Effectiveness of Lifestyle Nutrition and Physical Activity Interventions for Childhood Obesity and Associated Comorbidities among Children from Minority Ethnic Groups: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria for Studies
2.3. Study Selection, Quality Assessment and Data Extraction
2.3.1. Selection Process
2.3.2. Risk of Bias in Individual Studies
2.3.3. Data Extraction Process (Selection and Coding)
2.4. Data Synthesis
2.5. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Characteristics of Included Studies
| Study | Study Design, Setting and Sample | Study Participants (Sample, Ethnicity and Age) | Intervention (Type, Duration, Frequency and Theory Base) | Comparator Control | Main Results | Comments on Key Strengths/Limitations |
|---|---|---|---|---|---|---|
| Yli-Piipari et al., 2018, USA [50]. | Quasi-experimental one-arm pre- and post-test design, conducted in a primary care setting. | 22 high-risk overweight and obese Hispanic children (BMI ≥85th United States CDC BMI percentile for age and sex); Mean age 11.7 years; 27% female. | 12 weeks PA and nutrition behaviour programme: 60 min twice per week (for a total of 24 h) of moderate- to vigorous-intensity boxing exercise, 12 h of nutrition education for guardians and a 30-min paediatrician appointment. | None | BMI (kg/m2) change: t(15) = −2; BMI% change: t(15) = −2.53, p = 0.023, d = 0.20, p = 0.044, d = 0.5; BM z-score change: t(15) = −3.64, p = 0.002, d = 0.19; WC change: t(17) = −2.57, p = 0.020; Fasting glucose change: t(15) = −6.43, p < 0.001, d = 1.67. | In Hispanic children with severe obesity, the multicomponent supervised exercise and nutrition intensive programme was effective in the short term, reducing obesity and metabolic risk (fasting glucose). However, long-term adherence to this programme is unknown. |
| Yin et al., 2012, USA [49]. | Quasi-experimental pre- and post-test design with two groups, conducted in community Head Start centres and home settings. | 384 predominantly Hispanic children; 52% female; attending community Head Start centres; aged 3 to 5 (mean = 4.1) years. | 18-week PA and nutrition intervention. Centre-based intervention: (i) PA: 60 min of structured (15–20 min) and free play (30–45 min) per day; (ii) Nutrition promotion. Home-based intervention: (i) Peer-led parent obesity education; (ii) Healthy snacks for their children (<150 calories). | The control group received the intervention materials and implementation training upon completing the study. | Adjusted difference in BMI z-score for age and gender between centre-based + home-based intervention and comparator = –0.09, p < 0.09; Adjusted difference in BMI z-score for age and gender between comparison and centre-based intervention = −0.04 (not significant). | This large study with intervention in both centre and home settings targeting both PA and nutrition showed improvements in BMI z-scores, though not statistically significant. The participants were children not described as overweight or obese; therefore, nonsignificant reductions in zBMI were to be expected. |
| Yin et al., 2005, USA [51]. | Quasi-experimental pre- and post-test design with two groups, conducted in an elementary school setting. | 601 predominantly Black (61%) elementary school children; Mean age 8.7 years; 52% female. | 24-week (8-month) after-school programme: (i) 40 min academic enrichment; (ii) Healthy snack; (iii) 80 min of PA. | 265 children served as the control, who only received health screening (no after-school activities). | BMI (kg/m2) change: −0.16 (−0.40, 0.07), p = 0.18; % Body fat (BF): 0.76 (1.42, 0.09), p = 0.027; Fat mass (FM) (kg): −0.29 (−0.70, 0.13), p = 0.17; Free fat mass (FFM) (kg): 0.18 (−0.04, 0.40), p = 0.12; WC (cm): −0.4 (−1.1, 0.4), p = 0.32; SBP (mmHg): −1.8 (−4.2, 0.6), p = 0.15; DBP (mmHg): −1.1 (−3.6, 1.5), p = 0.41; TC (mg/dl): −0.2 (−6.2, 5.7), p = 0.94; HDL (mg/dl): 0.7 (−2.1, 3.5), p = 0.64. | After-school intervention programme had some effects on BMI, body fat and lipid profile in Black communities, but not statically significant. The interventions were difficult to adhere to in home and community settings because they lacked parental involvement. |
| Wylie-Rosett et al., 2018, USA [52]. | RCT, conducted in a safety-net paediatric primary care setting in the Bronx, New York. | 360 predominantly Hispanic (73%) children with BMI ≥85th United States CDC BMI percentile for age and sex; aged 7 to 12 (mean = 9.3) years; 33% female. | A 12-month 8-weekly programme: (i) Standard care; (ii) Enhanced programme (skill building core: food preparation or other skill activity for parents/guardians and children, PA session for the children and discussion session for parents/guardians regarding their role in weight management + post-core programme support) | Standard care participants had quarterly visits to see a paediatrician for weight management. | BMI Z-score change: The mean BMI Z-score decreased in both programmes (0.12 kg within the standard care group (p < 0.01) and 0.15 kg within both standard care + enhanced care groups (p < 0.01). No significant differences between the two programmes. Older children had a greater decline in BMI Z-score than younger children (beta = −0.04 units per additional year of age; p < 0.01). Girls exhibited a greater decline in BMI Z-score than boys (β = 0.09; p = 0.03). TC (mmol/L) change: −0.1, p = 0.05; HDL (mmol/L) change: 0.01, p = 0.67; LDL (mmol/L): −0.07, p = 0.04; Triglyceride (mmol/L): −0.06, p = 0.08. | In high-risk overweight/obese children, the enhanced care programme was not more effective than the standard care programme, although the clinical care in both groups reduced weight and improved lipid profiles. |
| Wong et al., 2016, USA [53]. | A non-randomised trial, conducted in community centres located in low-income neighbourhoods within the city. | 877 Hispanic and African American children; aged 9 to 12 years; 47% female. | 9-month programme: (i) 90 min of structured PA twice a week for 6 weeks in the fall, early spring and at the end of the school year; (ii) 30 min of nutrition or healthy habits lessons twice a week during each of the three 6-week sessions. | Regular after-school childcare enrichment programmes at community centres offered by the site staff, such as homework time, arts and crafts activities and supervised free play. | There were no significant intervention effects on BMI (p = 0.94), BMI z-score (p = 0.88) or BMI percentile (p = 0.23). | Structured 90 min PA plus nutrition education was not more effective than supervised free play in reducing weight but helped to enhance regular exercise. |
| Wilson et al., 2022, USA [54]. | RCT; Online setting. | 241 African American child/care giver dyads; Children aged 11 to 16 years, with BMI ≥85th United States CDC BMI percentile for age and sex. | 24-week (6-month) programme: (i) 8-week tailored online education on parenting, nutrition, PA and decreasing screen time. This was followed by 3 online booster sessions, 1 every 2 months. | Control online programme | There were no significant intervention effects on BMI but there was a significant effect of the group intervention on light physical activity among the parents at 16 weeks (B = 33.017, SE = 13.115, p = 0.012) and a similar trend for adolescents. | The online programme was not effective for BMI but had a useful impact on physical activity. Actual data were not shown on BMI mean differences between intervention and comparators for both children and parents. |
| Williford et al., 1996, USA [55]. | Quasi-experimental with pre- and post-test analyses, conducted in a school setting. | 17 African American male children in 7th grade from a physical education class; Aged 11 to 13 (mean = 12.8) years. | 15-week programme: 5 days/week for 45 min session of PE class + conditioning programme (aerobic training on 3 days and weight training on 2 days). | PE class as usual | Sum of 7 skin fold thickness (mm): 99.01 ± 67.8 to 97.7 ± 67.4, p = 0.09; TC (mmol/L): 4.03 ± 0.81 to 4.03 ± 0.77, p = 0.98; HDL (mmol/L) change intervention group: 1 ± 0.18 to 1.28 ± 0.17, p < 0.05; LDL (mmol/L) change intervention group: 2.73 ± 0.74 to 2.41 ± 0.81, p < 0.05 | Small improvement in HDL and LDL from the PA intervention. However, the sample was small and it was not clear how effective this PA alone intervention was on overweight/obesity. |
| Williamson et al., 2006, USA [56]. | RCT; Internet-based interactive behaviour therapy. | 57 African American girls; Aged 11 to 15 (mean = 13.2) years, with BMI >85th percentile for age and gender based on 1999 National Health and Nutrition Examination Study normative data and with a biological parent with BMI > 30. | A 96-week (24-month) Internet programme: (i) An interactive behavioural Internet programme; (ii) Face-to-face sessions and e-mail correspondence from a counsellor. | An Internet health education programme (a passive (non-interactive) programme that provided useful health education for parents and adolescents through electronic links to other health-related websites.) | BMI, F (3,54) = 3.13, p < 0.04; BF % change: 0.08 ± 0.71 vs. 0.84 ± 0.72 BF, p < 0.05. | The Internet-based intervention was effective in reducing weight in overweight/obese girls. However, the girls appeared to be a highly motivated group as they were willing to purchase their own computers for at least $300.00. |
| Van der Heijden et al. 2010, USA [57]. | Quasi-experimental with pre- and post-test analyses; recruitment was conducted in a community setting, while checks for good health were carried out in hospital. | 29 Hispanic adolescents; Median age 15 years, obese and lean (obese participants had BMI > 95th and lean participants had BMI <85th percentile for age, according to CDC growth chart; 48% females. | A 12-week PA programme supervised by an experienced exercise physiologist: (i) 30 min aerobic exercise session at ≥70% of peak oxygen consumption (VO2peak) twice a week at a hospital physical therapy unit. | None | In obese participants, intramyocellular fat remained unchanged, whereas hepatic fat content decreased from 8.9 ± 3.2 to 5.6 ± 1.8% (p < 0.05) and visceral fat content from 54.7 ± 6.0 to 49.6 ± 5.5 cm2 (p < 0.05). No significant changes were observed in lean participants. Insulin resistance: Decreased fasting insulin (21.8 ± 2.7 to 18.2 ± 2.4 μ/mL, p < 0.01) and homeostasis model assessment of insulin resistance (HOMAIR) (4.9 ± 0.7 to 4.1 ± 0.6, p < 0.01). No significant changes were observed in lean participants. | Aerobic exercise in a controlled environment reduced hepatic fat, visceral fat and insulin resistance in obese participants. The sample was small and involved selected individuals with severe adiposity; therefore, the results may not be generalisable. |
| Tomayko et al., 2018, USA [58]. | A modified crossover design, conducted in 4 tribal reservations and 1 urban clinic setting. | 450 American Indian adult/child dyads; Children were aged 2 to 5 (mean = 3.3) years; 50% female. | A 52-week (12-month) programme: Monthly mailed healthy lifestyle lessons, items and children’s books addressing six targets: increased fruit and vegetable consumption, decreased sugar consumption, increased PA, decreased screen time, improved sleep habits and decreased stress (adults only). | Active control-crossover. | BMI z-score at 1 year: Intervention = 0.80 ± 1.10; Comparator = 0.76 ± 1.04, p = 0.513. | The unsupervised mailed education materials were not effective in reducing BMI z-scores. The extent to which the materials were used was unknown. |
| Taveras et al., 2017, USA [59]. | RCT, conducted in 6 paediatric practices in an urban setting. | 721 predominantly (65%) non-White children; Children aged 2 to 12 (mean = 8) years, with BMI ≥85th percentile for age, according to CDC growth chart; 51% females. | A 12-month programme: Enhanced primary care plus contextually tailored individual health coaching lasting 15–20 min via telephone, videoconference (Vidyo) or in-person visits. | Enhanced primary care Two monthly educational materials focusing on healthy lifestyle behavioural changes. | BMI z-score: In the enhanced primary care group, there was an adjusted mean (SD) BMI z-score improvement of −0.06 BMI z-score units (95% CI = −0.10 to −0.02) from baseline to 1 year. In the enhanced primary care plus coaching group, there was an improvement of −0.09 BMI z-score units (95% CI = −0.13 to −0.05). However, there was no significant difference between the 2 intervention arms (difference = −0.02; 95% CI = −0.08 to 0.03; p = 0.39). | Advanced clinical care improved BMI z-scores in high-risk children (overweight/obese), but additional individual coaching did not add any effect. |
