Relationship between Serum Selenium Level and Self-Reported History of Kidney Stone
Abstract
1. Introduction
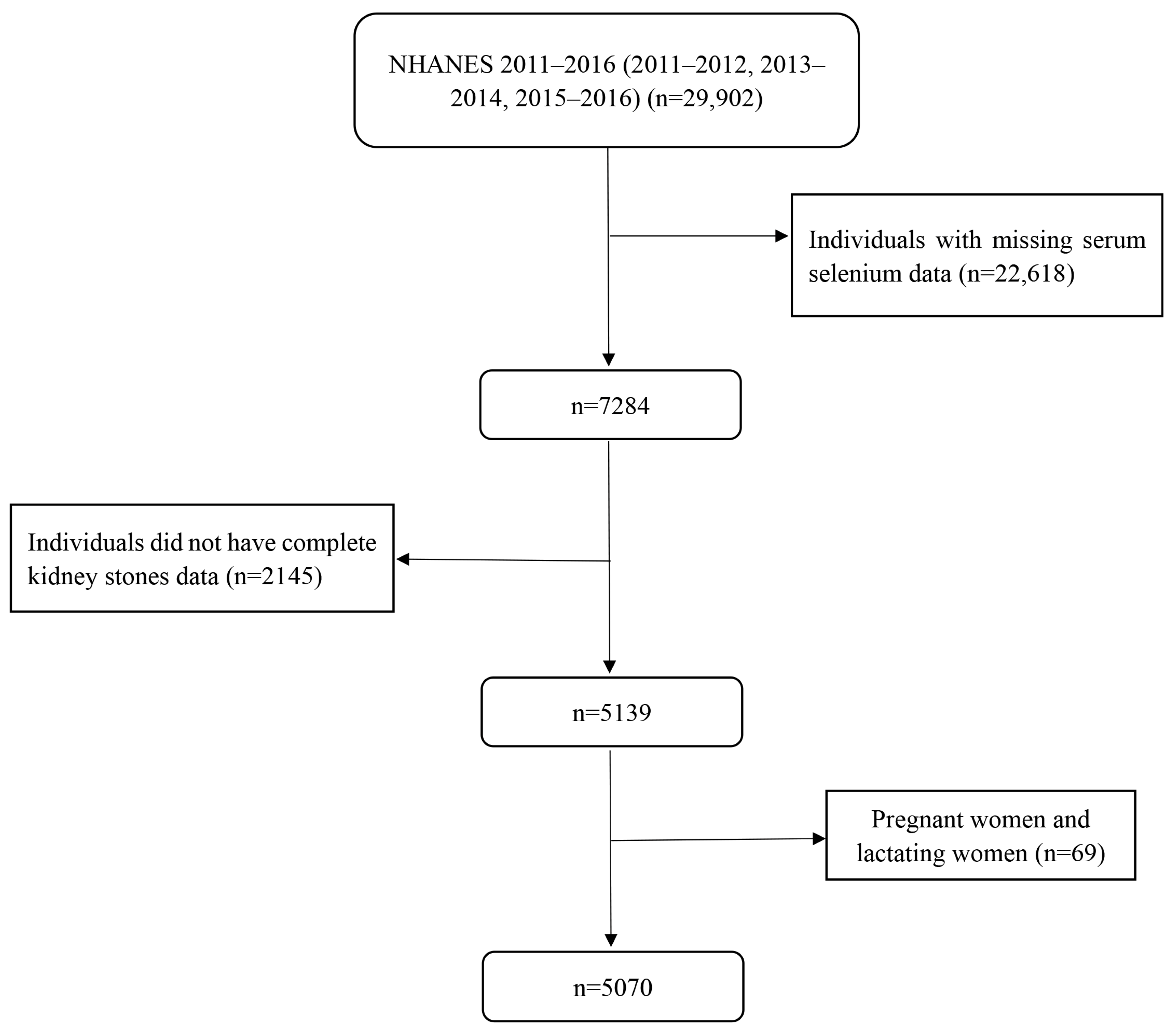
2. Materials and Methods
2.1. Study Population
2.2. Assessment of Kidney Stones
2.3. Evaluation of Serum Selenium Level
2.4. Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abufaraj, M.; Xu, T.; Cao, C.; Waldhoer, T.; Seitz, C.; D’Andrea, D.; Siyam, A.; Tarawneh, R.; Fajkovic, H.; Schernhammer, E.; et al. Prevalence and trends in kidney stone among adults in the USA: Analyses of national health and nutrition examination survey 2007–2018 data. Eur. Urol. Focus 2021, 7, 1468–1475. [Google Scholar] [CrossRef]
- Scales, C.D., Jr.; Smith, A.C.; Hanley, J.M.; Saigal, C.S. Prevalence of kidney stones in the united states. Eur. Urol. 2012, 62, 160–165. [Google Scholar] [CrossRef]
- Kartha, G.; Calle, J.C.; Marchini, G.S.; Monga, M. Impact of stone disease: Chronic kidney disease and quality of life. Urol. Clin. N. Am. 2013, 40, 135–147. [Google Scholar] [CrossRef]
- Halbritter, J. Genetics of kidney stone disease-polygenic meets monogenic. Nephrol. Ther. 2021, 17, S88–S94. [Google Scholar] [CrossRef] [PubMed]
- Abeywickarama, B.; Ralapanawa, U.; Chandrajith, R. Geoenvironmental factors related to high incidence of human urinary calculi (kidney stones) in central highlands of sri lanka. Environ. Geochem. Health 2016, 38, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.Z.; Li, C.; Xia, Q.D.; Lu, J.L.; Wan, Z.C.; Hu, L.; Lv, Y.M.; Lei, X.M.; Guan, W.; Xun, Y.; et al. Sex disparities and the risk of urolithiasis: A large cross-sectional study. Ann. Med. 2022, 54, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Al Salhi, Y.; Tasca, A.; Palleschi, G.; Fuschi, A.; De Nunzio, C.; Bozzini, G.; Mazzaferro, S.; Pastore, A.L. Obesity and kidney stone disease: A systematic review. Minerva Urol. E. Nefrol. Ital. J. Urol. Nephrol. 2018, 70, 393–400. [Google Scholar] [CrossRef]
- Khalili, P.; Jamali, Z.; Sadeghi, T.; Esmaeili-Nadimi, A.; Mohamadi, M.; Moghadam-Ahmadi, A.; Ayoobi, F.; Nazari, A. Risk factors of kidney stone disease: A cross-sectional study in the southeast of iran. BMC Urol. 2021, 21, 141. [Google Scholar] [CrossRef]
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.G. Kidney stones. Nat. Rev. Dis. Prim. 2016, 2, 16008. [Google Scholar] [CrossRef]
- Khan, A. Prevalence, pathophysiological mechanisms and factors affecting urolithiasis. Int. Urol. Nephrol. 2018, 50, 799–806. [Google Scholar] [CrossRef]
- Wagner, C.A. Etiopathogenic factors of urolithiasis. Arch. Esp. Urol. 2021, 74, 16–23. [Google Scholar]
- Siener, R. Nutrition and kidney stone disease. Nutrients 2021, 13, 1917. [Google Scholar] [CrossRef]
