Hydrangea serrata Hot Water Extract and Its Major Ingredient Hydrangenol Improve Skin Moisturization and Wrinkle Conditions via AP-1 and Akt/PI3K Pathway Upregulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Extract, Chemicals, and Antibodies
2.2. Cell Culture
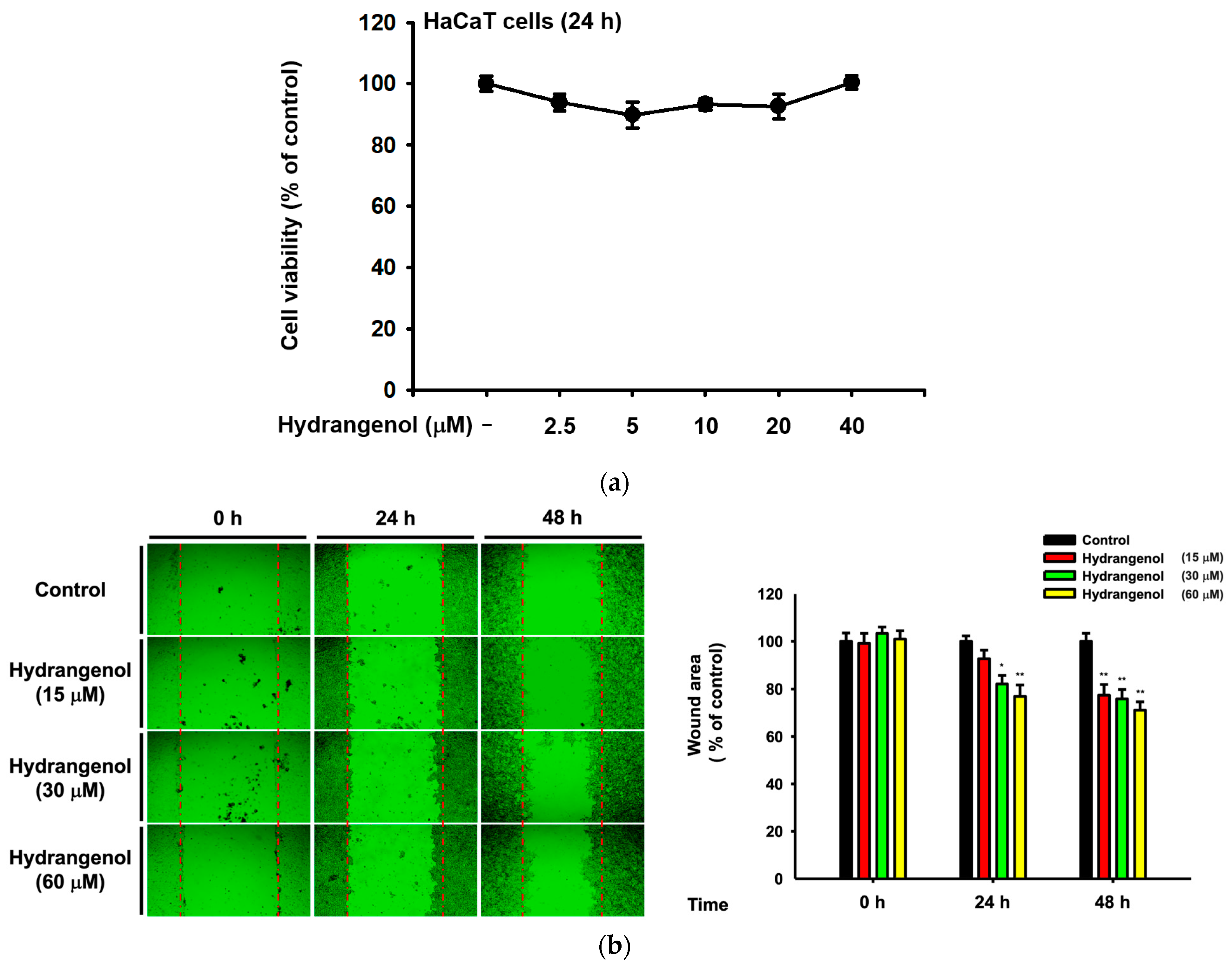
2.3. Cell Viability
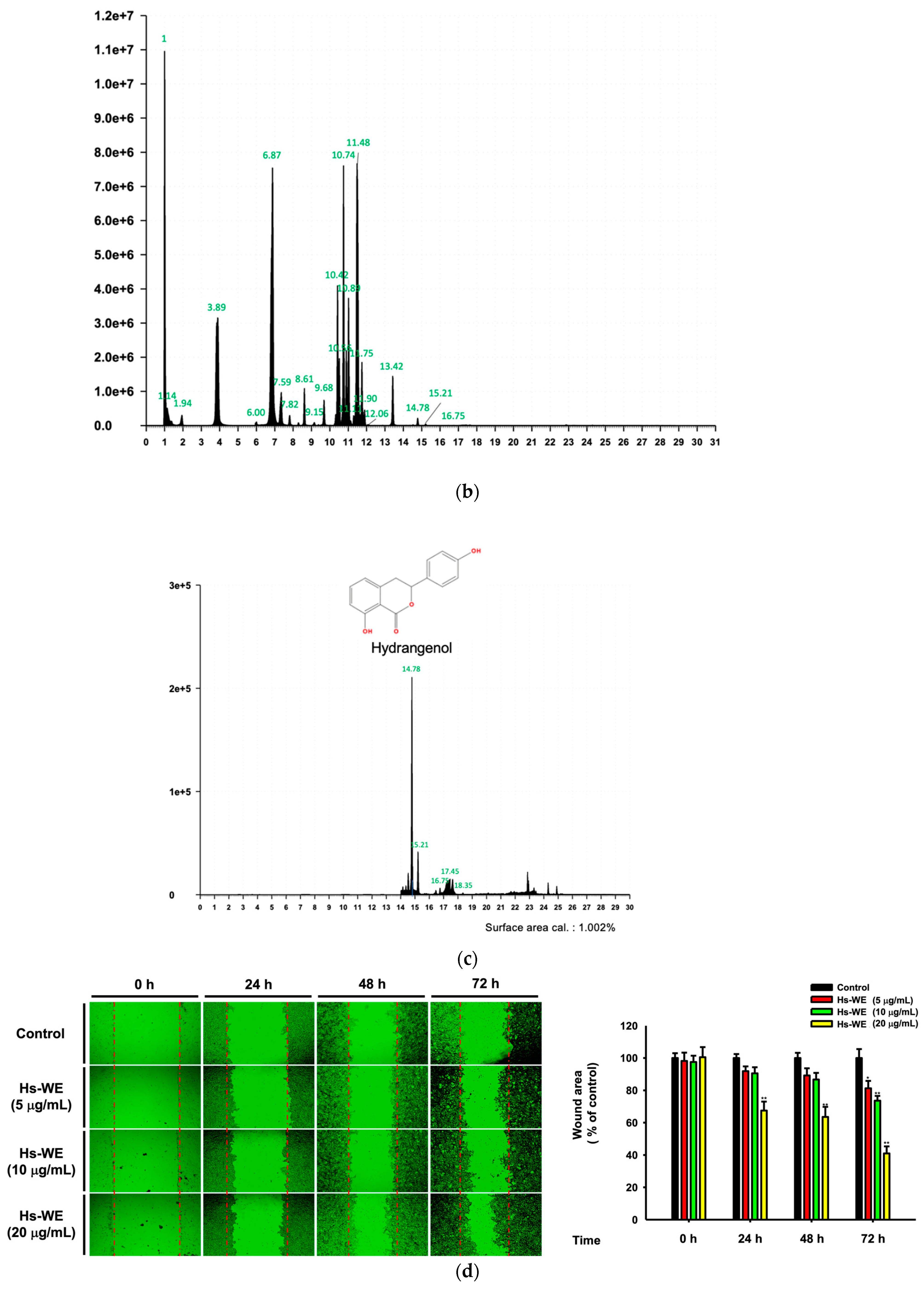
2.4. Wound Healing Assay
2.5. qRT-PCR
2.6. Luciferase Reporter Assay
2.7. Western Blotting
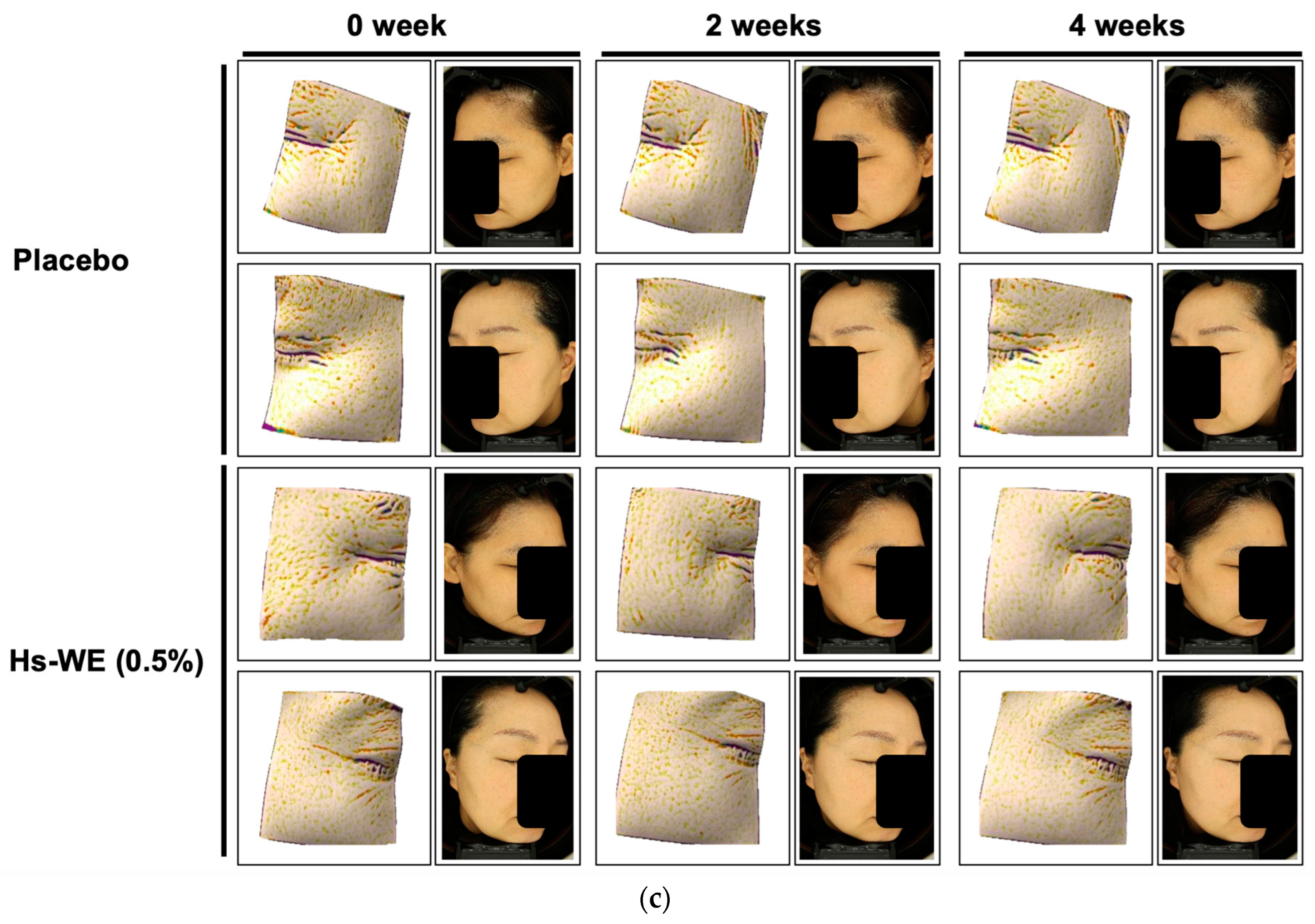
2.8. Clinical Trial
2.9. LC-MS/MS Analysis
2.10. Statistical Analysis
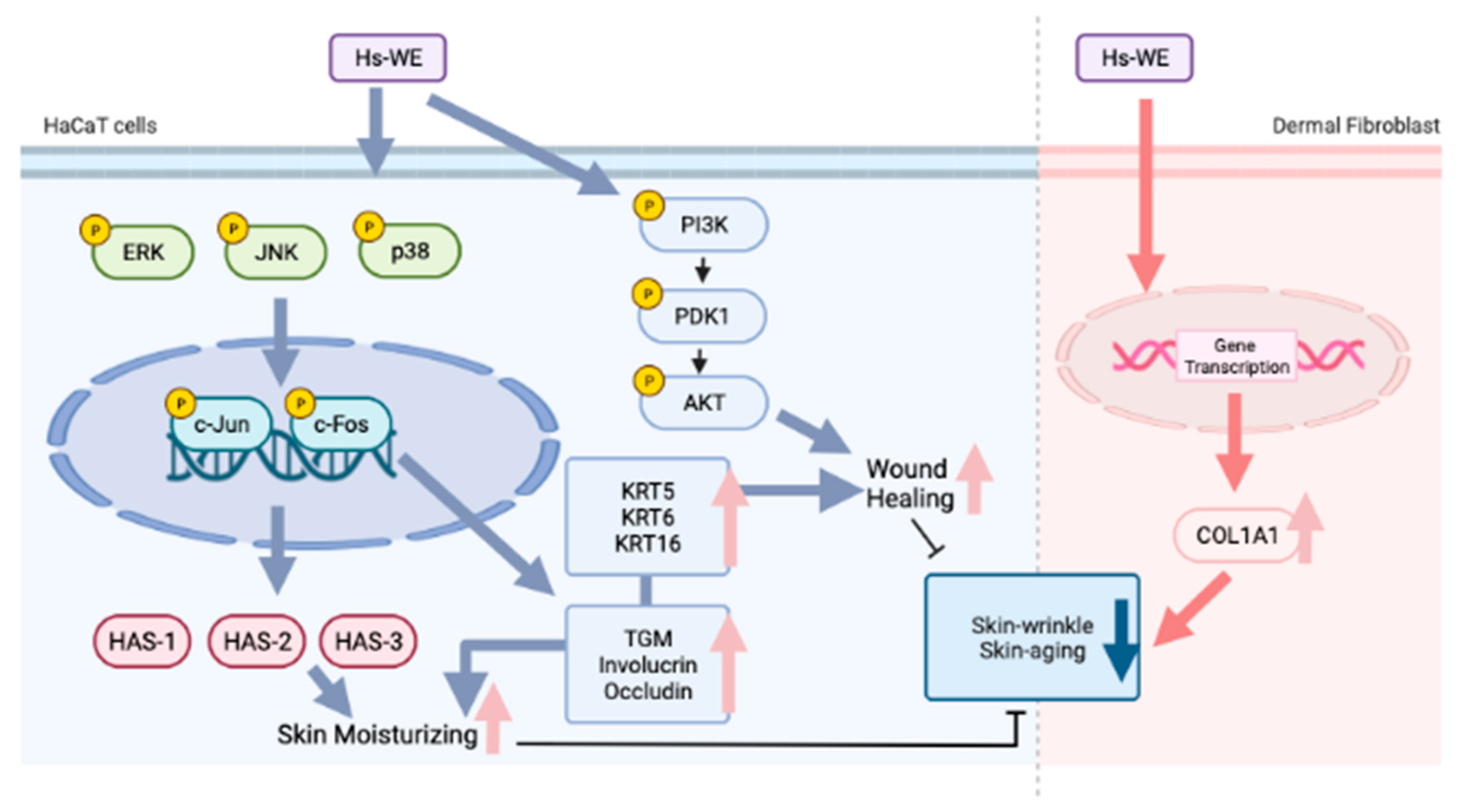
3. Results
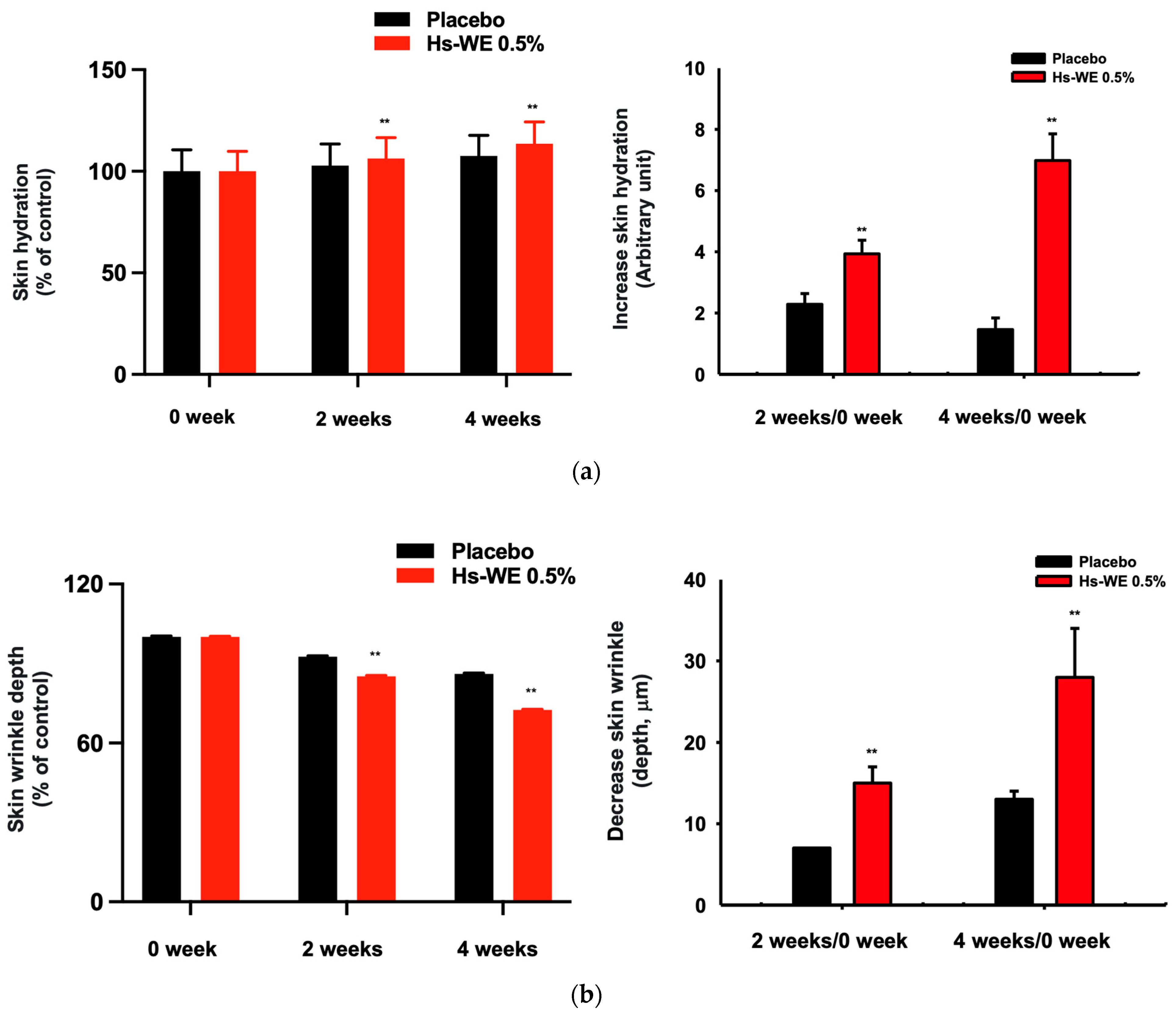
3.1. Skin Moisturizing and Anti-Wrinkle Effects of Hs-WE
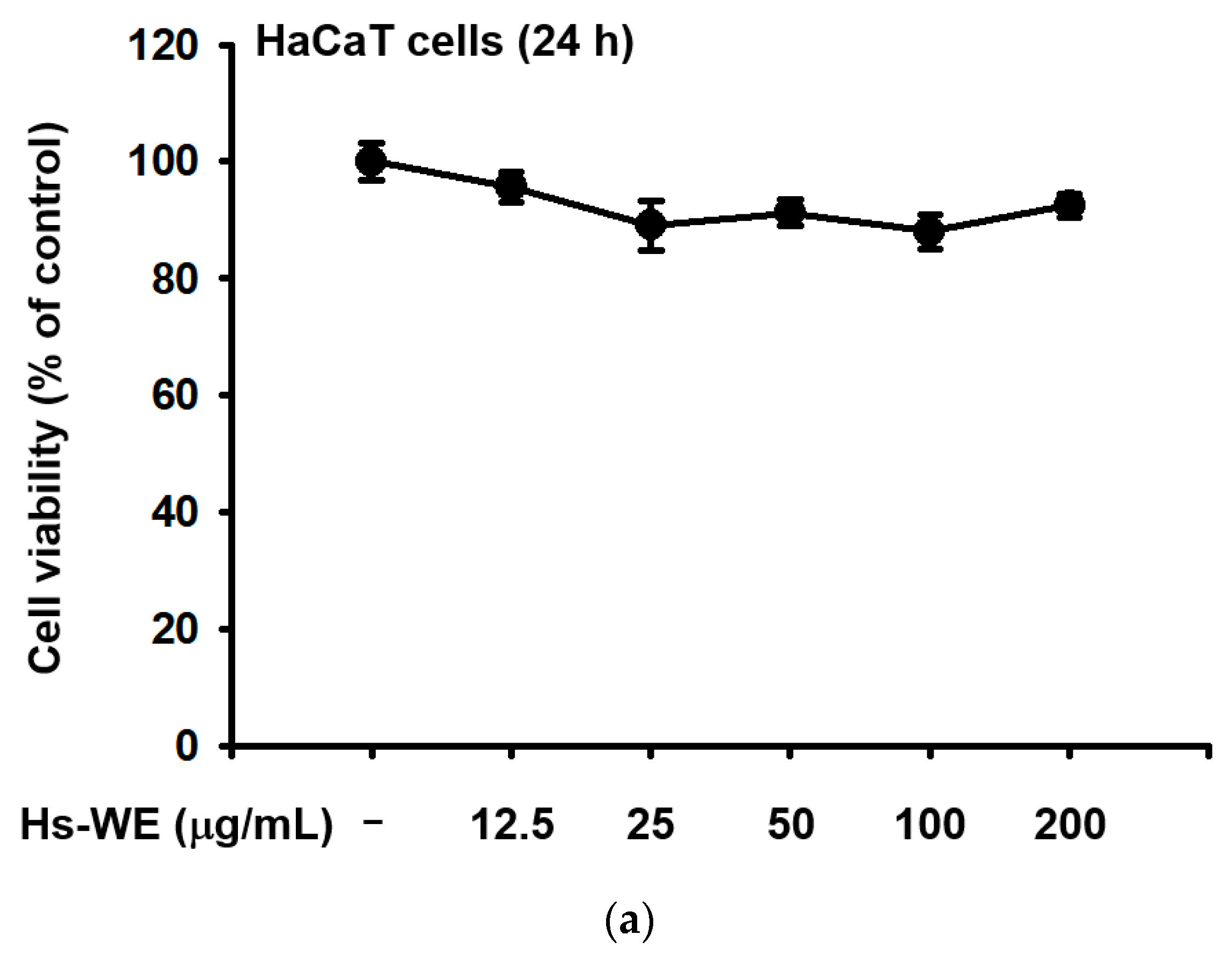
3.2. Hs-WE Shows No Toxicity in HaCaT Cells
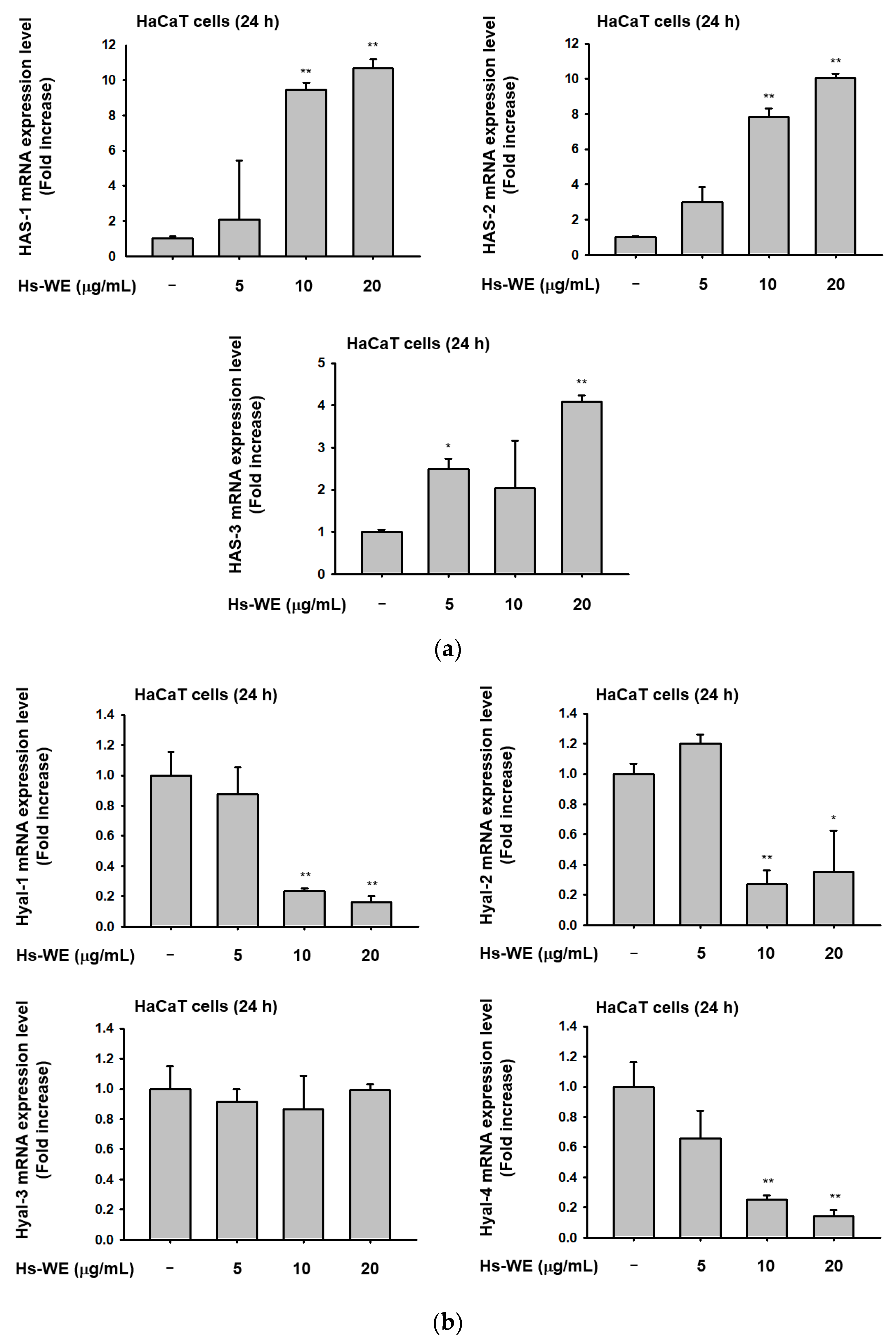
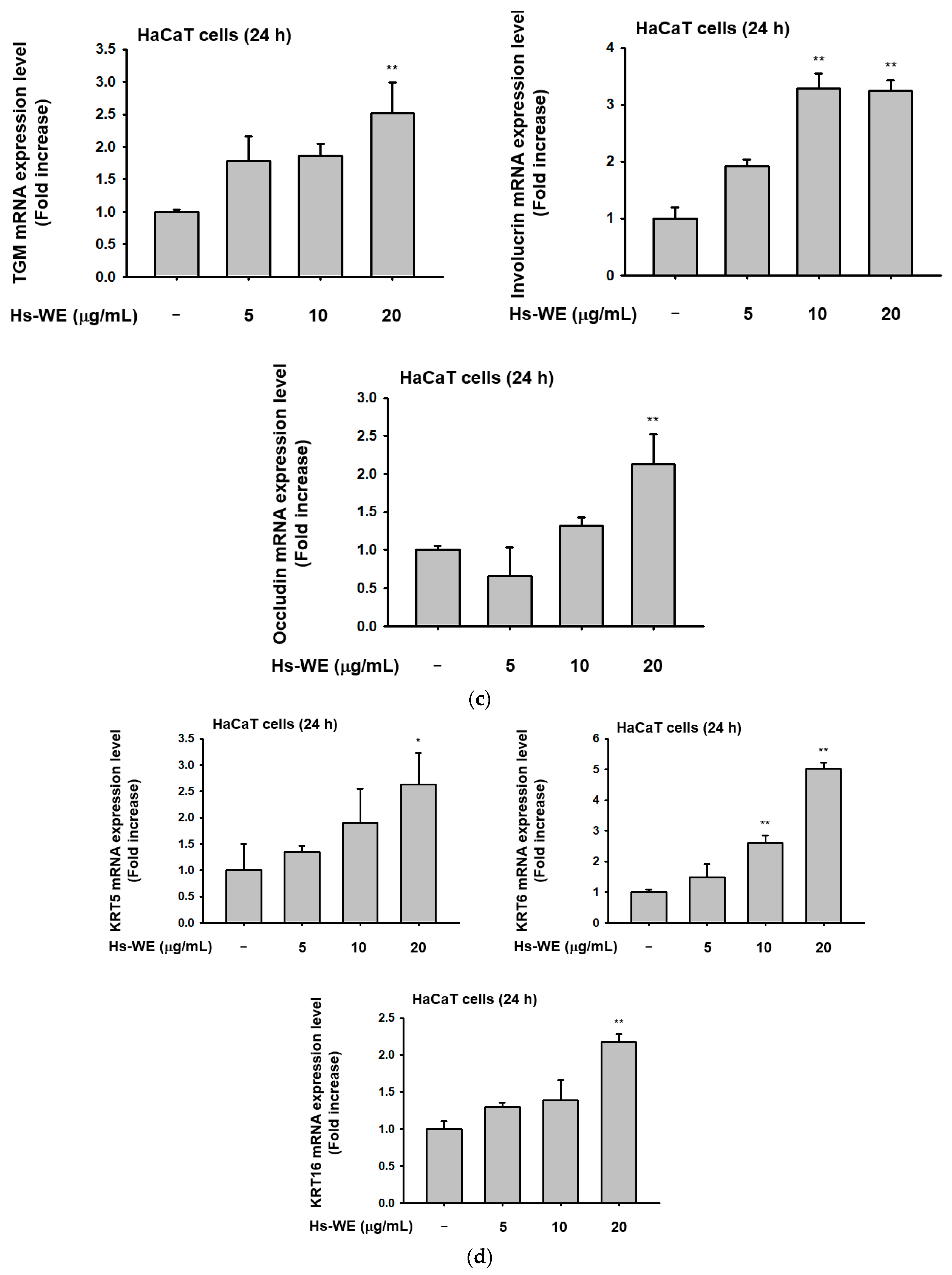
3.3. Hs-WE Skin Moisturizing Potential at the Transcription Level
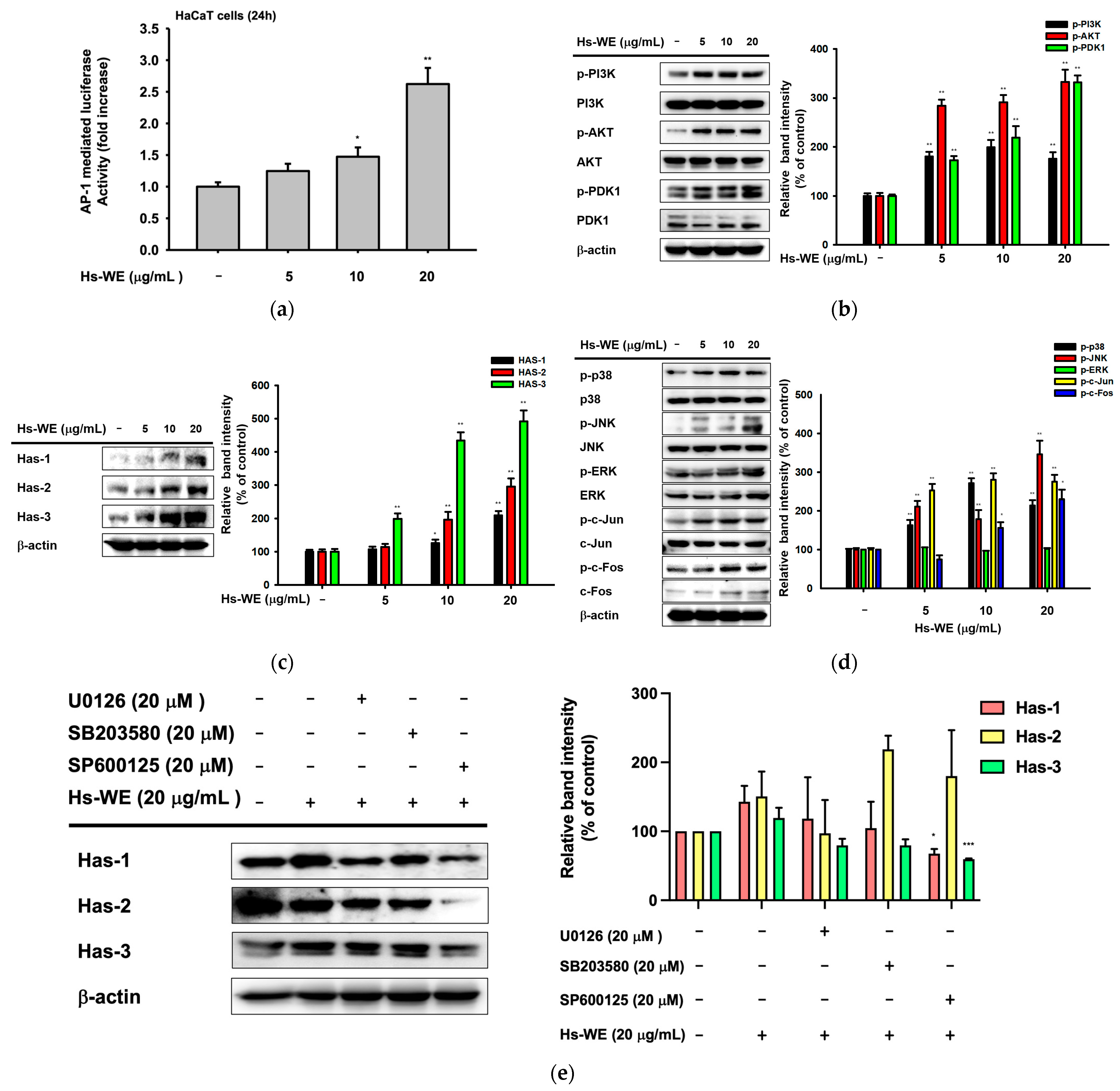
3.4. Hs-WE Upregulates the Expression of AP-1 Pathway Proteins
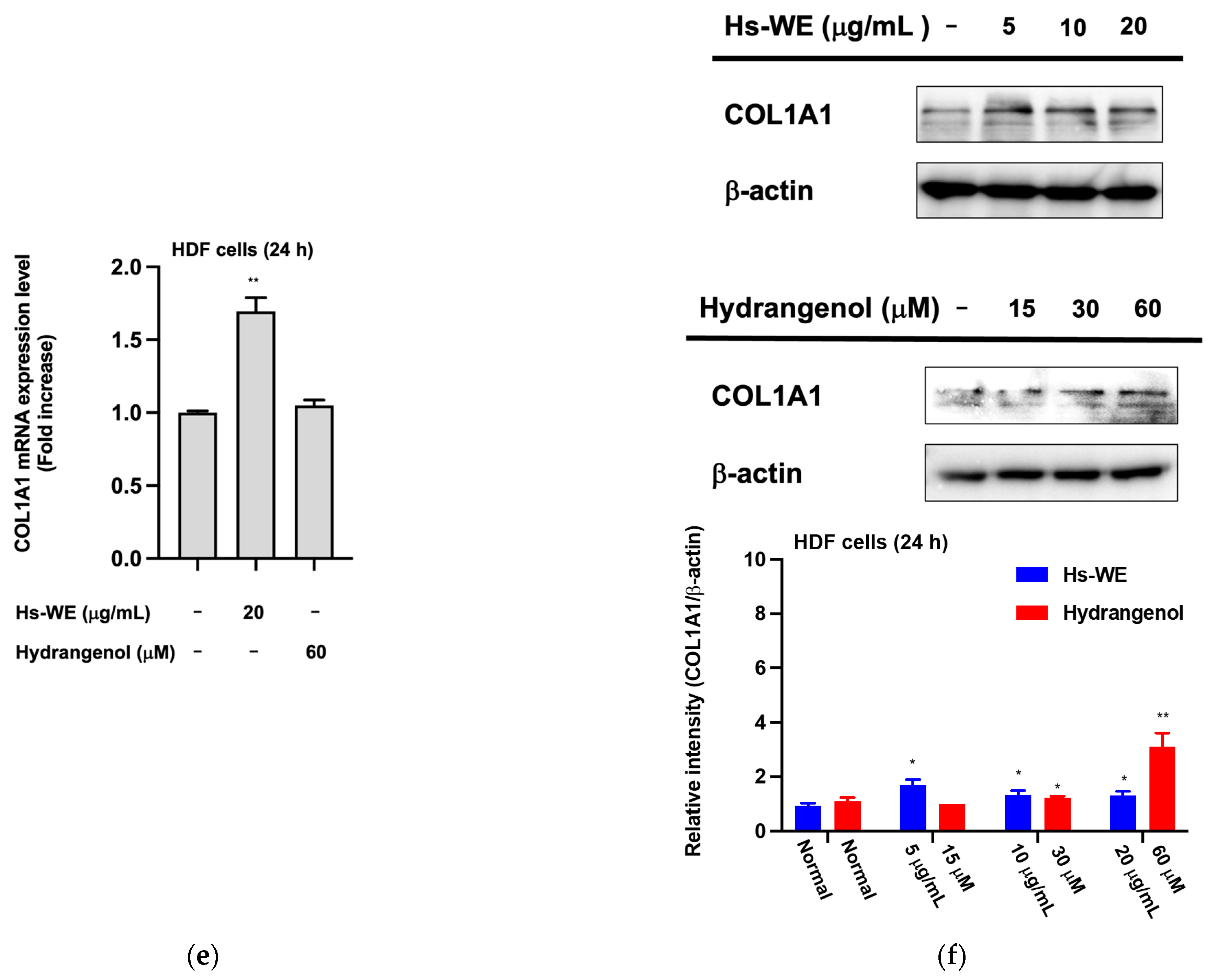
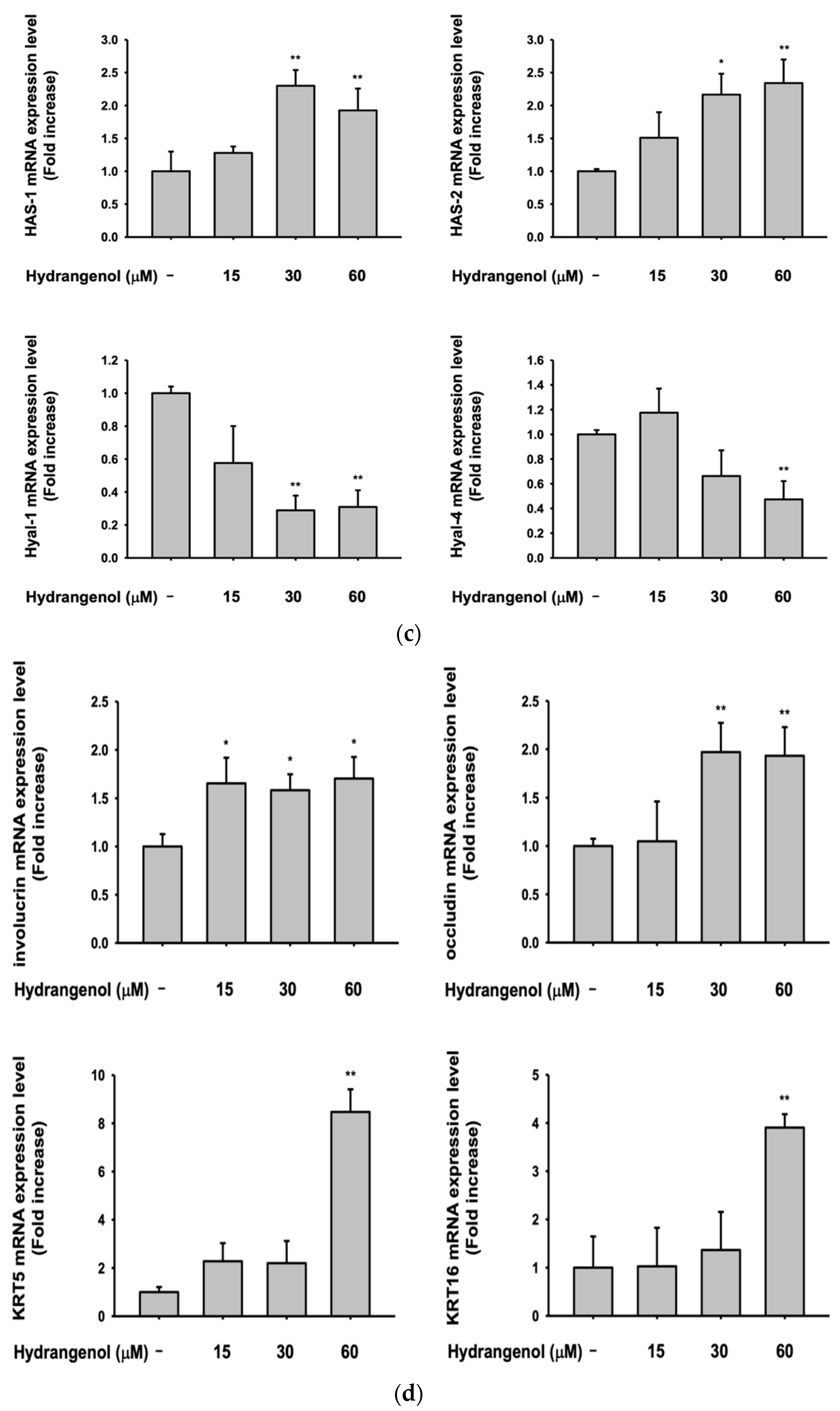
3.5. Hydrangenol Shows Skin Improvement Effects at the mRNA Level
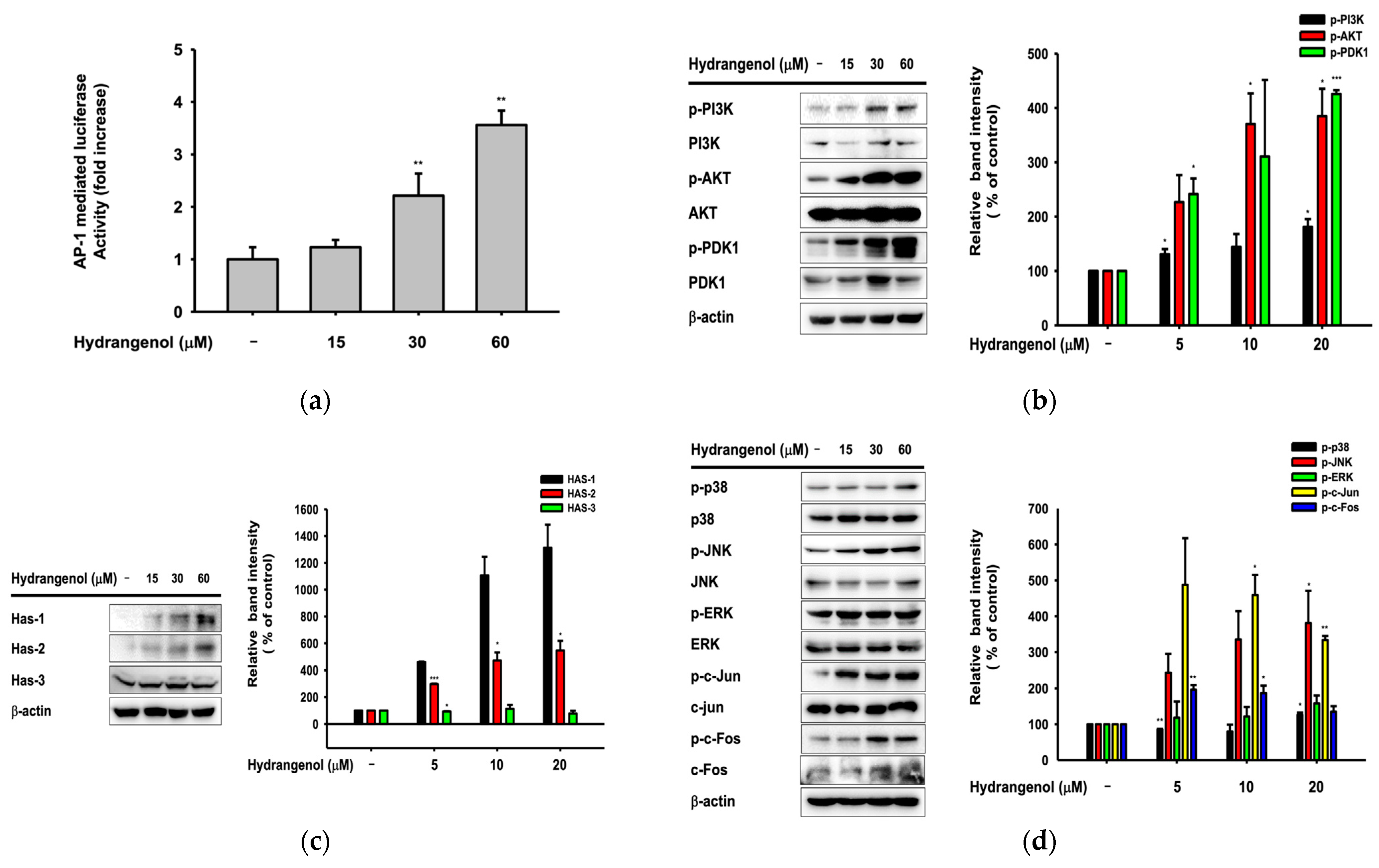
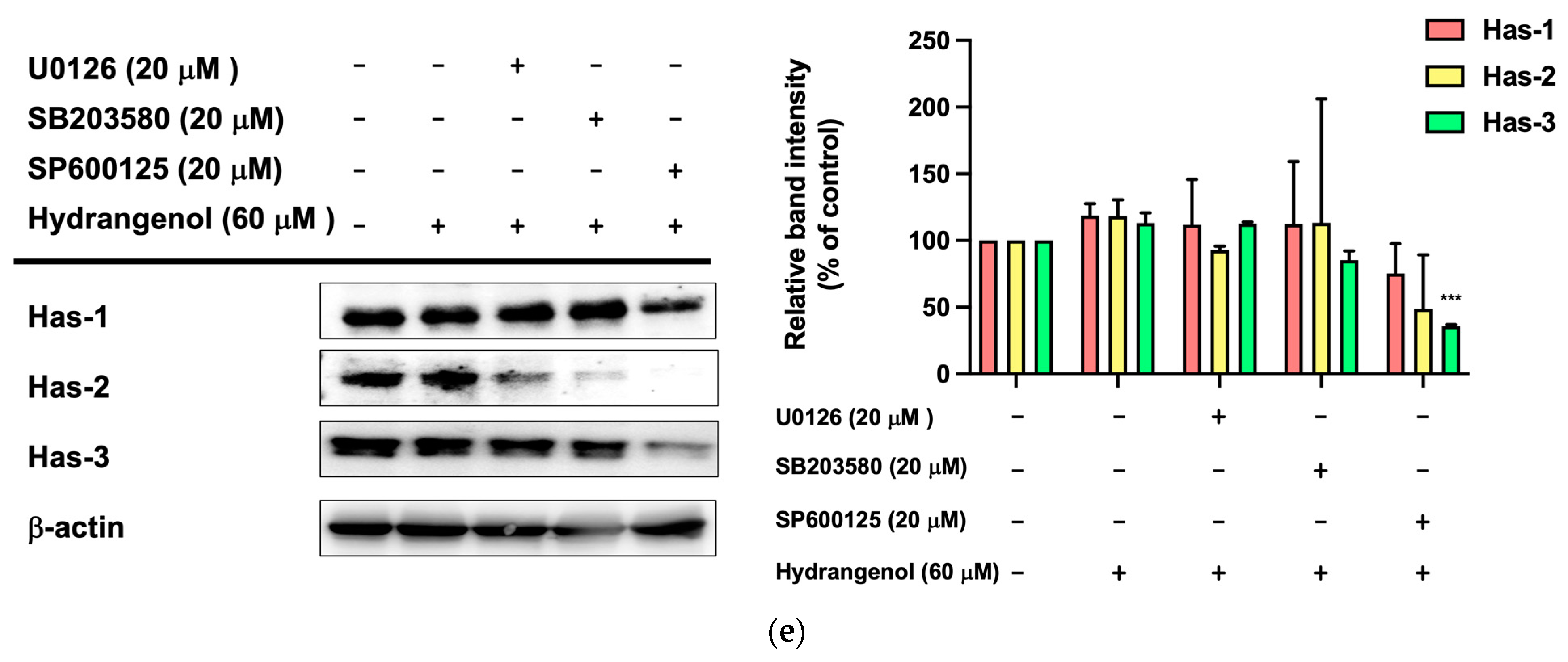
3.6. Hydrangenol Improves Skin as the Main Component of Hs-WE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| ROS | reactive oxygen species |
| HDF | human dermal fibroblast |
| HYAL | hyaluronidase |
| HAS-1 | hyaluronic acid synthase 1 |
| HAS-2 | hyaluronic acid synthase 2 |
| HAS-3 | hyaluronic acid synthase 3 |
| INVN | involucrin |
| OCLN | occludin |
| TGM | transglutaminase 1 |
| FLG | filaggrin |
| AP-1 | activator protein 1 |
| MAPK | mitogen-activated protein kinase |
| ERK | extracellular signal-regulated kinases |
| JNK | c-Jun NH2-terminal kinases |
| HaCaT cells | human keratinocyte cell line |
| DMEM | Dulbecco’s modified eagle’s medium |
| PC/SM | penicillin/streptomycin solution |
| FBS | fetal bovine serum |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
References
- Huertas, A.C.M.; Schmelzer, C.E.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular-level insights into aging processes of skin elastin. Biochimie 2016, 128, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passeron, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Sutopo, N.C.; Kim, J.H.; Cho, J.Y. Role of histone methylation in skin cancers: Histone methylation–modifying enzymes as a new class of targets for skin cancer treatment. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188865. [Google Scholar] [CrossRef]
