Dioscorea esculenta Intake with Resistance Training Improves Muscle Quantity and Quality in Healthy Middle-Aged and Older Adults: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Resistance Training Intervention
2.4. Dioscorea esculenta or Placebo Intake
2.5. Outcome Measures
2.5.1. Body Composition
2.5.2. Physical Activity, Energy Intake, and Nutritional Status
2.5.3. Muscle Quantity and Quality Indexes
2.5.4. Cardiometabolic Parameters
2.6. Statistical Analysis
3. Results
3.1. Participant Characteristics and Nutritional Status
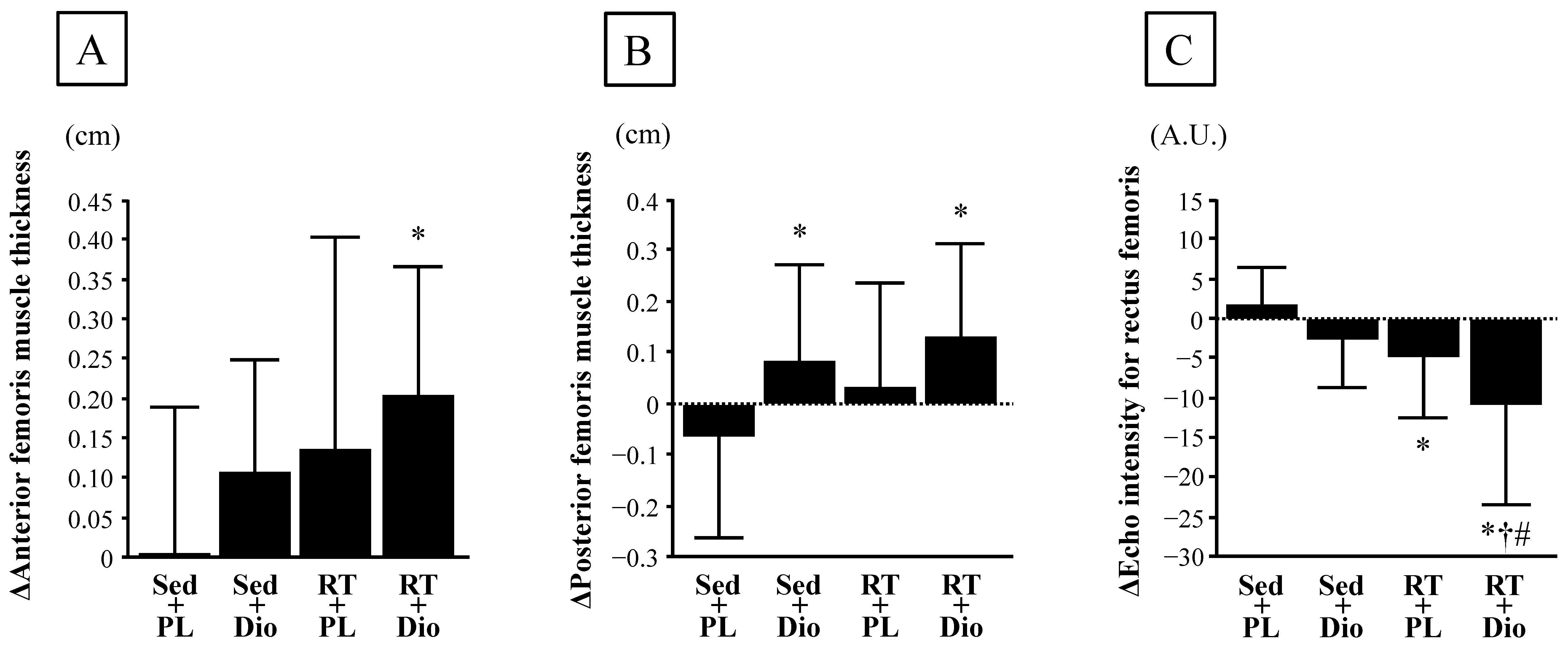
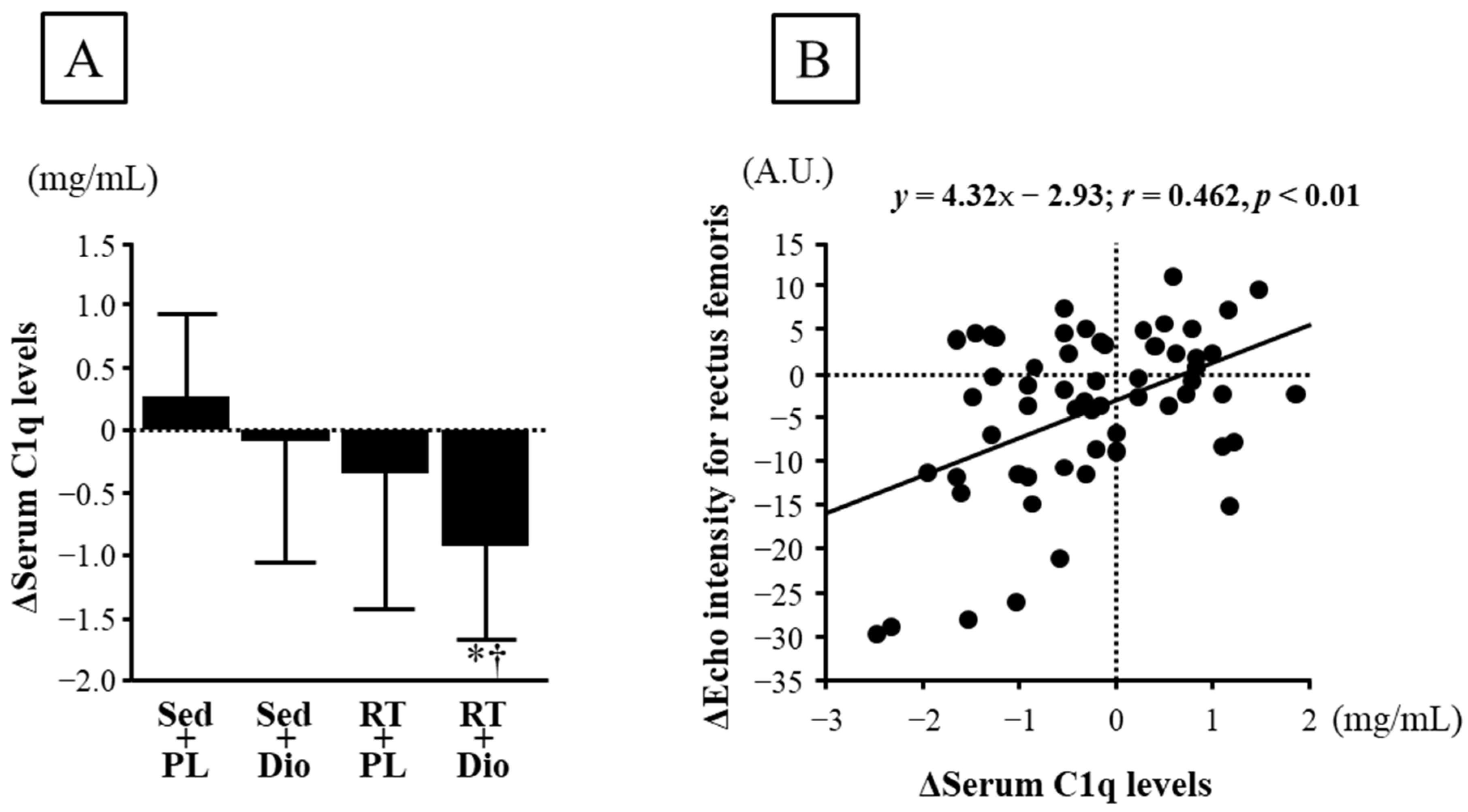
3.2. Muscle Quantity and Quality Indices
3.3. Cardiometabolic Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gale, C.R.; Martyn, C.N.; Cooper, C.; Sayer, A.A. Grip strength, body composition, and mortality. Int. J. Epidemiol. 2007, 36, 228–235. [Google Scholar] [CrossRef]
- Visser, M.; Schaap, L.A. Consequences of sarcopenia. Clin. Geriatr. Med. 2011, 27, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Dennison, E.M.; Syddall, H.E.; Gilbody, H.J.; Phillips, D.I.; Cooper, C. Type 2 diabetes, muscle strength, and impaired physical function: The tip of the iceberg? Diabetes Care 2005, 28, 2541–2542. [Google Scholar] [CrossRef]
- Ensrud, K.E.; Ewing, S.K.; Taylor, B.C.; Fink, H.A.; Stone, K.L.; Cauley, J.A.; Tracy, J.K.; Hochberg, M.C.; Rodondi, N.; Cawthon, P.M.; et al. Frailty and risk of falls, fracture, and mortality in older women: The study of osteoporotic fractures. J. Gerontol. Biol. Sci. Med. Sci. 2007, 62, 744–751. [Google Scholar] [CrossRef]
- Janssen, I.; Baumgartner, R.N.; Ross, R.; Rosenberg, I.H.; Roubenoff, R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am. J. Epidemiol. 2004, 159, 413–421. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.D.; Han, K.; Shin, K.E.; Lee, H.; Kim, T.R.; Cho, K.H.; Kim, D.H.; Kim, Y.H.; Kim, H.; et al. Optimal cutoffs for low skeletal muscle mass related to cardiovascular risk in adults: The Korea National Health and Nutrition Examination Survey 2009–2010. Endocrine 2015, 50, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef]
- Csapo, R.; Malis, V.; Sinha, U.; Du, J.; Sinha, S. Age-associated differences in triceps surae muscle composition and strength—An MRI-based cross-sectional comparison of contractile, adipose and connective tissue. BMC Musculoskelet. Disord. 2014, 15, 209. [Google Scholar] [CrossRef]