- Sorensen, M.D.; Kahn, A.J.; Reiner, A.P.; Tseng, T.Y.; Shikany, J.M.; Wallace, R.B.; Chi, T.; Wactawski-Wende, J.; Jackson, R.D.; O’Sullivan, M.J.; et al. Impact of nutritional factors on incident kidney stone formation: A report from the whi os. J. Urol. 2012, 187, 1645–1649. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Bargagli, M. Dietetic and lifestyle recommendations for stone formers. Arch. Esp. Urol. 2021, 74, 112–122. [Google Scholar]
- Sun, Y.; Wang, D.; Zhou, Q. Caffeine intake and the risk of recurrent kidney stones in adults, an analysis of 2007–2014 national health and nutrition examination surveys. Eur. J. Nutr. 2020, 59, 2683–2692. [Google Scholar] [CrossRef]
- Ferraro, P.M.; Curhan, G.C.; Gambaro, G.; Taylor, E.N. Total, dietary, and supplemental vitamin c intake and risk of incident kidney stones. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2016, 67, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Holoch, P.A.; Tracy, C.R. Antioxidants and self-reported history of kidney stones: The national health and nutrition examination survey. J. Endourol. 2011, 25, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Harb, A.A.; Han, D.S.; Lee, J.A.; Schulster, M.L.; Shah, O. Micronutrient inadequacy and urinary stone disease: An analysis of the national health and nutrition examination survey 2007–2018. Urolithiasis 2023, 51, 59. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Wang, D.; Zhou, Q. Dietary zinc intake, supplemental zinc intake and serum zinc levels and the prevalence of kidney stones in adults. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. (GMS) 2020, 57, 126410. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Gambaro, G.; Curhan, G.C.; Taylor, E.N. Intake of trace metals and the risk of incident kidney stones. J. Urol. 2018, 199, 1534–1539. [Google Scholar] [CrossRef]
- Matouschek, E.; Huber, R.; Schneider, J.; Vogg, H. Quantitative element investigations in urine, serum, kidney and muscle tissue of calcium oxalate stone patients. Eur. Urol. 1978, 4, 206–211. [Google Scholar] [CrossRef]
- Priyadarshini; Negi, A.; Faujdar, C.; Nigam, L.; Subbarao, N. Exploring the molecular level interaction of human serum albumin with calcium oxalate monohydrate crystals. Protein Pept. Lett. 2021, 28, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, Z.; Wei, J.; Zeng, C.; Wang, Y.; Yang, T. Association between serum magnesium and the prevalence of kidney stones: A cross-sectional study. Biol. Trace Elem. Res. 2020, 195, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Avery, J.C.; Hoffmann, P.R. Selenium, selenoproteins, and immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef]
- Köhrle, J. Selenium and the thyroid. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 392–401. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Mojadadi, A.; Au, A.; Salah, W.; Witting, P.; Ahmad, G. Role for selenium in metabolic homeostasis and human reproduction. Nutrients 2021, 13, 3256. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium-fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef]