- Farage, M.; Miller, K.; Elsner, P.; Maibach, H. Intrinsic and extrinsic factors in skin ageing: A review. Int. J. Cosmet. Sci. 2008, 30, 87–95. [Google Scholar] [CrossRef]
- Gilchrest, B.A. Skin aging and photoaging: An overview. J. Am. Acad. Dermatol. 1989, 21, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Kligman, L.; Kligman, A. The nature of photoaging: Its prevention and repair. Photodermatology 1986, 3, 215–227. [Google Scholar]
- Kazanci, A.; Kurus, M.; Atasever, A. Analyses of changes on skin by aging. Skin Res. Technol. 2017, 23, 48–60. [Google Scholar] [CrossRef]
- Lee, J.-O.; Hwang, S.-H.; Shen, T.; Kim, J.H.; You, L.; Hu, W.; Cho, J.Y. Enhancement of skin barrier and hydration-related molecules by protopanaxatriol in human keratinocytes. J. Ginseng Res. 2021, 45, 354–360. [Google Scholar] [CrossRef]
- Li, L.; Asteriou, T.; Bernert, B.; Heldin, C.-H.; Heldin, P. Growth factor regulation of hyaluronan synthesis and degradation in human dermal fibroblasts: Importance of hyaluronan for the mitogenic response of PDGF-BB. Biochem. J. 2007, 404, 327–336. [Google Scholar] [CrossRef]
- Chinenov, Y.; Kerppola, T.K. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 2001, 20, 2438–2452. [Google Scholar] [CrossRef] [PubMed]
- Vogt, P.K. Fortuitous convergences: The beginnings of JUN. Nat. Rev. Cancer 2002, 2, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Angel, P.; Karin, M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim. Biophys. Acta Rev. Cancer 1991, 1072, 129–157. [Google Scholar] [CrossRef]
- Hunter, T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Karin, M. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 1995, 270, 16483–16486. [Google Scholar] [CrossRef]
- Waskiewicz, A.J.; Cooper, J.A. Mitogen and stress response pathways: MAP kinase cascades and phosphatase regulation in mammals and yeast. Curr. Opin. Cell Biol. 1995, 7, 798–805. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Sounding the alarm: Protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 1996, 271, 24313–24316. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.J.; Cobb, M.H. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 1997, 9, 180–186. [Google Scholar] [CrossRef]
- Chen, H.; Hossain, M.A.; Kim, J.H.; Cho, J.Y. Kahweol Exerts Skin Moisturizing Activities by Upregulating STAT1 Activity. Int. J. Mol. Sci. 2021, 22, 8864. [Google Scholar] [CrossRef]
