Blueberries Improve Abdominal Symptoms, Well-Being and Functioning in Patients with Functional Gastrointestinal Disorders
Abstract
1. Introduction
2. Materials and Methods
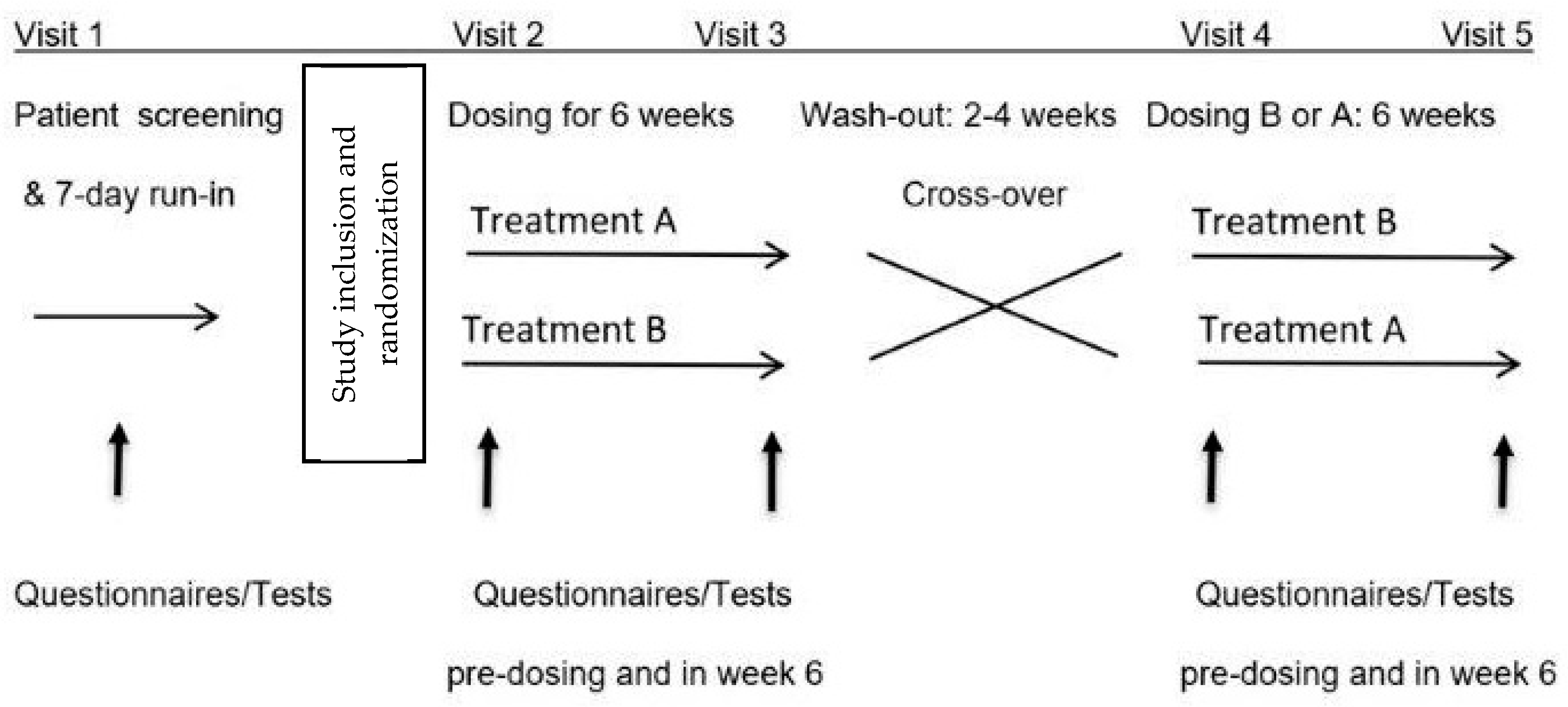
2.1. Design
2.2. Patients
2.3. Study Procedures
2.4. Questionnaires, Tests, and Biological Samples
2.5. Primary Outcome Variables
2.6. Secondary Outcome Variables
- Bristol Stool Scale (BSS). The proportion of patients with normal stool consistency was compared.