- Horii, N.; Uchida, M.; Hasegawa, N.; Fujie, S.; Oyanagi, E.; Yano, H.; Hashimoto, T.; Iemitsu, M. Resistance training prevents muscle fibrosis and atrophy via down-regulation of C1q-induced Wnt signaling in senescent mice. FASEB J. 2018, 32, 3547–3559. [Google Scholar] [CrossRef]
- DeFreitas, J.M.; Beck, T.W.; Stock, M.S.; Dillon, M.A.; Kasishke, P.R. 2nd. An examination of the time course of training-induced skeletal muscle hypertrophy. Eur. J. Appl. Physiol. 2011, 111, 2785–2790. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Shen, Y.; Pink, H.; Triantafillou, J.; Stimpson, S.A.; Turnbull, P.; Han, B. Phosphorylation of p70s6 kinase is implicated in androgen-induced levator ani muscle anabolism in castrated rats. J. Steroid Biochem. Mol. Biol. 2004, 92, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Iemitsu, M.; Aizawa, K.; Ajisaka, R. DHEA improves impaired activation of Akt and PKC zeta/lambda-GLUT4 pathway in skeletal muscle and improves hyperglycaemia in streptozotocin-induced diabetes rats. Acta Physiol. 2009, 197, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Iemitsu, M.; Aizawa, K.; Mesaki, N.; Ajisaka, R.; Fujita, S. DHEA administration and exercise training improves insulin resistance in obese rats. Nutr. Metab. 2012, 9, 47. [Google Scholar] [CrossRef]
- Horii, N.; Sato, K.; Mesaki, N.; Iemitsu, M. DHEA Administration Activates Transcription of Muscular Lipid Metabolic Enzymes via PPARα and PPARδ in Obese Rats. Horm. Metab. Res. 2016, 48, 207–212. [Google Scholar] [CrossRef]
- Sato, K.; Iemitsu, M.; Aizawa, K.; Ajisaka, R. Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E961–E968. [Google Scholar] [CrossRef]
- Liang, X.; Glowacki, J.; Hahne, J.; Xie, L.; LeBoff, M.S.; Zhou, S. Dehydroepiandrosterone Stimulation of Osteoblastogenesis in Human MSCs Requires IGF-I Signaling. J. Cell. Biochem. 2016, 117, 1769–1774. [Google Scholar] [CrossRef]
- Pluchino, N.; Drakopoulos, P.; Bianchi-Demicheli, F.; Wenger, J.M.; Petignat, P.; Genazzani, A.R. Neurobiology of DHEA and effects on sexuality, mood and cognition. J. Steroid Biochem. Mol. Biol. 2015, 145, 273–280. [Google Scholar] [CrossRef]
- Aizawa, K.; Iemitsu, M.; Maeda, S.; Jesmin, S.; Otsuki, T.; Mowa, C.N.; Miyauchi, T.; Mesaki, N. Expression of steroidogenic enzymes and synthesis of sex steroid hormones from DHEA in skeletal muscle of rats. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E577–E584. [Google Scholar] [CrossRef] [PubMed]
