Neuroprotective Potential of Seed Extracts: Review of In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Methodology
3. Results
3.1. In Vitro Models
3.1.1. Cassia obtusifolia L.
3.1.2. Alpinia katsumadai
3.1.3. Glycine max L.
3.1.4. Perilla frutescens L.
3.1.5. Litchi chinensis
3.1.6. Cannabis sativa L.
3.1.7. Camellia sinensis
3.1.8. Hibiscus sabdariffa
3.1.9. Reynoutria elliptica
3.1.10. Vitis vinifera L.
3.1.11. Celosia argentea L.
3.1.12. Mucuna pruriens (L.)
3.1.13. Celastrus paniculatus
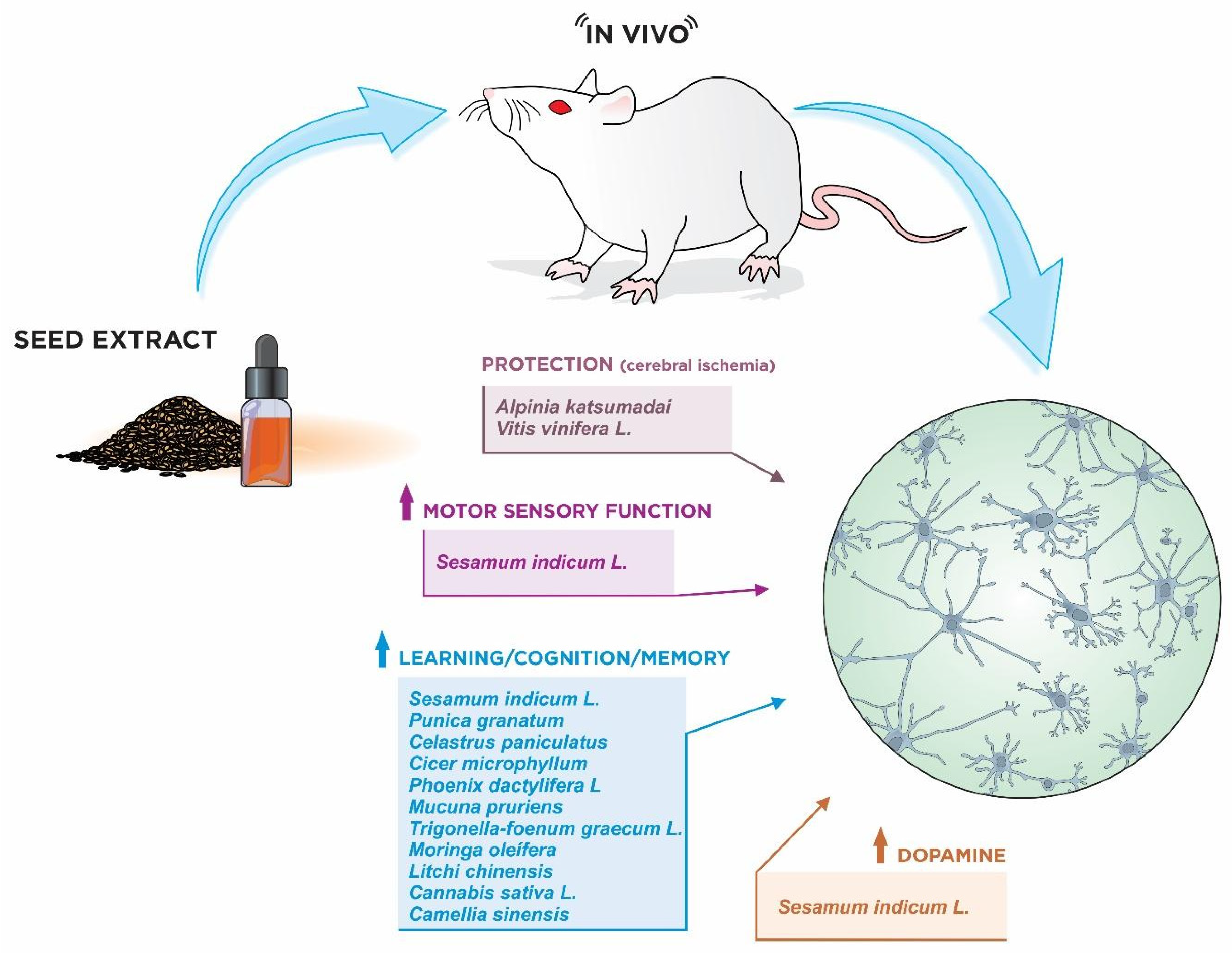
3.2. In Vivo Models
3.2.1. Alpinia katsumadai
3.2.2. Cassia obtusifolia L.
3.2.3. Sesamum indicum L.
3.2.4. Punica granatum
3.2.5. Camellia oleífera
3.2.6. Nigella sativa
3.2.7. Celastrus paniculatus
3.2.8. Cicer microphyllum
3.2.9. Phoenix dactylifera L.
3.2.10. Mucuna pruriens
3.2.11. Trigonella-foenum graecum L.
3.2.12. Moringa oleífera
3.2.13. Litchi chinensis
3.2.14. Cannabis sativa L.
3.2.15. Camellia sinensis
3.2.16. Vitis vinifera L.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prusiner, S.B. Biology and genetics of prions causing neurodegeneration. Annu. Rev. Genet. 2013, 47, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.C.; Giles, K.; Oehler, A.; Middleton, L.; Dexter, D.T.; Gentleman, S.M.; DeArmond, S.J.; Prusiner, S.B. Transmission of multiple system atrophy prions to transgenic mice. Proc. Natl. Acad. Sci. USA 2013, 110, 19555–19560. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.C.; Jucker, M. Neurodegenerative Diseases: Expanding the Prion Concept. Annu. Rev. Neurosci. 2015, 38, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Kim, J.E.; Rhie, S.J.; Yoon, S. The Role of Oxidative Stress in Neurodegenerative Diseases. Exp. Neurobiol. 2015, 24, 325–340. [Google Scholar] [CrossRef]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef]
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006239. [Google Scholar] [CrossRef]
- Sosa-Ortiz, A.L.; Acosta-Castillo, I.; Prince, M.J. Epidemiology of Dementias and Alzheimer’s Disease. Arch. Med. Res. 2012, 43, 600–608. [Google Scholar] [CrossRef]
- Vega, I.E.; Cabrera, L.Y.; Wygant, C.M.; Velez-Ortiz, D.; Counts, S.E. Alzheimer’s Disease in the Latino Community: Intersection of Genetics and Social Determinants of Health. J. Alzheimer’s Dis. 2017, 58, 979–992. [Google Scholar] [CrossRef]
- Marras, C.; Beck, J.C.; Bower, J.H.; Roberts, E.; Ritz, B.; Ross, G.W.; Abbott, R.D.; Savica, R.; Van Den Eeden, S.K.; Willis, A.W.; et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinson’s Dis. 2018, 4, 21. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49. [Google Scholar] [PubMed]
- Milenkovic, I.; Kovacs, G.G. Incidental corticobasal degeneration in a 76-year-old woman. Clin. Neuropathol. 2013, 32, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Varenika, V.; Forsayeth, J.; Bankiewicz, K. Future Applications: Gene Therapy. Neurosurg. Clin. N. Am. 2009, 20, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Lyros, E.; Bakogiannis, C.; Liu, Y.; Fassbender, K. Molecular links between endothelial dysfunction and neurodegeneration in Alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kandel, E.R.; Dudai, Y.; Mayford, M.R. The Molecular and Systems Biology of Memory. Cell 2014, 157, 163–186. [Google Scholar] [CrossRef]
- Dehghanian, F.; Kalantaripour, T.P.; Esmaeilpour, K.; Elyasi, L.; Oloumi, H.; Pour, F.M.; Asadi-Shekaari, M. Date seed extract ameliorates β-amyloid-induced impairments in hippocampus of male rats. Biomed. Pharmacother. 2017, 89, 221–226. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J.; Yu, C.; Tang, Y.; Liu, J.; Chen, H.; Jin, B.; Mei, Q.; Cao, S.; Qin, D. Lychee Seed Saponins Improve Cognitive Function and Prevent Neuronal Injury via Inhibiting Neuronal Apoptosis in a Rat Model of Alzheimer’s Disease. Nutrients 2017, 9, 105. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, S.K.; Kang, J.Y.; Bin Park, S.; Yoo, S.K.; Han, H.J.; Cho, K.H.; Kim, J.C.; Heo, H.J. Green Tea Seed Oil Suppressed Aβ1–42-Induced Behavioral and Cognitive Deficit via the Aβ-Related Akt Pathway. Int. J. Mol. Sci. 2019, 20, 1865. [Google Scholar] [CrossRef]