- Fructose breath tests. Hydrogen and methane breath concentrations were measured before, 1 and 2 h following ingestion of 35 g fructose dissolved in 300 mL tap water (Quintron BreathTracker SC®, Quintron Instruments, Milwaukee, Brookfield, WI, USA). The following GI and extra-GI symptoms were scored hourly and rated for intensity (none = 0, mild = 1, intense = 2) concurrently with the collection of the breath samples: abdominal pain, arthralgia, bloating, borborygmi, diarrhea, diminished concentration, epigastric pain/heartburn, flatulence, fullness, headache, myalgia, nausea, and tiredness [5,31]. The fructose test was performed in accordance with previous studies [5,31].
2.7. Treatments and Blinding
2.8. Statistics
2.9. Ethics
3. Results
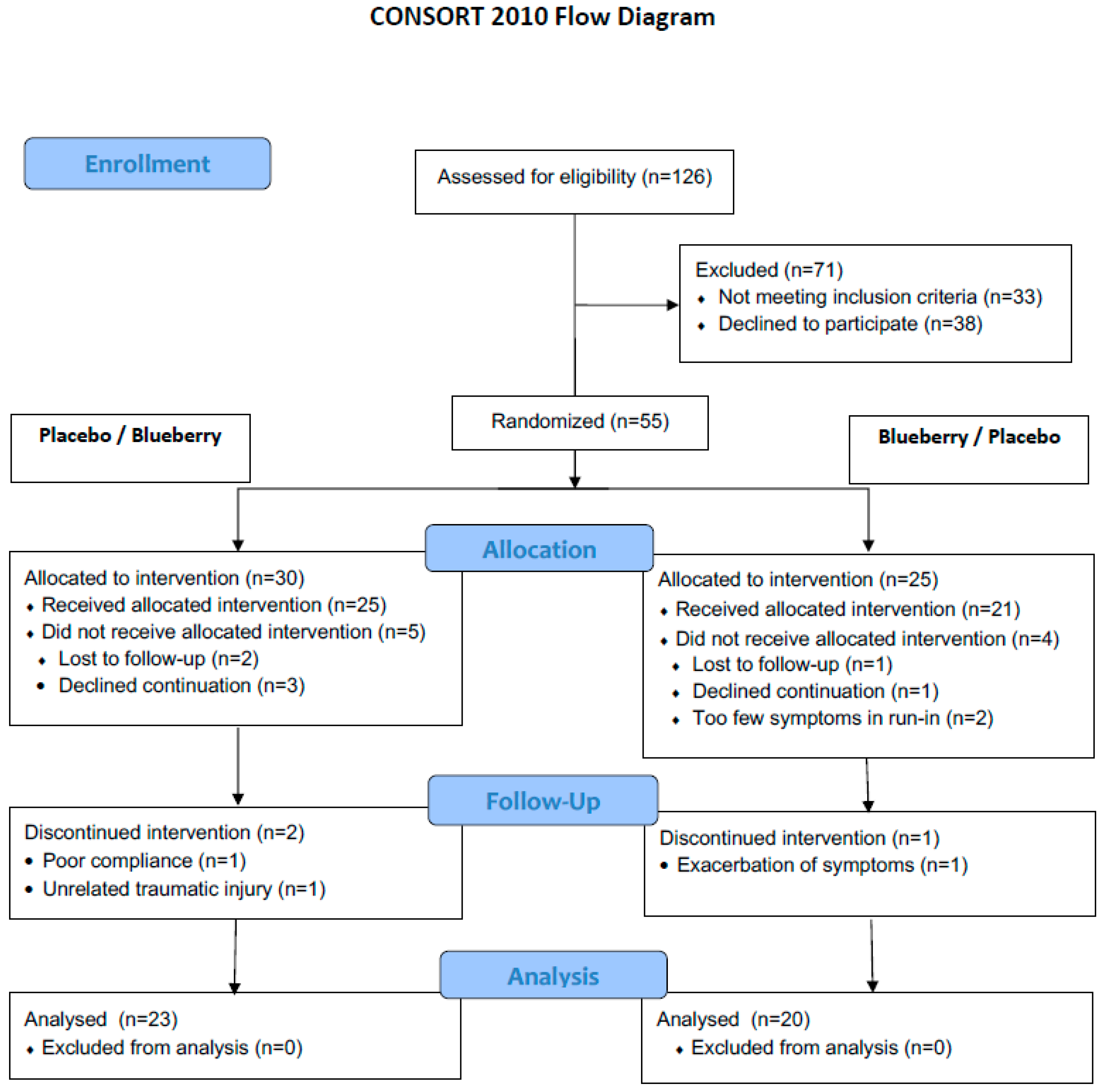
3.1. Treatment Compliance
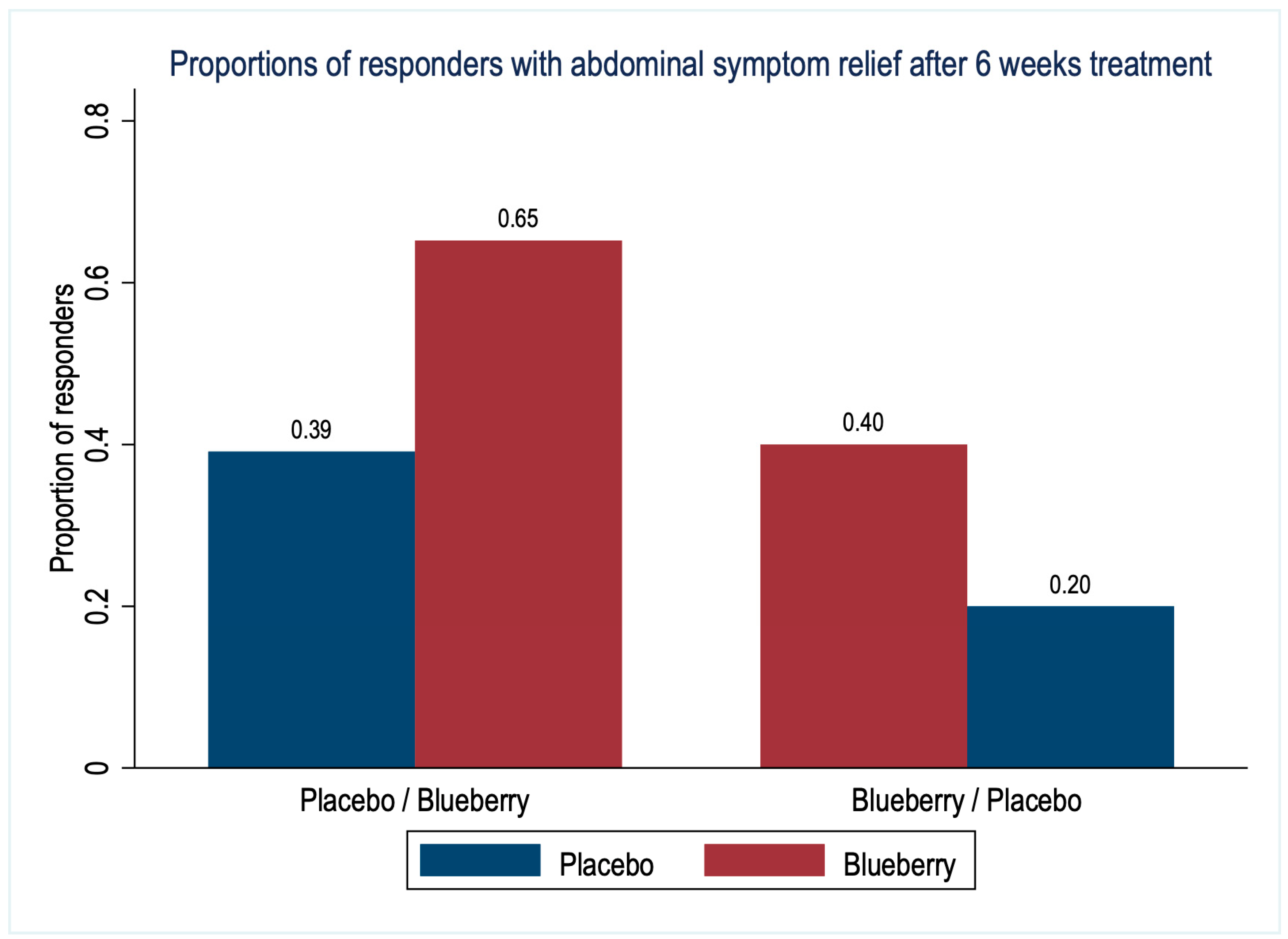
3.2. Primary Outcomes
3.3. Secondary Outcomes
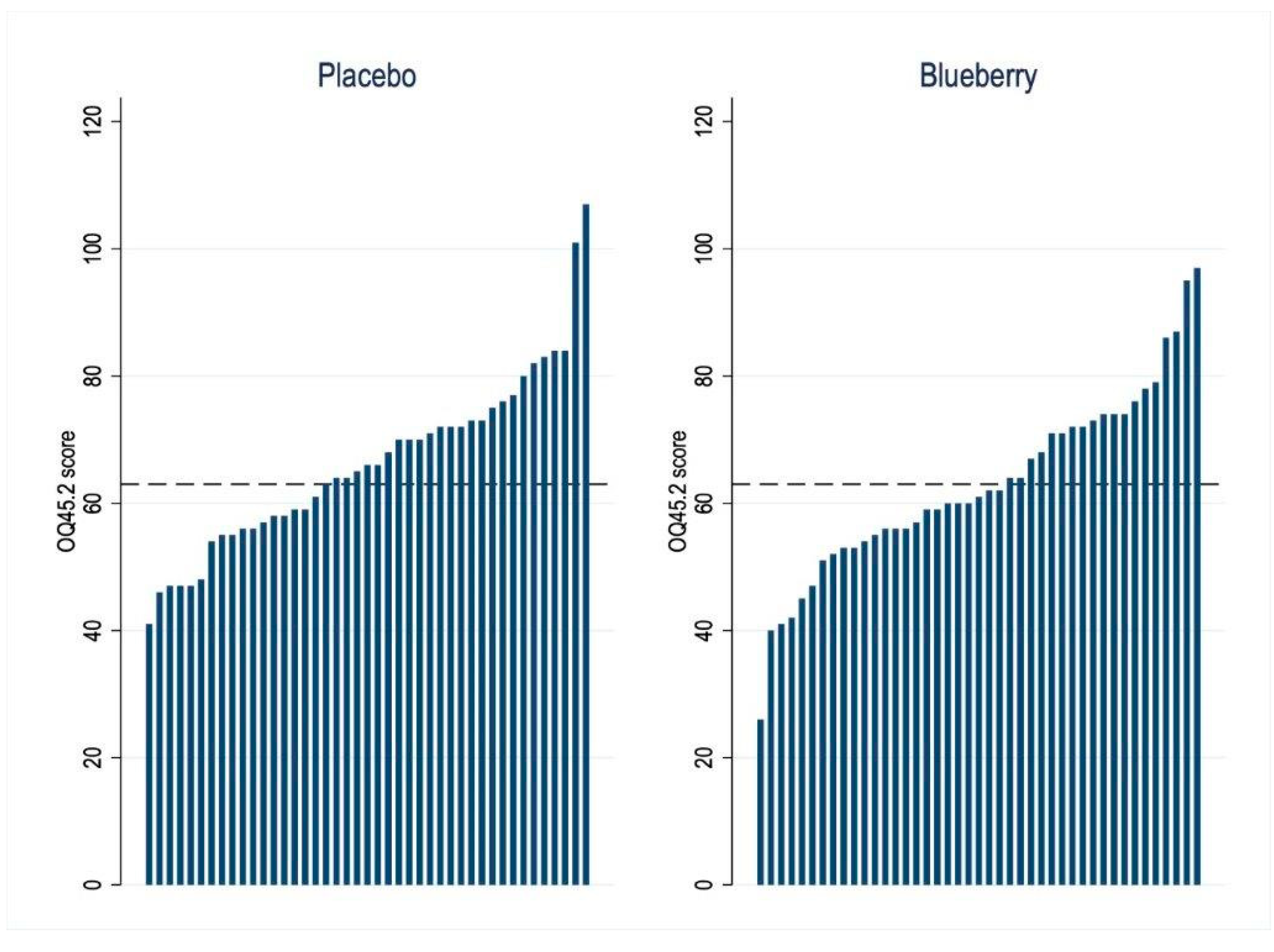
3.4. Quality of Life and Life Functioning Outcome
3.5. Adverse Events
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | area-under-the-curve |
| DGBI | Disorders of gut–brain interaction |
| FD | Functional dyspepsia |
| FGID | Functional gastrointestinal disorders |
| GI | Gastrointestinal |
| GSRS | Gastrointestinal Symptom Rating Scale |
| HADS | Hospital Anxiety and Depression Scale |
| IBS | Irritable bowel syndrome |
| IPAQ | International Physical Activity Questionnaire |
| PHQ-15 | Patient Health Questionnaire-15 |
| OQ45.2 | Outcome Questionnaire 45.2 |
| STAI-S/T | State-Trait Anxiety Inventory, -S: State, -T: Trait |
References
