The Impact and Effectiveness of Weight Loss on Kidney Transplant Outcomes: A Narrative Review
Abstract
1. Introduction
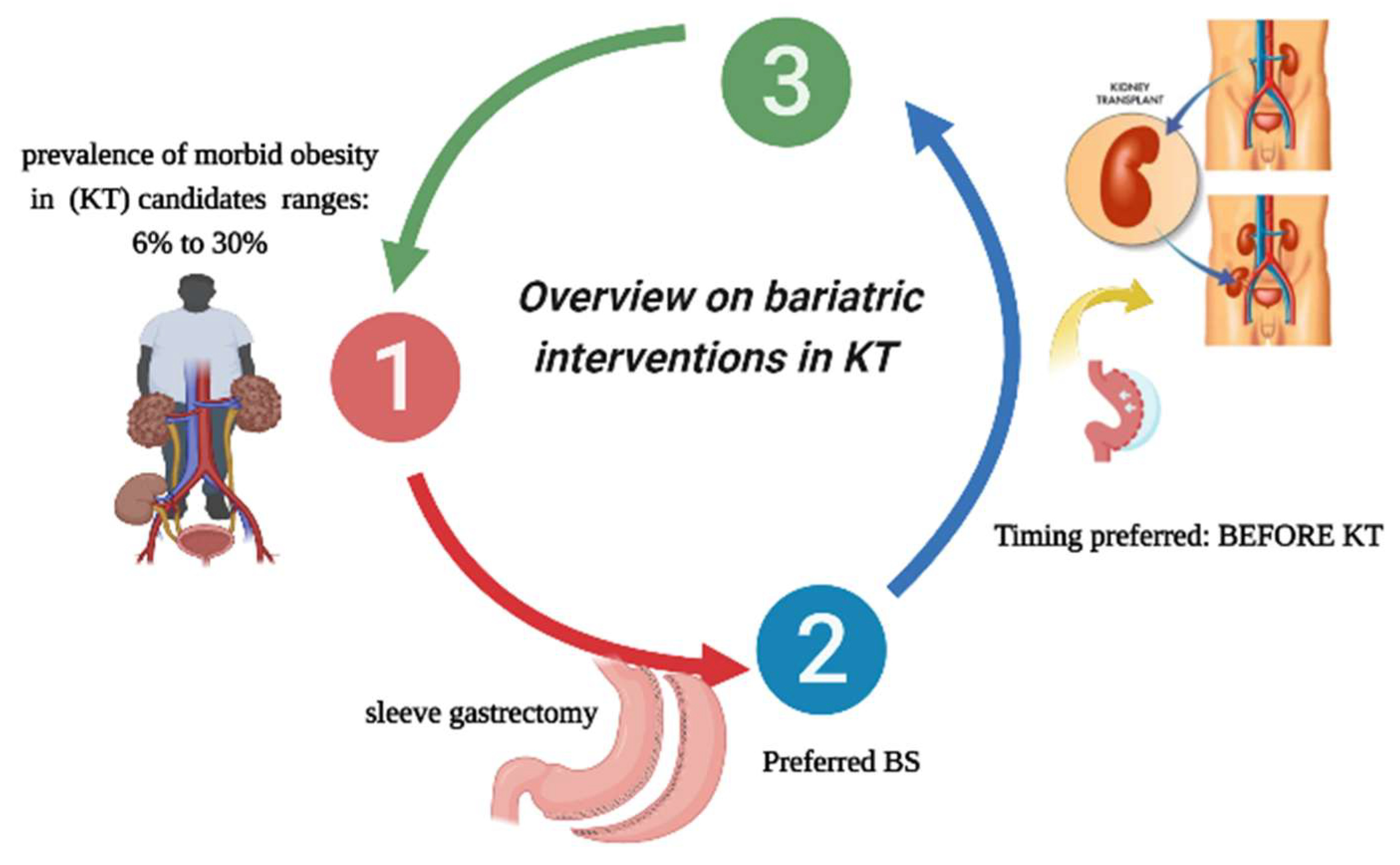
2. Overview of Bariatric Interventions in Kidney Transplant Candidates/Recipients
2.1. Incidence of Morbid Obesity in This Class of Patients
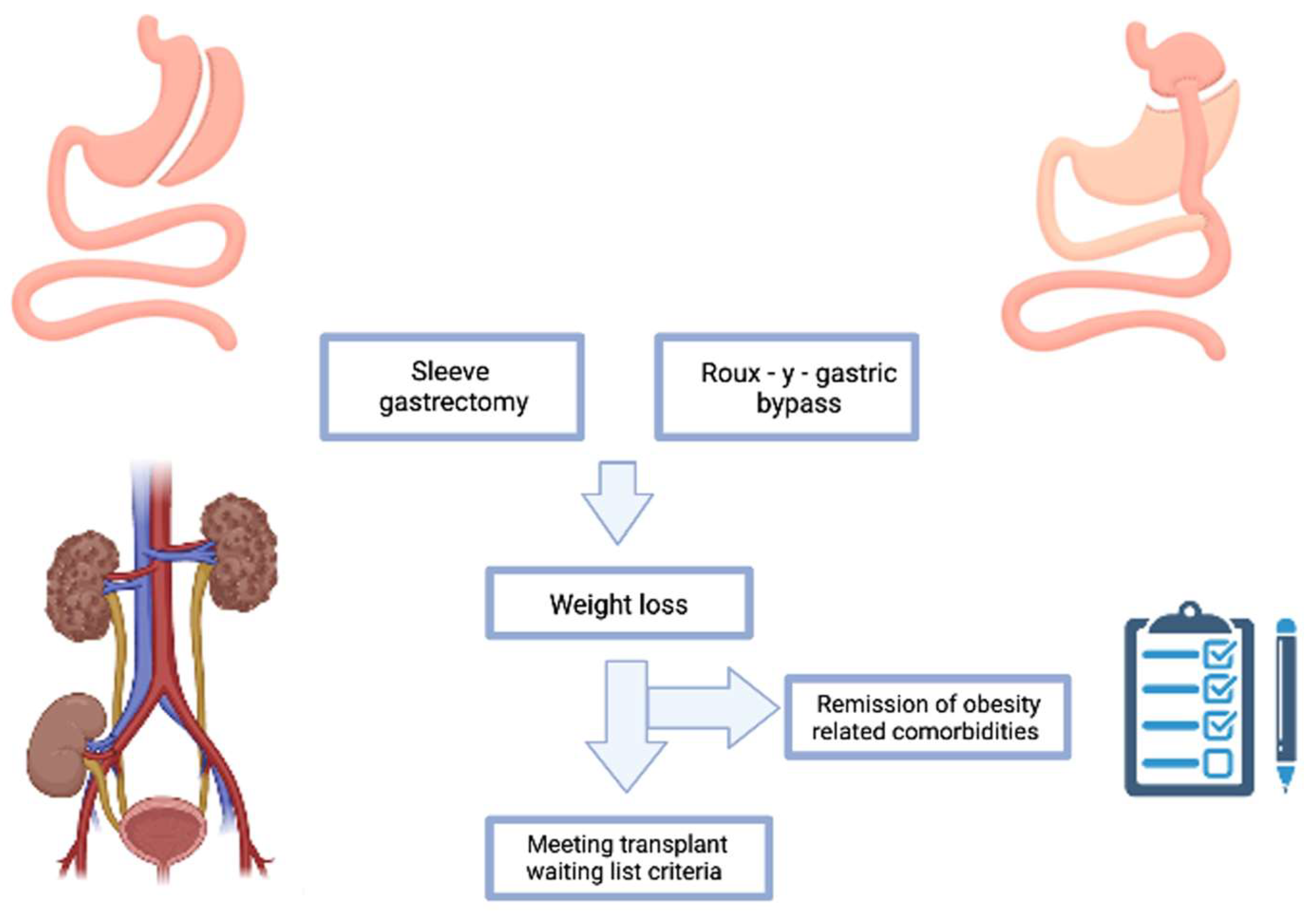
2.2. Type of Bariatric Interventions Performed
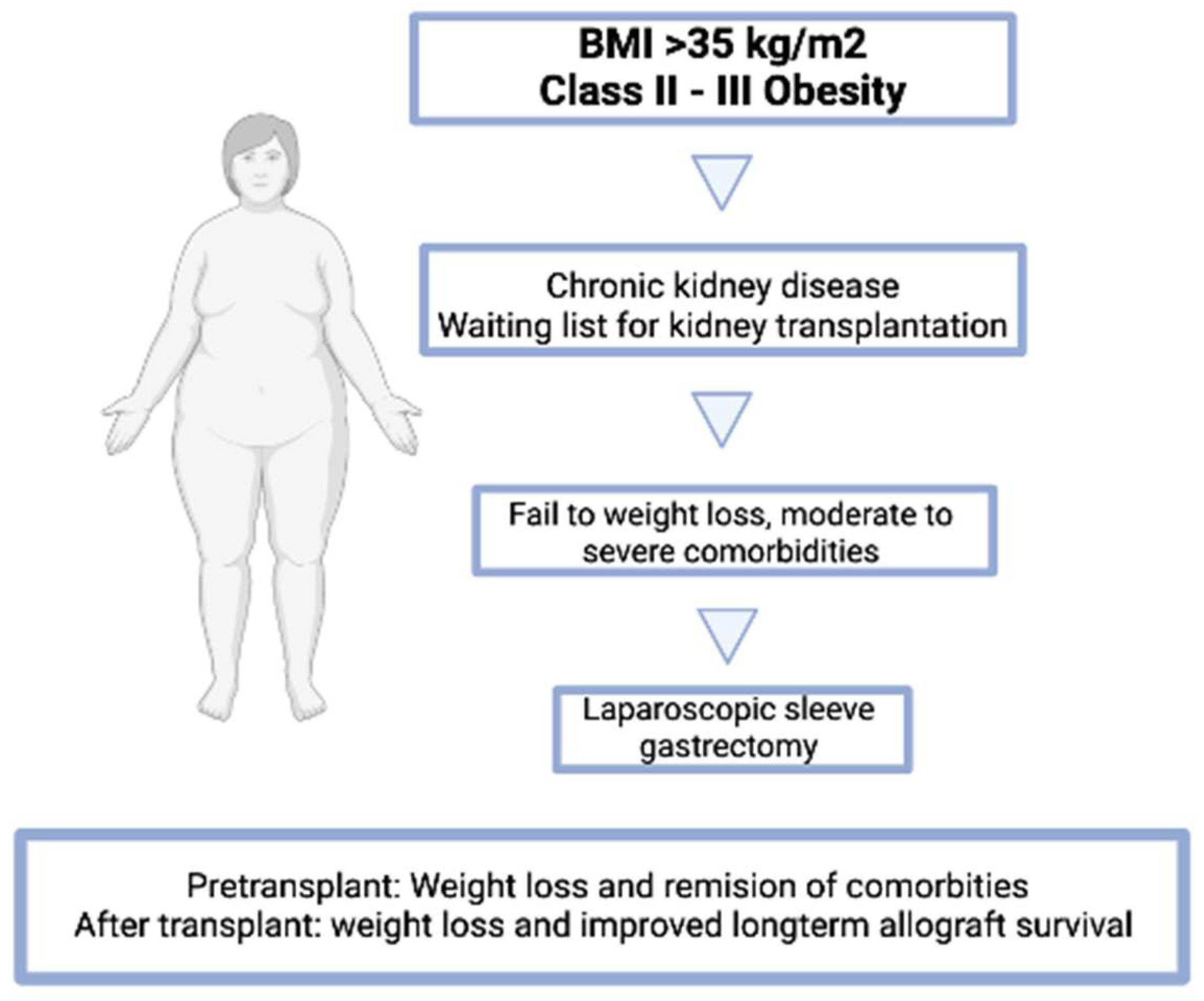
2.3. Is a Specific BMI Cut-Off Point in Patients That Makes Bariatric Surgery More Effective?
3. Metabolic Changes Related to Bariatric Surgery
3.1. Impact of Bariatric Surgery on the Absorption and Effectiveness of Immunosuppressive Drugs
3.2. Outcomes and Complications of Transplant Surgery. Impact of Obesity on KT Outcomes
3.3. Impact of BS on Weight Loss and Outcomes in KT Patients
3.4. Diet Regimens and Therapeutic Strategies to Avoid or Minimize Weight Regain
3.5. Nutritional Behavior
4. Cost-Effectiveness of Bariatric Surgery in Kidney Transplantation
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayoral, L.P.; Andrade, G.M.; Mayoral, E.P.; Huerta, T.H.; Canseco, S.P.; Canales, F.J.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Santiago, A.D.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Mousapour, P.; Ling, J.; Zimbudzi, E. Comparison of Kidney Transplantation Outcomes Between Patients with and Without Pre-transplantation Bariatric Surgery: A Systematic Review. Obes. Surg. 2022, 32, 4066–4081. [Google Scholar] [CrossRef] [PubMed]
- Chintam, K.; Chang, A.R. Strategies to treat obesity in patients with CKD. Am. J. Kidney Dis. 2021, 77, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dabbas, W.; Gangemi, A.; Benedetti, E.; Lash, J.; Finn, P.W.; Perkins, D.L. Obesity Management and Chronic Kidney Disease. Semin. Nephrol. 2021, 41, 392–402. [Google Scholar] [CrossRef]
- Montgomery, J.R.; Telem, D.A.; Waits, S.A. Bariatric surgery for prospective living kidney donors with obesity? Am. J. Transplant. 2019, 19, 2415–2420. [Google Scholar] [CrossRef]
- Kostro, J.Z.; Bzoma, B.; Proczko-Stepaniak, M.; Hellmann, A.R.; Hać, S.; Kaska, Ł.; Dębska-Ślizień, A. Kidney Transplantation in Patients After Bariatric Surgery: High-Volume Bariatric and Transplant Center Experience. Transplant. Proc. 2022, 54, 955–959. [Google Scholar] [CrossRef]