- Ijaz, S.; Shoaib Khan, H.M.; Anwar, Z.; Talbot, B.; Walsh, J.J. HPLC profiling of Mimosa pudica polyphenols and their non-invasive biophysical investigations for anti-dermatoheliotic and skin reinstating potential. Biomed. Pharmacother. 2019, 109, 865–875. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. NEJM 1997, 337, 1419–1429. [Google Scholar] [CrossRef]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking older: Fibroblast collapse and therapeutic implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef]
- Quan, T.; Shao, Y.; He, T.; Voorhees, J.J.; Fisher, G.J. Reduced expression of connective tissue growth factor (CTGF/CCN2) mediates collagen loss in chronologically aged human skin. J. Investig. Dermatol. 2010, 130, 415–424. [Google Scholar] [CrossRef]
- Qin, Z.; Balimunkwe, R.; Quan, T. Age-related reduction of dermal fibroblast size upregulates multiple matrix metalloproteinases as observed in aged human skin in vivo. Br. J. Dermatol. 2017, 177, 1337–1348. [Google Scholar] [CrossRef]
- Tewari, A.; Grys, K.; Kollet, J.; Sarkany, R.; Young, A.R. Upregulation of MMP12 and its activity by UVA1 in human skin: Potential implications for photoaging. J. Investig. Dermatol. 2014, 134, 2598–2609. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Protein oxidation and aging. Science 1992, 257, 1220–1224. [Google Scholar] [CrossRef] [PubMed]
- Golden, T.R.; Hinerfeld, D.A.; Melov, S. Oxidative stress and aging: Beyond correlation. Aging Cell 2002, 1, 117–123. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin: Roles of Age-Dependent Alteration in Fibroblast Function and Defective Mechanical Stimulation. Am. J. Clin. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.-E.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of Skin Collagen Metabolism in Aged and Photoaged Human Skin In Vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Kim, Y.; Kim, M.S.; Lee, S.; Yoo, S.H. The establishment of efficient bioconversion, extraction, and isolation processes for the production of phyllodulcin, a potential high intensity sweetener, from sweet hydrangea leaves (Hydrangea macrophylla Thunbergii). Phytochem. Anal. 2016, 27, 140–147. [Google Scholar] [CrossRef]
- Han, H.-S.; Chung, K.-S.; Shin, Y.-K.; Lee, S.H.; Lee, K.-T. Standardized Hydrangea serrata (Thunb.) Ser. Extract Ameliorates Obesity in db/db Mice. Nutrients 2021, 13, 3624. [Google Scholar] [CrossRef]
- Han, H.S.; Shin, J.S.; Myung, D.B.; Ahn, H.S.; Lee, S.H.; Kim, H.J.; Lee, K.T. Hydrangea serrata (Thunb.) Ser. Extract Attenuate UVB-Induced Photoaging through MAPK/AP-1 Inactivation in Human Skin Fibroblasts and Hairless Mice. Nutrients 2019, 11, 533. [Google Scholar] [CrossRef]
- Hashimoto, T.; Tori, M.; Asakawa, Y. Three dihydroisocoumarin glucosides from Hydrangea macrophylla subsp. serrata. Phytochemistry 1987, 26, 3323–3330. [Google Scholar] [CrossRef]