- Koppen, I.J.; Vriesman, M.H.; Saps, M.; Rajindrajith, S.; Shi, X.; van Etten-Jamaludin, F.S.; Di Lorenzo, C.; Benninga, M.A.; Tabbers, M.M. Prevalence of Functional Defecation Disorders in Children: A Systematic Review and Meta-Analysis. J. Pediatr. 2018, 198, 121–130.e6. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV—Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Spiller, R. Irritable bowel syndrome: New insights into symptom mechanisms and advances in treatment. F1000Research 2016, 5, 780–789. [Google Scholar] [CrossRef]
- Spiller, R. Impact of Diet on Symptoms of the Irritable Bowel Syndrome. Nutrients 2021, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, C.H.; Olesen, S.S.; Materna, A.; Drewes, A.M. Fermentable Sugar Ingestion, Gas Production, and Gastrointestinal and Central Nervous System Symptoms in Patients with Functional Disorders. Gastroenterology 2018, 155, 1034–1044.e6. [Google Scholar] [CrossRef] [PubMed]
- Sperber, A.D.; Dekel, R. Irritable Bowel Syndrome and co-morbid gastrointestinal and extra-gastrointestinal functional syn-dromes. J. Neurogastroenterol. Motil. 2010, 16, 113–119. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; Jonkers, D.M.; Salonen, A.; Hanevik, K.; Raes, J.; Jalanka, J.; De Vos, W.M.; Manichanh, C.; Golic, N.; Enck, P.; et al. Intestinal microbiota and diet in IBS: Causes, consequences, or epiphe-nomena? Am. J. Gastroenterol. 2015, 110, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Yu, L.W.; Agirman, G.; Hsiao, E.Y. The Gut Microbiome as a Regulator of the Neuroimmune Landscape. Annu. Rev. Immunol. 2022, 40, 143–167. [Google Scholar] [CrossRef]