| Story et al., 2012, USA [60]. | RCT, conducted in schools in reservations. | 454 American Indian children attending Kindergarten and first grade; Mean age 5.8 years; 49% female. | A 45-week programme: (i) PA: school-based PA of at least 60 min daily; (ii) Nutrition: healthy eating at school; (iii) Family-focused intervention: improving nutrition and PA and reducing sedentary lifestyle; (iv) Parents received telephone motivational encouragement. | Usual school activities and no change in family environment. | Mean BMI (kg/m2) net difference (I vs. C): 0.34, p = 0.057; zBMI net difference: 0.01, p = 0.904. %BF net difference: 0.9, p = 0.122; Prevalence of overweight (BMI ≥85th percentile and <95th): net difference: 10.14, p = 0.019; Prevalence of obesity (BMI ≥95th percentile): 2.11, p = 0.503. | Interestingly, this multicomponent programme reduced the prevalence of overweight children, although participants were young children and not described as overweight. |
| Stolley et al., 2003, USA [61]. | RCT, conducted in public school settings. | 618 African American preschool children; Aged 3 to 5 (mean = 4.3) years; 53% female. | A 14-week programme: (i) Education: two lessons each week on healthy eating and exercise; (ii) Two 20 min session of PA each week; (iii) Parents received a weekly newsletter. | Usual preschool activities. | Adjusted BMI (kg/m2) difference: −0.08, p = 0.28; Adjusted BMI z-scores: −0.05, p = 0.23. | This predominantly nutrition and PA education intervention reduced BMI z-scores but was not more effective than usual school activities. |
| Soltero et al., 2018, USA [62]. | RCT, recruitment conducted through schools, community centres and healthcare organisations but intervention was administered at YMCA centres. | 160 Hispanic children; Aged 14 to 16 years, with BMI ≥95th percentile for age and sex, according to CDC growth chart, or a BMI ≥ 30 kg/m2; 46% females. | A 52-week (12 months) programme: (i). Nutrition and health education one days/week, 60 min. (ii). PA: exercise curriculum was delivered by fitness instructors three days/week for 60 min (iii). Behaviour changes strategies. | Handout with general information on healthy lifestyle behaviours. | Changes in insulin sensitivity (using insulin and glucose sensitivity during OGTT): Intervention: 0.8 ± 0.1 to 2.2 ± 0.1, p < 0.01; Comparator: 1.7 ± 0.2 to 1.7 ± 0.1, p > 0.05. Between-group difference (delta difference) Δ = 0.37, p < 0.05, at 12 weeks; Δ = 0.21, p > 0.05, at 12 months (no difference). Within-group changes in intervention group at 12 months: BMI (kg/m2) = 1.16, p < 0.001; BMI% = −0.1, p = 0.95; %BF = −0.63, p = 0.65; WC (cm) = 1.68, p = 0.29. At 12 months, between-group differences in BMI and percentage body fat remained significant (all p < 0.01); however, changes in WC was not (p = 0.078). | This seemed to be effective in increasing insulin sensitivity in the short term but there was no difference in the long term in this high-risk group with obesity. The intervention was shown to be effective in reducing adiposity parameters, which were sustained at 12 months. This long duration nutrition education and PA intervention improved insulin resistance, but only in the short term. |
| Slusser et al., 2012, USA [63]. | RCT, conducted in a family clinic and wellness centre and community sites, serving a low-income predominantly Hispanic community. | 161 Hispanic children; Aged 2 to 4 years, living in the home. | A 17-week programme: 9 sessions lasting 90 min of parent training, based on social learning theory. | Care as usual and a standard nutritional informational pamphlet. | BMI percentile changes: Intervention = −3.85; Comparator = 1.33. BMI z-score difference between intervention group and control: −2.4, p = 0.04. Children in the intervention group decreased their BMI z-scores significantly; on average, by 0.20 (se = 0.08) compared to children in the control group, who increased z-scores on average by 0.04 (se = 0.09) at 1 year (p < 0.05). | Only 9 sessions of parent training over 17 weeks were effective in reducing overweight/obesity in preschool children from low-income families. It was not clear whether this would be the same in a larger population or over a longer period. |
| Shaibi et al., 2006, USA [64]. | RCT; Participants were recruited through medical clinics, advertisements and local schools, while the intervention was conducted at girls’ and boys’ clubs. | 22 Hispanic male adolescents; Mean age = 15.3 years, with BMI ≥85th percentile for age, according to CDC growth chart. | A 17-week programme: (i) resistance training twice per week. | Non-exercising control group. | Changes in insulin sensitivity (×10−4 min−1 mL−1, using insulin and glucose sensitivity during OGTT): I = 0.9 ± 0.1, p < 0.05; C = 0.1 ± 0.3. The intervention group significantly increased insulin sensitivity compared to the comparator group (p < 0.05). | Resistance training alone significantly reduced the metabolic risk factor of insulin sensitivity within 3 months in overweight/obese children. However, its effect on adiposity was not reported. |
| Robinson et al., 2021, USA [65]. | RCT, recruitment was conducted through medical clinics, advertisements and schools, while the administration of the intervention was carried out at the Los Angeles Boys’ and Girls’ Club. | 241 primarily Hispanic children; Aged 7 to 11 years, with BMI ≥85th percentile for age and sex, according to CDC growth chart; 56% females. | A 3-year community-based, multilevel, multi-setting, multicomponent programme: (i) Home environment changes and behavioural counselling; (ii) Community after-school team sports; (iii) Reports to primary health-care providers. | General health education. | Mean adjusted difference in BMI trajectory over 3 years between multicomponent and HE = −0.25 (95% CI = −0.90, 0.40) kg/m2, Cohen’s d = −0.10, p = 0.45. | The multicomponent and multilevel intervention did not reduce BMI gain in low-socioeconomic overweight Hispanic children, despite the long duration of intervention. However, there was a drop in participation over time. |
| Rieder et al., 2013, USA [66]. | Quasi-experimental with pre- and post-test analyses, conducted in a community setting. | 349 majority minority ethnic group (52% Black and 44% Hispanic); Mean age = 15 years; 54% females. | A 9-month programme: (i) Teaching healthy lifestyle principles; (ii) 60 min per week of moderate PA; (iii) Monthly family healthy behaviour education. | No comparator intervention. | Decreases in BMI (kg/m2) (−0.07 per month; p < 0.001); Percent overweight (−0.002%/month; p < 0.001); BMI z-score (−0.003/month; p < 0.01); Decrease in BMI percentile (−0.006 percentile/month; p = 0.06). | This 9-month education and PA showed a small effect in reducing overweight/obesity in adolescents. However, their pre-intervention weights were unknown. |
| Resnicow et al., 2005, USA [67]. | RCT, conducted in churches in a rural setting. | 147 African American female children; Aged 12 to 16 years, with BMI >90th percentile for age and sex, according to CDC growth chart. | A 26-week (6-month) multicomponent programme tailored to the population: High-intensity PA (24 to 26 sessions). (i) At least 30 min of moderate to vigorous PA; (ii) Preparation and/or consumption of low-fat, portion-controlled meals or snacks; (iii) Parental involvement. | Moderate-intensity intervention. Six sessions of education, with topics including fat facts, barriers to physical activity, fad diets, neophobia (i.e., the fear of new foods) and the benefits of PA. | 0.5 BMI units of difference. This difference was not statistically significant (p = 0.20). | There was no difference between high-intensity and moderate-intensity PA over 6 months in the group of obese African American adolescent girls. However, both groups showed some improvements in adiposity. |
| Prado et al., 2020, USA [68]. | RCT, conducted in a community setting. | 22 Hispanic children; Mean age = 13.1 years (in 7th/8th grade), with BMI >85th percentile for age and sex, according to CDC growth chart; 88% females. | A 12-week programme of 2.5-h sessions: 1.5 h of lifestyle education, involving families and children, and 1 h of PA for the children. PA was supervised by a coach in local park. | Prevention as usual. Participants were referred to their local health department’s health initiative Internet page and the usual programmes they offer to reflect the typical services that overweight and obese adolescents may receive in their own community. | BMI (kg/m2) difference between baseline and 2 years: −0.3 (95% CI = −0.7 to 0.1), p = 0.15 (not significant). | The small sample and short duration of this lifestyle education intervention had no effect and the results are not generalisable because of the small sample. |
| Polonsky et al., 2019, USA [69]. | RCT, conducted in communities and schools. | 1362 predominantly Black 4th through 6th grade students; Mean age = 10.8 years; 51% females. | A 2-year programme: Free school breakfasts and 18 45-min sessions of nutrition education, plus items with a healthy breakfast logo. | Control schools served breakfasts free of charge in the cafeterias before school and existing SNAP-Ed nutrition education continued. | There was no significant difference in the combined incidence of overweight and obesity between intervention schools (11.7%) and control schools (9.1%) after 2.5 years (odds ratio (OR) = 1.42; 95% CI = 0.82–2.44; p = 0.21). | Healthy school breakfasts and education alone without PA or home environment changes were not shown to be effective in preventing overweight or obese children. Moreover, the incidence of overweight and obese children was slightly higher in the intervention group. |
| Pena et al., 2022, USA [70]. | RCT, conducted in community YCMA centres. | 117 Hispanic youths; Aged 12 to 16, with prediabetes (fasting glucose 100 to 125 mg/dL or HbA1c level of 5.7% to 6.4%) and BMI >95th percentile for age and sex, according to CDC growth chart; 40% females. | A 52-week (12-month) programme: (i) One day/week of nutrition and health education with behaviour change skills training; (ii) Three days/week of physical activity. | Comparator group met with a paediatric endocrinologist and a bilingual, bicultural registered dietitian to discuss laboratory results and develop SMART goals for making healthy lifestyle changes. | The intervention led to significant decreases in mean 2-h glucose level (baseline: 144 mg/dL; 6 months: 132 mg/dL; p = 0.002) and increases in mean insulin sensitivity (baseline: 1.9 (0.2); 6 months: 2.6 (0.3); p = 0.001). | The 1-year education and structured PA intervention was effective in decreasing NCD metabolic risk in a high-risk group. However, there was no information on its effect on overweight/obesity. |
| Novotny et al., 2015, USA [71]. | RCT, conducted in a clinical setting. | 85 predominantly Asian children; Aged 5 to 8 years, with BMI between the 50th and 99th percentile for age and sex, according to CDC growth chart; 62% females. | A 39-week (9-month) programme: (i) Handout on recommended eating patterns and Dietary Approaches to Stop Hypertension (DASH) of Aloha cookbook; (ii) Farmers’ market locations; (iii) A PA location/map inthe study informational packet. | Received a welcome letter and attention control mailings on unrelated health topics, such as the importance of hand washing, sun protection and dental hygiene, at 2, 5 and 8 months. | There was no significant effect of the DASH intervention on changes in BMI z-score, SBP, waist circumference, total body fat by skinfolds, PA level or total Healthy Eating Index score (p > 0.05). DBP percentile was 12.2 points lower in the treatment group than the control group (p = 0.01). | The only effect was on DBP. However, as participants were in a clinical setting, there could have been ongoing clinical care. |
| Norman et al., 2016, USA [72]. | RCT, conducted in a clinical setting. | 106 predominantly Hispanic (82%) children; Aged 11–13 years, with BMI >95th percentile for age and sex, according to CDC growth chart; 51% females. | A 17-week (4-month) ‘steps’ programme, beginning with the most intensive contact followed by reduced contact if treatment goals were met. Based on the Chronic Care Model and social cognitive theory: (i) Counselling by a physician on healthy dietary and PA changes; (ii) Health educator visits to discuss weight management, barriers to healthy eating and PA; (iii) Follow-up phone calls. | Participants received an initial counselling visit by the physician, one visit with a health educator, materials on how to improve weight-related behaviours and monthly follow-up mailings on weight-related issues. | BMI (kg/m2) change differences between the intervention group and comparator: boys = 1.3, p = 0.003; girls = 0.7, p = 0.15. zBMI change differences between the intervention group and comparator: boys = 0.1, p = 0.008; girls = −0.2, p = 0.42. BF (kg): no difference (boys: p = 0.26; girls: p = 0.11. Fasting lipid profile and BP: no difference. | The intervention was shown to be effective in reducing BMI among boys but not girls. However, the intervention was tested in the age group of 11 to 13 years, a period of growth spurts in girls. |