- Pöllänen, E.; Sipilä, S.; Alen, M.; Ronkainen, P.H.; Ankarberg-Lindgren, C.; Puolakka, J.; Suominen, H.; Hämäläinen, E.; Turpeinen, U.; Konttinen, Y.T.; et al. Differential influence of peripheral and systemic sex steroids on skeletal muscle quality in pre- and postmenopausal women. Aging Cell. 2011, 10, 650–660. [Google Scholar] [CrossRef]
- Sato, K.; Iemitsu, M.; Matsutani, K.; Kurihara, T.; Hamaoka, T.; Fujita, S. Resistance training restores muscle sex steroid hormone steroidogenesis in older men. FASEB J. 2014, 28, 1891–1897. [Google Scholar] [CrossRef] [PubMed]
- Horii, N.; Sato, K.; Mesaki, N.; Iemitsu, M. Increased Muscular 5α-Dihydrotestosterone in Response to Resistance Training Relates to Skeletal Muscle Mass and Glucose Metabolism in Type 2 Diabetic Rats. PLoS ONE 2016, 11, e0165689. [Google Scholar] [CrossRef]
- Raju, J.; Patlolla, J.M.; Swamy, M.V.; Rao, C.V. Diosgenin, a steroid saponin of Trigonella foenum graecum (Fenugreek), inhibits azoxymethane-induced aberrant crypt foci formation in F344 rats and induces apoptosis in HT-29 human colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1392–1398. [Google Scholar] [CrossRef]
- Sato, K.; Fujita, S.; Iemitsu, M. Dioscorea esculenta-induced increase in muscle sex steroid hormones is associated with enhanced insulin sensitivity in a type 2 diabetes rat model. FASEB J. 2017, 31, 793–801. [Google Scholar] [CrossRef]
- Horii, N.; Hasegawa, N.; Fujie, S.; Iemitsu, K.; Uchida, M.; Hamaoka, T.; Iemitsu, M. Effects of Dioscorea esculenta intake with resistance training on muscle hypertrophy and strength in sprint athletes. J. Clin. Biochem. Nutr. 2020, 67, 338–343. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, P.H.; Lin, L.F.; Liao, C.D.; Liou, T.H.; Huang, S.W. Effects of progressive elastic band resistance exercise for aged osteosarcopenic adiposity women. Exp. Gerontol. 2021, 147, 111272. [Google Scholar] [CrossRef]
- Huang, S.W.; Ku, J.W.; Lin, L.F.; Liao, C.D.; Chou, L.C.; Liou, T.H. Body composition influenced by progressive elastic band resistance exercise of sarcopenic obesity elderly women: A pilot randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2017, 53, 556–563. [Google Scholar] [CrossRef]