- O’Keefe, S.J. Diet, microorganisms and their metabolites, and colon cancer. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 691–706. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. Int. Rev. J. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Sun, Z.; Zeng, Y.; Luo, M.; Yang, J. Molecular Mechanism and Health Role of Functional Ingredients in Blueberry for Chronic Disease in Human Beings. Int. J. Mol. Sci. 2018, 19, 2785. [Google Scholar] [CrossRef]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Berry Phenolic Antioxidants—Implications for Human Health? Front. Pharmacol. 2018, 26, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Travica, N.; D’Cunha, N.M.; Naumovski, N.; Kent, K.; Mellor, D.D.; Firth, J.; Georgousopoulou, E.N.; Dean, O.M.; Loughman, A.; Jacka, F.; et al. The effect of blueberry interventions on cognitive performance and mood: A systematic review of randomized controlled trials. Brain, Behav. Immun. 2019, 85, 96–105. [Google Scholar] [CrossRef]
- Cutler, B.R.; Petersen, C.; Babu, P.V.A. Mechanistic insights into the vascular effects of blueberries: Evidence from recent studies. Mol. Nutr. Food Res. 2017, 61, 1600271. [Google Scholar] [CrossRef]
- Lee, S.; Keirsey, K.I.; Kirkland, R.; Grunewald, Z.I.; Fischer, J.G.; De La Serre, C.B. Blueberry Supplementation Influences the Gut Microbiota, Inflammation, and Insulin Resistance in High-Fat-Diet–Fed Rats. J. Nutr. 2018, 148, 209–219. [Google Scholar] [CrossRef]
- Osman, N.; Adawi, D.; Ahrné, S.; Jeppsson, B.; Molin, G. Probiotics and Blueberry Attenuate the Severity of Dextran Sulfate Sodium (DSS)-Induced Colitis. Dig. Dis. Sci. 2008, 53, 2464–2473. [Google Scholar] [CrossRef]