- Uesato, S.; Takeda, Y.; Hashimoto, T.; Uobe, K.; Inouye, H.; Taguchi, H.; Endo, T. Studies on Monoterpene Glucosides and Related Natural Products Part 51. Absolute Structures of Hydrangenosides A, B, C, D, E, F, and G. Novel Type Secoiridoid Glucosides from Two Hydrangea Plants. Helv. Chim. Acta 1984, 67, 2111–2127. [Google Scholar] [CrossRef]
- Nozawa, K.; Yamada, M.; Tsuda, Y.; Kawai, K.; Nakajima, S. Antifungal activity of oosponol, oospolactone, phyllodulcin, hydrangenol, and some other related compounds. Chem. Pharm. Bull. 1981, 29, 2689–2691. [Google Scholar] [CrossRef]
- Kakegawa, H.; Matsumoto, H.; Satoh, T. Inhibitory effects of hydrangenol derivatives on the activation of hyaluronidase and their antiallergic activities. Planta Med. 1988, 54, 385–389. [Google Scholar] [CrossRef]
- Zhang, H.; Matsuda, H.; Kumahara, A.; Ito, Y.; Nakamura, S.; Yoshikawa, M. New type of anti-diabetic compounds from the processed leaves of Hydrangea macrophylla var. thunbergii (Hydrangeae Dulcis Folium). Bioorg. Med. Chem. Lett. 2007, 17, 4972–4976. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kang, C.-H.; Jayasooriya, R.G.P.T.; Dilshara, M.G.; Lee, S.; Choi, Y.H.; Seo, Y.T.; Kim, G.-Y. Hydrangenol inhibits lipopolysaccharide-induced nitric oxide production in BV2 microglial cells by suppressing the NF-κB pathway and activating the Nrf2-mediated HO-1 pathway. Int. Immunopharmacol. 2016, 35, 61–69. [Google Scholar] [CrossRef]
- Gho, Y.; Shin, S.-S.; Choi, Y.H.; Ko, K.; Kim, W.-J.; Moon, S.-K. Hydrangenol suppresses VEGF-stimulated angiogenesis by targeting p27KIP1-dependent G1-cell cycle arrest, VEGFR-2-mediated signaling, and MMP-2 expression. Anim. Cells Syst. 2019, 23, 72–81. [Google Scholar] [CrossRef]
- Myung, D.B.; Han, H.S.; Shin, J.S.; Park, J.Y.; Hwang, H.J.; Kim, H.J.; Ahn, H.S.; Lee, S.H.; Lee, K.T. Hydrangenol Isolated from the Leaves of Hydrangea serrata Attenuates Wrinkle Formation and Repairs Skin Moisture in UVB-Irradiated Hairless Mice. Nutrients 2019, 11, 2354. [Google Scholar] [CrossRef] [PubMed]
- Myung, D.B.; Lee, J.H.; Han, H.S.; Lee, K.Y.; Ahn, H.S.; Shin, Y.K.; Song, E.; Kim, B.H.; Lee, K.H.; Lee, S.H.; et al. Oral Intake of Hydrangea serrata (Thunb.) Ser. Leaves Extract Improves Wrinkles, Hydration, Elasticity, Texture, and Roughness in Human Skin: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2020, 12, 1588. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-Y.; Jang, S.-I. The Anti-Inflammatory Effect from Lipopolysaccharide-Stimulated RAW 264.7 of Extracts of Hydrangea serrata Seringe. J. Korean Soc. Food Sci. Nutr. 2021, 50, 236–245. [Google Scholar] [CrossRef]