- Barbosa, C.D.; Costa, J.G.; Giolo, J.S.; Rossato, L.T.; Nahas, P.C.; Mariano, I.M.; Batista, J.P.; Puga, G.M.; de Oliveira, E.P. Isoflavone supplementation plus combined aerobic and resistance exercise do not change phase angle values in postmenopausal women: A randomized placebo-controlled clinical trial. Exp. Gerontol. 2019, 117, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Honda, S.; Murakami, K.; Sasaki, S.; Okubo, H.; Hirota, N.; Notsu, A.; Fukui, M.; Date, C. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J. Epidemiol. 2012, 22, 151–159. [Google Scholar] [CrossRef]
- Akima, H.; Yoshiko, A.; Tomita, A.; Ando, R.; Saito, A.; Ogawa, M.; Kondo, S.; Tanaka, N.I. Relationship between quadriceps echo intensity and functional and morphological characteristics in older men and women. Arch. Gerontol. Geriatr. 2017, 70, 105–111. [Google Scholar] [CrossRef]
- Caresio, C.; Molinari, F.; Emanuel, G.; Minetto, M.A. Muscle echo intensity: Reliability and conditioning factors. Clin. Physiol. Funct. Imaging 2015, 35, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Mayhew, A.J.; So, H.Y.; Ma, J.; Beauchamp, M.; Griffith, L.E.; Kuspinar, A.; Lang, J.J.; Raina, P. Normative values for grip strength, gait speed, timed up and go, single leg balance, and chair rise derived from the Canadian longitudinal study on ageing. Age Ageing 2023, 52, afad054. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N.; Kurihara, T.; Sato, K.; Homma, T.; Fujie, S.; Fujita, S.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Intramyocellular and Extramyocellular Lipids Are Associated with Arterial Stiffness. Am. J. Hypertens. 2015, 28, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Brook, M.S.; Wilkinson, D.J.; Mitchell, W.K.; Lund, J.N.; Szewczyk, N.J.; Greenhaff, P.L.; Smith, K.; Atherton, P.J. Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J. 2015, 29, 4485–4496. [Google Scholar] [CrossRef] [PubMed]
- Horii, N.; Hasegawa, N.; Fujie, S.; Uchida, M.; Iemitsu, M. Resistance exercise-induced increase in muscle 5α-dihydrotestosterone contributes to the activation of muscle Akt/mTOR/p70S6K- and Akt/AS160/GLUT4-signaling pathways in type 2 diabetic rats. FASEB J. 2020, 34, 11047–11057. [Google Scholar] [CrossRef]
- Naito, A.T.; Sumida, T.; Nomura, S.; Liu, M.L.; Higo, T.; Nakagawa, A.; Okada, K.; Sakai, T.; Hashimoto, A.; Hara, Y.; et al. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell 2012, 149, 1298–1313. [Google Scholar] [CrossRef]
