Effects of a Low-FODMAP Diet on Irritable Bowel Syndrome in Both Children and Adults—A Narrative Review
Abstract
1. Introduction
2. Materials and Methods
3. Irritable Bowel Syndrome
3.1. Overview
3.2. Pathophysiology
3.3. Diagnosis
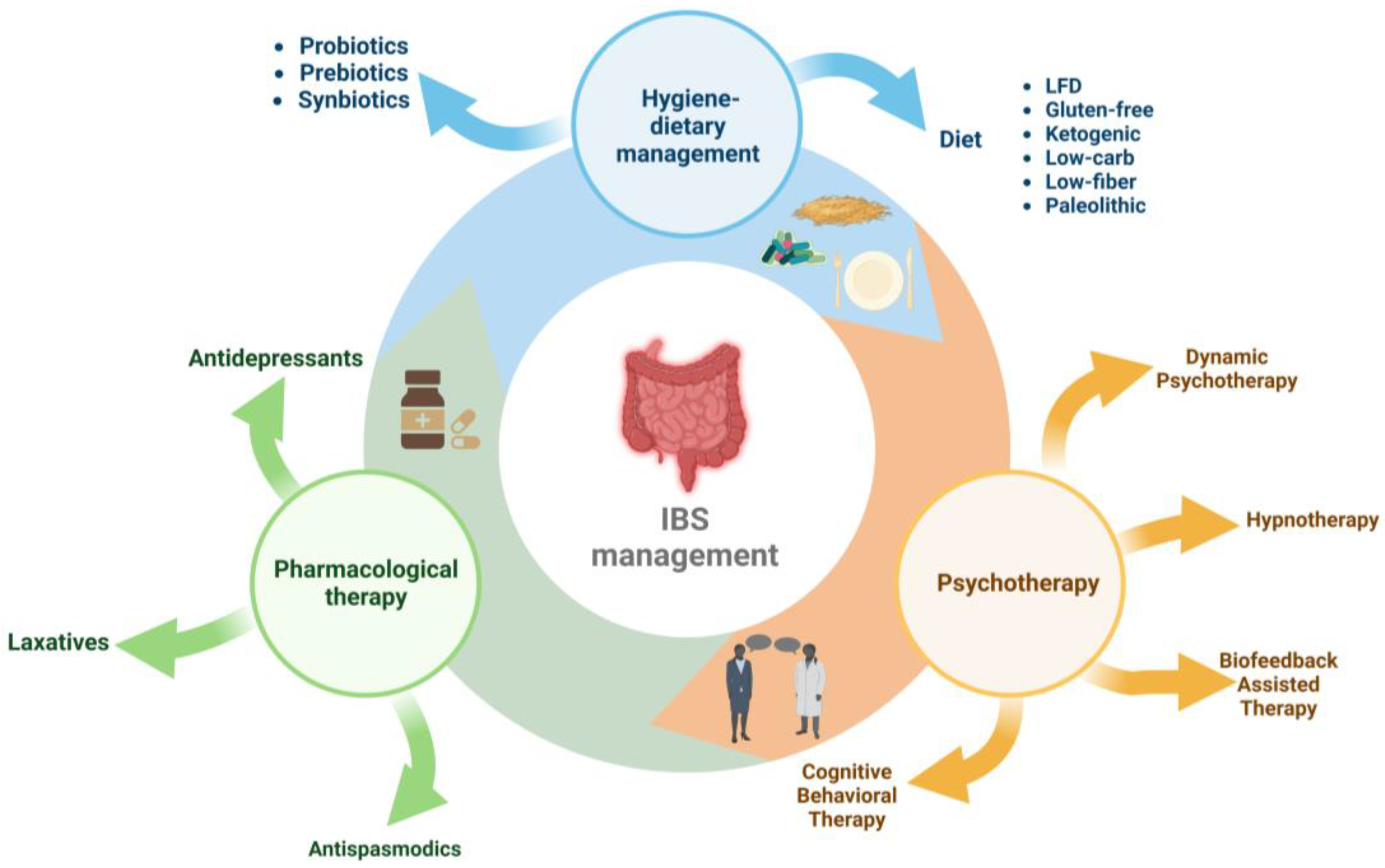
3.4. Therapeutic and Nutritional Management
4. Low-FODMAP Diet
5. Results and Discussion
5.1. Results and Discussion in Adults
5.1.1. Effects on Global Symptoms, Abdominal Pain, and Bloating in Adults
5.1.2. Effects on Quality of Life in Adults
5.1.3. Effects on Bowel Water Content in Adults
5.1.4. Effects on Biochemical Markers of Disease Activity in Adults
5.1.5. Effects on Nutrient Intake in Adults
5.2. Results and Discussion in Children
5.2.1. Effects on Global Symptoms, Abdominal Pain, and Bloating in Children
5.2.2. Effects on Quality of Life in Children
5.2.3. Effects on Bowel Water Content in Children
5.2.4. Effects on Biochemical Markers of Disease Activity in Children
5.2.5. Effects on Nutrient Intake in Children
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional Bowel Disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Brusaferro, A.; Farinelli, E.; Zenzeri, L.; Cozzali, R.; Esposito, S. The Management of Paediatric Functional Abdominal Pain Disorders: Latest Evidence. Pediatr. Drugs 2018, 20, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Chang, L. Review Article: Epidemiology and Quality of Life in Functional Gastrointestinal Disorders. Aliment. Pharmacol. Ther. 2004, 20, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Maxion-Bergemann, S.; Thielecke, F.; Abel, F.; Bergemann, R. Costs of Irritable Bowel Syndrome in the UK and US. Pharmacoeconomics 2006, 24, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Müller-Lissner, S.A.; Bollani, S.; Brummer, R.-J.; Coremans, G.; Dapoigny, M.; Marshall, J.K.; Muris, J.W.M.; Oberndorff-Klein Wolthuis, A.; Pace, F.; Rodrigo, L.; et al. Epidemiological Aspects of Irritable Bowel Syndrome in Europe and North America. Digestion 2001, 64, 200–204. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; Jonkers, D.M.; Salonen, A.; Hanevik, K.; Raes, J.; Jalanka, J.; de Vos, W.M.; Manichanh, C.; Golic, N.; Enck, P.; et al. Intestinal Microbiota and Diet in IBS: Causes, Consequences, or Epiphenomena? Am. J. Gastroenterol. 2015, 110, 278–287. [Google Scholar] [CrossRef]
- Shaikh, S.D.; Sun, N.; Canakis, A.; Park, W.Y.; Weber, H.C. Irritable Bowel Syndrome and the Gut Microbiome: A Comprehensive Review. J. Clin. Med. 2023, 12, 2558. [Google Scholar] [CrossRef]
- Radovanovic-Dinic, B.; Tesic-Rajkovic, S.; Grgov, S.; Petrovic, G.; Zivkovic, V. Irritable Bowel Syndrome—From Etiopathogenesis to Therapy. Biomed. Pap. 2018, 162, 1–9. [Google Scholar] [CrossRef]
- Camilleri, M.; Boeckxstaens, G. Dietary and Pharmacological Treatment of Abdominal Pain in IBS. Gut 2017, 66, 966–974. [Google Scholar] [CrossRef]
- Barrett, J.S.; Gearry, R.B.; Muir, J.G.; Irving, P.M.; Rose, R.; Rosella, O.; Haines, M.L.; Shepherd, S.J.; Gibson, P.R. Dietary Poorly Absorbed, Short-Chain Carbohydrates Increase Delivery of Water and Fermentable Substrates to the Proximal Colon. Aliment. Pharmacol. Ther. 2010, 31, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Varney, J.; Malakar, S.; Muir, J.G. Food Components and Irritable Bowel Syndrome. Gastroenterology 2015, 148, 1158–1174.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, P.; Zhang, L.; Hou, X. A Low-FODMAP Diet Improves the Global Symptoms and Bowel Habits of Adult IBS Patients: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 683191. [Google Scholar] [CrossRef]
- Morariu, I.D.; Chirila, I.; Avasilcai, L.; Panainte, A.D.; Vieriu, M.; Bibire, N.; Drug, V.L. Impact of Nutritional Supplements and Food for Weight Reduction on Body Composition of Adults. Rev. Cercet. Interv. Soc. 2018, 62, 140–150. [Google Scholar]
- Farré, R.; Tack, J. Food and Symptom Generation in Functional Gastrointestinal Disorders: Physiological Aspects. Am. J. Gastroenterol. 2013, 108, 698–706. [Google Scholar] [CrossRef]
- Agarwal, N.; Spiegel, B.M.R. The Effect of Irritable Bowel Syndrome on Health-Related Quality of Life and Health Care Expenditures. Gastroenterol. Clin. N. Am. 2011, 40, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Reznikov, E.A.; Suskind, D.L. Current Nutritional Therapies in Inflammatory Bowel Disease: Improving Clinical Remission Rates and Sustainability of Long-Term Dietary Therapies. Nutrients 2023, 15, 668. [Google Scholar] [CrossRef]