- Flores-Mateo, G.; Navas-Acien, A.; Pastor-Barriuso, R.; Guallar, E. Selenium and coronary heart disease: A meta-analysis. Am. J. Clin. Nutr. 2006, 84, 762–773. [Google Scholar] [CrossRef]
- Wang, X.L.; Yang, T.B.; Wei, J.; Lei, G.H.; Zeng, C. Association between serum selenium level and type 2 diabetes mellitus: A non-linear dose-response meta-analysis of observational studies. Nutr. J. 2016, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Seo, Y.A.; Park, S.K. Serum selenium and non-alcoholic fatty liver disease (nafld) in u.S. Adults: National health and nutrition examination survey (nhanes) 2011–2016. Environ. Res. 2021, 197, 111190. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xue, S.; Zhang, L.; Chen, G. Trace elements and the thyroid. Front. Endocrinol. 2022, 13, 904889. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, Y. Systems biology of selenium and complex disease. Biol. Trace Elem. Res. 2019, 192, 38–50. [Google Scholar] [CrossRef]
- Vinceti, M.; Filippini, T.; Cilloni, S.; Crespi, C.M. The epidemiology of selenium and human cancer. Adv. Cancer Res. 2017, 136, 1–48. [Google Scholar]
- Sakly, R.; Chaouch, A.; el Hani, A.; Najjar, M.F. Effects of intraperitoneally administered vitamin e and selenium on calcium oxalate renal stone formation: Experimental study in rat. Ann. D’urologie 2003, 37, 47–50. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, H.; Zhong, W.; Shen, Q.; Zhuang, T.; Huang, K. Organic selenium alleviated the formation of ethylene glycol-induced calcium oxalate renal calculi by improving osteopontin expression and antioxidant capability in dogs. Biol. Trace Elem. Res. 2015, 168, 392–400. [Google Scholar] [CrossRef]
- Qi, Q.; Hu, Y.; Chen, Y.; Xu, Y.; Hao, Z. Dietary selenium intake and kidney stones in old adults: An analysis from nhanes 2011 to 2018. Biol. Trace Elem. Res. 2023, 201, 1588–1595. [Google Scholar] [CrossRef]
- Liu, M.; Cui, Z.; Chen, J.; Gao, M.; Zhu, Z.; Chen, H. Dietary selenium intake and the risk of kidney stones in adults, an analysis of 2007-2018 national health and nutrition examination survey, a cross-sectional study. Front. Nutr. 2022, 9, 877917. [Google Scholar] [CrossRef]
- Bailey, R.L.; Gahche, J.J.; Lentino, C.V.; Dwyer, J.T.; Engel, J.S.; Thomas, P.R.; Betz, J.M.; Sempos, C.T.; Picciano, M.F. Dietary supplement use in the united states, 2003–2006. J. Nutr. 2011, 141, 261–266. [Google Scholar] [CrossRef]
- Oishi, J.; Doi, H.; Kawakami, N. Nutrition and depressive symptoms in community-dwelling elderly persons in japan. Acta Med. Okayama 2009, 63, 9–17. [Google Scholar] [PubMed]
- Chen, S.; Cui, K.; Luo, J.; Zhang, D. Association of urinary iodine concentration with depressive symptoms among adults: Nhanes 2007–2018. Nutrients 2022, 14, 4165. [Google Scholar] [CrossRef]
- Kidney Conditions. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/KIQ_U_I.htm (accessed on 12 March 2023).