- Sato, K.; Iemitsu, M.; Aizawa, K.; Mesaki, N.; Fujita, S. Increased muscular dehydroepiandrosterone levels are associated with improved hyperglycemia in obese rats. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E274–E280. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Iemitsu, M. The Role of Dehydroepiandrosterone (DHEA) in Skeletal Muscle. Vitam. Horm. 2018, 108, 205–221. [Google Scholar] [PubMed]
- Peixoto, P.; Vieira-Alves, I.; Couto, G.K.; Lemos, V.S.; Rossoni, L.V.; Bissoli, N.S.; Santos, R.L.D. Sex differences in the participation of endothelial mediators and signaling pathways involved in the vasodilator effect of a selective GPER agonist in resistance arteries of gonadectomized Wistar rats. Life Sci. 2022, 308, 120917. [Google Scholar] [CrossRef]
- Oliver-Martínez, P.A.; Ramos-Campo, D.J.; Martínez-Aranda, L.M.; Martínez-Rodríguez, A.; Rubio-Arias, J.Á. Chronic effects and optimal dosage of strength training on SBP and DBP: A systematic review with meta-analysis. J. Hypertens. 2020, 38, 1909–1918. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Fagard, R.H. Effect of resistance training on resting blood pressure: A meta-analysis of randomized controlled trials. J. Hypertens. 2005, 23, 251–259. [Google Scholar] [CrossRef] [PubMed]
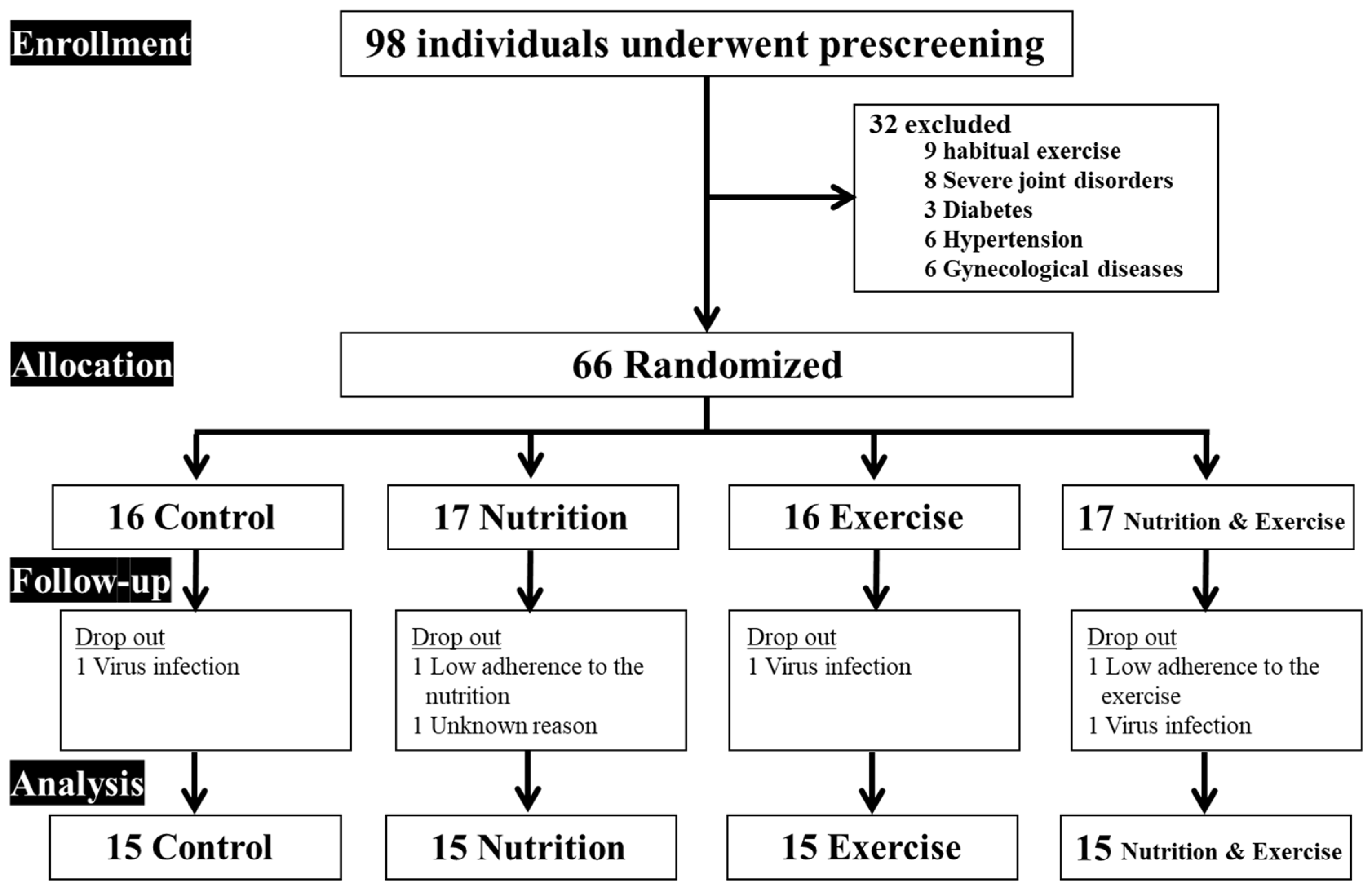
| Sed+PL | Sed+Dio | RT+PL | RT+Dio | Two-Way ANOVA | ΔSed+PL | ΔSed+Dio | ΔRT+PL | ΔRT+Dio | One-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |||||||
| Age, year | 53 ± 5 | 53 ± 5 | 55 ± 7 | 55 ± 7 | 52 ± 5 | 53 ± 6 | 53 ± 5 | 53 ± 5 | 1.000 | |||||
| Height, cm | 161 ± 8 | 161 ± 7 | 159 ± 9 | 159 ± 9 | 160 ± 9 | 160 ± 9 | 161 ± 5 | 161 ± 5 | 1.000 | |||||
| Body weight, kg | 59 ± 9 | 59 ± 9 | 61 ± 12 | 61 ± 11 | 61 ± 14 | 60 ± 14 | 64 ± 10 | 63 ± 9 | 1.000 | 0.2 ± 1.2 | 0.3 ± 1.0 | −0.4 ± 0.8 | −0.1 ± 1.0 | 0.289 |
| BMI, kg/cm2 | 23 ± 3 | 23 ± 3 | 24 ± 4 | 24 ± 4 | 23 ± 4 | 23 ± 4 | 24 ± 4 | 25 ± 4 | 0.998 | 0.1 ± 0.4 | 0.3 ± 0.4 | −0.1 ± 0.4 | 0.1 ± 0.4 | 0.104 |
| Body fat, % | 28 ± 6 | 28 ± 6 | 31 ± 8 | 32 ± 8 | 30 ± 7 | 31 ± 7 | 30 ± 10 | 31 ± 9 | 0.991 | 0.3 ± 1.7 | 0.6 ± 1.6 | 0.3 ± 1.7 | 0.3 ± 1.1 | 0.949 |
| PA, kcal/day | 789 ± 392 | 824 ± 415 | 817 ± 374 | 710 ± 267 | 731 ± 451 | 716 ± 362 | 656 ± 312 | 688 ± 338 | 0.865 | 35 ± 221 | −107 ± 369 | −15 ± 260 | 32 ± 191 | 0.439 |