- Andrews, E.B.; Eaton, S.C.; Hollis, K.A.; Hopkins, J.S.; Ameen, V.; Hamm, L.R.; Cook, S.F.; Tennis, P.; Mangel, A.W. Prevalence and Demographics of Irritable Bowel Syndrome: Results from a Large Web-Based Survey. Aliment. Pharmacol. Ther. 2005, 22, 935–942. [Google Scholar] [CrossRef]
- Lovell, R.M.; Ford, A.C. Global Prevalence of and Risk Factors for Irritable Bowel Syndrome: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef]
- Testa, A.; Imperatore, N.; Rispo, A.; Rea, M.; Tortora, R.; Nardone, O.M.; Lucci, L.; Accarino, G.; Caporaso, N.; Castiglione, F. Beyond Irritable Bowel Syndrome: The Efficacy of the Low Fodmap Diet for Improving Symptoms in Inflammatory Bowel Diseases and Celiac Disease. Dig. Dis. 2018, 36, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Rhys-Jones, D.; Varney, J.E.; Muir, J.G.; Gibson, P.R.; Halmos, E.P. Application of The FODMAP Diet in a Paediatric Setting. Nutrients 2022, 14, 4369. [Google Scholar] [CrossRef] [PubMed]
- Vasant, D.H.; Paine, P.A.; Black, C.J.; Houghton, L.A.; Everitt, H.A.; Corsetti, M.; Agrawal, A.; Aziz, I.; Farmer, A.D.; Eugenicos, M.P.; et al. British Society of Gastroenterology Guidelines on the Management of Irritable Bowel Syndrome. Gut 2021, 70, 1214–1240. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.P.; Chin, V.K.; Looi, C.Y.; Wong, W.F.; Madhavan, P.; Yong, V.C. The Microbiome and Irritable Bowel Syndrome—A Review on the Pathophysiology, Current Research and Future Therapy. Front. Microbiol. 2019, 10, 1136. [Google Scholar] [CrossRef]
- Holtmann, G.; Shah, A.; Morrison, M. Pathophysiology of Functional Gastrointestinal Disorders: A Holistic Overview. Dig. Dis. 2017, 35, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Manabe, N.; Tanaka, T.; Hata, J.; Kusunoki, H.; Haruma, K. Pathophysiology Underlying Irritable Bowel Syndrome -From the Viewpoint of Dysfunction of Autonomic Nervous System Activity-. J. Smooth Muscle Res. 2009, 45, 15–23. [Google Scholar] [CrossRef]
- Lacy, B.E.; Pimentel, M.; Brenner, D.M.; Chey, W.D.; Keefer, L.A.; Long, M.D.; Moshiree, B. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 17–44. [Google Scholar] [CrossRef]
- Ancona, A.; Petito, C.; Iavarone, I.; Petito, V.; Galasso, L.; Leonetti, A.; Turchini, L.; Belella, D.; Ferrarrese, D.; Addolorato, G.; et al. The Gut–Brain Axis in Irritable Bowel Syndrome and Inflammatory Bowel Disease. Dig. Liver Dis. 2021, 53, 298–305. [Google Scholar] [CrossRef]
- Mamieva, Z.; Poluektova, E.; Svistushkin, V.; Sobolev, V.; Shifrin, O.; Guarner, F.; Ivashkin, V. Antibiotics, Gut Microbiota, and Irritable Bowel Syndrome: What Are the Relations? World J. Gastroenterol. 2022, 28, 1204–1219. [Google Scholar] [CrossRef]
- Singh, R.; Salem, A.; Nanavati, J.; Mullin, G.E. The Role of Diet in the Treatment of Irritable Bowel Syndrome. Gastroenterol. Clin. N. Am. 2018, 47, 107–137. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Simrén, M. Nutrient Intake in Patients with Irritable Bowel Syndrome Compared with the General Population. Neurogastroenterol. Motil. 2013, 25, 23–30.e1. [Google Scholar] [CrossRef]
- Chirila, I.; Drug, V.L.; Morariu, I.D. Food Related to Functional Digestive Disorders in Working Age Adults. Neurogastroenterol. Motil. 2017, 29, 133–134. [Google Scholar]
- Simpson, C.A.; Mu, A.; Haslam, N.; Schwartz, O.S.; Simmons, J.G. Feeling down? A Systematic Review of the Gut Microbiota in Anxiety/Depression and Irritable Bowel Syndrome. J. Affect. Disord. 2020, 266, 429–446. [Google Scholar] [CrossRef]
- Shiha, M.G.; Aziz, I. Review Article: Physical and Psychological Comorbidities Associated with Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2021, 54, S12–S23. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Shackelford, K. Irritable Bowel Syndrome; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Black, C.J. Review Article: Diagnosis and Investigation of Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2021, 54, S33–S43. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, J.; Bullock, I. Diagnosis and Management of Irritable Bowel Syndrome in Adults in Primary Care: Summary of NICE Guidance. BMJ 2008, 336, 556–558. [Google Scholar] [CrossRef]
- Nakov, R.; Snegarova, V.; Dimitrova-Yurukova, D.; Velikova, T. Biomarkers in Irritable Bowel Syndrome: Biological Rationale and Diagnostic Value. Dig. Dis. 2022, 40, 23–32. [Google Scholar] [CrossRef]