- Gioco, R.; Sanfilippo, C.; Veroux, P.; Corona, D.; Privitera, F.; Brolese, A.; Ciarleglio, F.; Volpicelli, A.; Veroux, M. Abdominal wall complications after kidney transplantation: A clinical review. Clin. Transplant. 2021, 35, e14506. [Google Scholar] [CrossRef]
- Oniscu, G.C.; Abramowicz, D.; Bolignano, D.; Gandolfini, I.; Hellemans, R.; Maggiore, U.; Nistor, I.; O’Neill, S.; Sever, M.S.; Koobasi, M.; et al. Management of obesity in kidney transplant candidates and recipients: A clinical practice guideline by the DESCARTES Working Group of ERA. Nephrol. Dial. Transplant. 2021, 37 (Suppl. S1), i1–i15. [Google Scholar] [CrossRef]
- Dziodzio, T.; Hillebrandt, K.H.; Knitter, S.; Nösser, M.; Globke, B.; Ritschl, P.V.; Biebl, M.; Denecke, C.; Raakow, J.; Lurje, G.; et al. Body Mass Index Thresholds and the Use of Bariatric Surgery in the Field of Kidney Transplantation in Germany. Obes. Surg. 2022, 32, 1641–1648. [Google Scholar] [CrossRef]
- Choudhury, R.A.; Hoeltzel, G.; Prins, K.; Chow, E.; Moore, H.B.; Lawson, P.J.; Yoeli, D.; Pratap, A.; Abt, P.L.; Dumon, K.R.; et al. Sleeve Gastrectomy Compared with Gastric Bypass for Morbidly Obese Patients with End Stage Renal Disease: A Decision Analysis. J. Gastrointest. Surg. 2020, 24, 756–763. [Google Scholar] [CrossRef]
- Schindel, H.; Winkler, J.; Yemini, R.; Carmeli, I.; Nesher, E.; Keidar, A. Survival benefit in bariatric surgery kidney recipients may be mediated through effects on kidney graft function and improvement of co-morbidities: A case-control study. Surg. Obes. Relat. Dis. 2019, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Zappa, M.A.; Zese, M.; Bardi, U.; Carbonelli, M.G.; Carrano, F.M.; Casella, G.; Chianelli, M.; Chiappetta, S.; Iossa, A.; et al. Development of the Italian Clinical Practice Guidelines on Bariatric and Metabolic Surgery: Design and Methodological Aspects. Nutrients 2022, 15, 189. [Google Scholar] [CrossRef] [PubMed]
- Nöhre, M.; Schieffer, E.; Hanke, A.; Pape, L.; Schiffer, L.; Schiffer, M.; de Zwaan, M. Obesity After Kidney Transplantation-Results of a KTx360°Substudy. Front. Psychiatry 2020, 11, 399. [Google Scholar] [CrossRef]
- Tarsitano, M.G.; Porchetti, G.; Caldara, R.; Secchi, A.; Conte, C. Obesity and lifestyle habits among kidney transplant recipients. Nutrients 2022, 14, 2892. [Google Scholar] [CrossRef]