- Ye, Y.; Fang, F.; Li, Y. Isolation of the Sapogenin from Defatted Seeds of Camellia oleifera and Its Neuroprotective Effects on Dopaminergic Neurons. J. Agric. Food Chem. 2014, 62, 6175–6182. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Q.; Fan, P. Cannabisin F from Hemp (Cannabis sativa) Seed Suppresses Lipopolysaccharide-Induced Inflammatory Responses in BV2 Microglia as SIRT1 Modulator. Int. J. Mol. Sci. 2019, 20, 507. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Ji, J.; Lou, H.; Fan, P. Hemp (Cannabis sativa L.) Seed Phenylpropionamides Composition and Effects on Memory Dysfunction and Biomarkers of Neuroinflammation Induced by Lipopolysaccharide in Mice. ACS Omega 2018, 3, 15988–15995. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.-R.; Ren, S.-J.; Li, J. A new flavonone from seeds of Alpinia katsumadai and its neuroprotective effect on PC12 cells. China J. Chin. Mater. Medica 2014, 39, 2674–2678. [Google Scholar]
- Shao, R.; Xiao, J. Natural Products for Treatment of Alzheimer’s Disease and Related Diseases: Understanding their Mechanism of Action. Curr. Neuropharmacol. 2013, 11, 337. [Google Scholar]
- Akram, M.; Nawaz, A. Effects of medicinal plants on Alzheimer’s disease and memory deficits. Neural Regen. Res. 2017, 12, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.D.C. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019, 20, 2313. [Google Scholar] [CrossRef]
- Godkar, P.; Gordon, R.K.; Ravindran, A.; Doctor, B.P. Celastrus paniculatus seed water soluble extracts protect cultured rat forebrain neuronal cells from hydrogen peroxide-induced oxidative injury. Fitoterapia 2003, 74, 658–669. [Google Scholar] [CrossRef]
- Godkar, P.B.; Gordon, R.K.; Ravindran, A.; Doctor, B.P. Celastrus paniculatus seed water soluble extracts protect against glutamate toxicity in neuronal cultures from rat forebrain. J. Ethnopharmacol. 2004, 93, 213–219. [Google Scholar] [CrossRef]
- Hwang, I.K.; Yoo, K.-Y.; Kim, D.S.; Jeong, Y.-K.; Kim, J.D.; Shin, H.-K.; Lim, S.S.; Yoo, I.-D.; Kang, T.-C.; Kim, D.-W.; et al. Neuroprotective effects of grape seed extract on neuronal injury by inhibiting DNA damage in the gerbil hippocampus after transient forebrain ischemia. Life Sci. 2004, 75, 1989–2001. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Y.-M.; Leblanc, M.H.; Bhatt, A.J.; Rhodes, P.G. Grape Seed Extract Given Three Hours After Injury Suppresses Lipid Peroxidation and Reduces Hypoxic-Ischemic Brain Injury in Neonatal Rats. Pediatr. Res. 2007, 61, 295–300. [Google Scholar] [CrossRef]
- Fujishita, K.; Ozawa, T.; Shibata, K.; Tanabe, S.; Sato, Y.; Hisamoto, M.; Okuda, T.; Koizumi, S. Grape Seed Extract Acting on Astrocytes Reveals Neuronal Protection Against Oxidative Stress via Interleukin-6-mediated Mechanisms. Cell. Mol. Neurobiol. 2009, 29, 1121–1129. [Google Scholar] [CrossRef]
- Kasture, S.; Pontis, S.; Pinna, A.; Schintu, N.; Spina, L.; Longoni, R.; Simola, N.; Ballero, M.; Morelli, M. Assessment of Symptomatic and Neuroprotective Efficacy of Mucuna Pruriens Seed Extract in Rodent Model of Parkinson’s Disease. Neurotox. Res. 2009, 15, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.S.; Kim, H.G.; Choi, J.G.; Ryu, J.H.; Hur, J.; Kim, Y.J.; Oh, M.S. Cassiae semen, a seed of Cassia obtusifolia, has neuroprotective effects in Parkinson’s disease models. Food Chem. Toxicol. 2010, 48, 2037–2044. [Google Scholar] [CrossRef]
- Jamarkattel-Pandit, N.; Pandit, N.R.; Kim, M.Y.; Park, S.H.; Kim, K.S.; Choi, H.; Bu, Y. Neuroprotective effect of defatted sesame seeds extract against in vitro and in vivo ischemic neuronal damage. Planta Med. 2010, 76, 20–26. [Google Scholar] [CrossRef]
- Li, H.; Park, J.H.; Yan, B.; Yoo, K.-Y.; Lee, C.H.; Choi, J.H.; Hwang, I.K.; Won, M.-H. Neuroprotection ofAlpinia katsumadaiSeed Extract against Neuronal Damage in the Ischemic Gerbil Hippocampus is Linked to Altered Brain-Derived Neurotrophic Factor. Lab. Anim. Res. 2011, 27, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, M.I.H.; Kim, J.Y.; Ha, T.J.; Kim, S.Y.; Cho, K.-O. Anthocyanins Extracted from Black Soybean Seed Coat Protect Primary Cortical Neurons against in Vitro Ischemia. Biol. Pharm. Bull. 2012, 35, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Yao-Yue, C.; Qin, G.-W.; Guo, L.-H. Luteolin from Purple Perilla mitigates ROS insult particularly in primary neurons. Neurobiol. Aging 2012, 33, 176–186. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, M.B.; Hoda, M.N.; Bhatia, K.; Haque, R.; Fazili, I.S.; Jamal, A.; Khan, J.S.; Katare, D.P. Neuroprotective Effect of Sesame Seed Oil in 6-Hydroxydopamine Induced Neurotoxicity in Mice Model: Cellular, Biochemical and Neurochemical Evidence. Neurochem. Res. 2011, 37, 516–526. [Google Scholar] [CrossRef]
- Sarkaki, A.; Rezaiei, M.; Naseri, M.G.; Rafieirad, M. Improving active and passive avoidance memories deficits due to permanent cerebral ischemia by pomegranate seed extract in female rats. Malays. J. Med. Sci. 2013, 20, 25–34. [Google Scholar]