- Linedale, E.C.; Andrews, J.M. Diagnosis and Management of Irritable Bowel Syndrome: A Guide for the Generalist. Med. J. Aust. 2017, 207, 309–315. [Google Scholar] [CrossRef]
- van Rheenen, P.F.; Van de Vijver, E.; Fidler, V. Faecal Calprotectin for Screening of Patients with Suspected Inflammatory Bowel Disease: Diagnostic Meta-Analysis. BMJ 2010, 341, c3369. [Google Scholar] [CrossRef]
- Bonetto, S.; Fagoonee, S.; Battaglia, E.; Grassini, M.; Saracco, G.M.; Pellicano, R. Recent Advances in the Treatment of Irritable Bowel Syndrome. Pol. Arch. Intern. Med. 2021, 131, 709–715. [Google Scholar] [CrossRef]
- Chirila, I.; Morariu, I.D.; Barboi, O.B.; Mihai, C.; Cijevschi-Prelipcean, C.; Drug, V.L. The Role of Diet in the Gastro-Esophageal Reflux and Dyspepsia Overlap. Eur. J. Clin. Investig. 2015, 45, 33. [Google Scholar]
- Chirila, I.; Morariu, I.D.; Barboi, O.B.; Drug, V.L. The Role of Diet in the Overlap between Gastroesophageal Reflux Disease and Functional Dyspepsia. Turk. J. Gastroenterol. 2016, 27, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Boeckxstaens, G. Irritable Bowel Syndrome: Treatment Based on Pathophysiology and Biomarkers. Gut 2023, 72, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Exarchopoulou, K.; Papageorgiou, A.; Bacopoulou, F.; Koumantarou Malisiova, E.; Vlachakis, D.; Chrousos, G.P.; Darviri, C. A Biofeedback-Assisted Stress Management Program for Patients with Irritable Bowel Syndrome: A Randomised Controlled Trial. EMBnet J. 2021, 26, e980. [Google Scholar] [CrossRef]
- Galica, A.N.; Galica, R.; Dumitrașcu, D.L. Diet, Fibers, and Probiotics for Irritable Bowel Syndrome. J. Med. Life 2022, 15, 174–179. [Google Scholar] [CrossRef]
- Moayyedi, P.; Simrén, M.; Bercik, P. Evidence-Based and Mechanistic Insights into Exclusion Diets for IBS. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.; Eslick, E.M.; Eslick, G.D. Does a Diet Low in FODMAPs Reduce Symptoms Associated with Functional Gastrointestinal Disorders? A Comprehensive Systematic Review and Meta-Analysis. Eur. J. Nutr. 2016, 55, 897–906. [Google Scholar] [CrossRef]
- Altobelli, E.; Del Negro, V.; Angeletti, P.; Latella, G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients 2017, 9, 940. [Google Scholar] [CrossRef]
- Black, C.J.; Staudacher, H.M.; Ford, A.C. Efficacy of a Low FODMAP Diet in Irritable Bowel Syndrome: Systematic Review and Network Meta-Analysis. Gut 2022, 71, 1117–1126. [Google Scholar] [CrossRef]
- Zhan, Y.; Zhan, Y.; Dai, S. Is a Low FODMAP Diet Beneficial for Patients with Inflammatory Bowel Disease? A Meta-Analysis and Systematic Review. Clin. Nutr. 2018, 37, 123–129. [Google Scholar] [CrossRef]
- Ispiryan, L.; Zannini, E.; Arendt, E.K. FODMAP Modulation as a Dietary Therapy for IBS: Scientific and Market Perspective. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1491–1516. [Google Scholar] [CrossRef]
- Valeur, J.; Røseth, A.G.; Knudsen, T.; Malmstrøm, G.H.; Fiennes, J.T.; Midtvedt, T.; Berstad, A. Fecal Fermentation in Irritable Bowel Syndrome: Influence of Dietary Restriction of Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols. Digestion 2016, 94, 50–56. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Lomer, M.C.E.; Gibson, P.R. Short-Chain Carbohydrates and Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2013, 108, 707–717. [Google Scholar] [CrossRef]
- Varney, J.; Barrett, J.; Scarlata, K.; Catsos, P.; Gibson, P.R.; Muir, J.G. FODMAPs: Food Composition, Defining Cutoff Values and International Application. J. Gastroenterol. Hepatol. 2017, 32, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Hayes, P.; Corish, C.; O’Mahony, E.; Quigley, E.M.M. A Dietary Survey of Patients with Irritable Bowel Syndrome. J. Hum. Nutr. Diet. 2014, 27, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Grez, C.; Vega, Á.; Araya, M. Consumo de Mono, Di, Oligo Sacáridos y Polioles Fermentables (FODMAPs), Una Nueva Fuente de Sintomatología Gastrointestinal. Rev. Med. Chile 2019, 147, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Baranguán Castro, M.L.; Ros Arnal, I.; García Romero, R.; Rodríguez Martínez, G.; Ubalde Sainz, E. Implantación de La Dieta Baja En FODMAP Para El Dolor Abdominal Funcional. An. Pediatr. 2019, 90, 180–186. [Google Scholar] [CrossRef]
- San Mauro Martín, I.; Garicano Vilar, E.; López Oliva, S.; Sanz Rojo, S. Existing Differences between Available Lists of FODMAP Containing Foods. Rev. Española Enferm. Dig. 2022. [Google Scholar] [CrossRef]