- Pervin, M.; Hasnat, A.; Lim, J.-H.; Lee, Y.-M.; Kim, E.O.; Um, B.-H.; Lim, B.O. Preventive and therapeutic effects of blueberry (Vaccinium corymbosum) extract against DSS-induced ulcerative colitis by regulation of antioxidant and inflammatory mediators. J. Nutr. Biochem. 2016, 28, 103–113. [Google Scholar] [CrossRef]
- Shukitt-Hale, B. Blueberries and Neuronal Aging. Gerontology 2012, 58, 518–523. [Google Scholar] [CrossRef]
- Schmulson, M.J.; Drossman, D.A. What is new in Rome IV? J. Neurogastroenterol. Motil. 2017, 23, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, S.K.; Weidner, K.J.; Hoppner, J.; Becker, N.; Friedrich, D.; Stokes, C.; Lammert, F.; Köllner, V. Design and validation of a German version of the GSRS-IBS—An analysis of its psychometric quality and factorial structure. BMC Gastroenterol. 2017, 17, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Kulich, K.R.; Madisch, A.; Pacini, F.; Piqué, J.M.; Regula, J.; Van Rensburg, C.J.; Újszászy, L.; Carlsson, J.; Halling, K.; Wiklund, I.K. Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: A six-country study. Health Qual. Life Outcomes 2008, 6, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Spielberger, C.D. Manual for the State-Trait Anxiety Inventory STAI; Mind Garden: Palo Alto, CA, USA, 1983. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-15: Validity of a New Measure for Evaluating the Severity of Somatic Symptoms. Psychosom. Med. 2002, 64, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.L.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Guidance for Industry Irritable Bowel Syndrome—Clinical Evaluation of Drugs for Treatment. U.S. Department of Health and Human Services Food and Drug Administration. Available online: https://www.fda.gov/downloads/Drugs/Guidances/UCM205269.pdf (accessed on 4 April 2023).