- Shoag, J.; Eisner, B.H. Relationship between c-reactive protein and kidney stone prevalence. J. Urol. 2014, 191, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.E.; Steck, S.E.; George, R.R.; Steffens, D.C. Fruit, vegetable, and antioxidant intakes are lower in older adults with depression. J. Acad. Nutr. Diet. 2012, 112, 2022–2027. [Google Scholar] [CrossRef]
- Huang, Z. Association between blood lead level with high blood pressure in us (nhanes 1999–2018). Front. Public Health 2022, 10, 836357. [Google Scholar] [CrossRef]
- Prohan, M.; Amani, R.; Nematpour, S.; Jomehzadeh, N.; Haghighizadeh, M.H. Total antioxidant capacity of diet and serum, dietary antioxidant vitamins intake, and serum hs-crp levels in relation to depression scales in university male students. Redox Rep. Commun. Free. Radic. Res. 2014, 19, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Beydoun, H.A.; Boueiz, A.; Shroff, M.R.; Zonderman, A.B. Antioxidant status and its association with elevated depressive symptoms among us adults: National health and nutrition examination surveys 2005–6. Br. J. Nutr. 2013, 109, 1714–1729. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Luo, J.; Zhang, L.; Kang, X.; Zhang, D. Association of pyridoxal 5’-phosphate with sleep-related problems in a general population. Nutrients 2022, 14, 3516. [Google Scholar] [CrossRef]
- Santhosh Kumar, M.; Selvam, R. Supplementation of vitamin e and selenium prevents hyperoxaluria in experimental urolithic rats. J. Nutr. Biochem. 2003, 14, 306–313. [Google Scholar] [CrossRef]
- Selvam, R. Calcium oxalate stone disease: Role of lipid peroxidation and antioxidants. Urol. Res. 2002, 30, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Thamilselvan, S.; Byer, K.J.; Hackett, R.L.; Khan, S.R. Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to llc-pk1 and mdck cells. J. Urol. 2000, 164, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Costa-Bauzá, A.; Bonarriba, C.R.; Pieras, E.C.; Fernández, R.A.; Rodríguez, A. On the origin of calcium oxalate monohydrate papillary renal stones. Urolithiasis 2015, 43 (Suppl. 1), 33–39. [Google Scholar] [CrossRef] [PubMed]

| Classification | Covariates |
|---|---|
| No; Yes | Smoker a, drinker b, hypertensive c, diabetic d, stroke patient e |
| Continuous | Protein, water, calcium, magnesium, potassium, and sodium intake (mg/day) |
| Vigorous f; moderate g; other | Occupational and recreational physical activity |
| Mexican American; other Hispanic; non-Hispanic White; non-Hispanic Black; other race | Race |
| 20–39; 40–59; ≥60 | Age (year) |
| ≤0.99; and ≥1 | Poverty–income ratio (PIR) |
| Male; female | Sex |
| Married/living with partner; widowed/divorced/separated/never married | Marital status |
| ≤25; 25 to <30; ≥30 | Body mass index (BMI) (kg/m2) |
| Trait | Ever Had Kidney Stones (No) | Ever Had Kidney Stones (Yes) | p-Value |