- Sultan, N.; Varney, J.E.; Halmos, E.P.; Biesiekierski, J.R.; Yao, C.K.; Muir, J.G.; Gibson, P.R.; Tuck, C.J. How to Implement the 3-Phase FODMAP Diet Into Gastroenterological Practice. J. Neurogastroenterol. Motil. 2022, 28, 343–356. [Google Scholar] [CrossRef]
- Ankersen, D.V.; Weimers, P.; Bennedsen, M.; Haaber, A.B.; Fjordside, E.L.; Beber, M.E.; Lieven, C.; Saboori, S.; Vad, N.; Rannem, T.; et al. Long-Term Effects of a Web-Based Low-FODMAP Diet Versus Probiotic Treatment for Irritable Bowel Syndrome, Including Shotgun Analyses of Microbiota: Randomized, Double-Crossover Clinical Trial. J. Med. Internet Res. 2021, 23, e30291. [Google Scholar] [CrossRef]
- Bodini, G.; Zanella, C.; Crespi, M.; Lo Pumo, S.; Demarzo, M.G.; Savarino, E.; Savarino, V.; Giannini, E.G. A Randomized, 6-Wk Trial of a Low FODMAP Diet in Patients with Inflammatory Bowel Disease. Nutrition 2019, 67–68, 110542. [Google Scholar] [CrossRef]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome as Well as Traditional Dietary Advice: A Randomized Controlled Trial. Gastroenterology 2015, 149, 1399–1407.e2. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Dolan, R.D.; Ball, S.C.; Jackson, K.; Chey, W. The Impact of a 4-Week Low-FODMAP and MNICE Diet on Nutrient Intake in a Sample of US Adults with Irritable Bowel Syndrome with Diarrhea. J. Acad. Nutr. Diet. 2020, 120, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Krieger-Grübel, C.; Hutter, S.; Hiestand, M.; Brenner, I.; Güsewell, S.; Borovicka, J. Treatment Efficacy of a Low FODMAP Diet Compared to a Low Lactose Diet in IBS Patients: A Randomized, Cross-over Designed Study. Clin. Nutr. ESPEN 2020, 40, 83–89. [Google Scholar] [CrossRef]
- Guerreiro, M.M.; Santos, Z.; Carolino, E.; Correa, J.; Cravo, M.; Augusto, F.; Chagas, C.; Guerreiro, C.S. Effectiveness of Two Dietary Approaches on the Quality of Life and Gastrointestinal Symptoms of Individuals with Irritable Bowel Syndrome. J. Clin. Med. 2020, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of Varying Dietary Content of Fermentable Short-Chain Carbohydrates on Symptoms, Fecal Microenvironment, and Cytokine Profiles in Patients with Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2017, 29, e12969. [Google Scholar] [CrossRef]
- McIntosh, K.; Reed, D.E.; Schneider, T.; Dang, F.; Keshteli, A.H.; De Palma, G.; Madsen, K.; Bercik, P.; Vanner, S. FODMAPs Alter Symptoms and the Metabolome of Patients with IBS: A Randomised Controlled Trial. Gut 2017, 66, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Menees, S.B.; Jackson, K.; Baker, J.R.; Fenner, D.E.; Eswaran, S.; Nojkov, B.; Saad, R.; Lee, A.A.; Chey, W.D. A Randomized Pilot Study to Compare the Effectiveness of a Low FODMAP Diet vs Psyllium in Patients with Fecal Incontinence and Loose Stools. Clin. Transl. Gastroenterol. 2022, 13, e00454. [Google Scholar] [CrossRef]
- Naseri, K.; Dabiri, H.; Rostami-Nejad, M.; Yadegar, A.; Houri, H.; Olfatifar, M.; Sadeghi, A.; Saadati, S.; Ciacci, C.; Iovino, P.; et al. Influence of Low FODMAP-Gluten Free Diet on Gut Microbiota Alterations and Symptom Severity in Iranian Patients with Irritable Bowel Syndrome. BMC Gastroenterol. 2021, 21, 292. [Google Scholar] [CrossRef]
- Patcharatrakul, T.; Juntrapirat, A.; Lakananurak, N.; Gonlachanvit, S. Effect of Structural Individual Low-FODMAP Dietary Advice vs. Brief Advice on a Commonly Recommended Diet on IBS Symptoms and Intestinal Gas Production. Nutrients 2019, 11, 2856. [Google Scholar] [CrossRef]
- Pedersen, N.; Ankersen, D.V.; Felding, M.; Wachmann, H.; Végh, Z.; Molzen, L.; Burisch, J.; Andersen, J.R.; Munkholm, P. Low-FODMAP Diet Reduces Irritable Bowel Symptoms in Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 2017, 23, 3356–3366. [Google Scholar] [CrossRef]
- Tuck, C.J.; Reed, D.E.; Muir, J.G.; Vanner, S.J. Implementation of the Low FODMAP Diet in Functional Gastrointestinal Symptoms: A Real-world Experience. Neurogastroenterol. Motil. 2020, 32, e13730. [Google Scholar] [CrossRef]
- Wong, Z.; Mok, C.-Z.; Majid, H.A.; Mahadeva, S. Early Experience with a Low FODMAP Diet in Asian Patients with Irritable Bowel Syndrome. JGH Open 2018, 2, 178–181. [Google Scholar] [CrossRef]
- Zahedi, M.J.; Behrouz, V.; Azimi, M. Low Fermentable Oligo-Di-Mono-Saccharides and Polyols Diet versus General Dietary Advice in Patients with Diarrhea-Predominant Irritable Bowel Syndrome: A Randomized Controlled Trial. J. Gastroenterol. Hepatol. 2018, 33, 1192–1199. [Google Scholar] [CrossRef]