- Li, H.; Park, J.H.; Lee, J.C.; Yoo, K.Y.; Hwang, I.K.; Lee, C.H.; Choi, J.H.; Kim, J.-D.; Kang, I.-J.; Won, M.H. Neuroprotective effects of Alpinia katsumadai against experimental ischemic damage via control of oxidative stress. Pharm. Biol. 2013, 51, 197–205. [Google Scholar] [CrossRef]
- Yadav, S.K.; Prakash, J.; Chouhan, S.; Singh, S.P. Mucuna pruriens seed extract reduces oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in paraquat-induced Parkinsonian mouse model. Neurochem. Int. 2013, 62, 1039–1047. [Google Scholar] [CrossRef]
- Noor, N.A.; Fahmy, H.M.; Mohammed, F.F.; Elsayed, A.A.; Radwan, N.M. Nigella sativa amliorates inflammation and demyelination in the experimental autoimmune encephalomyelitis-induced Wistar rats. Int. J. Clin. Exp. Pathol. 2015, 8, 6269–6286. [Google Scholar] [PubMed]
- Bhagya, V.; Christofer, T.; Rao, B.S. Neuroprotective effect of Celastrus paniculatus on chronic stress-induced cognitive impairment. Indian J. Pharmacol. 2016, 48, 687–693. [Google Scholar] [CrossRef] [PubMed]
- El-Tarras, A.E.-S.; Attia, H.; Soliman, M.M.; El Awady, M.; Amin, A.A. Neuroprotective effect of grape seed extract against cadmium toxicity in male albino rats. Int. J. Immunopathol. Pharmacol. 2016, 29, 398–407. [Google Scholar] [CrossRef]
- Yi, J.H.; Park, H.J.; Lee, S.; Jung, J.W.; Kim, B.C.; Lee, Y.C.; Ryu, J.H.; Kim, D.H. Cassia obtusifolia seed ameliorates amyloid β-induced synaptic dysfunction through anti-inflammatory and Akt/GSK-3β pathways. J. Ethnopharmacol. 2015, 178, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.; Karan, M.; Dogra, R. Ameliorating effect of Celastrus paniculatus standardized extract and its fractions on 3-nitropropionic acid induced neuronal damage in rats: Possible antioxidant mechanism. Pharm. Biol. 2017, 55, 980–990. [Google Scholar] [CrossRef]
- Sharma, D.; Biswal, S.N.; Kumar, K.; Bhardwaj, P.; Barhwal, K.K.; Kumar, A.; Hota, S.K.; Chaurasia, O.P. Estrogen Receptor β Mediated Neuroprotective Efficacy of Cicer microphyllum Seed Extract in Global Hypoxia. Neurochem. Res. 2017, 42, 3474–3489. [Google Scholar] [CrossRef]
- Rai, S.N.; Birla, H.; Singh, S.S.; Zahra, W.; Patil, R.R.; Jadhav, J.P.; Gedda, M.R.; Singh, S.P. Mucuna pruriens Protects against MPTP Intoxicated Neuroinflammation in Parkinson’s Disease through NF-κB/pAKT Signaling Pathways. Front. Aging Neurosci. 2017, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Guan, J.; Gong, S.; Wang, R. Neuroprotective Effects of Grape Seed Procyanidin Extract on Ischemia-Reperfusion Brain Injury. Chin. Med. Sci. J. 2017, 32, 92–99. [Google Scholar]
- Wang, X.; Zhang, H.; Liu, J.; Chen, R.; Tang, Y.; Chen, H.; Gu, L.; Li, M.; Cao, S.; Qin, D.; et al. Inhibitory Effect of Lychee Seed Saponins on Apoptosis Induced by Aβ25-35 through Regulation of the Apoptotic and NF-κB Pathways in PC12 Cells. Nutrients 2017, 9, 337. [Google Scholar] [CrossRef]
- Assad, T.; Alam Khan, R.; Rajput, M.A. Effect of Trigonella foenum-graecum Linn. seeds methanol extract on learning and memory. Metab. Brain Dis. 2018, 33, 1275–1280. [Google Scholar] [CrossRef]
- Luo, L.; Bai, R.; Zhao, Y.; Li, J.; Wei, Z.; Wang, F.; Sun, B. Protective Effect of Grape Seed Procyanidins against H2 O2 -Induced Oxidative Stress in PC-12 Neuroblastoma Cells: Structure-Activity Relationships. J. Food Sci. 2018, 83, 2622–2628. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, W.-S.; Suo, D.-Q.; Li, Y.; Peng, L.; Xu, L.-X.; Zeng, K.-Y.; Ren, T.; Wang, Y.; Zhou, Y.; et al. Moringa oleifera Seed Extract Alleviates Scopolamine-Induced Learning and Memory Impairment in Mice. Front. Pharmacol. 2018, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Chen, L.; Miao, L.; Guo, Y.; Zhang, W.; Bai, Y. Grape Seed Proanthocyanidins Protect N2a Cells against Ischemic Injury via Endoplasmic Reticulum Stress and Mitochondrial-associated Pathways. CNS Neurol. Disord.-Drug Targets 2019, 18, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Luo, Q.; Zhou, Y.; Fan, P. CLG from Hemp Seed Inhibits LPS-Stimulated Neuroinflammation in BV2 Microglia by Regulating NF-κB and Nrf-2 Pathways. ACS Omega 2019, 4, 16517–16523. [Google Scholar] [CrossRef] [PubMed]