| Messito et al., 2020, USA [46]. | RCT, conducted in a clinical setting. | 643 Hispanic pregnant mothers with a single uncomplicated pregnancy and postpartum infants; 54% of the infants were female. | 33-month programme based on social cognitive theory to promote healthy behaviours: (i) Prenatal nutrition counselling; (ii) Postpartum lactation support; (iii) Nutrition and parenting support groups coordinated with paediatric visits. | Standard prenatal, postpartum and paediatric primary care. | Intervention infants had significantly lower mean weight for age z-scores at 18 months (0.49 vs. 0.73, p = 0.04) and 2 years (0.56 vs. 0.81, p = 0.03) but not at 3 years (0.63 vs. 0.59, (p = 0.76). Obesity prevalence was not significantly different between groups at any age point (33.5% vs. 39.4%, p = 0.11). | The intervention targeting mothers was only effective up to 18 months and was not sustained at 3 years. |
| Johnston et al., 2007, USA [73]. | RCT, conducted in a school serving an urban student population. | 60 Mexican American children; Aged between 10 and 14 years, with BMI ≥85th percentile for age and sex, according to CDC growth chart; 45% females. | A 6-month programme: (i) A 12-week instructor/trainer-led PA intervention 4 days per week, lasting 35 to 40 min at the school location; (ii) Nutrition instruction (1 day/week); (iii) Monthly meetings for parents to teach them how to adapt family meals and activities to facilitate healthy changes. | 6-month parent-guided manual intended to promote child weight loss and the long-term maintenance of changes. | zBMI in the intervention group significantly reduced compared to the comparator group (F = 11.72, p < 0.001), with significant differences in zBMI at both 3 and 6 months (F = 16.50, p < 0.001 and F = 22.01, p < 0.001, respectively). Children in the intervention group significantly reduced their total cholesterol (F = 5.27, p = 0.027) and LDL cholesterol (F = 7.43, p = 0.01) compared to the children in the comparison group at 6 months. | In an urban setting, structured PA and nutrition was more effective than parental education alone over the short duration of 12 weeks. However, it was uncertain whether this improvement could be sustained long term. |
| Johnston et al., 2013, USA [74]. | RCT, conducted in a school serving an urban student population. | 71 Mexican American adolescents; Aged 10 to 14 years; 55% females. | 12-week programme: 12 weeks of daily instructor/trainer-led healthy eating and PA behaviour change intervention sessions, followed by 12 weeks of biweekly follow-up sessions. Based on behaviour theory. | Given a parent-guided manual for the prevention and treatment of childhood obesity. The manual provided a 12-week weight management plan and instructions for the long-term maintenance of changes. | Repeated measures analyses revealed that adolescents in the intervention group significantly reduced their BMI z-scores compared to the adolescents in the control (F = 8.34, p < 0.001). Similar results for BMI (overall: F = 6.0, p < 0.01; 1 year: F = 6.6, p < 0.05; 2 years: F = 7.0, p < 0.05) and BMI percentile (overall: F = 5.8, p < 0.01; 1 year: F = 5.6, p < 0.05; 2 years: F = 6.6, p < 0.05). TC: F = 5.27, p = 0.027; LDL: F = 7.43, p = 0.01; HDL: F = 0.5, p > 0.05; TG: F = 0.5, p > 0.05. | Structured PA, nutrition education and long-term follow-ups were more effective than parental education in reducing both overweight/obesity and metabolic NCD risks. This effect was sustained for over 2 years. |
| Hull et al., 2018, USA [75]. | RCT, conducted in a home setting in a metropolitan area. | 318 Hispanic children; Aged 5 to 7 years, with at least one adult parent of Hispanic origin (self-identified) and BMI ≥25th <-35/kg/m2 percentile; 52% females. | 52-week (12-month) programme aimed to increase PA, decrease sedentary behaviour and improve healthy eating behaviours. Used parental modelling and experiential learning for children. Based on social cognitive theory, behavioural choice theory and food preference theory. | Focused on oral health. | Intervention short-term effects: zBMI: 0.068, p = 0.11; BMI: 0.084, p = 0.42; WC to height ratio: −0.004, p = 0.15; WC to hip ratio: 0.005, p = 0.24. Intervention long-term effects: zBMI: 0.023, p = 0.25; BMI (Kg/m2): 0.067, p = 0.27; WC to height ratio: 0.006, p = 0.02; WC to hip ratio: −0.004, p = 0.15. | The purely education and behaviour change intervention showed no effect. |
| Hughes et al., 2021, USA [76]. | RCT, conducted in community childcare centres. | 25 predominantly Hispanic children; Aged 3 to 5 years; 50% females. | A 7-week programme: Weekly teaching sessions on nutrition and PA. | The control arm received no sessions. | BMI z-scores showed no significant changes (F = 0.18, p = 0.91). | Short duration nutrition education and infrequent PA showed no effect. |
| Hollar et al., 2010, USA [77]. | Quasi-experimental with pre- and post-test analyses, conducted in elementary schools. | 1197 predominantly Hispanic children; mean age = 7.8 years. | A 2-year programme: (i) Dietary intervention: Modifications to school breakfasts, lunches and extended-day snacks in the intervention schools; (ii) PA: Opportunities for PA during the school day. | Usual practice. | Significantly more children in the intervention schools stayed within the normal BMI percentile range for both years of the study than those in the control school (p = 0.02). | Long duration actual dietary changes and PA reduced BMI. |
| Heerman at al., 2019, USA [78]. | RCT, conducted in physicians’ offices and community settings. | 117 majority Hispanic child–parent pairs; Children aged 3 to 5 years and Spanish speaking, with BMI >50th percentile for age and sex, according to CDC growth chart; 54% females. | A 15-week programme: (i) Weekly 90-min education and PA sessions, followed by twice-monthly health coaching calls for 3 months. | The control group received a twice-monthly school readiness curriculum for 3 months. | After adjusting for covariates, the intervention effect on linear child BMI growth was −0.41 (kg/m2) per year (95% CI = −0.82 to 0.01, p = 0.05). | Surprisingly, health coaching alone showed effects in young children; however, the study was underpowered and not generalisable. |
| Hasson et al., 2012, USA [79]. | RCT, conducted in a clinical setting. | 100 African American and Latino children; Aged 14 to 18 years, with BMI > 95th percentile for age and sex, according to CDC growth chart; 61% females. | 16-week programme: Intervention 1: Nutrition education once per week and four motivational interviews during the 16 weeks; Intervention 2: Nutrition + strength training: Participants also received strength training twice per week (~60 min/session) for 16 weeks supervised in a Human Laboratory. | Control group. pre- and post-intervention data were compared | Interventions were ineffective in reducing BMI, BMI z-score and BMI percentile, with no between group difference. Nutrition group reported better improvements in insulin sensitivity compared than the combined nutrition and strength training or Control groups (+16.5% vs. −32.3% vs. −6.9%, respectively; p < 0.01) and disposition index (+15.5% vs. −14.2% vs. −13.7%, respectively; p < 0.01). Hepatic fat fraction decreased by 27.3% in the Nutrition combined with Strength training group compared to 4.3% in the Control group and 0% in the Nutrition group, p < 0.01. Ethnicity by intervention interaction effects showed better response in Hispanic group to Nutrition intervention compared with African American who showed worsened fat mass and glucose control post intervention. | Neither type of the 16-week lifestyle intervention was effective in changing primary obesity BMI outcomes. However, interventions improved secondary metabolic outcomes (e.g. insulin sensitivity, hepatic fat, inflammation). Hispanic ethnicity responded well to nutrition education, which contrasted the counterproductive response found in African Americans. The latter may benefit from a more direct approach involving multiple exercise and nutritional components. Culturally contextualised and ethnically tailored lifestyle intervention approaches are needed. |
| Haines et al., 2016, USA [80]. | RCT, conducted in community health centres and community agencies. | 112 predominantly Hispanic parents/child dyads; Children aged 2–5 years; 50% females. | A 39-week programme: A total of 9 sessions on parenting skills, children’s education and homework assignments (based on social contextual framework theory). | Mailed publicly available educational materials on promoting healthy behaviours among preschoolers each week for 9 weeks. | BMI (kg/m2) decreased by a mean of 0.13 among children in the intervention arm and increased by 0.21 among children in the control arm, with an unadjusted difference of 20.34 (95% CI = 21.21, 0.53). After adjusting for child sex and age, the difference was minimal (20.36; 95% CI = 21.23, 0.51; p = 0.41). | The predominantly nutrition education programme was not effective on adiposity. |
| Gatto et al., 2017, USA [81]. | RCT, conducted in elementary schools. | 319 Hispanic children in 3rd, 4th and 5th grade in schools that offered after-school programmes. | 12-week programme (LA Sprout). Weekly: (i) 45-min interactive cooking/nutrition lessons; (ii) 45-min gardening lessons; (iii) Parallel bimonthly classes were offered to parents. The intervention was based on self-efficiency theory. | Did not receive any nutrition, cooking or gardening information from investigators. | Intervention group had significantly greater reductions in BMI z-score than the control group (−0.1 (9.9%) vs. −0.04 (3.8%), respectively; p = 0.01). Intervention group had a 1.2 cm (1.7%) reduction in WC, while the control group had a 0.1 cm (0.1%) increase after the intervention (p < 0.001). Fewer children had metabolic syndrome (n = 1) after the intervention than before (n = 7), while the number of children in the control group with metabolic syndrome remained essentially the same between pre- (n = 3) and post-intervention (n = 4). | The predominantly school-based nutrition programme reduced both BMI and metabolic risks in the short term; however, it was not clear whether this could be sustained. |
| Fiechtner et al., 2021, USA [82]. | RCT, conducted in clinical and community settings. | 4044 Hispanic, low-income children; Aged 6 to 12 years, with BMI >85th percentile for age and sex, according to CDC growth chart; 48% females. | Two intervention groups: Intervention I: Healthy Weight Clinic: 30 h of multidisciplinary team nutrition and PA education for parents/guardians and children, alternating between group and individual sessions; Intervention II: YMCA Modified Healthy Weight and Your Child: A total of 25 education sessions were offered to parent/guardians and children over 1 year of age. Each session was 2 h long. Both groups were exposed to primary care provider weight management training and text messages to parents/guardians for self-guided behaviour change support. | Eight demographically matched comparison community health centres were chosen as control sites. | The mean difference in the % of children in the 95th percentile for BMI between Intervention II and intervention I was 0.75 (90% CI = 0.07 to 1.43), which did not support noninferiority. Compared to the control sites, children in Intervention I had a −0.23 (95% CI = −0.36 to −0.10) decrease in BMI (kg/m2) per year and there was a −1.03 (95% CI = −1.61 to −0.45) decrease in the % of children in 95th percentile for BMI. There was no significant effect on BMI in Intervention II. | There was no difference between offering the education (nutrition and PA) programme in a multidisciplinary clinical setting or a community YMCA setting in a large sample of low-income high-risk children. Both approaches reduced the percentage of children in the 95th percentile for BMI. |
| Eichner et al., 2016, USA [83]. | Quasi-experimental with pre- and post-test analyses, conducted in a school setting. | 353 predominantly America Indian children in 6th, 7th and 8th grade; Aged 12 to 15 years; 50% females. | A 5-year programme (Middle School Opportunity for Vigorous Exercise): (i) PA: walked or ran 1 mile each school day and then engaged in a team activity, such as basketball, soccer, football, dodgeball or volleyball. | Baseline data and second outcome data were collected from students who did not participate in the PA programme for comparison. | Mean BMI z-scores remained the same among girls participating in MOVE (from 0.7 to 0.7) and increased for nonparticipating girls (from 1.1 to 1.2). Mean BMI z-scores decreased among boys participating in MOVE (from 0.8 to 0.7) and increased among nonparticipating boys (from 1.1 to 1.2). Overall, MOVE participants had significantly lower BMI z-scores than nonparticipants (p = 0.01). | The 5-year PA programme was shown to prevent increases in BMI but as most of the children were not in the high-risk category, it did not reduce BMI z-scores. |