- Jang, Y.J.; Ahn, J.; Son, H.J.; Jung, C.H.; Ahn, J.; Ha, T.Y. Hydrangea serrata Tea Enhances Running Endurance and Skeletal Muscle Mass. Mol. Nutr. Food Res. 2019, 63, e1801149. [Google Scholar] [CrossRef]
- Mitra, A.; Rahmawati, L.; Lee, H.P.; Kim, S.A.; Han, C.K.; Hyun, S.H.; Cho, J.Y. Korean Red Ginseng water extract inhibits cadmium-induced lung injury via suppressing MAPK/ERK1/2/AP-1 pathway. J. Ginseng Res. 2022, 46, 690–699. [Google Scholar] [CrossRef]
- Bellacosa, A.; De Feo, D.; Godwin, A.K.; Bell, D.W.; Cheng, J.Q.; Altomare, D.A.; Wan, M.; Dubeau, L.; Scambia, G.; Masciullo, V. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int. J. Cancer 1995, 64, 280–285. [Google Scholar] [CrossRef]
- Bellacosa, A.; Kumar, C.C.; Di Cristofano, A.; Testa, J.R. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv. Cancer Res. 2005, 94, 29–86. [Google Scholar]
- Bergfeld, W.F. The aging skin. Int. J. Fertil. Womens Med. 1997, 42, 57–66. [Google Scholar]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Blank, I.H. Factors which influence the water content of the stratum corneum. J. Invest. Dermatol. 1952, 18, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Kim, M.Y.; Cho, J.Y. Apigenin: A Therapeutic Agent for Treatment of Skin Inflammatory Diseases and Cancer. Int. J. Mol. Sci. 2023, 24, 1498. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-K.; Atigadda, V.R.; Brzeminski, P.; Fabisiak, A.; Tang, E.K.Y.; Tuckey, R.C.; Reiter, R.J.; Slominski, A.T. Detection of Serotonin, Melatonin, and Their Metabolites in Honey. Food Sci. Technol. 2021, 1, 1228–1235. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Janjetovic, Z.; Kim, T.-K.; Böhm, M.; Steinbrink, K.; Reiter, R.J.; Kleszczyński, K.; Slominski, A.T. Protective Role of Melatonin and Its Metabolites in Skin Aging. Int. J. Mol. Sci. 2022, 23, 1238. [Google Scholar] [CrossRef] [PubMed]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. The Impact of Vitamin D on Skin Aging. Int. J. Mol. Sci. 2021, 22, 9097. [Google Scholar] [CrossRef]
- Kim, T.-K.; Atigadda, V.; Brzeminski, P.; Fabisiak, A.; Tang, E.K.Y.; Tuckey, R.C.; Slominski, A.T. Detection of 7-Dehydrocholesterol and Vitamin D3 Derivatives in Honey. Molecules 2020, 25, 2583. [Google Scholar] [CrossRef]
- Eckert, R.L.; Yaffe, M.B.; Crish, J.F.; Murthy, S.; Rorke, E.A.; Welter, J.F. Involucrin—Structure and role in envelope assembly. J. Investig. Dermatol. 1993, 100, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Steinert, P.M.; Cantieri, J.S.; Teller, D.C.; Lonsdale-Eccles, J.D.; Dale, B.A. Characterization of a class of cationic proteins that specifically interact with intermediate filaments. Proc. Natl. Acad. Sci. USA 1981, 78, 4097–4101. [Google Scholar] [CrossRef]