- Schold, J.D.; Augustine, J.J.; Huml, A.M.; Fatica, R.; Nurko, S.; Wee, A.; Poggio, E.D. Effects of body mass index on kidney transplant outcomes are significantly modified by patient characteristics. Am. J. Transplant. 2021, 21, 751–765. [Google Scholar] [CrossRef]
- Veasey, T.M.; Fleming, J.N.; Strout, S.E.; Miller, R.; Pilch, N.A.; Meadows, H.B.; Mardis, C.R.; Mardis, B.A.; Shenvi, S.; McGillicuddy, J.; et al. Morbid obesity and functional status as predictors of surgical complication after renal transplantation. Am. J. Surg. 2018, 215, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.N. Obesity in patients undergoing dialysis and kidney transplantation. Adv. Chronic Kidney Dis. 2013, 20, 128–134. [Google Scholar] [CrossRef]
- Ladhani, M.; Craig, J.C.; Irving, M.; Clayton, P.A.; Wong, G. Obesity and the risk of cardiovascular and all-cause mortality in chronic kidney disease: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2017, 32, 439–449. [Google Scholar] [CrossRef]
- Spaggiari, M.; Di Cocco, P.; Tulla, K.; Kaylan, K.B.; Masrur, M.A.; Hassan, C.; Alvarez, J.A.; Benedetti, E.; Tzvetanov, I. Simultaneous robotic kidney transplantation and bariatric surgery for morbidly obese patients with end-stage renal failure. Am. J. Transplant. 2021, 21, 1525–1534. [Google Scholar] [CrossRef]
- Vilallonga, R.; Fort, J.M.; Caubet, E.; Gonzalez, O.; Armengol, M. Robotic sleeve gastrectomy versus laparoscopic sleeve gastrectomy: A comparative study with 200 patients. Obes. Surg. 2013, 23, 1501–1507. [Google Scholar] [CrossRef]
- Kienzl-Wagner, K.; Weissenbacher, A.; Gehwolf, P.; Wykypiel, H.; Öfner, D.; Schneeberger, S. Laparoscopic sleeve gastrectomy: Gateway to kidney transplantation. Surg. Obes. Relat. Dis. 2017, 13, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Bailey, A.J.; Morris, M.C.; Kassam, A.F.; Shah, S.A.; Diwan, T.S. Kidney transplantation after sleeve gastrectomy in the morbidly obese candidate: Results of a 2-year experience. Surg. Obes. Relat. Dis. 2020, 16, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Tsapepas, D.; Sandra, V.; Dale, L.A.; Drexler, Y.; King, K.L.; Yu, M.; Toma, K.; Van Bever, J.; Sanichar, N.; Husain, S.A.; et al. Retrospective analysis of the impact of severe obesity on kidney transplant outcomes. Nephrol. Dial. Transplant. 2022, 38, 472–480. [Google Scholar] [CrossRef]