| EI, kcal/day | 1496 ± 480 | 1515 ± 500 | 1446 ± 476 | 1622 ± 525 | 1677 ± 626 | 1604 ± 627 | 1508 ± 505 | 1666 ± 486 | 0.773 | 19 ± 484 | 176 ± 256 | −73 ± 299 | 159 ± 276 | 0.155 |
| Sed+PL | Sed+Dio | RT+PL | RT+Dio | Two-Way ANOVA | ΔSed+PL | ΔSed+Dio | ΔRT+PL | ΔRT+Dio | One-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |||||||
| Protein (g/day) | 60 ± 26 | 57 ± 23 | 62 ± 26 | 62 ± 24 | 60 ± 18 | 60 ± 22 | 65 ± 23 | 62 ± 19 | 0.992 | −2.6 ± 13.7 | −0.1 ± 14.3 | −0.3 ± 12.7 | −3.1 ± 17.9 | 0.924 |
| Fat (g/day) | 48 ± 20 | 46 ± 16 | 57 ± 19 | 57 ± 19 | 53 ± 18 | 49 ± 17 | 54 ± 17 | 51 ± 15 | 0.966 | −2.1 ± 12.3 | 0.6 ± 11.9 | −3.8 ± 14.3 | −3.3 ± 12.8 | 0.794 |
| Carbohydrate (g/day) | 190 ± 66 | 193 ± 59 | 200 ± 84 | 192 ± 95 | 204 ± 111 | 183 ± 82 | 201 ± 59 | 209 ± 69 | 0.913 | 2 ± 28 | −8 ± 58 | −20 ± 62 | 8 ± 46 | 0.448 |
| Sodium (g/day) | 4 ± 1 | 3 ± 1 | 4 ± 1 | 3 ± 1 | 3 ± 1 | 3 ± 1 | 4 ± 1 | 4 ± 1 | 0.995 | −0.1 ± 0.8 | −0.1 ± 0.8 | −0.2 ± 0.7 | −0.1 ± 1.0 | 0.950 |
| Potassium (mg/day) | 2120 ± 947 | 2185 ± 855 | 2102 ± 808 | 1976 ± 740 | 2280 ± 815 | 2243 ± 939 | 2263 ± 949 | 2084 ± 664 | 0.948 | 65 ± 463 | −126 ± 413 | −37 ± 500 | −180 ± 627 | 0.575 |
| Calcium (mg/day) | 434 ± 177 | 385 ± 134 | 465 ± 230 | 423 ± 196 | 474 ± 175 | 499 ± 189 | 472 ± 234 | 416 ± 152 | 0.991 | −49 ± 131 | −42 ± 162 | −25 ± 95 | −56 ± 145 | 0.935 |
| Magnesium (mg/day) | 214 ± 91 | 216 ± 83 | 224 ± 89 | 210 ± 83 | 230 ± 68 | 221 ± 86 | 221 ± 86 | 211 ± 69 | 0.986 | 2 ± 38 | −14 ± 43 | −9 ± 45 | −9 ± 61 | 0.832 |
| Iron (mg/day) | 7 ± 4 | 7 ± 3 | 7 ± 3 | 7 ± 3 | 7 ± 3 | 7 ± 3 | 7 ± 3 | 7 ± 2 | 0.997 | −0.4 ± 2.1 | −0.3 ± 1.3 | −0.1 ± 1.5 | −0.4 ± 2.3 | 0.971 |
| Phosphorus (mg/day) | 894 ± 383 | 841 ± 313 | 924 ± 390 | 901 ± 366 | 915 ± 263 | 886 ± 330 | 966 ± 367 | 912 ± 277 | 0.997 | 53 ± 219 | −23 ± 218 | −29 ± 160 | −53 ± 251 | 0.970 |
| Vitamin D (μg/day) | 12 ± 10 | 9 ± 5 | 10 ± 8 | 9 ± 7 | 9 ± 6 | 10 ± 9 | 10 ± 8 | 11 ± 5 | 0.592 | −3.8 ± 8.2 | −0.6 ± 4.7 | 1.1 ± 6.0 | 0.2 ± 5.9 | 0.179 |
| Vitamin E (mg/day) | 7 ± 3 | 7 ± 2 | 7 ± 2 | 7 ± 3 | 7 ± 3 | 7 ± 4 | 7 ± 3 | 7 ± 3 | 0.998 | −0.1 ± 1.8 | 0.2 ± 1.4 | −0.1 ± 1.7 | −0.1 ± 2.1 | 0.971 |
| Vitamin C (mg/day) | 86 ± 47 | 101 ± 52 | 73 ± 29 | 74 ± 34 | 88 ± 58 | 99 ± 56 | 85 ± 50 | 91 ± 49 | 0.948 | 15 ± 34 | 1 ± 24 | 11 ± 41 | 6 ± 34 | 0.701 |
| Folic acids (μg/day) | 307 ± 192 | 291 ± 110 | 286 ± 110 | 266 ± 112 | 305 ± 176 | 310 ± 166 | 308 ± 176 | 284 ± 103 | 0.982 | −16 ± 153 | −19 ± 61 | 5 ± 87 | −23 ± 112 | 0.894 |