| Dos Santos et al., 2020, USA [84]. | Quasi-experimental with pre- and post-test analyses, conducted in a school setting. | 46 predominantly Hispanic parent–child dyads; Children aged from 10 to 16 years old, with a BMI ≥85th percentile for age and sex, according to CDC growth chart; 45% females. | An 8-week programme: (i) Joint education for parents and children on nutrition, PA and lifestyle issues; (ii) PA classes: Adolescents engaged in moderate to vigorous PA (e.g., lap runs). | None. | Mean BMI (kg/m2): Pre-intervention = 29.95 (SD = 5.82); post-intervention = 29.44 (SD = 5.78, p = 0.012). Participant waist–hip ratio from pre-intervention (mean = 1.00, SD = 0.06) to post intervention (mean = 0.99, SD = 0.06, p < 0.001). | Education and moderate PA had a small effect in reducing adiposity; however, the sample size was small and the intervention had a short duration. Long-term sustainability was uncertain. |
| De Heer et al., 2011, USA [48]. | RCT, conducted in a school setting. | 901 Hispanic students in 3rd, 4th and 5th grade; Mean age = 9.2 years; 45% females. | 12-weeks after-school programme run twice weekly, based on social cognitive theory: (i) Education: 20- to 30-min health education component; (ii) PA: 45 to 60 min of PA. | Control and spill over groups received 4th grade health workbooks and incentives at pre-test and follow-up measurements, but they did not attend the after-school sessions. | BMI percentile reduction: Intervention group = 2.8% (p = 0.015); Spill over group = 2.0% (p = 0.085); Control group = 1.4% (p = 0.249). | This education and PA programme was shown to reduce BMI; however, a comparative analysis was not conducted. Therefore, it was unclear to what extent it was effective. |
| Davis et al., 2016, USA [85]. | RCT, conducted in community and school settings. | 1898 predominantly American Indian and Hispanic children; Aged 3 years, enrolled in Head Start (HS) centres; 47% females. | 5-year programme based on a socioecological approach. The 6-component programme comprised nutrition and PA education and increasing the availability of healthier food options. | Participated in measurements but not the intervention. | No effect of the intervention on zBMI was observed: difference in slopes = −0.006 (95% CI = −0.031 to 0.020, p = 0.69). | This large and long-term predominantly prevention education intervention did not show any effect on BMI, although there were some reductions in zBMI. |
| Davis et al., 2012, USA [86]. | RCT, conducted in schools, community centres and health clinics. | 53 African American and Latino children in 9th through 12th grade; Mean age = 15.3 years, with BMI ≥85th percentile for age and sex, according to CDC growth chart; 55% females. | 12-month maintenance programme (newsletter group) following a 4-month nutrition and strength training intervention: Received a monthly newsletter in the mail that matched their 4-month intervention group assignment. | Maintenance group class: Met monthly (classes lasted 90 min) and received a monthly class that was similar to the 4-month intervention classes. | Fasting insulin and acute insulin response decreased by 26% and 16% (p < 0.001 and p = 0.046), respectively, while HDL and insulin sensitivity improved by 5% and 14% (p = 0.042 and p = 0.039), respectively. | 12-month programme of newsletters followed by nutrition and resistance training improved insulin and lipid metabolic profiles, although its effect on overweight/obesity was not assessed. |
| Davis et al., 2021, USA [87]. | RCT, conducted in schools. | 3135 predominantly Hispanic 3rd–5th grade students; Mean age = 9.2 years; 53% females. | 9-month programme (Sprout): (i) Garden Leadership Committee formation; (ii) A 0.25-acre outdoor teaching garden; (iii) 18 student gardening, nutrition and cooking lessons; (iv) 9 monthly parent lessons. Based on a social ecological transactional model. | The control schools received a delayed intervention (identical intervention) in the year after the post-testing for that wave. | BMI change, mean (kg/m2): I = 4.12, C = 3.71, p = 0.006; BMI z-score change, mean: I = −0.04, C = −0.02, p = 0.51; BMI percentile change: I = −0.82, C = −0.39, p = 0.53; WC change, mean (cm): I = 1.16, C = −1.53, p = 0.34; % BF change: I = −0.34, C = −0.49, p = 0.40; SBP change, mean (mmHg): I = −0.39, C = 0.20, p = 0.64; DBP change, mean (mmHg): I = −1.33, C = 0.32, p = 0.18. | The nutrition intervention did not show any effects on most overweight/obesity parameters or blood pressure, except there was a difference in mean BMI change. |
| Davis et al., 2009, USA [47]. | RCT, conducted in a clinical setting. | 54 overweight Hispanic children; Aged 14 to 18 years (mean = 15.5), with BMI ≥85th percentile for age and sex, according to CDC growth chart. | 16-week nutrition + strength training programme: In addition to the nutrition education classes described under comparator, participants in the intervention group also received strength training twice per week (~60 min/session) for 16 weeks. | Nutrition only group received culturally tailored dietary intervention once per week (~90 min) for 16 weeks. | There were no significant intervention effects on insulin sensitivity, body composition or most glucose/insulin indices, with the exception of glucose incremental area under the curve (p = 0.05), which decreased in the nutrition and nutrition + strength training groups by 18 and 6.3%, respectively, compared to a 32% increase in the control group. | The short duration and small size nutrition education and PA programme had no effects on adiposity or metabolic risk. |
| Davis et al., 2011, USA [88]. | RCT, conducted in a school setting. | 38 Hispanic females in grades 9–12; Aged 14 to 18 years, with BMI ≥85th percentile for age and sex, according to CDC growth chart. | 16-week intervention: Intervention group I (circuit training): aerobic + strength training two times/week for 60–90 min per session; Intervention group II (circuit training + motivational interviews on behaviour change). | Control group was offered abbreviated circuit training intervention after post-test data collection. | No changes in BMI. Children in all groups increased their overall mean BMI z-score over the course of the study. Circuit training participants showed decreased waist circumference (−3% vs. +3%, p = 0.001). % BF: Subcutaneous adipose tissue (10% vs. 8%, p = 0.04) and visceral adipose tissue (10% vs. +6%, p = 0.05). Fasting insulin (24% vs. +6%, p = 0.03) and insulin resistance (−21% vs. −4%, p = 0.05). | The 16-week PA (aerobic and strength training) programme was effective in reducing fat depots and improving insulin resistance in Latino youths who were overweight/obese. The additional motivational interviews showed no additional benefits. Neither intervention had an effect on overweight/obesity. |
| Crespo et al., 2012, USA [89]. | RCT, conducted in schools and community settings. | 808 Hispanic parent–child dyads; Children mean age = 5.9 years; 50% females. | 4-year intervention programme: Intervention I (Family group): Home visits (newsletters, recipe cards and goal setting) and follow-up phone calls; Intervention II (Community group): Improvements to nutrition and PA environments in school playgrounds and community parks. Distribution of education materials; Intervention 3 (Family + Community group): Involved in both family and community interventions. | Participants in the control group were asked to maintain their regular lifestyles and complete the yearly measurements. | No changes in any weight measures were statistically significant. Children in all groups increased their overall mean BMI z-score over the course of the study. | Despite the long duration, the nutrition education and support programme in family, school and combined family and school settings was not effective in reducing BMI. However, the effect of the intervention on other metabolic parameters was not assessed. |
| Chen et al., 2011, USA [90]. | RCT, conducted in a web-based setting. | 54 Chinese American adolescents; Aged 12 to 15 (mean = 12.5) years old and normal weight or overweight, with BMI ≥85th percentile for age and sex, according to CDC growth chart; 70% females. | An 8-week web-based programme (based on the transtheoretical model of stages of change): (i) Nutrition, PA and coping techniques; (ii) Internet sessions to coach parents on imparting the skills. | Participants in the control group also logged on to the website using a preassigned username and password. Every week for 8 weeks, adolescents also received general health information. | No reductions in BMI (Kg/m2) were observed in either the intervention (t0 = 20.79, T3 = 20.76) or control (t0 = 20.25, t3 = 20.21) groups. Significantly more adolescents in the intervention group decreased their waist to hip ratio than in the control group (Effect size = 0.01, p = 0.02). DBP (Effect size = 1.12, p = 0.02). | This short duration (8 weeks) web-based education programme was not shown to be effective in reducing BMI. However, as most of the participants were of normal weight, the intervention could have played a preventive role. |
| Chen et al., 2019, USA [91]. | RCT, conducted in a community setting. | 40 Chinese American children; Aged 13 to 18 years of age, with BMI ≥85th percentile for age and sex, according to CDC growth chart. | 12-week intervention (based on social cognitive theory): (i) Used a wearable sensor (Fitbit Flex) for 6 months; (ii) Reviewed 8 online educational modules for 3 months and after completing the modules, they received tailored, biweekly text messages for 3 months. | After the completion of the baseline assessments, control group participants were given an Omron HJ-105 pedometer and a blank food and activity diary. The adolescents were asked to record and track their physical activity, sedentary activity and food intake in the diary for 3 months. | BMI (kg/m2) difference: −4.89 (p < 0.001); BMI z-score difference: −4.72 (p < 0.001). | With overweight/obese Chinese American children, the online education programme was effective in reducing BMI. |
| Chen et al., 2010, USA [92]. | RCT, conducted in a community setting. | 67 Chinese American children; Aged 8 to 10 years and were normal weight or overweight, with BMI ≥85th percentile for age and sex, according to CDC growth chart; 44% females. | An 8-week programme (based on social cognitive theory): (i) Children participated in a 45-min session of education- and play-based activities once each week for 8 weeks; (ii) Parents participated in two sessions that lasted 2 h each during the 8 weeks. The parents took part in ‘Healthy Eating and Healthy Family: A Hands-on Workshop’. Follow-up was 8 months. | Waiting-list control group received intervention after the follow-up period. | Significant decreases in BMI (kg/m2) were observed in the intervention group (19.74 to 19.32, p < 0.05) but not in the control group (18.65 to 18.42, p > 0.05). No changes in waist to hip ratio were reported in the intervention group (0.88 to 0.88, p > 0.05). Significant reductions in DBP were seen in the intervention group (61.03 to 59.27, p < 0.05). | Surprisingly, small reductions in BMI and CVD risk among Chinese American were shown after a few sessions of child and parent education and play activities. However, the results may not be generalisable because of convenient sampling. |
| Caballero et al., 2003, USA [93]. | RCT, conducted in a school serving American Indian children. | 1704 American Indian children in 3rd to 5th grade; Mean age = 7.6 years. | The 3-year intervention had 4 components: (i) Change in dietary intake; (ii) Increase in PA; (iii) A classroom curriculum focused on healthy eating and lifestyle; (iv) A family involvement programme. | The control group participated in measurements but not the intervention. | Mean differences at follow-up: %BMI: −0.2, p = 0.30; %BF: 0.2, p = 0.66; Triceps skinfold thickness (mm): 0.1, p = 0.84; Scapula skinfold thickness (mm): −0.1, p = 0.85. | Despite the long duration of implementation, the predominantly education and low-intensity PA programme did have effects on BMI/adiposity among American Indian children. However, there were indications that their calorie intake improved. |