- Chan, G.; Soucisse, M. Survey of Canadian Kidney Transplant Specialists on the Management of Morbid Obesity and the Transplant Waiting List. Can. J. Kidney Health Dis. 2016, 3, 2054358116675344. [Google Scholar] [CrossRef]
- Hajjar, R.; Marcotte, C.; Chan, G. Conservative Management of Obesity in Kidney Transplant Candidates. J. Ren. Nutr. 2022, 32, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Sarno, G.; Calabrese, P.; Frias-Toral, E.; Ceriani, F.; Fuchs-Tarlovsky, V.; Spagnuolo, M.; Cucalón, G.; Córdova, L.Á.; Schiavo, L.; Pilone, V. The relationship between preoperative weight loss and intra and post-bariatric surgery complications: An appraisal of the current preoperative nutritional strategies. Crit. Rev. Food Sci. Nutr. 2022, 12, 1–9. [Google Scholar] [CrossRef]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Iannelli, A. The Role of the Nutritionist in a Multidisciplinary Bariatric Surgery Team. Obes. Surg. 2019, 29, 1028–1030. [Google Scholar] [CrossRef]
- Elli, E.F.; Gonzalez-Heredia, R.; Sanchez-Johnsen, L.; Patel, N.; Garcia-Roca, R.; Oberholzer, J. Sleeve gastrectomy surgery in obese patients post-organ transplantation. Surg. Obes. Relat. Dis. 2016, 12, 528–534. [Google Scholar] [CrossRef]
- Gazzetta, P.G.; Bissolati, M.; Saibene, A.; Ghidini, C.G.A.; Guarneri, G.; Giannone, F.; Adamenko, O.; Secchi, A.; Rosati, R.; Socci, C. Bariatric Surgery to Target Obesity in the Renal Transplant Population: Preliminary Experience in a Single Center. Transplant. Proc. 2017, 49, 646–649. [Google Scholar] [CrossRef]
- Yemini, R.; Nesher, E.; Winkler, J.; Carmeli, I.; Azran, C.; Ben David, M.; Mor, E.; Keidar, A. Bariatric surgery in solid organ transplant patients: Long-term follow-up results of outcome, safety, and effect on immunosuppression. Am. J. Transplant. 2018, 18, 2772–2780. [Google Scholar] [CrossRef]
- Pané, A.; Molina-Andujar, A.; Olbeyra, R.; Romano-Andrioni, B.; Boswell, L.; Montagud-Marrahi, E.; Jiménez, A.; Ibarzabal, A.; Viaplana, J.; Ventura-Aguiar, P.; et al. Bariatric Surgery Outcomes in Patients with Kidney Transplantation. J. Clin. Med. 2022, 11, 6030. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Hajjar, R.; Boutin, L.; Garneau, P.Y.; Pichette, V.; Lafrance, J.P.; Elftouh, N.; Michaud, J.; du Souich, P. Prospective study of the changes in pharmacokinetics of immunosuppressive medications after laparoscopic sleeve gastrectomy. Am. J. Transplant. 