- Segre, J.A. Epidermal barrier formation and recovery in skin disorders. J. Clin. Investig. 2006, 116, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.-D.; Kang, T.J.; Lee, C.H.; Lee, A.-Y.; Noh, M. HaCaT Keratinocytes and Primary Epidermal Keratinocytes Have Different Transcriptional Profiles of Cornified Envelope-Associated Genes to T Helper Cell Cytokines. Biomol. Ther. 2012, 20, 171–176. [Google Scholar] [CrossRef]
- Jiang, Z.; Wei, J.; Yang, W.; Li, W.; Liu, F.; Yan, X.; Yan, X.; Hu, N.; Li, J. MicroRNA-26a inhibits wound healing through decreased keratinocytes migration by regulating ITGA5 through PI3K/AKT signaling pathway. Biosci. Rep. 2020, 40, BSR20201361. [Google Scholar] [CrossRef]
- Varshney, P.; Narasimhan, A.; Mittal, S.; Malik, G.; Sardana, K.; Saini, N. Transcriptome profiling unveils the role of cholesterol in IL-17A signaling in psoriasis. Sci. Rep. 2016, 6, 19295. [Google Scholar] [CrossRef]
| Gene Name | Sequence (5′–3′) | |
|---|---|---|
| HAS-1 | Forward | GTATCCTGCATCAGCGGTCC |
| Reverse | TGCCGGTCATCCCCAAAAG | |
| HAS-2 | Forward | CCTCCTGGGTGGTGTGATTT |
| Reverse | GCGTCAAAAGCATGACCCAA | |
| HAS-3 | Forward | CAAGTGCCTCACAGAGACCC |
| Reverse | GGAAGAAACCCGTGACCACT | |
| HYAL-1 | Forward | CAGAATGCAGCCTGATTGC |
| Reverse | CCGGTGTAGTTGGGGCTTAG | |
| HYAL-2 | Forward | TACACCACAAGCACGGAGAC |
| Reverse | ATGCAGGAAGGGTACTGGCAC | |
| HYAL-3 | Forward | CCAGGATGACCTTGTGCAGT |
| Reverse | CCATCTGTCCTGGATCTCGC | |
| HYAL-4 | Forward | TGACCTCTCTTGGCTCTGGA |
| Reverse | AGGCAGCACTTTCTCCTATGG | |
| Ker5 | Forward | GAGATCAGTGACTTGTGCGT |
| Reverse | ATTGCTGAGTTGCTCAGGTG | |
| Ker6 | Forward | TCCTCTTCGAGCCGTCAGA |
| Reverse | TGGTAGAGGCAGCTCAGTTC | |
| Ker16 | Forward | CCAGGGACTGATTGGCAGTGT |
| Reverse | AAGGGTCTGGGAGGCAGAACT | |
| TGM-1 | Forward | ACAGGCTCATCTGGTTGGTG |
| Reverse | TTCCCGATGCTTGTGGTCTC | |
| FLG | Forward | TGAGGCATACCCAGAGGACT |
| Reverse | CTGTATCGCGGTGAGAGGAT | |
| OCLN | Forward | TGGCCTACAGGAATACAAGAGC |
| Reverse | AAAGGATGCTGTACCTCCACAG | |
| IVLN | Forward | CCAGAAGGTGCCTGTCGAG |
| Reverse | TCAGGCAGTCCCTTTACAGC | |
| COL1A1 | Forward | CAGGTACCATGACCGAGACG |
| Reverse | AGCACCATCATTTCCACGAG |
| Criteria | No. | Contents |
|---|---|---|
| Inclusion criteria | 1 | Females with dried skin, aged 30–59 years (average = 49.2 years). |
| 2 | The subject has eye wrinkles (crow’s feet). | |
| 3 | A person who has voluntarily signed consent after the test purpose and content were fully explained. | |
| 4 | Those who can follow up during the test period. | |