| Barkin et al., 2011, USA [94]. | RCT; participants were identified from primary care clinics, radio advertising and local churches. | 72 mostly Hispanic parent–child dyads; Children aged 8 to 11 years, with BMI ≥85% for age and sex, according to CDC growth chart; 54% females. | 6-month programme (based on a transtheoretical model): (i) Counselling by a physician trained in the brief principles of motivational interviewing; (ii) 45-min group health education session; (iii) 5 monthly 1-hour sessions on the topic of increasing PA for both parents and children. | Families in the control group received standard of care counselling from physicians trained using American Academy of Pediatrics guidelines, addressing both nutrition and activity. | Participants that had a higher baseline BMI were more likely to decrease their absolute BMI (Kg/m2) (β= −0.22, p< 0.0001). | The counselling and education were effective for obese children but less so for normal weight or overweight children. |
| Barkin et al., 2012, USA [95]. | RCT, conducted in a community setting. | 75 majority Hispanic parent–child dyads; Children aged 2 to 6 years; 48% females. | A programme: (i) Weekly 90-min skills-building sessions for parents and preschool-aged children designed to improve family nutritional habits, increase weekly PA and reduce sedentary activity. | A brief school readiness programme was conducted as an alternative to the active intervention because there was no standard care condition for comparison. | The effect of the treatment on post-intervention absolute BMI (Kg/m2) was B = –0.59 (p = 0.001). | This skills-building intervention programme targeting both parents and children with obesity had a small but significant effect on BMI. |
| Arlinghaus et al., 2017, USA [96]. | RCT, conducted in a school setting. | 189 Hispanic adolescent students in grades 6 through 12, with BMI ≥85% for age and sex, according to CDC growth chart; 47% females. | 6-month programme: Trained peer-led discussion on the selected topic with their group of middle-school students during PE classes, e.g., what they were going to eat for lunch that day or their favourite vegetables. | Usual PE classes. | Significant differences were found between groups across time (F = 4.58, p = 0.01). After the 6-month intervention, the intervention group had a larger decrease in zBMI (F = 6.94, p = 0.01) than the control. | Adding peer-led nutritional education to PE classes reduced adiposity in high-risk Hispanic children. |
| Arlinghaus et al., 2021, USA [97]. | RCT, conducted in a school setting. | 491 Hispanic American middle-school students enrolled in PE classes; 53% females. | A 12-month programme: (i) The PA component of an obesity intervention with established efficacy at reducing standardised BMI among this population. | Control was physical education (PE) class as traditionally taught in the district. | Intervention decreased zBMI significantly more than the control (F (1, 56) = 6.16, p < 0.05). | The addition of the PA component to PE classes reduced overweight/obesity after 12 months; however, it was uncertain whether this was sustainable. |
| Adab et al., 2018, UK [45]. | RCT, conducted in primary schools. | 1392 non-White multi-ethnic children; Aged 5 to 6 years in year 1 of primary school; 51% females. | A 12-month programme: (i) Encouraged healthy eating and PA; (ii) Additional 30 min of school time every day for PA opportunities; (iii) A 6-week interactive skills-based programme in conjunction with a football club; (iv) Signposting of families to local PA places; (v) School-led family workshops on healthy cooking skills. | Ongoing health-related activities for pupils in year 2 study. In addition, citizenship education resources, excluding topics related to healthy eating and physical activity, were provided. | At 15 months, mean difference in BMI z-score: −0.075 (95% CI = −0.183 to 0.033, p = 0.18). At 30 months, mean difference: −0.027 (95% CI = −0.137 to 0.083, p = 0.63). There were no statistically significant differences between groups. | No significant effects of intervention on adiposity were observed in either the short or longer term. Although there were improvements in BMI, the differences were smaller the longer the duration of intervention. |
3.3. Risk of Bias within Studies
3.4. Effectiveness of Interventions
3.4.1. Quasi-Experimental Pre- and Post-Intervention Studies
3.4.2. Randomised Control Studies
4. Discussion
4.1. Findings
4.2. Implications for Practice and Research
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Non-Communicable Disease; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- WHO. Preventing Noncommunicable Diseases (NCDs) by Reducing Environmental Risk Factors; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Geneau, R.; Stuckler, D.; Stachenko, S.; McKee, M.; Ebrahim, S.; Basu, S.; Chockalingham, A.; Mwatsama, M.; Jamal, R.; Alwan, A.; et al. Raising the priority of preventing chronic diseases: A political process. Lancet 2010, 376, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.S.; Yusuf, S. Stemming the global tsunami of cardiovascular disease. Lancet 2011, 377, 529–532. [Google Scholar] [CrossRef]
- Twig, G. A spotlight on obesity prevention. Lancet Diabetes Endocrinol. 2021, 9, 645–646. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Strategy on Diet, Physical Activity and Health; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Xiao, C.; Graf, S. Overweight, poor diet and physical activity: Analysis of trends and patterns. In The Heavy Burden of Obesity: The Economics of Prevention; OECD Publishing: Paris, France, 2019. [Google Scholar]
- Ng, S.W.; Popkin, B.M. Time use and physical activity: A shift away from movement across the globe. Obes. Rev. 2012, 13, 659–680. [Google Scholar] [CrossRef] [PubMed]
- Zvolinskaia, E.I.; Rozanov, V.; Aleksandrov, A.A.; Shugaeva, E.N.; Avakov, M.S. Relationship between carotid artery intima media thickness and risk factors of cardiovascular diseases in young men. Kardiologiia 2012, 52, 55–60. [Google Scholar]
- Yates, T.; Summerfield, A.; Razieh, C.; Banerjee, A.; Chudasama, Y.; Davies, M.J.; Gillies, C.; Islam, N.; Lawson, C.; Mirkes, E.; et al. A population-based cohort study of obesity, ethnicity and COVID-19 mortality in 12.6 million adults in England. Nat. Commun. 2022, 13, 624. [Google Scholar] [CrossRef]
- World Obesity Federation. World Obesity Atlas 2023; World Obesity Federation: London, UK, 2023. [Google Scholar]
- Morales Camacho, W.J.; Molina Díaz, J.M.; Plata Ortiz, S.; Plata Ortiz, J.E.; Morales Camacho, M.A.; Calderón, B.P. Childhood obesity: Aetiology, comorbidities, and treatment. Diabetes Metab. Res. Rev. 2019, 35, e3203. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tuomilehto, J. Lifestyle Diabetes Prevention. In Encyclopedia of Endocrine Diseases, 2nd ed.; Huhtaniemi, I., Martini, L., Eds.; Academic Press: Oxford, UK, 2019; pp. 148–159. [Google Scholar]
- Reinehr, T. Effectiveness of lifestyle intervention in overweight children. Proc. Nutr. Soc. 2011, 70, 494–505. [Google Scholar] [CrossRef]
- Wijtzes, A.I.; van de Gaar, V.M.; van Grieken, A.; de Kroon, M.L.; Mackenbach, J.P.; van Lenthe, F.J.; Jansen, W.; Raat, H. Effectiveness of interventions to improve lifestyle behaviors among socially disadvantaged children in Europe. Eur. J. Public Health 2017, 27, 240–247. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Tzanninis, S.; Ntanasis-Stathopoulos, I.; Panotopoulos, G.; Kostopoulou, M.; Tzanninis, I.-G.; Tsagianni, A.; Sergentanis, T.N. Prevention and treatment of childhood and adolescent obesity: A systematic review of meta-analyses. World J. Pediatr. 2019, 15, 350–381. [Google Scholar] [CrossRef]
- Weihrauch-Blüher, S.; Kromeyer-Hauschild, K.; Graf, C.; Widhalm, K.; Korsten-Reck, U.; Jödicke, B.; Markert, J.; Müller, M.J.; Moss, A.; Wabitsch, M.; et al. Current Guidelines for Obesity Prevention in Childhood and Adolescence. Obes. Facts 2018, 11, 263–276. [Google Scholar] [CrossRef]
- Ballon, M.; Botton, J.; Charles, M.A.; Carles, S.; de Lauzon-Guillain, B.; Forhan, A.; Cameron, A.J.; Heude, B.; Lioret, S.; EDEN Mother–Child Cohort Study Group. Socioeconomic inequalities in weight, height and body mass index from birth to 5 years. Int. J. Obes. 2018, 42, 1671–1679. [Google Scholar] [CrossRef]
- Falconer, C.L.; Park, M.H.; Croker, H.; Kessel, A.S.; Saxena, S.; Viner, R.M.; Kinra, S. Can the relationship between ethnicity and obesity-related behaviours among school-aged children be explained by deprivation? A cross-sectional study. BMJ Open 2014, 4, e003949. [Google Scholar] [CrossRef]
- Public Health England. Differences in Child Obesity by Ethnic Group; Public Health England: London, UK, 2019.
- Obita, G.; Alkhatib, A. Disparities in the Prevalence of Childhood Obesity-Related Comorbidities: A Systematic Review. Front. Public Health 2022, 10, 923744. [Google Scholar] [CrossRef]
- Gortmaker, S.L.; Swinburn, B.A.; Levy, D.; Carter, R.; Mabry, P.L.; Finegood, D.T.; Huang, T.; Marsh, T.; Moodie, M.L. Changing the future of obesity: Science, policy, and action. Lancet 2011, 378, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Hillier-Brown, F.C.; Bambra, C.L.; Cairns, J.-M.; Kasim, A.; Moore, H.J.; Summerbell, C.D. A systematic review of the effectiveness of individual, community and societal level interventions at reducing socioeconomic inequalities in obesity amongst children. BMC Public Health 2014, 14, 834. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.R.; Acholonu, N.O.; Dolan, A.C.; Krishnan, A.; Wang, E.H.-C.; Stanford, F.C. Racial Disparities in Obesity Treatment Among Children and Adolescents. Curr. Obes. Rep. 2021, 10, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Salam, R.A.; Padhani, Z.A.; Das, J.K.; Shaikh, A.Y.; Hoodbhoy, Z.; Jeelani, S.M.; Lassi, Z.S.; Bhutta, Z.A. Effects of Lifestyle Modification Interventions to Prevent and Manage Child and Adolescent Obesity: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2208. [Google Scholar] [CrossRef]
- Waters, E.; De Silva-Sanigorski, A.; Burford, B.J.; Brown, T.; Campbell, K.J.; Gao, Y.; Armstrong, R.; Prosser, L.; Summerbell, C.D. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 2011, CD001871. [Google Scholar] [CrossRef]
- Brown, T.; Moore, T.H.; Hooper, L.; Gao, Y.; Zayegh, A.; Ijaz, S.; Elwenspoek, M.; Foxen, S.C.; Magee, L.; O’Malley, C.; et al. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 2019, CD001871. [Google Scholar] [CrossRef]
- Brown, T.; Smith, S.; Bhopal, R.; Kasim, A.; Summerbell, C. Diet and physical activity interventions to prevent or treat obesity in South Asian children and adults: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2015, 12, 566–594. [Google Scholar] [CrossRef]
- White, M.; Adams, J.; Heywood, P. How and why do interventions that increase health overall widen inequalities within populations? In Social Inequality and Public Health; Policy Press: Bristol, UK, 2009; pp. 65–81. [Google Scholar]
- Bhopal, R. Health education and ethnic minorities. Br. Med. J. 1991, 302, 1338. [Google Scholar] [CrossRef]
- Liu, J.; Davidson, E.; Bhopal, R.; White, M.; Johnson, M.; Netto, G.; Deverill, M.; Sheikh, A. Adapting health promotion interventions to meet the needs of ethnic minority groups: Mixed-methods evidence synthesis. Health Technol. Assess. 2012, 16, 1–469. [Google Scholar] [CrossRef]
- Murphy, M.; Johnson, R.; Parsons, N.; Robertson, W. Understanding local ethnic inequalities in childhood BMI through cross-sectional analysis of routinely collected local data. BMC Public Health 2019, 19, 1585. [Google Scholar] [CrossRef]
- Smith, J.D.; Fu, E.; Kobayashi, M.A. Prevention and Management of Childhood Obesity and Its Psychological and Health Comorbidities. Annu. Rev. Clin. Psychol. 2020, 16, 351–378. [Google Scholar] [CrossRef]
- Bhavnani, R.; Mirza, H.S.; Meetoo, V. Tackling the Roots of Racism: Lessons for Success; Policy Press: Bristol, UK, 2005. [Google Scholar]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Italian J. Public Health 2009, 6, 354–391. [Google Scholar]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef]
- Mazzucca, S.; Tabak, R.G.; Pilar, M.; Ramsey, A.T.; Baumann, A.A.; Kryzer, E.; Lewis, E.M.; Padek, M.; Powell, B.J.; Brownson, R.C. Variation in Research Designs Used to Test the Effectiveness of Dissemination and Implementation Strategies: A Review. Front. Public Health 2018, 6, 32. [Google Scholar] [CrossRef]
- Moher, D.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Higgins, J.; Altman, D. Assessing Risk of Bias in Included 11 Studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0; Higgins, J., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2008; Available online: http://www.cochrane-handbook.org/ (accessed on 14 January 2023).