2020, 20, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Foucher, Y.; Lorent, M.; Albano, L.; Roux, S.; Pernin, V.; Le Quintrec, M.; Legendre, C.; Buron, F.; Morelon, E.; Girerd, S.; et al. Renal transplantation outcomes in obese patients: A French cohort-based study. BMC Nephrol. 2021, 22, 79. [Google Scholar] [CrossRef] [PubMed]
- Scheuermann, U.; Babel, J.; Pietsch, U.C.; Weimann, A.; Lyros, O.; Semmling, K.; Hau, H.M.; Seehofer, D.; Rademacher, S.; Sucher, R. Recipient obesity as a risk factor in kidney transplantation. BMC Nephrol. 2022, 23, 37. [Google Scholar] [CrossRef] [PubMed]
- Liese, J.; Bottner, N.; Büttner, S.; Reinisch, A.; Woeste, G.; Wortmann, M.; Hauser, I.A.; Bechstein, W.O.; Ulrich, F. Influence of the recipient body mass index on the outcomes after kidney transplantation. Langenbecks Arch. Surg. 2018, 403, 73–82. [Google Scholar] [CrossRef]
- Yemini, R.; Rahamimov, R.; Nesher, E.; Anteby, R.; Ghinea, R.; Hod, T.; Mor, E. The Impact of Obesity and Associated Comorbidities on the Outcomes after Renal Transplantation with a Living Donor vs. Deceased Donor Grafts. J. Clin. Med. 2022, 11, 3069. [Google Scholar]
- Hill, C.J.; Courtney, A.E.; Cardwell, C.R.; Maxwell, A.P.; Lucarelli, G.; Veroux, M.; Furriel, F.; Cannon, R.M.; Hoogeveen, E.K.; Doshi, M.; et al. Recipient obesity and outcomes after kidney transplantation: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2015, 30, 1403–1411. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Mao, S.A.; Jadlowiec, C.C.; Mao, M.A.; Leeaphorn, N.; Kaewput, W.; Vaitla, P.; Pattharanitima, P.; Tangpanithandee, S.; Krisanapan, P.; et al. Machine Learning Consensus Clustering of Morbidly Obese Kidney Transplant Recipients in the United States. J. Clin. Med. 2022, 11, 3288. [Google Scholar] [CrossRef]
- Grèze, C.; Pereira, B.; Boirie, Y.; Guy, L.; Millet, C.; Clerfond, G.; Garrouste, C.; Heng, A.E. Impact of obesity in kidney transplantation: A prospective cohort study from French registries between 2008 and 2014. Nephrol. Dial. Transplant. 2022, 37, 584–594. [Google Scholar] [CrossRef]
- Jarrar, F.; Tennankore, K.K.; Vinson, A.J. Combined Donor-Recipient Obesity and the Risk of Graft Loss After Kidney Transplantation. Transpl. Int. 2022, 35, 10656. [Google Scholar] [CrossRef]