| 5 | A healthy person without an acute or chronic physical disease including skin diseases. | |
| Exclusion criteria | 1 | Pregnant or lactating women and women of childbearing age who do not agree to the contraceptive method prescribed by the protocol. |
| 2 | A person with a lesion at the test site or suffering from an infectious skin disease. | |
| 3 | People with allergies, hypersensitivity, or irritation to cosmetics, pharmaceuticals, or daily exposure to light. | |
| 4 | Those who have received systemic steroids or phototherapy within 1 month of participating in the trial, or who have received skin treatments (scaling/Botox/filler/laser/tattoo) within 3 months of participating in the trial. | |
| 5 | Those who have used drugs with similar functions at the research site within 3 months before the start of the study, or have a mental illness or mental retardation disorder. | |
| 6 | Other than the above, a person who will make it difficult to conduct a human test based on the judgment of the responsible researcher or the person in charge of the study. |
| No. | Ingredient Name |
|---|---|
| 1 | Water |
| 2 | Glycerin |
| 3 | Butylene glycol |
| 4 | Dipropylene glycol |
| 5 | Disodium EDTA |
| 6 | Triethanolamine |
| 7 | Cetearyl alcohol |
| 8 | Caprylic/capric triglyceride |
| 9 | Glyceryl stearate |
| 10 | PEG-100 stearate |
| 11 | Carbomer |
| 12 | 1,2-hexanediol |
| 13 | Phenoxyethanol |
| 14 | Sodium acrylate/sodium acryloyldimethyl taurate copolymer |
| 15 | Polyisobutene |
| 16 | Sorbitan oleate |
| 17 | Caprylyl/capryl glucoside |
| No. | Ingredient Name |
|---|---|
| 1 | Water |
| 2 | Glycerin |
| 3 | Butylene glycol |
| 4 | Dipropylene glycol |
| 5 | Disodium EDTA |
| 6 | Triethanolamine |
| 7 | Cetearyl alcohol |
| 8 | Caprylic/capric triglyceride |
| 9 | Glyceryl stearate |
| 10 | PEG-100 stearate |
| 11 | Carbomer |
| 12 | 1,2-hexanediol |
| 13 | Phenoxyethanol |
| 14 | Sodium acrylate/sodium acryloyldimethyl taurate copolymer |
| 15 | Polyisobutene |
| 16 | Sorbitan oleate |
| 17 | Caprylyl/capryl glucoside |
| 18 | Hydrangea serrata leaf extract |
| 0.5% Hs-WE | Placebo | ||
|---|---|---|---|
| Moisturizing measurement value (A.U) | 0 week | 51.544 ± 9.862 | 51.771 ± 10.593 |
| after 2 weeks | 54.828 ± 10.214 | 53.230 ± 10.631 | |
| after 4 weeks | 58.525 ± 10.735 | 55.705 ± 10.147 | |
| Variance a | 0 week–after 2 weeks | 3.284 | 1.459 |
| 0 week–after 4 weeks | 6.981 | 3.934 | |
| Improvement rate b (%) | 0 week–after 2 weeks | 6.371 | 2.818 |
| 0 week–after 4 weeks | 13.544 | 7.599 | |
| p-Value within group | 0 week–after 2 weeks | <0.001 | <0.001 |
| 0 week–after 4 weeks | <0.001 | <0.001 | |