- Jüni, P.; Altman, D.G.; Egger, M. Assessing the quality of controlled clinical trials. BMJ 2001, 323, 42–46. [Google Scholar] [CrossRef]
- West, S.; King, V.; Carey, T.S.; Lohr, K.N.; McKoy, N.; Sutton, S.F.; Lux, L. Systems to Rate the Strength of Scientific Evidence: Summary. In AHRQ Evidence Report Summaries; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2002; pp. 1998–2005. Available online: http://www.ncbi.nlm.nih.gov/books/NBK11930/ (accessed on 25 January 2023).
- The-Cochrane-Collaboration. Review Manager (RevMan); The Cochrane Collaboration: London, UK, 2020; Available online: www.cochrane.org/online-learning/core-software-cochrane-reviews/revman/ (accessed on 14 January 2023).
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Adab, P.; Pallan, M.J.; Lancashire, E.R.; Hemming, K.; Frew, E.; Barrett, T.; Bhopal, R.; Cade, J.E.; Canaway, A.; Clarke, J.; et al. Effectiveness of a childhood obesity prevention programme delivered through schools, targeting 6 and 7 year olds: Cluster randomised controlled trial (WAVES study). BMJ 2018, 360, k211. [Google Scholar] [CrossRef] [PubMed]
- Messito, M.J.; Mendelsohn, A.L.; Katzow, M.W.; Scott, M.A.; Vandyousefi, S.; Gross, R. Prenatal and Pediatric Primary Care-Based Child Obesity Prevention Program: A Randomized Trial. Pediatrics 2020, 146, e20200709. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.N.; Kelly, L.A.; Lane, C.J.; Ventura, E.E.; Byrd-Williams, C.E.; Alexandar, K.A.; Azen, S.P.; Chou, C.-P.; Spruijt-Metz, D.; Weigensberg, M.J.; et al. Randomized Control Trial to Improve Adiposity and Insulin Resistance in Overweight Latino Adolescents. Obesity 2009, 17, 1542–1548. [Google Scholar] [CrossRef]
- De Heer, H.D.; Koehly, L.; Pederson, R.; Morera, O. Effectiveness and Spillover of an After-School Health Promotion Program for Hispanic Elementary School Children. Am. J. Public Health 2011, 101, 1907–1913. [Google Scholar] [CrossRef]
- Yin, Z.; Parra-Medina, D.; Cordova, A.; He, M.; Trummer, V.; Sosa, E.; Gallion, K.J.; Sintes-Yallen, A.; Huang, Y.; Wu, X.; et al. Míranos! Look at us, we are healthy! An environmental approach to early childhood obesity prevention. Child. Obes. 2012, 8, 429–439. [Google Scholar] [CrossRef]
- Yli-Piipari, S.; Berg, A.; Laing, E.M.; Hartzell, D.L.; Parris, K.O.; Udwadia, J.; Lewis, R.D. A Twelve-Week Lifestyle Program to Improve Cardiometabolic, Behavioral, and Psychological Health in Hispanic Children and Adolescents. J. Altern. Complement. Med. 2018, 24, 132–138. [Google Scholar] [CrossRef]
- Yin, Z.; Gutin, B.; Johnson, M.H.; Hanes, J.; Moore, J.B.; Cavnar, M.; Thornburg, J.; Moore, D.; Barbeau, P. An environmental approach to obesity prevention in children: Medical College of Georgia FitKid Project year 1 results. Obes. Res. 2005, 13, 2153–2161. [Google Scholar] [CrossRef]
- Wylie-Rosett, J.; Groisman-Perelstein, A.E.; Diamantis, P.M.; Jimenez, C.C.; Shankar, V.; Conlon, B.A.; Mossavar-Rahmani, Y.; Isasi, C.R.; Martin, S.N.; Ginsberg, M.; et al. Embedding weight management into safety-net pediatric primary care: Randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 12. [Google Scholar] [CrossRef]
- Wong, W.W.; Ortiz, C.L.; Stuff, J.E.; Mikhail, C.; Lathan, D.; Moore, L.A.; Alejandro, M.E.; Butte, N.F.; Smith, E.O. A Community-based Healthy Living Promotion Program Improved Self-esteem Among Minority Children. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 106–112. [Google Scholar] [CrossRef]
- Wilson, D.K.; Sweeney, A.M.; Van Horn, M.L.; Kitzman, H.; Law, L.H.; Loncar, H.; Kipp, C.; Brown, A.; Quattlebaum, M.; McDaniel, T.; et al. The Results of the Families Improving Together (FIT) for Weight Loss Randomized Trial in Overweight African American Adolescents. Ann. Behav. Med. Publ. Soc. Behav. Med. 2022, 56, 1042–1055. [Google Scholar] [CrossRef]
- Williford, H.N.; Blessing, D.L.; Duey, W.J.; Barksdale, J.M.; Wang, N.; Olson, M.S.; Teel, S. Exercise training in black adolescents: Changes in blood lipids and Vo2max. Ethn. Dis. 1996, 6, 279–285. [Google Scholar]
- Williamson, D.A.; Walden, H.M.; White, M.; York-Crowe, E.; Newton, R.; Alfonso, A.; Gordon, S.; Ryan, D. Two-year internet-based randomized controlled trial for weight loss in African-American girls. Obesity 2006, 14, 1231–1243. [Google Scholar] [CrossRef]
- Van der Heijden, G.J.; Wang, Z.J.; Chu, Z.D.; Sauer, P.J.; Haymond, M.W.; Rodriguez, L.M.; Sunehag, A.L. A 12-week aerobic exercise program reduces hepatic fat accumulation and insulin resistance in obese, Hispanic adolescents. Obesity 2010, 18, 384–390. [Google Scholar] [CrossRef]
- Tomayko, E.J.; Prince, R.J.; Cronin, K.A.; Kim, K.; Parker, T.; Adams, A.K. The Healthy Children, Strong Families 2 (HCSF2) Randomized Controlled Trial Improved Healthy Behaviors in American Indian Families with Young Children. Curr. Dev. Nutr. 2018, 3 (Suppl. S2), 53–62. [Google Scholar] [CrossRef]
- Taveras, E.M.; Marshall, R.; Sharifi, M.; Avalon, E.; Fiechtner, L.; Horan, C.; Gerber, M.W.; Orav, E.J.; Price, S.N.; Sequist, T.; et al. Comparative Effectiveness of Clinical-Community Childhood Obesity Interventions: A Randomized Clinical Trial. JAMA Pediatr. 2017, 171, e171325. [Google Scholar] [CrossRef]
- Story, M.; Hannan, P.J.; Fulkerson, J.A.; Rock, B.H.; Smyth, M.; Arcan, C.; Himes, J.H. Bright Start: Description and main outcomes from a group-randomized obesity prevention trial in American Indian children. Obesity 2012, 20, 2241–2249. [Google Scholar] [CrossRef]
- Stolley, M.R.; Fitzgibbon, M.L.; Dyer, A.; Van Horn, L.; KauferChristoffel, K.; Schiffer, L. Hip-Hop to Health Jr., an obesity prevention program for minority preschool children: Baseline characteristics of participants. Prev. Med. 2003, 36, 320–329. [Google Scholar] [CrossRef]
- Soltero, E.G.; Olson, M.L.; Williams, A.N.; Konopken, Y.P.; Castro, F.G.; Arcoleo, K.J.; Keller, C.S.; Patrick, D.L.; Ayers, S.L.; Barraza, E.; et al. Effects of a Community-Based Diabetes Prevention Program for Latino Youth with Obesity: A Randomized Controlled Trial. Obesity 2018, 26, 1856–1865. [Google Scholar] [CrossRef]
- Slusser, W.; Frankel, F.; Robison, K.; Fischer, H.; Cumberland, W.G.; Neumann, C. Pediatric overweight prevention through a parent training program for 2–4 year old Latino children. Child. Obes. 2012, 8, 52–59. [Google Scholar] [CrossRef]
- Shaibi, G.Q.; Cruz, M.L.; Ball, G.D.C.; Weigensberg, M.J.; Salem, G.J.; Crespo, N.C.; Goran, M.I. Effects of resistance training on insulin sensitivity in overweight Latino adolescent males. Med. Sci. Sports Exerc. 2006, 38, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.N.; Matheson, D.; Wilson, D.M.; Weintraub, D.L.; Banda, J.A.; McClain, A.; Sanders, L.M.; Haskell, W.L.; Haydel, K.F.; Kapphahn, K.I.; et al. A community-based, multi-level, multi-setting, multi-component intervention to reduce weight gain among low socioeconomic status Latinx children with overweight or obesity: The Stanford GOALS randomised controlled trial. The lancet. Diabetes Endocrinol. 2021, 9, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Rieder, J.; Khan, U.I.; Heo, M.; Mossavar-Rahmani, Y.; Blank, A.E.; Strauss, T.; Viswanathan, N.; Wylie-Rosett, J. Evaluation of a community-based weight management program for predominantly severely obese, difficult-to-reach, inner-city minority adolescents. Child. Obes. 2013, 9, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Resnicow, K.; Taylor, R.; Baskin, M.; McCarty, F. Results of go girls: A weight control program for overweight African-American adolescent females. Obes. Res. 2005, 13, 1739–1748. [Google Scholar] [CrossRef]
- Prado, G.; Fernandez, A.; George, S.M.S.; Lee, T.K.; Lebron, C.; Tapia, M.I.; Velazquez, M.R.; Messiah, S.E. Results of a Family-Based Intervention Promoting Healthy Weight Strategies in Overweight Hispanic Adolescents and Parents: An RCT. Am. J. Prev. Med. 2020, 59, 658–668. [Google Scholar] [CrossRef]
- Polonsky, H.M.; Bauer, K.W.; Fisher, J.O.; Davey, A.; Sherman, S.; Abel, M.L.; Hanlon, A.; Ruth, K.J.; Dale, L.C.; Foster, G.D. Effect of a Breakfast in the Classroom Initiative on Obesity in Urban School-aged Children: A Cluster Randomized Clinical Trial. JAMA Pediatr. 2019, 173, 326–333. [Google Scholar] [CrossRef]
- Peña, A.; Olson, M.L.; Hooker, E.; Ayers, S.L.; Castro, F.G.; Patrick, D.L.; Corral, L.; Lish, E.; Knowler, W.C.; Shaibi, G.Q. Effects of a Diabetes Prevention Program on Type 2 Diabetes Risk Factors and Quality of Life Among Latino Youths with Prediabetes: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2231196. [Google Scholar] [CrossRef]
- Novotny, R.; Davis, J.; Butel, J.; Boushey, C.J.; Fialkowski, M.K.; Nigg, C.R.; Braun, K.L.; Guerrero, R.T.L.; Coleman, P.; Bersamin, A.; et al. Effect of the Children’s Healthy Living Program on Young Child Overweight, Obesity, and Acanthosis Nigricans in the US-Affiliated Pacific Region: A Randomized Clinical Trial. JAMA Netw. Open 2018, 1, e183896. [Google Scholar] [CrossRef]
- Norman, G.; Huang, J.; Davila, E.P.; Kolodziejczyk, J.K.; Carlson, J.; Covin, J.R.; Gootschalk, M.; Patrick, K. Outcomes of a 1-year randomized controlled trial to evaluate a behavioral ‘stepped-down’ weight loss intervention for adolescent patients with obesity. Pediatr. Obes. 2016, 11, 18–25. [Google Scholar] [CrossRef]