- Krishnan, N.; Higgins, R.; Short, A.; Zehnder, D.; Pitcher, D.; Hudson, A.; Raymond, N.T. Kidney Transplantation Significantly Improves Patient and Graft Survival Irrespective of BMI: A Cohort Study. Am. J. Transplant. 2015, 15, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Hasanain, M.; Rathore, S.S.; Iqbal, A.; Kazmi, S.K.; Yasmin, F.; Koritala, T.; Thongprayoon, C.; Surani, S. Incidence, predictors, and outcomes of early hospital readmissions after kidney transplantation: Systemic review and meta-analysis. Front. Med. 2022, 9, 1038315. [Google Scholar] [CrossRef] [PubMed]
- Chierici, A.; Alromayan, M.; Defatico, S.; Céline, D.R.; Vinci, D.; Rodolphe, A.N.; Schiavo, L.; Iannelli, A. Is bariatric surgery safer before, during, or after liver transplantation? A systematic review and meta-analysis. J. Liver Transplant. 2023, 9, 100139. [Google Scholar] [CrossRef]
- Cohen, J.B.; Lim, M.A.; Tewksbury, C.M.; Torres-Landa, S.; Trofe-Clark, J.; Abt, P.L.; Williams, N.N.; Dumon, K.R.; Goral, S. Bariatric surgery before and after kidney transplantation: Long-term weight loss and allograft outcomes. Surg. Obes. Relat. Dis. 2019, 15, 935–941. [Google Scholar] [CrossRef]
- Forte, C.C.; Pedrollo, E.F.; Nicoletto, B.B.; Lopes, J.B.; Manfro, R.C.; Souza, G.C.; Leitão, C.B. Risk factors associated with weight gain after kidney transplantation: A cohort study. PLoS ONE 2020, 15, e0243394. [Google Scholar] [CrossRef]
- Altheaby, A.; Alajlan, N.; Shaheen, M.F.; Abosamah, G.; Ghallab, B.; Aldawsari, B.; Rashidi, A.; Gafar, M.; Arabi, Z. Weight gain after renal transplant: Incidence, risk factors, and outcomes. PLoS ONE 2022, 17, e0268044. [Google Scholar] [CrossRef]
- de Oliveira, C.M.; Moura, Á.E.; Gonçalves, L.; Pinheiro, L.S.; Pinheiro, F.M., Jr.; Esmeraldo, R.M. Post-transplantation weight gain: Prevalence and the impact of steroid-free therapy. Transplant. Proc. 2014, 46, 1735–1740. [Google Scholar] [CrossRef]
- Sayilar, E.I.; Ersoy, A.; Ersoy, C.; Oruc, A.; Ayar, Y.; Sigirli, D. The effect of calcineurin inhibitors on anthropometric measurements in kidney transplant recipients. BMC Nephrol. 2022, 23, 375. [Google Scholar] [CrossRef]
- Costa, B.; Moratelli, L.; Silva, L.B.; Paiva, A.C.; Silva, A.N.; Carminatti, M.; Bastos, M.G.; Sanders-Pinheiro, H. Body mass index in the first year after kidney transplantation. Transplant. Proc. 2014, 46, 1750–1752. [Google Scholar] [CrossRef]
