu15112577