| p-Value between groups | 0 week–after 2 weeks | <0.001 | |
| 0 week–after 4 weeks | <0.001 | ||
| 0.5% Hs-WE | Placebo | ||
|---|---|---|---|
| Measurement of skin wrinkles (depth, mm) | 0 week | 0.102 ± 0.029 | 0.094 ± 0.030 |
| after 2 weeks | 0.087 ± 0.027 | 0.087 ± 0.030 | |
| after 4 weeks | 0.074 ± 0.023 | 0.081 ± 0.029 | |
| Variance a | 0 week–after 2 weeks | −0.015 | −0.007 |
| 0 week–after 4 weeks | −0.028 | −0.013 | |
| Improvement rate b (%) | 0 week–after 2 weeks | 14.706 | 7.447 |
| 0 week–after 4 weeks | 27.451 | 13.830 | |
| p-value within group | 0 week–after 2 weeks | <0.001 | <0.001 |
| 0 week–after 4 weeks | <0.001 | <0.001 | |
| p-value between groups | 0 week–after 2 weeks | 0.002 | |
| 0 week–after 4 weeks | <0.001 | ||
| Peak No. | RT | Name of the Compound |
|---|---|---|
| 1 | 1 | GABA |
| 2 | 1.94 | Gallic acid |
| 3 | 3.85 | Protocatechuic acid |
| 4 | 6.87 | Chlorogenic acid |
| 5 | 7.59 | Vanillic acid |
| 6 | 7.8 | Caffeic acid |
| 7 | 8.61 | Loganin_A |
| 8 | 9.68 | p-Coumaric acid |
| 9 | 10.42 | Rutin |
| 10 | 10.56 | Ferulic acid |
| 11 | 10.74 | Hyperoside |
| 12 | 10.89 | Isoquercitrin |
| 13 | 11.11 | Salicylic acid |
| 14 | 11.8 | Astragalin |
| 15 | 13.42 | Resveratrol |
| 16 | 14.78 | Hydrangenol |
| 17 | 16.75 | Kaempferol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.H.; Park, S.H.; Yoon, S.E.; Hong, S.Y.; Lee, J.B.; Lee, J.; Cho, J.Y. Hydrangea serrata Hot Water Extract and Its Major Ingredient Hydrangenol Improve Skin Moisturization and Wrinkle Conditions via AP-1 and Akt/PI3K Pathway Upregulation. Nutrients 2023, 15, 2436. https://doi.org/10.3390/nu15112436
Yoon JH, Park SH, Yoon SE, Hong SY, Lee JB, Lee J, Cho JY. Hydrangea serrata Hot Water Extract and Its Major Ingredient Hydrangenol Improve Skin Moisturization and Wrinkle Conditions via AP-1 and Akt/PI3K Pathway Upregulation. Nutrients. 2023; 15(11):2436. https://doi.org/10.3390/nu15112436
Chicago/Turabian StyleYoon, Ji Hye, Sang Hee Park, Si Eun Yoon, Seong Yoon Hong, Jun Bae Lee, Jongsung Lee, and Jae Youl Cho. 2023. "Hydrangea serrata Hot Water Extract and Its Major Ingredient Hydrangenol Improve Skin Moisturization and Wrinkle Conditions via AP-1 and Akt/PI3K Pathway Upregulation" Nutrients 15, no. 11: 2436. https://doi.org/10.3390/nu15112436
APA StyleYoon, J. H., Park, S. H., Yoon, S. E., Hong, S. Y., Lee, J. B., Lee, J., & Cho, J. Y. (2023). Hydrangea serrata Hot Water Extract and Its Major Ingredient Hydrangenol Improve Skin Moisturization and Wrinkle Conditions via AP-1 and Akt/PI3K Pathway Upregulation. Nutrients, 15(11), 2436. https://doi.org/10.3390/nu15112436