- Pilone, V.; Tramontano, S.; Renzulli, M.; Romano, M.; Cobellis, L.; Berselli, T.; Schiavo, L. Metabolic effects, safety, and acceptability of very low-calorie ketogenic dietetic scheme on candidates for bariatric surgery. Surg. Obes. Relat. Dis. 2018, 14, 1013–1019. [Google Scholar] [CrossRef]
- Schiavo, L.; Scalera, G.; Pilone, V.; De Sena, G.; Iannelli, A.; Barbarisi, A. Preservation of Fat-Free Mass After Bariatric Surgery: Our Point of View. Obes. Surg. 2017, 27, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, L.; Scalera, G.; Pilone, V.; De Sena, G.; Quagliariello, V.; Iannelli, A.; Barbarisi, A. A Comparative Study Examining the Impact of a Protein-Enriched Vs Normal Protein Postoperative Diet on Body Composition and Resting Metabolic Rate in Obese Patients after Sleeve Gastrectomy. Obes. Surg. 2017, 27, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, L.; Di Rosa, M.; Tramontano, S.; Rossetti, G.; Iannelli, A.; Pilone, V. Long-Term Results of the Mediterranean Diet After Sleeve Gastrectomy. Obes. Surg. 2020, 30, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Kluch, M.; Kurnatowska, I.; Matera, K.; Łokieć, K.; Puzio, T.; Czkwianianc, E.; Grzelak, P. Nutrition Trends in Patients Over the Long Term After Kidney Transplantation. Transplant. Proc. 2020, 52, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Heldal, K.; Midtvedt, K.; Lønning, K.; Iversen, T.; Hernæs, K.H.; Tsarpali, V.; Reisæter, A.V.; Bernklev, T. Kidney transplantation: An attractive and cost-effective alternative for older patients? A cost-utility study. Clin. Kidney J. 2019, 12, 888–894. [Google Scholar] [CrossRef]
- Al-Bahri, S.; Fakhry, T.K.; Gonzalvo, J.P.; Murr, M.M. Bariatric Surgery as a Bridge to Renal Transplantation in Patients with End-Stage Renal Disease. Obes. Surg. 2017, 27, 2951–2955. [Google Scholar] [CrossRef]
- Orandi, B.J.; Purvis, J.W.; Cannon, R.M.; Smith, A.B.; Lewis, C.E.; Terrault, N.A.; Locke, J.E. Bariatric surgery to achieve transplant in end-stage organ disease patients: A systematic review and meta-analysis. Am. J. Surg. 2020, 220, 566–579. [Google Scholar] [CrossRef]
- Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020, 104 (Suppl. S1), S11–S103. [Google Scholar] [CrossRef]