- Johnston, C.A.; Tyler, C.; McFarlin, B.K.; Poston, W.S.; Haddock, C.K.; Reeves, R.; Foreyt, J.P. Weight loss in overweight Mexican American children: A randomized, controlled trial. Pediatrics 2007, 120, e1450–e1457. [Google Scholar] [CrossRef]
- Johnston, C.A.; Moreno, J.P.; El-Mubasher, A.; Gallagher, M.; Tyler, C.; Woehler, D. Impact of a School-Based Pediatric Obesity Prevention Program Facilitated by Health Professionals. J. Sch. Health 2013, 83, 171–181. [Google Scholar] [CrossRef]
- Hull, P.C.; Buchowski, M.; Canedo, J.R.; Beech, B.M.; Du, L.; Koyama, T.; Zoorob, R. Childhood obesity prevention cluster randomized trial for Hispanic families: Outcomes of the healthy families study. Pediatr. Obes. 2018, 13, 686–696. [Google Scholar] [CrossRef]
- Hughes, S.O.; Power, T.G.; Beck, A.D.; Betz, D.; Goodell, L.S.; Hopwood, V.; Jaramillo, J.A.; Lanigan, J.; Martinez, A.D.; Micheli, N.; et al. Twelve-Month Efficacy of an Obesity Prevention Program Targeting Hispanic Families With Preschoolers From Low-Income Backgrounds. J. Nutr. Educ. Behav. 2021, 53, 677–690. [Google Scholar] [CrossRef]
- Hollar, D.; Lombardo, M.; Lopez-Mitnik, G.; Hollar, T.L.; Almon, M.; Agatston, A.S.; Messiah, S.E. Effective multi-level, multi-sector, school-based obesity prevention programming improves weight, blood pressure, and academic performance, especially among low-income, minority children. J. Health Care Poor Underserved 2010, 21, 93–108. [Google Scholar] [CrossRef]
- Heerman, W.J.; Teeters, L.; Sommer, E.C.; Burgess, L.E.; Escarfuller, J.; Van Wyk, C.; Barkin, S.L.; Duhon, A.A.; Cole, J.; Samuels, L.R.; et al. Competency-Based Approaches to Community Health: A Randomized Controlled Trial to Reduce Childhood Obesity among Latino Preschool-Aged Children. Child. Obes. 2019, 15, 519–531. [Google Scholar] [CrossRef]
- Hasson, R.E.; Adam, T.C.; Davis, J.N.; Kelly, L.A.; Ventura, E.E.; Byrd-Williams, C.E.; Toledo-Corral, C.M.; Roberts, C.K.; Lane, C.J.; Azen, S.P.; et al. Randomized controlled trial to improve adiposity, inflammation, and insulin resistance in obese African-American and Latino youth. Obesity 2012, 20, 811–818. [Google Scholar] [CrossRef]
- Haines, J.; Rifas-Shiman, S.L.; Gross, D.; McDonald, J.; Kleinman, K.; Gillman, M.W. Randomized trial of a prevention intervention that embeds weight-related messages within a general parenting program. Obesity 2016, 24, 191–199. [Google Scholar] [CrossRef]
- Gatto, N.M.; Martinez, L.; Spruijt-Metz, D.; Davis, J. LA sprouts randomized controlled nutrition, cooking and gardening programme reduces obesity and metabolic risk in Hispanic/Latino youth. Pediatr. Obes. 2017, 12, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Fiechtner, L.; Perkins, M.; Biggs, V.; Langhans, N.; Sharifi, M.; Price, S.; Luo, M.; Locascio, J.J.; Hohman, K.H.; Hodge, H.; et al. Comparative Effectiveness of Clinical and Community-Based Approaches to Healthy Weight. Pediatrics 2021, 148, e2021050405. [Google Scholar] [CrossRef] [PubMed]
- Eichner, J.E.; Folorunso, O.A.; Moore, W.E. A Physical Activity Intervention and Changes in Body Mass Index at a Middle School With a Large American Indian Population, Oklahoma, 2004–2009. Prev. Chronic Dis. 2016, 13, E163. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, H.; Walbolt, M.M.; Arthur, K.N.; Sherman, C.; Dehorn, S.; Herring, R.P.; Reis, W. The effect of the shapedown program on BMI, waist-hip ratio and family functioning—A family-based weigh-reduction intervention serviceable to low-income, minority communities. J. Cult. Divers. 2020, 27, 22–28. [Google Scholar]
- Davis, S.M.; Myers, O.B.; Cruz, T.H.; Morshed, A.B.; Canaca, G.F.; Keane, P.C.; O’Donald, E.R. CHILE: Outcomes of a group randomized controlled trial of an intervention to prevent obesity in preschool Hispanic and American Indian children. Prev. Med. 2016, 89, 162–168. [Google Scholar] [CrossRef]
- Davis, J.N.; Ventura, E.E.; Tung, A.; Munevar, M.A.; Hasson, R.E.; Byrd-Williams, C.; Vanni, A.K.; Spruijt-Metz, D.; Weigensberg, M.; Goran, M.I. Effects of a randomized maintenance intervention on adiposity and metabolic risk factors in overweight minority adolescents. Pediatr. Obes. 2012, 7, 16–27. [Google Scholar] [CrossRef]
- Davis, J.N.; Pérez, A.; Asigbee, F.M.; Landry, M.J.; Vandyousefi, S.; Ghaddar, R.; Hoover, A.; Jeans, M.; Nikah, K.; Fischer, B.; et al. School-based gardening, cooking and nutrition intervention increased vegetable intake but did not reduce BMI: Texas sprouts—A cluster randomized controlled trial. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 18. [Google Scholar] [CrossRef]
- Davis, J.N.; Ventura, E.E.; Cook, L.T.; Gyllenhammer, L.E.; Gatto, N.M. LA Sprouts: A gardening, nutrition, and cooking intervention for Latino youth improves diet and reduces obesity. J. Am. Diet. Assoc. 2011, 111, 1224–1230. [Google Scholar] [CrossRef]
- Crespo, N.C.; Elder, J.P.; Ayala, G.X.; Slymen, D.J.; Campbell, N.R.; Sallis, J.F.; McKenzie, T.L.; Baquero, B.; Arredondo, E.M. Results of a multi-level intervention to prevent and control childhood obesity among Latino children: The Aventuras Para Niños Study. Ann. Behav. Med. Publ. Soc. Behav. Med. 2012, 43, 84–100. [Google Scholar] [CrossRef]
- Chen, J.-L.; Weiss, S.; Heyman, M.B.; Cooper, B.; Lustig, R.H. The Efficacy of the Web-Based Childhood Obesity Prevention Program in Chinese American Adolescents (Web ABC Study). J. Adolesc. Health 2011, 49, 148–154. [Google Scholar] [CrossRef]
- Chen, J.-L.; Guedes, C.M.; Lung, A.E. Smartphone-based Healthy Weight Management Intervention for Chinese American Adolescents: Short-term Efficacy and Factors Associated with Decreased Weight. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med. 2019, 64, 443–449. [Google Scholar] [CrossRef]
- Chen, J.; Weiss, S.; Heyman, M.B.; Lustig, R.H. Efficacy of a child-centred and family-based program in promoting healthy weight and healthy behaviors in Chinese American children: A randomized controlled study. J. Public Health 2010, 32, 219–229. [Google Scholar] [CrossRef]
- Caballero, B.; Clay, T.; Davis, S.M.; Ethelbah, B.; Rock, B.H.; Lohman, T.; Norman, J.; Story, M.; Stone, E.J.; Stephenson, L.; et al. Pathways: A school-based, randomized controlled trial for the prevention of obesity in American Indian schoolchildren. Am. J. Clin. Nutr. 2003, 78, 1030–1038. [Google Scholar] [CrossRef]
- Barkin, S.L.; Gesell, S.B.; Póe, E.K.; Ip, E.H. Changing overweight Latino preadolescent body mass index: The effect of the parent-child dyad. Clin. Pediatr. 2011, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Barkin, S.L.; Gesell, S.B.; Po’e, E.K.; Escarfuller, J.; Tempesti, T. Culturally tailored, family-centered, behavioral obesity intervention for Latino-American preschool-aged children. Pediatrics 2012, 130, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, K.R.; Moreno, J.P.; Reesor, L.; Hernandez, D.C.; Johnston, C.A. Compañeros: High School Students Mentor Middle School Students to Address Obesity Among Hispanic Adolescents. Prev. Chronic Dis. 2017, 14, E92. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, K.R.; Ledoux, T.A.; Johnston, C.A. Randomized Controlled Trial to Increase Physical Activity Among Hispanic-American Middle School Students. J. Sch. Health 2021, 91, 307–317. [Google Scholar] [CrossRef]
- Van Der Heijden, G.J.; Wang, Z.J.; Chu, Z.; Toffolo, G.; Manesso, E.; Sauer, P.J.; Sunehag, A.L. Strength Exercise Improves Muscle Mass and Hepatic Insulin Sensitivity in Obese Youth. Med. Sci. Sports Exerc. 2010, 42, 1973–1980. [Google Scholar] [CrossRef]
- Johnston, C.A.; Moreno, J.P.; Gallagher, M.R.; Wang, J.; Papaioannou, M.A.; Tyler, C.; Foreyt, J.P. Achieving long-term weight maintenance in Mexican-American adolescents with a school-based intervention. J. Adolesc. Health Off. Publ. Soc. Adolesc. Med. 2013, 53, 335–341. [Google Scholar] [CrossRef]
- Johnston, C.A.; Tyler, C.; Fullerton, G.; Poston, W.S.C.; Haddock, C.K.; McFarlin, B.; Reeves, R.S.; Foreyt, J.P. Results of an intensive school-based weight loss program with overweight Mexican American children. Int. J. Pediatr. Obes. 2007, 2, 144–152. [Google Scholar] [CrossRef]
- Novotny, R.; Nigg, C.R.; Li, F.; Wilkens, L.R. Pacific kids DASH for health (PacDASH) randomized, controlled trial with DASH eating plan plus physical activity improves fruit and vegetable intake and diastolic blood pressure in children. Child. Obes. 2015, 11, 177–186. [Google Scholar] [CrossRef]
- Bender, M.S.; Clark, M.J. Cultural Adaptation for Ethnic Diversity: A Review of Obesity Interventions for Preschool Children. Calif. J. Health Promot. 2011, 9, 40. [Google Scholar] [CrossRef]
- Ngongalah, L.; Rankin, J.; Rapley, T.; Odeniyi, A.; Akhter, Z.; Heslehurst, N. Dietary and Physical Activity Behaviours in African Migrant Women Living in High Income Countries: A Systematic Review and Framework Synthesis. Nutrients 2018, 10, 1017. [Google Scholar] [CrossRef]
- Bhopal, R.S.; Douglas, A.; Wallia, S.; Forbes, J.F.; Lean, M.E.; Gill, J.M.; McKnight, J.A.; Sattar, N.; Sheikh, A.; Wild, S.H.; et al. Effect of a lifestyle intervention on weight change in south Asian individuals in the UK at high risk of type 2 diabetes: A family-cluster randomised controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 218–227. [Google Scholar] [CrossRef]