- van Lanen, A.-S.; de Bree, A.; Greyling, A. Efficacy of a Low-FODMAP Diet in Adult Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Eur. J. Nutr. 2021, 60, 3505–3522. [Google Scholar] [CrossRef]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in Inflammatory Bowel Disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef]
- Więcek, M.; Panufnik, P.; Kaniewska, M.; Lewandowski, K.; Rydzewska, G. Low-FODMAP Diet for the Management of Irritable Bowel Syndrome in Remission of IBD. Nutrients 2022, 14, 4562. [Google Scholar] [CrossRef]
- Staudacher, H.M.; Ralph, F.S.E.; Irving, P.M.; Whelan, K.; Lomer, M.C.E. Nutrient Intake, Diet Quality, and Diet Diversity in Irritable Bowel Syndrome and the Impact of the Low FODMAP Diet. J. Acad. Nutr. Diet. 2020, 120, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Boradyn, K.M.; Jarocka-Cyrta, E.; Przybyłowicz, K.E.; Obara-Gołębiowska, M. Parental Opinion about the Low FODMAP Diet in Dietary Treatment of Children with Functional Abdominal Pain. Int. J. Environ. Res. Public Health 2020, 17, 5554. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Irving, P.M.; Lomer, M.C.E.; Whelan, K. Mechanisms and Efficacy of Dietary FODMAP Restriction in IBS. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Masuy, I.; Biesiekierski, J.R.; Fitzke, H.E.; Parikh, C.; Schofield, L.; Shaikh, H.; Bhagwanani, A.; Aziz, Q.; Taylor, S.A.; et al. Gut-brain Axis Dysfunction Underlies FODMAP-induced Symptom Generation in Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2022, 55, 670–682. [Google Scholar] [CrossRef]
- Murray, K.; Wilkinson-Smith, V.; Hoad, C.; Costigan, C.; Cox, E.; Lam, C.; Marciani, L.; Gowland, P.; Spiller, R.C. Differential Effects of FODMAPs (Fermentable Oligo-, Di-, Mono-Saccharides and Polyols) on Small and Large Intestinal Contents in Healthy Subjects Shown by MRI. Am. J. Gastroenterol. 2014, 109, 110–119. [Google Scholar] [CrossRef]
- Hillilä, M.T.; Färkkilä, N.J.; Färkkilä, M.A. Societal Costs for Irritable Bowel Syndrome—A Population Based Study. Scand. J. Gastroenterol. 2010, 45, 582–591. [Google Scholar] [CrossRef]
- Sebastián Domingo, J.J.; Sánchez Sánchez, C. La Dieta Baja En FODMAP, ¿es Realmente Eficaz y Segura En El Síndrome Del Intestino Irritable?: Una Revisión Panorámica. Med. Fam. SEMERGEN 2020, 46, 566–576. [Google Scholar] [CrossRef]
- El Gendy, Y.G.A.; Abdel Wahed, M.A.; Ragab, M.H.H.; Awad, Y.M.M. Effects of a Low-Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyol Diet on Symptoms of Functional Abdominal Pain in Pediatric Patients. Pediatr. Gastroenterol. Hepatol. Nutr. 2022, 25, 510–518. [Google Scholar] [CrossRef]
- Joishy, M.; Davies, I.; Ahmed, M.; Wassel, J.; Davies, K.; Sayers, A.; Jenkins, H. Fecal Calprotectin and Lactoferrin as Noninvasive Markers of Pediatric Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Nogay, N.H.; Walton, J.; Roberts, K.M.; Nahikian-Nelms, M.; Witwer, A.N. The Effect of the Low FODMAP Diet on Gastrointestinal Symptoms, Behavioral Problems and Nutrient Intake in Children with Autism Spectrum Disorder: A Randomized Controlled Pilot Trial. J. Autism Dev. Disord. 2021, 51, 2800–2811. [Google Scholar] [CrossRef]
- Chiou, E.; Nurko, S. Functional Abdominal Pain and Irritable Bowel Syndrome in Children and Adolescents. Therapy 2011, 8, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Rexwinkel, R.; Vlieger, A.M.; Saps, M.; Tabbers, M.M.; Benninga, M.A. A Therapeutic Guide on Pediatric Irritable Bowel Syndrome and Functional Abdominal Pain-Not Otherwise Specified. Eur. J. Pediatr. 2022, 181, 2603–2617. [Google Scholar] [CrossRef]
- Devanarayana, N.M.; Rajindrajith, S. Irritable Bowel Syndrome in Children: Current Knowledge, Challenges and Opportunities. World J. Gastroenterol. 2018, 24, 2211–2235. [Google Scholar] [CrossRef]
- Adams, H.L.; Basude, D.; Kyle, A.; Sandmann, S.; Paul, S.P. Managing Irritable Bowel Syndrome in Children. Nurs. Stand. 2016, 31, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Popa, S.L.; Pop, C.; Dumitrascu, D.L. Diet Advice for Crohn’s Disease: FODMAP and Beyond. Nutrients 2020, 12, 3751. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.J.; Pettei, M.J.; Valderrama, E.; Gold, D.M.; Kessler, B.H.; Trachtman, H. Nitric Oxide and Inflammatory Bowel Disease: Evidence for Local Intestinal Production in Children with Active Colonic Disease. J. Pediatr. Gastroenterol. Nutr. 1998, 26, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Fodor, I.; Man, S.C.; Dumitrascu, D.L. Low Fermentable Oligosaccharides, Disaccharides, Monosaccharides, and Polyols Diet in Children. World J. Clin. Cases 2019, 7, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