- Veroux, M.; Mattone, E.; Cavallo, M.; Gioco, R.; Corona, D.; Volpicelli, A.; Veroux, P. Obesity and bariatric surgery in kidney transplantation: A clinical review. World J. Diabetes. 2021, 12, 1563–1575. [Google Scholar] [CrossRef]
- Montgomery, J.R.; Cohen, J.A.; Brown, C.S.; Sheetz, K.H.; Chao, G.F.; Waits, S.A.; Telem, D.A. Perioperative risks of bariatric surgery among patients with and without history of solid organ transplant. Am. J. Transplant. 2020, 20, 2530–2539. [Google Scholar] [CrossRef]
- Guggino, J.; Coumes, S.; Wion, N.; Reche, F.; Arvieux, C.; Borel, A.L. Effectiveness and Safety of Bariatric Surgery in Patients with End-Stage Chronic Kidney Disease or Kidney Transplant. Obesity 2020, 28, 2290–2304. [Google Scholar] [CrossRef] [PubMed]
- Favre, G.; Schiavo, L.; Lemoine, S.; Esnault, V.L.M.; Iannelli, A. Longitudinal assessment of renal function in native kidney after bariatric surgery. Surg. Obes. Relat. Dis. 2018, 14, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarno, G.; Frias-Toral, E.; Ceriani, F.; Montalván, M.; Quintero, B.; Suárez, R.; García Velasquèz, E.; Muscogiuri, G.; Iannelli, A.; Pilone, V.; et al. The Impact and Effectiveness of Weight Loss on Kidney Transplant Outcomes: A Narrative Review. Nutrients 2023, 15, 2508. https://doi.org/10.3390/nu15112508
Sarno G, Frias-Toral E, Ceriani F, Montalván M, Quintero B, Suárez R, García Velasquèz E, Muscogiuri G, Iannelli A, Pilone V, et al. The Impact and Effectiveness of Weight Loss on Kidney Transplant Outcomes: A Narrative Review. Nutrients. 2023; 15(11):2508. https://doi.org/10.3390/nu15112508
Chicago/Turabian StyleSarno, Gerardo, Evelyn Frias-Toral, Florencia Ceriani, Martha Montalván, Beatriz Quintero, Rosario Suárez, Eloísa García Velasquèz, Giovanna Muscogiuri, Antonio Iannelli, Vincenzo Pilone, and et al. 2023. "The Impact and Effectiveness of Weight Loss on Kidney Transplant Outcomes: A Narrative Review" Nutrients 15, no. 11: 2508. https://doi.org/10.3390/nu15112508
APA StyleSarno, G., Frias-Toral, E., Ceriani, F., Montalván, M., Quintero, B., Suárez, R., García Velasquèz, E., Muscogiuri, G., Iannelli, A., Pilone, V., & Schiavo, L. (2023). The Impact and Effectiveness of Weight Loss on Kidney Transplant Outcomes: A Narrative Review. Nutrients, 15(11), 2508. https://doi.org/10.3390/nu15112508