|---|---|---|---|
| Number of participants (%) a | 4580 (90.34) | 490 (9.66) | |
| Age (year) a | 0.0006 | ||
| 20–39 | 1639 (36.71) | 104 (21.87) | |
| 40–59 | 1521 (36.95) | 170 (43.52) | |
| ≥60 | 1420 (26.34) | 216 (34.61) | |
| PIR (%) a | 0.4001 | ||
| <1 | 923 (13.91) | 117 (15.56) | |
| ≥1 | 3657 (86.09) | 373 (84.44) | |
| Sex (%) a | 0.0334 | ||
| Male | 2249 (47.95) | 285 (55.67) | |
| Female | 2331 (52.05) | 205 (44.33) | |
| Material status (%) a | 0.5484 | ||
| Married/living with partner | 2885 (66.45) | 310 (68.42) | |
| Widowed/divorced/separated/never married | 1667 (33.55) | 176 (31.58) | |
| Educational level (%) a | 0.1549 | ||
| <High school | 970 (15.17) | 126 (18.54) | |
| High school | 956 (19.88) | 106 (20.70) | |
| >High school | 2478 (64.95) | 246 (60.76) | |
| Race/ethnicity (%) a | 0.0001 | ||
| Mexican American | 629 (8.79) | 62 (5.85) | |
| Other Hispanic | 489 (6.31) | 65 (6.21) | |
| Non-Hispanic White | 1705 (65.30) | 245 (75.93) | |
| Non-Hispanic Black | 1028 (11.42) | 63 (5.82) | |
| Other races | 729 (8.18) | 55 (6.19) | |
| BMI (kg/m2) (%) a | 0.0013 | ||
| <25 | 1381 (30.51) | 109 (21.79) | |
| 25 to <30 | 1453 (33.32) | 154 (31.40) | |
| ≥30 | 1688 (36.17) | 223 (46.81) | |
| Work activity (%) a | 0.6106 | ||
| Vigorous | 839 (20.97) | 89 (22.65) | |
| Moderate | 923 (22.31) | 85 (20.16) | |
| Other | 2814 (56.72) | 315 (57.19) | |
| Leisure activity (%) a | 0.0151 | ||
| Vigorous | 1106 (27.56) | 76 (18.90) | |
| Moderate | 1165 (27.68) | 125 (30.82) | |
| Other | 2306 (44.76) | 289 (50.28) | |
| Alcohol consumption (%) a | 2960 (78.42) | 345 (79.85) | 0.5554 |
| Smoker (%) a | 1929 (42.86) | 253 (53.04) | 0.0036 |
| Stroke (%) a | 157 (2.51) | 24 (2.38) | 0.8284 |
| Diabetes (%) a | 810 (17.69) | 162 (33.06) | <0.0001 |
| Hypertension (%) a | 2482 (50.57) | 320 (62.76) | 0.0002 |
| Serum selenium (μg/L) b | 128.05 (21.6) | 127.8 (22.1) | 0.9501 |
| Water intake (gm/d) b | 2468.75 (1261) | 2380 (1201) | 0.3692 |
| Protein intake (gm/d) b | 74.445 (41.49) | 72.145 (41.65) | 0.2648 |
| Calcium intake (mg/d) b | 822.25 (562.5) | 826.5 (508.5) | 0.4579 |
| Magnesium intake (mg/d) b | 276 (152) | 259 (157.5) | 0.0162 |
| Potassium intake (mg/d) b | 2473 (1255) | 2381 (1200.25) | 0.0334 |
| Sodium intake (mg/d) b | 3136.5 (1758.5) | 3135.5 (1569) | 0.6083 |
| Cases/Participants | Crude a | Model 1 b | Model 2 c | |
|---|---|---|---|---|
| Serum selenium (μg/L) | ||||
| Q1 (<118.1) | 126/1276 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Q2 (118.1 to <128.1) | 121/1261 | 0.97 (0.75–1.26) | 0.82 (0.56–1.20) | 0.78 (0.51–1.21) |
| Q3 (128.1 to <139.7) | 122/1272 | 0.97 (0.75–1.26) | 0.74 (0.48–1.13) | 0.67 (0.42–1.08) |
| Q4 (≥139.7) | 121/1261 | 0.97 (0.75–1.26) | 0.58 (0.38–0.90) * | 0.54 (0.33–0.88) * |
| Serum Selenium | Cases/Participants | Odds Ratio a | 95% CI | p-Value |