- Shalgum, A.; Govindarajulu, M.; Majrashi, M.; Ramesh, S.; Collier, W.E.; Griffin, G.; Amin, R.; Bradford, C.; Moore, T.; Dhanasekaran, M. Neuroprotective effects of Hibiscus Sabdariffa against hydrogen peroxide-induced toxicity. J. Herb. Med. 2019, 17–18, 100253. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, S.; Yu, J.S.; Park, D.H.; Kim, S.-Y.; Kang, K.S.; Lee, S.; Kim, K.H. Procyanidin B2 3″-O-gallate Isolated from Reynoutria elliptica Prevents Glutamate-Induced HT22 Cell Death by Blocking the Accumulation of Intracellular Reactive Oxygen Species. Biomolecules 2019, 9, 412. [Google Scholar] [CrossRef]
- Tu, X.; Wang, M.; Liu, Y.; Zhao, W.; Ren, X.; Li, Y.; Liu, H.; Gu, Z.; Jia, H.; Liu, J.; et al. Pretreatment of Grape Seed Proanthocyanidin Extract Exerts Neuroprotective Effect in Murine Model of Neonatal Hypoxic-ischemic Brain Injury by Its Antiapoptotic Property. Cell. Mol. Neurobiol. 2019, 39, 953–961. [Google Scholar] [CrossRef]
- Zeng, K.; Li, Y.; Yang, W.; Ge, Y.; Xu, L.; Ren, T.; Zhang, H.; Zhuo, R.; Peng, L.; Chen, C.; et al. Moringa oleifera seed extract protects against brain damage in both the acute and delayed stages of ischemic stroke. Exp. Gerontol. 2019, 122, 99–108. [Google Scholar] [CrossRef]
- Jin, W.; Sun, M.; Yuan, B.; Wang, R.; Yan, H.; Qiao, X. Neuroprotective Effects of Grape Seed Procyanidins on Ethanol-Induced Injury and Oxidative Stress in Rat Hippocampal Neurons. Alcohol Alcohol. 2020, 55, 357–366. [Google Scholar] [CrossRef]
- Qiu, W.Q.; Pan, R.; Tang, Y.; Zhou, X.G.; Wu, J.M.; Yu, L.; Law, B.Y.-K.; Ai, W.; Yu, C.-L.; Qin, D.-L.; et al. Lychee seed polyphenol inhibits Aβ-induced activation of NLRP3 inflammasome via the LRP1/AMPK mediated autophagy induction. Biomed. Pharmacother. 2020, 130, 110575. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, A.; Li, X.; Qin, D.; Jin, B.; Liu, J.; Tang, Y.; Wu, J.; Yu, C. The seed of Litchi chinensis fraction ameliorates hippocampal neuronal injury in an Aβ25-35-induced Alzheimer’s disease rat model via the AKT/GSK-3β pathway. Pharm. Biol. 2020, 58, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, M.Y.; Shin, T.S.; Kim, J.D. Green Tea Seed Isolated Theasaponin E1 Ameliorates AD Promoting Neurotoxic Pathogenesis by Attenuating Aβ Peptide Levels in SweAPP N2a Cells. Molecules 2020, 25, 2334. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Guo, X.; Ma, Z.; Li, Y.; Kang, J.; Zhang, G.; Gao, Y.; Liu, M.; Chen, H.; Kang, X. Grape seed proanthocyanidins protect PC12 cells from hydrogen peroxide-induced damage via the PI3K/AKT signaling pathway. Neurosci. Lett. 2021, 750, 13579. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Shen, S.; Zhang, X.; Wang, G.; Lu, Y.; Liu, X.; Wang, S.; Li, Q.; Cong, Y.; Shi, B. Chemical compounds with a neuroprotective effect from the seeds of Celosia argentea L. Food Funct. 2021, 12, 83–96. [Google Scholar] [CrossRef]
- Rachsee, A.; Chiranthanut, N.; Kunnaja, P.; Sireeratawong, S.; Khonsung, P.; Chansakaow, S.; Panthong, A. Mucuna pruriens (L.) DC. seed extract inhibits lipopolysaccharide-induced inflammatory responses in BV2 microglial cells. J. Ethnopharmacol. 2020, 267, 113518. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, J.; Song, G.L.; Niu, Q.; Xu, S.Z.; Feng, G.L.; Wang, H.X.; Li, Y.; Li, S.G.; Li, F. Grape Seed Procyanidin Extract Reduces Arsenic-Induced Renal Inflammatory Injury in Male Mice. Biomed. Environ. Sci. 2017, 30, 535–539. [Google Scholar]
- Peng, W.; Qin, R.; Li, X.; Zhou, H. Botany, phytochemistry, pharmacology, and potential application of Polygonum cuspidatum Sieb.et Zucc.: A review. J. Ethnopharmacol. 2013, 148, 729–745. [Google Scholar] [CrossRef]
- Fan, P.; Hostettmann, K.; Lou, H. Allelochemicals of the invasive neophyte Polygonum cuspidatum Sieb. & Zucc. (Polygonaceae). Chemoecology 2010, 20, 223–227. [Google Scholar]
- Kumar, R.; Deep, G.; Wempe, M.F.; Surek, J.; Kumar, A.; Agarwal, R.; Agarwal, C. Procyanidin B2 3,3″-di-O-gallate induces oxidative stress-mediated cell death in prostate cancer cells via inhibiting MAP kinase phosphatase activity and activating ERK1/2 and AMPK. Mol. Carcinog. 2018, 57, 57–69. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Dudek, M.K.; Pawłowska, K.A.; Pruś, A.; Ziaja, M.; Granica, S. The influence of procyanidins isolated from small-leaved lime flowers (Tilia cordata Mill.) on human neutrophils. Fitoterapia 2018, 127, 115–122. [Google Scholar] [CrossRef]
- Yin, C.; Luo, X.; Duan, Y.; Duan, W.; Zhang, H.; He, Y.; Sun, G.; Sun, X. Neuroprotective effects of lotus seedpod procyanidins on extremely low frequency electromagnetic field-induced neurotoxicity in primary cultured hippocampal neurons. Biomed. Pharmacother. 2016, 82, 628–639. [Google Scholar] [CrossRef] [PubMed]