| Food Products | High-FODMAP Content | Low-FODMAP Content |
|---|---|---|
| vegetables | asparagus, garlic, onions, broccoli, green peas, sugar snap peas, mushrooms, cabbage | capsicum, carrot, corn, cucumber, eggplant, green beans, lettuce, pumpkin, tomato, zucchini |
| fruits | apples, pears, mangos, watermelon, nectarines, peaches, plums, dried fruits | orange, mandarin, grapes, blueberries, lemon, kiwi, banana, strawberries |
| dairy and alternatives | milk (cow, goat, sheep), condensed milk, yoghurt, cream, ice cream, cheese (fresh), soy milk | lactose-free milk, almond/rice milk, lactose-free yogurts, ripened cheese, peanut butter, hard cheese, camembert/brie cheese |
| bread and cereals | rye, wheat-containing bread, wheat-based cereals with dried fruit, wheat pasta, breakfast cereals | rice, quinoa, gluten-free bread, gluten-free pasta, sourdough, spelt bread |
| nuts and seeds | pistachios and cashews | peanuts, walnuts, pumpkin seeds |
| Author | Type | Size of the Study | Study Characteristics | Conclusions |
|---|---|---|---|---|
| Ankersen et al. [60] | RCT | n = 29 | Adults diagnosed with IBS according to Rome IV criteria. Comparing LFD with a moderate FODMAP diet. Exclusion criteria: patients with previous GI surgery, cardiovascular, liver, psychiatric, and neurological diseases, and other GI disease; patients with allergies or intolerance to food; and patients who used antibiotics within a month before the start of the trial. | LFD decreased the intensity of GI symptoms, including less frequent and firmer stool, when compared with moderate portions of the FODMAP diet. LFD seemed more helpful for IBS patients (IBS-D/IBS-M) with frequent loose stools than those with IBS-C. |
| Bodini et al. [61] | RCT | n = 127 | RCT with adults diagnosed with IBS, according to Rome IV criteria, compares LFD with SD. Exclusion criteria: patients with moderate to severe disease, patients with previous GI surgery, and patients with coeliac disease, diabetes, and lactose intolerance. | The study highlighted the impact of LFD on the treatment of IBS and other intestinal diseases by evaluating some intestinal inflammatory markers (fCal and CRP dose in the beginning and after 6 weeks of the nutrition plan). A decrease in faecal biomarkers was observed, which was also associated with improvements in QoL. |
| Bohn et al. [62] | RCT | n = 67 | RCT with adults diagnosed with IBS according to Rome III criteria compared LFD with NICE. Exclusion criteria: patients with cardiac, neurological, liver, psychiatric, or IBD. | The study showed that offering food guidance to patients with IBS in a medical environment helped improve GI symptoms; however, there were no obvious distinctions between LFD and NICE, as both reduced IBS symptoms. |
| Eswaran et al. [63] | RCT | n = 84 | RCT with adults diagnosed with IBS-D according to Rome III criteria, compared LFD with mNICE. Exclusion criteria: patients with IBS-C, GI diseases, IBD, patients with previous GI surgery, pregnant patients, and patients using antibiotics or narcotics within a month before the beginning of the trial. | During a 4-week nutritional intervention, the low-FODMAP diet significantly exceeded the mNICE diet to improve disease-specific QoL across all dimensions of the IBS-QoL questionnaire, except eliminating food. Following the introduction of LFD, a decrease in the average daily consumption of some micronutrients was observed, although there were no changes in the amount of energy consumed. Therefore, LFD was not immediately associated with significant nutritional deficits. |
| Grubel et al. [64] | RCT | n = 39 | RCT with adults diagnosed with IBS, according to Rome IV criteria, which compared LFD with a low-lactose diet. Exclusion criteria: patients with coeliac disease, patients with food allergies, and patients using laxatives, antidiarrheal agents, and antibiotics. | LFD was associated with significantly fewer IBS symptoms than a low-lactose diet, highlighting the susceptibility of short-chain carbohydrates to poor digestion. That improvement was also due to the advice of the dietitian. Pain severity/frequency, bloating, and stool habits had better subscores when following an LFD. |