- Lambert, M.J.; Burlingame, G.M.; Umphress, V.; Hansen, N.B.; Vermeersch, D.A.; Clouse, G.C.; Yanchar, S.C. The reliability and validity of the Outcome Questionnaire. Clin. Psychol. Psychother. 1996, 3, 249–258. [Google Scholar] [CrossRef]
- Crameri, A.; Schuetz, C.; Andreae, A.; Koemeda, M.; Schulthess, P.; Tschuschke, V.; von Wyl, A. The Brief Symptom Inventory and the Outcome Questionnaire-45 in the Assessment of the Outcome Quality of Mental Health Interventions. Psychiatry J. 2016, 2016, 7830785. [Google Scholar] [CrossRef]
- Wilder-Smith, C.H.; Materna, A.; Wermelinger, C.; Schuler, J. Fructose and lactose intolerance and malabsorption testing: The relationship with symptoms in functional gastrointestinal disorders. Aliment. Pharmacol. Ther. 2013, 37, 1074–1083. [Google Scholar] [CrossRef]
- Taylor, A.M.; Phillips, K.; Patel, K.V.; Turk, D.C.; Dworkin, R.H.; Beaton, D.; Clauw, D.J.; Gignac, M.A.; Markman, J.D.; Williams, D.A.; et al. Assessment of physical function and participation in chronic pain clinical trials: IMMPACT/OMERACT recommendations. Pain 2016, 157, 1836–1850. [Google Scholar] [CrossRef]
- Guidance for Industry Irritable Bowel Syndrome—Clinical Evaluation of Drugs for Treatment. U.S. Department of Health and Human Services Food and Drug Administration. Available online: https://www.fda.gov/media/78622/download (accessed on 3 April 2023).
- Beckstead, D.J.; Hatch, A.L.; Lambert, M.J.; Eggett, D.L.; Goates, M.K.; Vermeersch, D.A. Clinical significance of the Outcome Questionnaire (OQ-45.2). Behav. Anal. Today 2003, 4, 86–97. [Google Scholar] [CrossRef]
- Mendonça, C.R.; Noll, M.; Castro, M.C.R.; Silveira, E.A. Effects of Nutritional Interventions in the Control of Musculoskeletal Pain: An Integrative Review. Nutrients 2020, 12, 3075. [Google Scholar] [CrossRef] [PubMed]
- Torri, E.; Lemos, M.; Caliari, V.; Kassuya, C.A.; Bastos, J.K.; Andrade, S.F. Anti-inflammatory and antinociceptive properties of blue-berry extract. J. Pharm. Pharmacol. 2007, 59, 591–596. [Google Scholar] [CrossRef]
- Ntemiri, A.; Ghosh, T.S.; Gheller, M.E.; Tran, T.T.; Blum, J.E.; Pellanda, P.; Vlckova, K.; Neto, M.C.; Howell, A.; Thalacker-Mercer, A.; et al. Whole Blueberry and Isolated Polyphenol-Rich Fractions Modulate Specific Gut Mi-crobes in an In Vitro Colon Model and in a Pilot Study in Human Consumers. Nutrients 2020, 12, 2800. [Google Scholar] [CrossRef] [PubMed]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-Week Consumption of a Wild Blueberry Powder Drink Increases Bifidobacteria in the Human Gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Chen, Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Nickerson, K.P.; Chanin, R.; McDonald, C. Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes 2015, 6, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Kaden-Volynets, V.; Rosa, L.F.; Guseva, D.; Seethaler, B. Regulation of the gut barrier by carbohydrates from diet—Underlying mechanisms and possible clinical implications. Int. J. Med. Microbiol. 2021, 311, 151499. [Google Scholar] [CrossRef]