- Yi, Y.; EL Khoudary, S.R.; Miller, R.G.; Rubinstein, D.; Matthews, K.A.; Orchard, T.J.; Costacou, T. Women with Type 1 diabetes (T1D) experience a shorter reproductive period compared with nondiabetic women: The Pittsburgh Epidemiology of Diabetes Complications (EDC) study and the Study of Women’s Health Across the Nation (SWAN). Menopause 2021, 28, 634–641. [Google Scholar] [CrossRef]
- Ho, M.; Garnett, S.P.; Baur, L.; Burrows, T.; Stewart, L.; Neve, M.; Collins, C. Effectiveness of lifestyle interventions in child obesity: Systematic review with meta-analysis. Pediatrics 2012, 130, e1647–e1671. [Google Scholar] [CrossRef]
- El-Medany, A.Y.M.; Birch, L.; Hunt, L.P.; Matson, R.I.B.; Chong, A.H.W.; Beynon, R.; Hamilton-Shield, J.; Perry, R. What Change in Body Mass Index Is Required to Improve Cardiovascular Outcomes in Childhood and Adolescent Obesity through Lifestyle Interventions: A Meta-Regression. Child. Obes. 2020, 16, 449–478. [Google Scholar] [CrossRef]
- González Cortés, C.A.; Cossío-Torres, P.E.; Vargas-Morales, J.M.; Vidal-Batres, M.; Galván Almazán, G.J.; Portales Pérez, D.P.; Aradillas-García, C. Prevalence of prediabetes and its comorbidities in the Mexican pediatric population. Nutr. Hosp. 2021, 38, 722–728. [Google Scholar]
- Fernandes, F.B.; Fernandes, A.B.; Febba, A.C.S.; Leite, A.P.O.; Leite, C.A.; Vitalle, M.S.S.; Jung, F.F.; Casarini, D.E. Association of Ang-(1-7) and des-Arg 9 BK as new biomarkers of obesity and cardiometabolic risk factors in adolescents. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2021, 44, 969–977. [Google Scholar] [CrossRef]
- Alkhatib, A. Optimizing Lifestyle Behaviors in Preventing Multiple Long-Term Conditions. Encyclopedia 2023, 3, 468–477. [Google Scholar] [CrossRef]
- Byrd, A.S.; Toth, A.T.; Stanford, F.C. Racial Disparities in Obesity Treatment. Curr. Obes. Rep. 2018, 7, 130–138. [Google Scholar] [CrossRef]
- Ford, M.C.; Gordon, N.P.; Howell, A.; Green, C.E.; Greenspan, L.C.; Chandra, M.; Mellor, R.G.; Lo, J.C. Obesity severity, dietary behaviors, and lifestyle risks vary by race/ethnicity and age in a Northern California cohort of children with obesity. J. Obes. 2016, 2016, 4287976. [Google Scholar] [CrossRef]
- Bauer, K.W.; Marcus, M.D.; Larson, N.; Neumark-Sztainer, D. Socioenvironmental, personal, and behavioral correlates of severe obesity among an ethnically/racially diverse sample of US adolescents. Child. Obes. 2017, 13, 470–478. [Google Scholar] [CrossRef]
- Wang, Y. Disparities in pediatric obesity in the United States. Adv. Nutr. 2011, 2, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, A.; Nnyanzi, L.A.; Mujuni, B.; Amanya, G.; Ibingira, C. Preventing Multimorbidity with Lifestyle Interventions in Sub-Saharan Africa: A New Challenge for Public Health in Low and Middle-Income Countries. Int. J. Environ. Res. Public Health 2021, 18, 12449. [Google Scholar] [CrossRef] [PubMed]


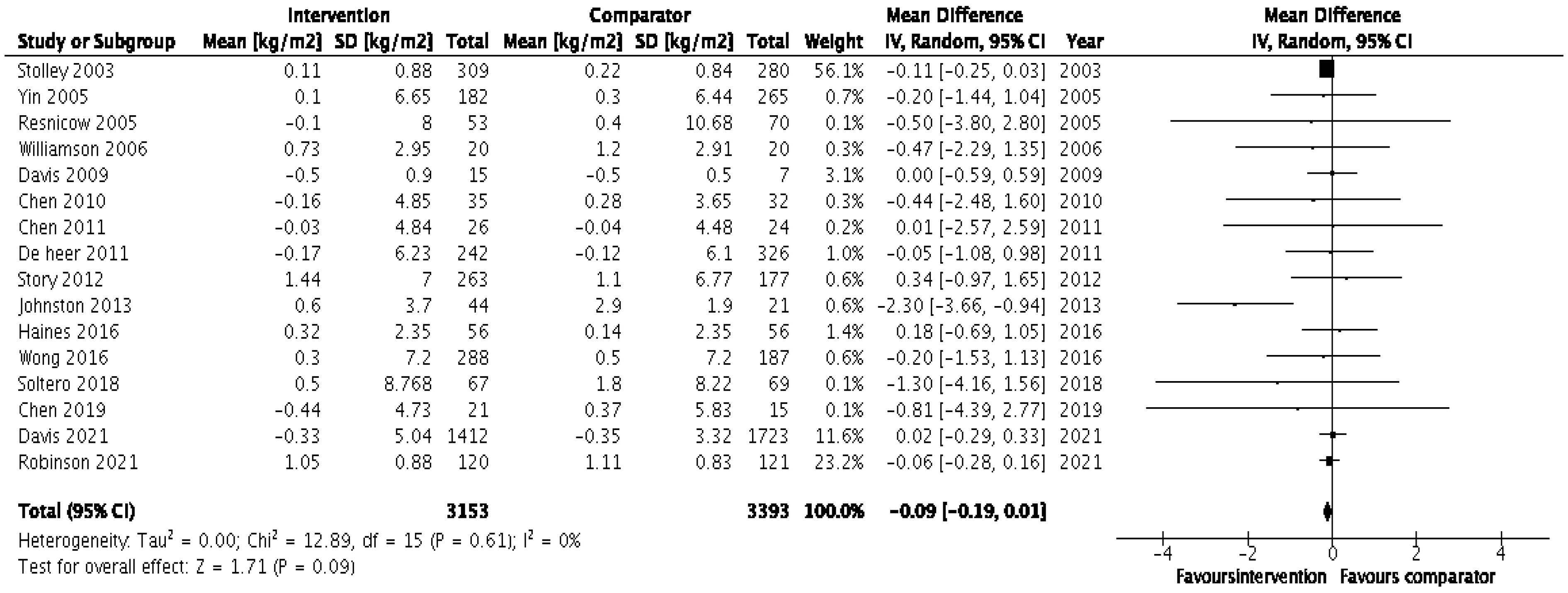
| Study, Year and Author | Intervention Type, Duration, Intensity and Time Characteristics | Intervention Setting | Age and Characteristics of Participating Children | Intervention Benefits on Obesity and Measured Comorbidity Outcomes | Recommendation for Effectiveness on Comorbidities |
|---|---|---|---|---|---|
| Effective interventions | |||||
| Yli-Piipari et al., 2018, USA [50]. | 12 weeks of supervised PA (moderate/vigorous, 60 min, twice a week) and nutrition education for parents/guardians. | Paediatric primary care setting. | Overweight/obese Hispanic children; Mean age = 11 years. | Change in mean BMI (kg/m2): −2.2, p = 0.04. | Short-term supervised high-intensity PA targeting high-risk adolescents was effective in reducing diabetes risk. |
| Change in mean BMI%: −2.53, p = 0.02. | |||||
| Change in mean BMI z-score: −3.64, p = 0.002. | |||||
| Change in mean WC (cm): −2.57, p = 0.02. | |||||
| Change in mean fasting glucose: −6.43, p < 0.001. | |||||
| Williford et al., 1996, USA [55]. | 15 weeks of supervised PA only (5 days/week, 45-min sessions of PE classes + conditioning programme). | School. | Predominantly African American children; Age range 12 to 13 (7th grade). | Change in sum of 7 skinfold thickness (mm): −1.31, p = 0.09. | Short-term, more frequent, moderate-intensity PA was effective in improving serum lipid profile, regardless of BMI. |
| Change in mean HDL (mmol/L): 0.28, p < 0.05. | |||||
| Change in mean LDL (mmol/L): −0.32, p < 0.05. | |||||
| Van der Heijden et al. 2010, USA [98]. | 12 weeks of supervised PA (twice a week, 30-min aerobic exercise session at ≥70% of peak oxygen consumption (VO2peak)). | Primary care (equipped laboratory in a hospital). | Lean and obese Hispanic children; Median age = 15 years. | Change in intrahepatic fats: Obese: −3.3, p < 0.05; Lean: No change. | Well-controlled short-term high-intensity exercise intervention was effective in reducing diabetes risk, only in high-risk obese children. |
| Change in visceral fats: Obese: −5.1, p < 0.05; Lean: No change. | |||||
| Change in fasting insulin: Obese: −3.6, p < 0.01; Lean: No change. | |||||
| Change in HOM-AIR: Obese: −0.8, p < 0.01; Lean: No change. | |||||
| Rieder et al., 2013, USA [66]. | 6 months of supervised PA (60 min/week, moderate) and lifestyle education. | Community. | Mixed ethnic minority children; Mean age = 15 years. | Change in mean BMI (kg/m2): −0.7/month, p < 0.001. | Medium-term, moderate-intensity supervised PA was effective at the community level in adolescents with obesity. |
| Change in mean BMI z-score: −0.003/month, p < 0.001. | |||||
| Hollar et al., 2010, USA [77]. | 2 years of unsupervised PA and dietary modifications. | School. | Predominantly Hispanic children; Mean age = 7.8 years. | Mean BMI: Maintained normal BMI, p = 0.02. | Longitudinal unsupervised PA with diet education was effective for the maintenance of healthy BMI in minority groups. |
| Eichner et al., 2016, USA [83]. | 5 years of unsupervised, unstructured PA (walk to school and team sports). | School. | Indian American children; Aged 12–15 years. | Change in BMI z-score: 0.7 to 0.7. | Very long duration, unsupervised, unstructured PA maintained BMI in American Indian adolescents. |
| Dos Santos et al., 2020, USA [84]. | 8 weeks of supervised PA (vigorous, e.g., lap runs). | School. | Overweight/obese mainly Hispanic children; Aged 10–16 years. | Change in BMI (kg/m2): −0.51, p = 0.012. | Short duration, supervised, high-intensity PA reduced obesity in high-risk Hispanic adolescents. |
| Change in waist to hip ratio: −0.01, p < 0.001. | |||||
| No significant effects of intervention | |||||
| Yin et al., 2012, USA [49]. | 18 weeks of supervised, unstructured and structured PA (free play and 15 – 20 min PA), nutrition promotion and parental education. | Community centres and homes. | Hispanic children; Mean age = 4.1 years and range = 3 to 5 years. | Change in mean BMI z-score: −0.09, p = 0.09. | Short-term low-intensity PA and nutrition education was ineffective in very young Hispanic children. |
| Yin et al., 2005, USA [51]. | 24 weeks of intervention, including 129 days of after-school PA (moderate intensity, 80 min per week) and the provision of healthy snacks. | School (school and after-school sessions). | Predominantly African American children; Mean age = 8.7 years. | Change in mean BMI (kg/m2): 0.1, p > 0.05. | Short-term moderate-intensity PA was ineffective in reducing obesity or its comorbidities among African Americans. |
| Change in %BF: −0.76, p = 0.027. | |||||
| Change in FM (Kg): −0.29, p = 0.17. | |||||
| Change in FFM (kg): 0.18, p = 0.12. | |||||
| Change in mean WC (cm): −0.4, p = 0.32. | |||||
| Change in mean SBP (mmHg): −1.8, p = 0.15. | |||||
| Change in mean DBP (mmHg): −1.1, p = 0.41. | |||||
| Change in mean TC (mg/dL): −0.2, p = 0.94. | |||||
| Change in mean HDL (mg/dL): 0.7, p = 0.64. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obita, G.; Alkhatib, A. Effectiveness of Lifestyle Nutrition and Physical Activity Interventions for Childhood Obesity and Associated Comorbidities among Children from Minority Ethnic Groups: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2524. https://doi.org/10.3390/nu15112524
Obita G, Alkhatib A. Effectiveness of Lifestyle Nutrition and Physical Activity Interventions for Childhood Obesity and Associated Comorbidities among Children from Minority Ethnic Groups: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(11):2524. https://doi.org/10.3390/nu15112524
Chicago/Turabian StyleObita, George, and Ahmad Alkhatib. 2023. "Effectiveness of Lifestyle Nutrition and Physical Activity Interventions for Childhood Obesity and Associated Comorbidities among Children from Minority Ethnic Groups: A Systematic Review and Meta-Analysis" Nutrients 15, no. 11: 2524. https://doi.org/10.3390/nu15112524
APA StyleObita, G., & Alkhatib, A. (2023). Effectiveness of Lifestyle Nutrition and Physical Activity Interventions for Childhood Obesity and Associated Comorbidities among Children from Minority Ethnic Groups: A Systematic Review and Meta-Analysis. Nutrients, 15(11), 2524. https://doi.org/10.3390/nu15112524