- Kornpointner, C.; Martinez, A.S.; Marinovic, S.; Haselmair-Gosch, C.; Jamnik, P.; Schröder, K.; Löfke, C.; Halbwirth, H. Chemical composition and antioxidant potential of Cannabis sativa L. roots. Ind. Crop. Prod. 2021, 165, 113422. [Google Scholar] [CrossRef]
- Hritcu, L.; Foyet, H.S.; Stefan, M.; Mihasan, M.; Asongalem, A.E.; Kamtchouing, P. Neuroprotective effect of the methanolic extract of Hibiscus asper leaves in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. J. Ethnopharmacol. 2011, 137, 585–591. [Google Scholar] [CrossRef]
- Ryz, N.R.; Remillard, D.J.; Russo, E.B. Cannabis Roots: A Traditional Therapy with Future Potential for Treating Inflammation and Pain. Cannabis Cannabinoid Res. 2017, 2, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xin, H.-L.; Guo, M.-L. Review on research of the phytochemistry and pharmacological activities of Celosia argentea. Rev. Bras. De Farm. 2016, 26, 787–796. [Google Scholar] [CrossRef]
- Worawattananutai, P.; Itharat, A.; Ruangnoo, S. In vitro antioxidant, anti-inflammatory, cytotoxic activities against prostate cancer of extracts from Hibiscus sabdariffa leaves. J. Med. Assoc. Thail. 2014, 97 (Suppl. 8), S81–S87. [Google Scholar]
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative stress and neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188. [Google Scholar] [CrossRef] [PubMed]
- American Association of Neurological Surgeons (AANS); American Society of Neuroradiology (ASNR); Cardiovascular and Interventional Radiology Society of Europe (CIRSE); Canadian Interventional Radiology Association (CIRA); Congress of Neurological Surgeons (CNS); European Society of Minimally Invasive Neurological Therapy (ESMINT); European Society of Neuroradiology (ESNR); European Stroke Organization (ESO); Society for Cardiovascular Angiography and Interventions (SCAI); Society of Interventional Radiology (SIR); et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke 2018, 13, 612–632. [Google Scholar]
- Gao, X.; Cassidy, A.; Schwarzschild, M.A.; Rimm, E.B.; Ascherio, A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 2012, 78, 1138–1145. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Chen, W.-W.; Zhang, X.; Huang, W.-J. Role of neuroinflammation in neurodegenerative diseases (Review). Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Yang, C.-H.; Chang, M.-S.; Ciou, Y.-P.; Huang, Y.-C. Foam Properties and Detergent Abilities of the Saponins from Camellia oleifera. Int. J. Mol. Sci. 2010, 11, 4417–4425. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Guo, J.-L.; Wang, L.; Zhong, X.; Yao, W.-F.; Gao, H.; Liu, M.-Y. Natural products as pharmacological modulators of mitochondrial dysfunctions for the treatments of Alzheimer’s disease: A comprehensive review. Eur. J. Med. Chem. 2021, 218, 113401. [Google Scholar] [CrossRef] [PubMed]
- Lanctôt, K.L.; Rajaram, R.D.; Herrmann, N. Therapy for Alzheimer’s Disease: How Effective are Current Treatments? Ther. Adv. Neurol. Disord. 2009, 2, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.K.; AlBalawi, S.M.; Athar, T.; Khan, A.Q.; Al-Shahrani, H.; Islam, M. Moringa oleifera as an Anti-Cancer Agent against Breast and Colorectal Cancer Cell Lines. PLoS ONE 2015, 10, e0135814. [Google Scholar] [CrossRef]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Mufson, E.J.; Counts, S.; Perez, S.; Ginsberg, S.D. Cholinergic system during the progression of Alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef]
- Khan, M.M.; Kempuraj, D.; Thangavel, R.; Zaheer, A. Protection of MPTP-induced neuroinflammation and neurodegeneration by Pycnogenol. Neurochem. Int. 2013, 62, 379–388. [Google Scholar] [CrossRef]
- Heinrich, M. Duke’s handbook of medicinal plants of Latin America. J. Ethnopharmacol. 2009, 126, 577–578. [Google Scholar]
- Sahu, P.; Pal, A.; Nanda, G.; Champatisingh, D. Anticataleptic and antiepileptic activity of ethanolic extract of leaves of Mucuna pruriens: A study on role of dopaminergic system in epilepsy in albino rats. Indian J. Pharmacol. 2011, 43, 197–199. [Google Scholar] [CrossRef]
- Gordon, R.; Anantharam, V.; Kanthasamy, A.G.; Kanthasamy, A. Proteolytic activation of proapoptotic kinase protein kinase Cδ by tumor necrosis factor α death receptor signaling in dopaminergic neurons during neuroinflammation. J. Neuroinflamm. 2012, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Kim, S.R.; Park, J.Y.; Chung, E.S.; Park, K.W.; Won, S.Y.; Bok, E.; Jin, M.; Park, E.S.; Yoon, S.-H.; et al. Fluoxetine prevents MPTP-induced loss of dopaminergic neurons by inhibiting microglial activation. Neuropharmacology 2011, 60, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, D.; Liu, Z. Synergistic, additive and antagonistic effects of Potentilla fruticosa combined with EGb761 on antioxidant capacities and the possible mechanism. Ind. Crop. Prod. 2015, 67, 227–238. [Google Scholar] [CrossRef]