|---|---|---|---|---|
| Men | ||||
| Q1 (<118.1) | 63/515 | 1 | 1 | |
| Q2 (118.1 to <128.1) | 66/600 | 0.826 | 0.41–1.65 | 0.581 |
| Q3 (128.1 to <139.7) | 75/663 | 0.881 | 0.48–1.62 | 0.680 |
| Q4 (≥139.7) | 81/756 | 0.596 | 0.31–1.14 | 0.115 |
| Women | ||||
| Q1 (<118.1) | 63/761 | 1 | 1 | |
| Q2 (118.1 to <128.1) | 55/661 | 0.770 | 0.45–1.31 | 0.327 |
| Q3 (128.1 to <139.7) | 47/609 | 0.499 | 0.24–1.03 | 0.059 |
| Q4 (≥139.7) | 40/505 | 0.528 | 0.24–1.03 | 0.059 |
| Serum Selenium | Cases/Participants | Odds Ratio a | 95% CI | p Value |
|---|---|---|---|---|
| 20–39 years old | ||||
| Q1 (<118.1) | 28/487 | 1 | 1 | |
| Q2 (118.1 to <128.1) | 30/468 | 0.724 | 0.35–1.49 | 0.347 |
| Q3 (128.1 to <139.7) | 27/406 | 1.068 | 0.46–2.47 | 0.875 |
| Q4 (≥139.7) | 19/382 | 0.494 | 0.24–1.03 | 0.059 |
| 40–59 years old | ||||
| Q1 (<118.1) | 43/430 | 1 | 1 | |
| Q2 (118.1 to <128.1) | 40/397 | 0.899 | 0.44–1.83 | 0.765 |
| Q3 (128.1 to <139.7) | 43/450 | 0.364 | 0.19–0.69 | 0.003 |
| Q4 (≥139.7) | 44/414 | 0.585 | 0.27–1.27 | 0.172 |
| ≥60 years old | ||||
| Q1 (<118.1) | 55/359 | 1 | 1 | |
| Q2 (118.1 to <128.1) | 51/396 | 0.757 | 0.38–1.49 | 0.412 |
| Q3 (128.1 to <139.7) | 52/416 | 1.239 | 0.59–2.61 | 0.564 |
| Q4 (≥139.7) | 58/465 | 0.657 | 0.31–1.40 | 0.271 |
| Serum Selenium (ug/L) | Odds Ratio | 95% CI |
|---|---|---|
| 75 | 0.57 | (0.44–0.76) |
| 85 | 0.41 | (0.27–0.64) |
| 95 | 0.30 | (0.16–0.55) |
| 105 | 0.22 | (0.10–0.46) |
| 115 | 0.16 | (0.06–0.40) |
| 125 | 0.12 | (0.04–0.35) |
| 135 | 0.11 | (0.04–0.34) |
| 145 | 0.11 | (0.04–0.34) |
| 155 | 0.12 | (0.04–0.36) |
| 166 | 0.13 | (0.04–0.39) |
| 175 | 0.14 | (0.05–0.42) |
| 187 | 0.16 | (0.06–0.47) |
| 197 | 0.18 | (0.06–0.52) |
| 207 | 0.19 | (0.07–0.57) |
| 218 | 0.22 | (0.07–0.65) |
| 224 | 0.23 | (0.08–0.69) |
| 230 | 0.24 | (0.08–0.74) |
| 258 | 0.31 | (0.09–1.06) |
| 262 | 0.33 | (0.10–1.12) |
| 278 | 0.38 | (0.10–1.39) |
| 298 | 0.46 | (0.11–1.85) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, A.; Wang, N.; Zhang, D.; Wen, J.; Wang, W. Relationship between Serum Selenium Level and Self-Reported History of Kidney Stone. Nutrients 2023, 15, 2549. https://doi.org/10.3390/nu15112549
Wang A, Wang N, Zhang D, Wen J, Wang W. Relationship between Serum Selenium Level and Self-Reported History of Kidney Stone. Nutrients. 2023; 15(11):2549. https://doi.org/10.3390/nu15112549
Chicago/Turabian StyleWang, Anni, Ningrui Wang, Dongfeng Zhang, Jing Wen, and Weijing Wang. 2023. "Relationship between Serum Selenium Level and Self-Reported History of Kidney Stone" Nutrients 15, no. 11: 2549. https://doi.org/10.3390/nu15112549
APA StyleWang, A., Wang, N., Zhang, D., Wen, J., & Wang, W. (2023). Relationship between Serum Selenium Level and Self-Reported History of Kidney Stone. Nutrients, 15(11), 2549. https://doi.org/10.3390/nu15112549