| Guerroiro et al. [65] | RCT | n = 70 | A clinical trial with adult patients with IBS according to Rome IV criteria. Comparing LFD with SD. Exclusion criteria: patients with previous GI diseases and surgery, patients using antibiotics, prebiotics, and probiotics within a month before the start of the trial. | The global symptom frequency scores of both groups decreased significantly compared with baseline. However, the LFD group had a greater decrease in magnitude. LFD has been suggested to be more efficient than SD in reducing pain and diarrhoea. Although SD decreased the frequency of constipation, there were no statistically significant differences between the diets. Furthermore, the overall score for QoL increased significantly in both groups compared with baseline, with no statistically significant differences between the groups. |
| Hustoft et al. [66] | RCT | n = 20 | A clinical trial with adult patients with IBS-D or IBD-M according to Rome III criteria, comparing LFD with FOS. Exclusion criteria: patients with IBS-C, pregnant women, and patients using probiotics or antibiotics. | In patients diagnosed with IBS-D or IBS-M, LFD was best at decreasing functional GI symptoms, and significantly more participants had symptom relief in response to a placebo (80%) than FOS (30%). |
| McIntosh et al. [67] | RCT | n = 37 | According to Rome III criteria, a clinical trial of adult patients with IBS compares LFD with a high-FODMAP diet. Exclusion criteria: patients with previous GI surgery, patients using antibiotics, stool bulking agents, narcotics, or lactulose. | After 3 weeks, comparing patients diagnosed with IBS who received LFD with those who received a high-FODMAP diet, an overall decrease in GI symptoms was observed. |
| Menees et al. [68] | RCT | n = 43 | According to Rome III criteria, adults diagnosed with IBS compare the effectiveness of an LFD vs. psyllium. Exclusion criteria: patients with dementia, diabetes, scleroderma, IBD, renal and hepatic disease, patients with previous GI surgery, and patients using antibiotics, prebiotics, probiotics, or narcotics. | The proportion of patients who reported a decrease of 50% in global symptoms was comparable for both groups. The psyllium group revealed a greater improvement in overall symptoms, but the LFD group reported a better QoL and stool consistency. |
| Naseri et al. [69] | RCT | n = 42 | According to Rome IV criteria, adults diagnosed with IBS associated LFD with GFD. Exclusion criteria: patients with coeliac disease, IBD, liver disease, patients with precedent GI surgery, cancer, and patients using NSAIDs and drinking alcohol. | IBS patients who ingested LFD with GFD saw a substantial decrease in IBS symptoms and an adjustment of their gut microbiome. Intestinal inflammation can be reduced by association, which decreases IBS-SSS. |
| Patcharatrakul et al. [70] | RCT | n = 62 | Adults diagnosed with IBS according to Rome III criteria, with moderate to severe GI symptoms, comparing LFD with BRD. Exclusion criteria: patients with previous GI surgery; coeliac disease; GI cancers; severe cardiovascular, liver, lung, neurological or mental diseases; and patients who used antibiotics, prebiotics, probiotics, or symbiotics within a month before the start of the study. | Compared with the BRD diet, the LFD proved its efficiency in decreasing VAS values. Following the LFD intervention, abdominal discomfort and bloating decreased considerably from their baseline values compared with those who received BRD. After both approaches, there were no significant improvements in belching or stool urgency. |
| Pederson et al. [71] | RCT | n = 123 | A clinical trial of adult patients with IBS according to Rome III criteria, comparing LFD with ND. Exclusion criteria: pregnant women, patients with GI surgery. | After 6 weeks of dietary intervention, patients who followed LFD compared with ND had a significant reduction in the IBS-SSS average. |
| Tuck et al. [72] | RCT | n = 80 | A questionnaire was used to gather information about how LFD impacts patients with IBS. | Half of the patients reported an improvement in GI symptoms, but many did not reach the therapeutic level of FODMAP intake level, especially in the absence of the diet physician’s guidance. |
| Wong et al. [73] | RCT | n = 16 | Adults diagnosed with IBS according to Rome III criteria analyse the impact of LFD in Asian patients. Exclusion criteria: patients with frequent organic diseases (cancer and inflammatory bowel disease). | 11 of 16 patients (68.8%) reported an improvement in their general symptoms, which were classified in the following order: abdominal pain (60%), bloating / distension (70%), and flatulence (87.5%). |