- Skroza, D.; Mekinić, I.G.; Svilović, S.; Šimat, V.; Katalinić, V. Investigation of the potential synergistic effect of resveratrol with other phenolic compounds: A case of binary phenolic mixtures. J. Food Compos. Anal. 2015, 38, 13–18. [Google Scholar] [CrossRef]
- Brusotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and characterization of bioactive compounds from plant resources: The role of analysis in the ethnopharmacological approach. J. Pharm. Biomed. Anal. 2013, 87, 218–228. [Google Scholar] [CrossRef]
- Medina-Franco, J.L.; Giulianotti, M.A.; Welmaker, G.S.; Houghten, R.A. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov. Today 2013, 18, 495–501. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Vandamme, T.F. Rodent models for human diseases. Eur. J. Pharmacol. 2015, 759, 84–89. [Google Scholar] [CrossRef]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef]
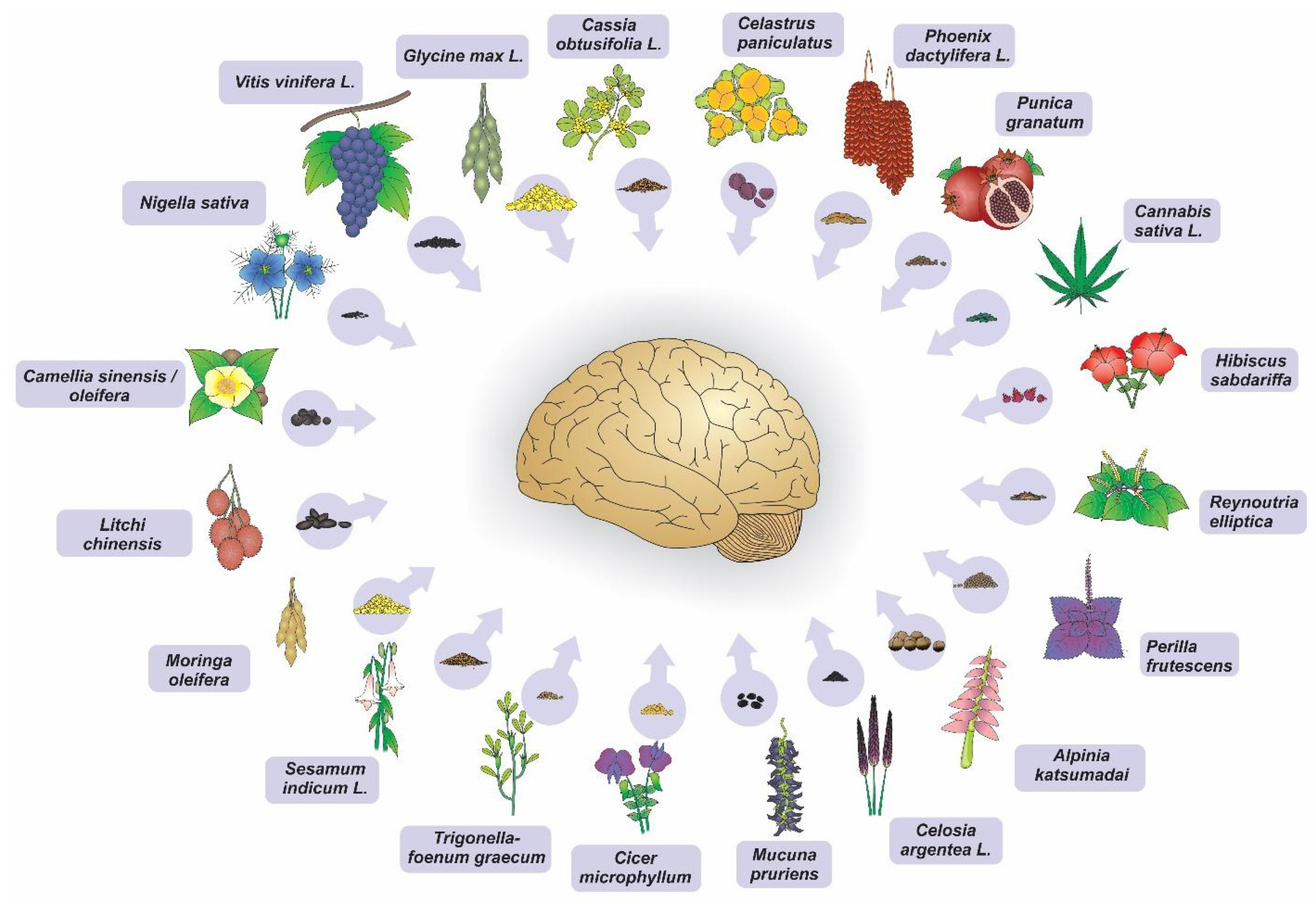


| Authorship and Publication Year | Anatomical/Cell Structure AnalyzeD | Experimental Model of Induced Neurodegeneration | Seeds | Extract | Most Relevant Results |
|---|---|---|---|---|---|
| Godkar et al. (2003) [26] | Neuronal cultures of rat forebrain | Hydrogen peroxide-induced oxidative damage | Celastrus paniculatus | Water soluble extract | Antioxidant |
| Godkar et al. (2004) [27] | Neuronal cultures of rat forebrain | Glutamate-induced toxicity model | Celastrus paniculatus | Water soluble extract | Attenuated toxicity and cell death |
| Hwang et al. (2004) [28] | Gerbil hippocampus | Neuronal injury induced by transient forebrain ischemia | Vitis vinifera L. | - | Protection from ischemic damage |
| Feng et al. (2007) [29] | Rats | Hypoxic-ischemic brain injury model | Vitis vinifera L. | - | Neuronal injury protection |
| Fujishita et al. (2009) [30] | Primary cultures of astrocytes in the hippocampus | Hydrogen peroxide-induced oxidative damage | Vitis vinifera | Polyphenols | Anti-apoptotic and antioxidant |
| Kasture et al. (2009) [31] | Rats | Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine | Mucuna pruriens | - | No significant antagonism on dopamine neuron degeneration and glial cell activity |
| Ju et al. (2010) [32] | Rat mesencephalic Pheochromocytoma-12 cells | Model of Parkinson’s disease induced by 6-hydroxydopamine | Cassia obtusifolia L. | Ethanolic extract with standardized concentration of rubrofusarin 6- O-gentiobioside | Antioxidant |
| Jamarkattel-Pandit et al. (2010) [33] | Rat’s brain | Ischemia-reperfusion injury model induced by occlusion of middle cerebral artery | Sesamum indicum L. | Defatted seed extract | Reduced the cerebral infarction volume and improved the sensorimotor function |
| Li et al. (2011) [34] | Neurons in the CA1 region of the rat’s hippocampus | Transient cerebral ischemia due to occlusion of bilateral carotid arteries | Alpinia katsumadai | Ethanolic extract | Protection from ischemic damage by neurotrophic factors |
| Bhuiyan et al. (2012) [35] | Primary cortical neurons of rats | Ischemia model by cell death induced by glutamate and oxygen-glucose deprivation | Glycine max L. | Crude ethanol extract of anthocyanins with three isolated components: delphindin-3-glucoside, petuidin-3-glucoside and C3G | Antioxidant and protection against neuronal cell death |
| Zhao et al. (2012) [36] | Primary cortical neurons of rats | Increased reactive oxygen species and hydrogen peroxide-induced cytotoxicity | Perilla frutescens L. | Luteolin isolated extract (BA-kuchiol, 1,3-hydroxybakuchiol, e 3,2-hydroxybaku-chiol) | Antioxidant |
| Ahmad et al. (2012) [37] | Striated body removed from mice | Model of Parkinson’s disease by 6-hydroxydopamine -induced neurotoxicity | Sesamum indicum L. | Oil extraction | Anti-inflammatory, antioxidant, and prevention of neuronal cell death |
| Sarkaki et al. (2013) [38] | Carotid arteries of female tats | Brain ischemia model induced by the bilateral permanent occlusion of carotid arteries | Punica granatum | Ethanolic extract | Antioxidant, significant improvement in passive and active memory and learning impairments |
| Li et al. (2013) [39] | CA1 region of the gerbil hippocampus | Ischemic damage model induced by transient cerebral ischemia | Alpinia katsumadai | - | Antioxidant |