| Zinc (mg/day) | 7 ± 3 | 7 ± 3 | 7 ± 2 | 7 ± 3 | 7 ± 2 | 7 ± 2 | 8 ± 2 | 7 ± 2 | 0.994 | −0.2 ± 1.6 | 0.1 ± 1.7 | −0.3 ± 1.4 | −0.3 ± 2.0 | 0.961 |
| Cholesterol (mg/day) | 344 ± 174 | 292 ± 116 | 411 ± 229 | 423 ± 246 | 360 ± 143 | 348 ± 159 | 442 ± 201 | 401 ± 128 | 0.899 | −53 ± 131 | 12 ± 95 | −13 ± 104 | −41 ± 147 | 0.460 |
| Dietary fiber (g/day) | 10 ± 4 | 11 ± 4 | 10 ± 4 | 9 ± 4 | 11 ± 5 | 11 ± 6 | 10 ± 5 | 10 ± 4 | 0.932 | 0.7 ± 1.7 | −0.9 ± 1.9 | −0.1 ± 2.7 | −0.1 ± 3.6 | 0.435 |
| Sed+PL | Sed+Dio | RT+PL | RT+Dio | Two-Way ANOVA | ΔSed+PL | ΔSed+Dio | ΔRT+PL | ΔRT+Dio | One-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |||||||
| Anterior femoris muscle thickness, cm | 4.3 ± 0.6 | 4.3 ± 0.6 | 4.3 ± 0.7 | 4.4 ± 0.7 | 3.9 ± 0.7 | 4.1 ± 0.7 | 4.3 ± 0.6 | 4.5 ± 0.6 | 0.948 | 0.01 ± 0.19 | 0.11 ± 0.14 | 0.14 ± 0.27 | 0.21 ± 0.16 * | 0.046 |
| Posterior femoris muscle thickness, cm | 4.9 ± 0.8 | 4.9 ± 0.8 | 5.3 ± 0.9 | 5.4 ± 0.9 | 5.1 ± 0.7 | 5.2 ± 0.6 | 5.1 ± 0.5 | 5.3 ± 0.6 | 0.959 | −0.07 ± 0.20 | 0.08 ± 0.19 * | 0.03 ± 0.20 | 0.13 ± 0.18 * | 0.044 |
| Echo intensity for rectus femoris, A.U. | 47.1 ± 14.1 | 49.0 ± 14.6 | 55.4 ± 22.3 | 52.8 ± 22.6 | 54.8 ± 16.8 | 50.2 ± 12.7 | 50.0 ± 23.0 | 39.3 ± 16.8 | 0.604 | 1.9 ± 4.7 | −2.6 ± 6.2 | −4.6 ± 7.9 * | −10.8 ± 12.7 *†# | 0.001 |
| Normal walking speed, m/s | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.1 | 1.5 ± 0.2 | 0.723 | 0.05 ± 0.16 | 0.01 ± 0.16 | 0.10 ± 0.14 | 0.05 ± 0.13 | 0.379 |
| Single leg stand test, min | 1.6 ± 0.6 | 1.8 ± 0.4 | 1.6 ± 0.7 | 1.6 ± 0.6 | 1.4 ± 0.7 | 1.8 ± 0.4 | 1.6 ± 0.6 | 1.8 ± 0.4 | 0.691 | 7.5 ± 28.4 | 2.0 ± 21.3 | 22.3 ± 30.5 | 12.4 ± 21.5 | 0.180 |
| Five times sit to stand test, sec | 7.8 ± 1.3 | 7.2 ± 1.1 | 7.8 ± 0.8 | 6.9 ± 1.1 | 8.6 ± 1.0 | 7.5 ± 1.0 | 8.5 ± 1.2 | 7.0 ± 1.2 | 0.426 | −0.6 ± 0.9 | −0.9 ± 0.8 | −1.1 ± 0.9 | −1.5 ± 1.1 * | 0.049 |
| Grip strength, kg | 26.3 ± 7.0 | 26.5 ± 5.9 | 25.8 ± 9.0 | 26.0 ± 9.2 | 26.3 ± 8.9 | 26.9 ± 8.0 | 27.7 ± 7.4 | 29.2 ± 7.1 | 0.987 | 0.2 ± 1.7 | 0.2 ± 2.0 | 0.6 ± 2.7 | 1.5 ± 2.4 | 0.349 |
| Sed+PL | Sed+Dio | RT+PL | RT+Dio | Two-Way ANOVA | ΔSed+PL | ΔSed+Dio | ΔRT+PL | ΔRT+Dio | One-Way ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |||||||
| SBP, mmHg | 123 ± 19 | 121 ± 21 | 121 ± 16 | 115 ± 21 | 123 ± 12 | 117 ± 22 | 127 ± 23 | 120 ± 19 | 0.968 | 0.2 ± 10.6 | −4.1 ± 8.9 | −4.5 ± 14.8 | −7.1 ± 11.6 | 0.394 |