- Yu, X.; Gurry, T.; Nguyen, L.T.T.; Richardson, H.S.; Alm, E.J. Prebiotics and Community Composition Influence Gas Production of the Human Gut Microbiota. mBio 2020, 11, e00217-20. [Google Scholar] [CrossRef]
- Pimentel, M.; Mathur, R.; Chang, C. Gas and the microbiome. Curr. Gastroenterol. Rep. 2013, 15, 356–362. [Google Scholar] [CrossRef]
- Major, G.; Pritchard, S.; Murray, K.; Alappadan, J.P.; Hoad, C.L.; Marciani, L.; Gowland, P.; Spiller, R. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produc-es Carbohydrate-Related Symptoms in Individuals With Irritable Bowel Syndrome. Gastroenterology 2017, 152, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Castelli, V.; Grassi, D.; Bocale, R.; d’Angelo, M.; Antonosante, A.; Cimini, A.; Ferri, C.; Desideri, G. Diet and Brain Health: Which Role for Polyphenols? Curr. Pharm. Des. 2018, 24, 227–238. [Google Scholar] [CrossRef] [PubMed]
- de Vries, K.; Medawar, E.; Korosi, A.; Witte, A.V. The Effect of Polyphenols on Working and Episodic Memory in Non-pathological and Pathological Aging: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 8, 720756. [Google Scholar] [CrossRef]
- Bonyadi, N.; Dolatkhah, N.; Salekzamani, Y.; Hashemian, M. Effect of berry-based supplements and foods on cognitive function: A systematic review. Sci. Rep. 2022, 12, 3239–3251. [Google Scholar] [CrossRef] [PubMed]
- Wellek, S.; Blettner, M. On the Proper Use of the Crossover Design in Clinical Trials. Dtsch. Arztebl. Int. 2012, 109, 276–281. [Google Scholar] [CrossRef]
| Sequence Placebo/Blueberry n = 23 | Sequence Blueberry/Placebo n = 20 | p-Value of Comparison | |
|---|---|---|---|
| Female, n (%) | 20 (87) | 17 (85) | 1.00 |
| Male, n (%) | 3 (13) | 3 (15) | 1.00 |
| Age, years ǂ | 31.3 ± 11.6 | 30.8 ± 9.0 | 0.87 |
| BMI (kg/m2) ǂ | 23.6 ± 4.2 | 21.9 ± 3.2 | 0.14 |
| IBS, n (%) * | 20 (87) | 16 (80) | 0.69 |
| FD, n (%) * | 18 (78) | 16 (80) | 1.00 |
| Smoker, n (%) | 3 (13) | 2 (10) | 1.00 |
| Food preference, n (%) | |||
| -Omnivore | 15 (65) | 15 (75) | 0.75 |
| -Vegetarian | 7 (30) | 4 (20) | |
| -Vegan | 1 (4) | 1 (5) | |
| STAI-S ǂ | 47.1 ± 3.6 | 46.2 ± 4.6 | 0.44 |
| STAI-T ǂ | 45.5 ± 6.4 | 46.5 ± 5.8 | 0.62 |
| HADS depression score ǂ | 10.3 ± 10.0 | 9.4 ± 5.1 | 0.70 |
| PHQ-15 ǂ | 5.3 ± 4.8 | 4.8 ± 2.8 | 0.68 |
| IPHQ, n (%) | |||
| -low | 5 (23) | 3 (15) | 0.70 |
| -moderate | 16 (73) | 17 (85) | |
| -high | 1 (5) | 0 |
| Outcome Variable | Sequence | n | Value after 6 Weeks’ Treatment Means ± SD | Blueberry vs. Placebo Means (95% CI) | Ptreatment | Pcarry-over | Pperiod | |
|---|---|---|---|---|---|---|---|---|
| Period 1 | Period 2 | |||||||