| Yadav et al. (2013) [40] | Mice | Parkinson’s disease model by chronic exposure to paraquat | Mucuna pruriens | Aqueous extract | Antioxidant and improvement of neurobehavioral activity |
| Ye et al. (2014) [19] | Brain striatum of rats | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease model | Camellia oleifera | Defatted sapogenin extract | Anti-neuroinflammatory and protection of dopaminergic neurons |
| Xin et al. (2014) [22] | Pheochromocytoma-12 cells | Corticosterone damage | Alpinia katsumadai | (2R, 3S) -pinobanksin-3-cinnamate isolated from ethanolic extract | Antioxidant |
| Noor et al. (2015) [41] | Wistar rats | Animal model of multiple sclerosis with demyelination induced by experimental autoimmune encephalomyelitis | Nigella sativa | Oil extraction | Anti-inflammatory and demyelinating |
| Bhagya et al. (2016) [42] | Rats | Chronic stress-induced cognitive impairment model | Celastrus paniculatus | Seed extract oil | Anxiety reduction, improvement in cognitive deficits, restoration of spatial learning and memory |
| El-Tarras et al. (2016) [43] | Albine rats | Cadmium chloride neurotoxicity model | Vitis vinifera L. | - | Decrease in histopathological changes in brain tissue |
| Yi et al. (2016) [44] | Hippocampus of mice | β amyloid-induced synaptic dysfunction model of Alzheimer’s disease | Cassia obtusifolia | Ethanolic extract | Anti-inflammatory effect and significant improvement in memory impairment |
| Malik et al. (2017) [45] | Cortex and striatum of the rat brain | Huntington’s disease-like symptom model of 3-nitropropionic acid-induced neuronal damage | Celastrus paniculatus | Ethanolic extract | Antioxidant |
| Dehghanian et al. (2017) [16] | Neurons of rat hippocampal subfield CA1 | β-amyloid-induced neurodegeneration model of Alzheimer’s disease | Phoenix dactylifera L. | Phenolic compounds from methanolic extract | Antioxidant; memory restoration and learning; protection against deterioration of neuronal morphology of the CA1 area of the hippocampus |
| Shama et al. (2017) [46] | CA1 region of the rat hippocampus | Neurodegeneration and hypoxia-induced oxidative stress | Cicer microphyllum | Crude ethanolic extract of compounds (biochanin A, formononetin, genistin) | Antioxidant, reduction of dendritic atrophy in CA1 neurons, significant memory improvement |
| Wang et al. (2017) [17] | Pheochromocytoma-12 cells neural cells of the rat adrenal medulla | Neuronal apoptotic model with β amyloid 25–35-induced Alzheimer’s Disease | Litchi chinensis | Ethanolic extract of saponins | Significant inhibition of apoptosis |
| Rai et al. (2017) [47] | Mice | Parkinson’s disease model with neuroinflammation induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxication | Mucuna pruriens | Aqueous extract | Anti-inflammatory, antioxidant, and anti-apoptotic |
| Kong et al. (2017) [48] | Mice | Cerebral injury by ischemia due to the transient occlusion of the middle cerebral artery | Vitis vinifera L. | Procyanidin extract | Antioxidant and anti-apoptotic neurotrophic effect |
| Wang et al. (2017) [49] | Pheochromocytoma-12 cells | Alzheimer’s disease model induced by neuronal apoptosis using β amyloid 25–35 | Litchi chinensis | Saponin extracts | Anti-apoptotic |
| Assad et al. (2018) [50] | Rats | Amnesia induced by scopolamine | Trigonella-foenum graecum L. | Methanolic extract | Antiamnesic, improvement in the learning and memory process |
| Luo et al. (2018) [51] | PC-12 neuroblastoma cells | Proteomic evaluation through a neurodegeneration model of oxidative stress induced by hydrogen peroxide | Vitis vinifera L. | Procyanidin extract | Antioxidant |
| Zhou et al. (2018) [52] | Mice’s hippocampus | Cognitive impairment induced by scopolamine injection | Moringa oleifera | Ethanolic extract | Antiamnesic, significant improvement in cognitive impairment, increased reactivity of the cholinergic system, hippocampal neurogenesis |
| Zhou et al. (2018) [21] | Mice | Memory dysfunction and neuroinflammation model induced by lipopolysaccharide | Cannabis sativa L. | Hemp seed extracts containing phenylpropionamides | Improved learning and alleviated damage to spatial memory |
| Fu et al. (2019) [53] | Neuroblastoma N2a cells of mice | Model of ischemic injury by oxygen-glucose deprivation/reoxygenation | Vitis vinifera L. | Procyanidin extract | Increased cell viability and anti-apoptotic activity |