| DBP, mmHg | 74 ± 10 | 75 ± 12 | 74 ± 15 | 68 ± 12 | 76 ± 7 | 68 ± 10 | 79 ± 16 | 74 ± 14 | 0.604 | 1.7 ± 9.3 | −4.5 ± 6.3 * | −7.0 ± 6.7* | −4.9 ± 9.3 * | 0.028 |
| HR, bpm | 76 ± 11 | 73 ± 6 | 74 ± 12 | 70 ± 13 | 74 ± 10 | 74 ± 9 | 76 ± 7 | 79 ± 7 | 0.535 | −2.9 ± 10.5 | −3.8 ± 10.6 | −0.9 ± 6.4 | 2.7 ± 7.0 | 0.194 |
| Total-Cho, mg/mL | 214 ± 43 | 205 ± 37 | 231 ± 45 | 235 ± 39 | 233 ± 30 | 223 ± 38 | 211 ± 24 | 202 ± 24 | 0.864 | −9.4 ± 26.2 | 3.8 ± 21.4 | −9.4 ± 19.3 | −8.6 ± 17.9 | 0.261 |
| HDL, mg/mL | 71 ± 15 | 69 ± 12 | 69 ± 20 | 71 ± 20 | 76 ± 16 | 73 ± 17 | 67 ± 21 | 64 ± 21 | 0.972 | −2.1 ± 7.3 | 1.1 ± 6.4 | −2.8 ± 5.0 | −2.7 ± 2.8 | 0.206 |
| TG, mg/mL | 92 ± 46 | 88 ± 46 | 93 ± 46 | 94 ± 36 | 99 ± 55 | 90 ± 50 | 101 ± 48 | 102 ± 44 | 0.972 | −3.9 ± 19.1 | 0.8 ± 30.2 | −8.7 ± 14.8 | 1.5 ± 12.1 | 0.492 |
| HbA1c, % | 5.3 ± 0.2 | 5.4 ± 0.3 | 5.3 ± 0.2 | 5.3 ± 0.2 | 5.3 ± 0.3 | 5.3 ± 0.3 | 5.3 ± 0.3 | 5.3 ± 0.3 | 0.648 | 0.09 ± 0.19 | −0.03 ± 0.14 * | −0.01 ± 0.14 | −0.08 ± 0.14 * | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iemitsu, K.; Fujie, S.; Uchida, M.; Inoue, K.; Shinohara, Y.; Iemitsu, M. Dioscorea esculenta Intake with Resistance Training Improves Muscle Quantity and Quality in Healthy Middle-Aged and Older Adults: A Randomized Controlled Trial. Nutrients 2023, 15, 2438. https://doi.org/10.3390/nu15112438
Iemitsu K, Fujie S, Uchida M, Inoue K, Shinohara Y, Iemitsu M. Dioscorea esculenta Intake with Resistance Training Improves Muscle Quantity and Quality in Healthy Middle-Aged and Older Adults: A Randomized Controlled Trial. Nutrients. 2023; 15(11):2438. https://doi.org/10.3390/nu15112438
Chicago/Turabian StyleIemitsu, Keiko, Shumpei Fujie, Masataka Uchida, Kenichiro Inoue, Yasushi Shinohara, and Motoyuki Iemitsu. 2023. "Dioscorea esculenta Intake with Resistance Training Improves Muscle Quantity and Quality in Healthy Middle-Aged and Older Adults: A Randomized Controlled Trial" Nutrients 15, no. 11: 2438. https://doi.org/10.3390/nu15112438
APA StyleIemitsu, K., Fujie, S., Uchida, M., Inoue, K., Shinohara, Y., & Iemitsu, M. (2023). Dioscorea esculenta Intake with Resistance Training Improves Muscle Quantity and Quality in Healthy Middle-Aged and Older Adults: A Randomized Controlled Trial. Nutrients, 15(11), 2438. https://doi.org/10.3390/nu15112438