| Overall GI symptoms GSRS total | Placebo/Blueberry | 23 | 2.7 ± 1.0 | 2.3 ± 0.9 | −0.3 (−0.6 to 0.0) | 0.09 | 0.15 | 0.32 |
| Blueberry/Placebo | 20 | 2.8 ± 1.0 | 2.9 ± 0.9 | |||||
| Bloating GSRS | Placebo/Blueberry | 23 | 3.3 ± 1.3 | 3.1 ± 1.5 | −0.2 (−0.7 to 0.3) | 0.49 | 0.13 | 0.80 |
| Blueberry/Placebo | 20 | 3.6 ± 1.5 | 3.9 ± 1.4 | |||||
| Diarrhea GSRS | Placebo/Blueberry | 23 | 2.4 ± 1.5 | 1.8 ± 0.9 | −0.2 (−0.7 to 0.2) | 0.34 | 0.26 | 0.08 |
| Blueberry/Placebo | 20 | 1.9 ± 0.8 | 1.7 ± 1.0 | |||||
| Constipation GSRS | Placebo/Blueberry | 23 | 2.3 ± 1.1 | 1.9 ± 0.8 | −0.3 (−0.7 to 0.2) | 0.19 | 0.06 | 0.68 |
| Blueberry/Placebo | 20 | 2.5 ± 1.1 | 2.7 ± 1.3 | |||||
| Abdominal pain GSRS | Placebo/Blueberry | 23 | 2.9 ± 1.3 | 2.5 ±1.2 | −0.3 (−0.6 to 0.0) | 0.08 | 0.26 | 0.18 |
| Blueberry/Placebo | 20 | 3.1 ± 1.3 | 3.1 ± 1.3 | |||||
| Functioning & QOL OQ 45.2 | Placebo/Blueberry | 23 | 64.5 ± 13.0 | 58.9 ± 15.4 | −3.2 (−5.6 to −0.7) | 0.01 | 0.13 | 0.05 |
| Blueberry/Placebo | 20 | 67.8 ± 12.5 | 68.4 ± 15.2 | |||||
| Fructose breath test—GI symptoms (AUC × 2 h) | Placebo/Blueberry | 23 | 2.5 ± 3.1 | 2.5 ± 3.1 | −0.1 (−0.9 to 0.7) | 0.85 | 0.91 | 0.80 |
| Blueberry/Placebo | 20 | 2.4 ± 3.1 | 2.5 ± 3.0 | |||||
| Fructose breath test—CNS symptoms (AUC × 2 h) | Placebo/Blueberry | 23 | 0.9 ± 1.3 | 1.2 ± 1.3 | 0.2 (−0.3 to 0.7) | 0.47 | 0.89 | 0.47 |
| Blueberry/Placebo | 20 | 1.1 ± 2.0 | 1.1 ± 1.7 | |||||
| Fructose breath test—hydrogen (AUC concentration ppm × 2 h) | Placebo/Blueberry | 23 | 28.0 ± 34.7 | 25.2 ± 34.5 | −6.6 (−15.3 to 2.1) | 0.14 | 0.21 | 0.38 |
| Blueberry/Placebo | 20 | 35.3 ± 38.8 | 45.7 ± 44.5 | |||||
| Fructose breath test—methane (AUC concentration ppm × 2 h) | Placebo/Blueberry | 23 | 3.3 ± 3.9 | 3.0 ± 4.9 | −1.1 (−2.5 to 0.3) | 0.13 | 0.04 | 0.25 |
| Blueberry/Placebo | 20 | 5.1 ± 5.0 | 7.0 ± 6.4 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilder-Smith, C.H.; Materna, A.; Olesen, S.S. Blueberries Improve Abdominal Symptoms, Well-Being and Functioning in Patients with Functional Gastrointestinal Disorders. Nutrients 2023, 15, 2396. https://doi.org/10.3390/nu15102396
Wilder-Smith CH, Materna A, Olesen SS. Blueberries Improve Abdominal Symptoms, Well-Being and Functioning in Patients with Functional Gastrointestinal Disorders. Nutrients. 2023; 15(10):2396. https://doi.org/10.3390/nu15102396
Chicago/Turabian StyleWilder-Smith, Clive H., Andrea Materna, and Søren S. Olesen. 2023. "Blueberries Improve Abdominal Symptoms, Well-Being and Functioning in Patients with Functional Gastrointestinal Disorders" Nutrients 15, no. 10: 2396. https://doi.org/10.3390/nu15102396
APA StyleWilder-Smith, C. H., Materna, A., & Olesen, S. S. (2023). Blueberries Improve Abdominal Symptoms, Well-Being and Functioning in Patients with Functional Gastrointestinal Disorders. Nutrients, 15(10), 2396. https://doi.org/10.3390/nu15102396