| Wang et al. (2019) [54] | Murine microglia (BV2) | Neuroinflammation induced by lipopolysaccharide | Cannabis sativa L. | Ethanolic extract of coumaroylaminobutanol glucopyranoside | Anti-inflammatory and antioxidant |
| Kim et al. (2019) [18] | Mice’s Pheochromocytoma-12 cells | Alzheimer’s disease model by cytotoxicity induced by hydrogen peroxide with behavioral and cognitive deficit induced by beta amyloid 1–42 | Camellia sinensis | Oil extract | Antioxidant and significant improvement in cognitive and behavioral dysfunction; protection of cholinergic function |
| Shalgum et al. (2019) [55] | SH-SY5Y neuroblastoma cells | Toxicicity induced by hydrogen peroxide | Hibiscus sabdariffa L. | Ethanolic extract | Antioxidant, lipid peroxidation activity, blocked mitochondrial dysfunction and apoptosis |
| Song et al. (2019) [56] | HT22 cells from the hippocampus | HT22 cell death by glutamate-induced excitotoxicity | Reynoutria elliptica | Isolated methanol extract of procyanidin B2 3″—O-gallate | Antioxidant, inhibition of kinase phosphorylation, protection against apoptotic death and toxicity |
| Tu et al. (2019) [57] | Murines | Neonatal hypoxic-ischemic brain injury model | Vitis vinifera L. | Procyanidin extract | Anti-apoptotic |
| Wang et al. (2019) [20] | BV2 microglial cells | Model of inflammatory response and lipopolysaccharide-induced oxidative stress | Cannabis sativa L. | Cannabisin F extract | Anti-inflammatory and antioxidant |
| Zeng et al. (2019) [58] | Rats | Ischemic stroke model by acute and late-stage brain damage | Moringa oleífera | - | Cerebrovascular protection |
| Jin et al. (2020) [59] | Primary cultures of hippocampal neurons | Ethanol-induced neuronal damage associated with oxidative stress | Vitis vinifera L. | Procyanidin ethanolic extract | Antioxidant |
| Qiu et al. (2020) [60] | BV-2 and Pheochromocytoma-12 cells | Alzheimer’s disease model of neuroinflammation induced by β amyloid (1–42) | Litchi chinensis | Seed polyphenols | Anti-neuroinflammatory and antiapoptotic |
| Sun et al. (2020) [61] | Rat’s hippocampus | Alzheimer’s disease model of hippocampus neuronal injury induced by β amyloid 25–35 | Litchi chinensis | - | Injury relief and improvement of cognitive functions via AKT/GSK-3β |
| Khan et al. (2020) [62] | Neuroblastoma cells (SweAPP N2a) of mice | Alzheimer’s disease model | Camellia sinensis | Saponin E1 extract | Reduction of β amyloid |
| He et al. (2021) [63] | Pheochromocytoma-12 cells | Neuronal damage induced by hydrogen peroxide | Vitis vinifera L. | Proanthocyanidin extract | Redução de reactive oxygen species intracelular e inibição de apoptose |
| Guo et al. (2021) [64] | NSC-34 cell line | Neuronal damage induced by tert-butyl hydro-peroxide | Celosia argentea L. | Ethanolic extract from nine saponins and two phenylacetonitrile glycosides | Decrease in reactive oxygen species and cell apoptosis |
| Rachesee et al. (2021) [65] | BV2 mouse microglial cells | Neuroinflammation induced by lipopolysaccharide | Mucuna pruriens (L.) DC. | Ethanolic extract | Suppression of inflammatory responses by inhibiting the nuclear factor kappa B signaling pathway |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, G.M.; de Araújo, F.E.A.; da Rocha, J.M.C.; Idalina Neta, F.; do Rego, A.C.M.; Araújo Filho, I.; Pinheiro, F.I.; de Azevedo, E.P.; Cobucci, R.N.; Guzen, F.P. Neuroprotective Potential of Seed Extracts: Review of In Vitro and In Vivo Studies. Nutrients 2023, 15, 2502. https://doi.org/10.3390/nu15112502
Duarte GM, de Araújo FEA, da Rocha JMC, Idalina Neta F, do Rego ACM, Araújo Filho I, Pinheiro FI, de Azevedo EP, Cobucci RN, Guzen FP. Neuroprotective Potential of Seed Extracts: Review of In Vitro and In Vivo Studies. Nutrients. 2023; 15(11):2502. https://doi.org/10.3390/nu15112502
Chicago/Turabian StyleDuarte, Gabriella Mendes, Francisco Emanoel Alves de Araújo, João Matheus Caé da Rocha, Francisca Idalina Neta, Amália Cinthia Meneses do Rego, Irami Araújo Filho, Francisco Irochima Pinheiro, Eduardo Pereira de Azevedo, Ricardo Ney Cobucci, and Fausto Pierdoná Guzen. 2023. "Neuroprotective Potential of Seed Extracts: Review of In Vitro and In Vivo Studies" Nutrients 15, no. 11: 2502. https://doi.org/10.3390/nu15112502
APA StyleDuarte, G. M., de Araújo, F. E. A., da Rocha, J. M. C., Idalina Neta, F., do Rego, A. C. M., Araújo Filho, I., Pinheiro, F. I., de Azevedo, E. P., Cobucci, R. N., & Guzen, F. P. (2023). Neuroprotective Potential of Seed Extracts: Review of In Vitro and In Vivo Studies. Nutrients, 15(11), 2502. https://doi.org/10.3390/nu15112502