| Zahedi et al. [74] | RCT | n = 101 | According to Rome III criteria, the study involved the clinical response in patients with IBS-D after LFD vs. GDA. Exclusion criteria: patients with coeliac disease; IBD; cardiovascular, liver, kidney, and neurological diseases; diabetes; and thyroid disorders. | After six weeks, patients with IBS-D had a satisfactory reduction in GI symptoms with both LFD and GDA. However, LFD had greater benefits in improving IBS, such as a reduction in the severity, frequency, and status of abdominal pain and abdominal distention. However, in contrast with the GDA group, LFD did not affect quality of life. |
| Author | Type | Size of Study | Study Characteristics | Conclusions |
|---|---|---|---|---|
| Boradyn et al. [79] | RCT | n = 29 | RCT with a parenteral opinion about LFD on children (age: 5–12 years) diagnosed with FAP, according to Rome III criteria. Exclusion criteria: patients with organic GI disorders, patients with food allergies, patients with acute infection, and patients with antibiotics, within two months of starting the study. | The effectiveness of LFD was evaluated after 4 weeks of dietary intervention based on parents’ opinions on the intensity of their children’s abdominal pain. LFD and BDA/NICE diets required the supervision of a paediatric dietician to obtain an effective result in children, thus avoiding nutritional deficiencies. |
| El Gendy et al. [85] | RCT | n = 50 | RCT evaluated the effects of LFD in children (age: 3–18 years) diagnosed with FAP, according to Rome IV. Exclusion criteria: patients with a family history of IBD, coeliac disease, peptic ulcer disease, dysphagia, vomiting, blood loss, odynophagia, diarrhea, arthritis, and weight loss. | After 2 months of LFD intervention, a decrease in pain intensity was observed in 74% of the patients, as well as an increase in quality of life, without detrimental effects on body weight. |
| Joishy et al. [86] | RCT | n = 74 | The RCT evaluates faecal calprotectin and lactoferrin in children (age 4–17 years) with IBD. | Faecal calprotectin and lactoferrin were evaluated as highly precise and non-invasive indicators for the preliminary identification of inflammatory bowel disease (IBD), Crohn’s disease, and ulcerative colitis in children. They could help distinguish between IBD and other non-inflammatory bowel diseases such as IBS. |
| Nogay et al. [87] | RCT | n = 15 | RCT evaluating the effect of LFD in children (age: 6–17 years) with ASD together with IBS according to Rome IV. Exclusion criteria: patients with previous GI surgery, patients with IBD, cystic fibrosis, liver and cardiovascular disease, and patients using antibiotics. | After 2 weeks, the LFD intervention had benefits in children diagnosed with autism with abdominal pain and/or constipation, as it was effective in reducing constipation and other GI problems without affecting the intake of nutrients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morariu, I.-D.; Avasilcai, L.; Vieriu, M.; Lupu, V.V.; Morariu, B.-A.; Lupu, A.; Morariu, P.-C.; Pop, O.-L.; Starcea, I.M.; Trandafir, L. Effects of a Low-FODMAP Diet on Irritable Bowel Syndrome in Both Children and Adults—A Narrative Review. Nutrients 2023, 15, 2295. https://doi.org/10.3390/nu15102295
Morariu I-D, Avasilcai L, Vieriu M, Lupu VV, Morariu B-A, Lupu A, Morariu P-C, Pop O-L, Starcea IM, Trandafir L. Effects of a Low-FODMAP Diet on Irritable Bowel Syndrome in Both Children and Adults—A Narrative Review. Nutrients. 2023; 15(10):2295. https://doi.org/10.3390/nu15102295
Chicago/Turabian StyleMorariu, Ionela-Daniela, Liliana Avasilcai, Madalina Vieriu, Vasile Valeriu Lupu, Branco-Adrian Morariu, Ancuța Lupu, Paula-Cristina Morariu, Oana-Lelia Pop, Iuliana Magalena Starcea, and Laura Trandafir. 2023. "Effects of a Low-FODMAP Diet on Irritable Bowel Syndrome in Both Children and Adults—A Narrative Review" Nutrients 15, no. 10: 2295. https://doi.org/10.3390/nu15102295
APA StyleMorariu, I.-D., Avasilcai, L., Vieriu, M., Lupu, V. V., Morariu, B.-A., Lupu, A., Morariu, P.-C., Pop, O.-L., Starcea, I. M., & Trandafir, L. (2023). Effects of a Low-FODMAP Diet on Irritable Bowel Syndrome in Both Children and Adults—A Narrative Review. Nutrients, 15(10), 2295. https://doi.org/10.3390/nu15102295








