Association of Maternal Gestational Vitamin D Supplementation with Respiratory Health of Young Children
Abstract
1. Introduction
2. Methods
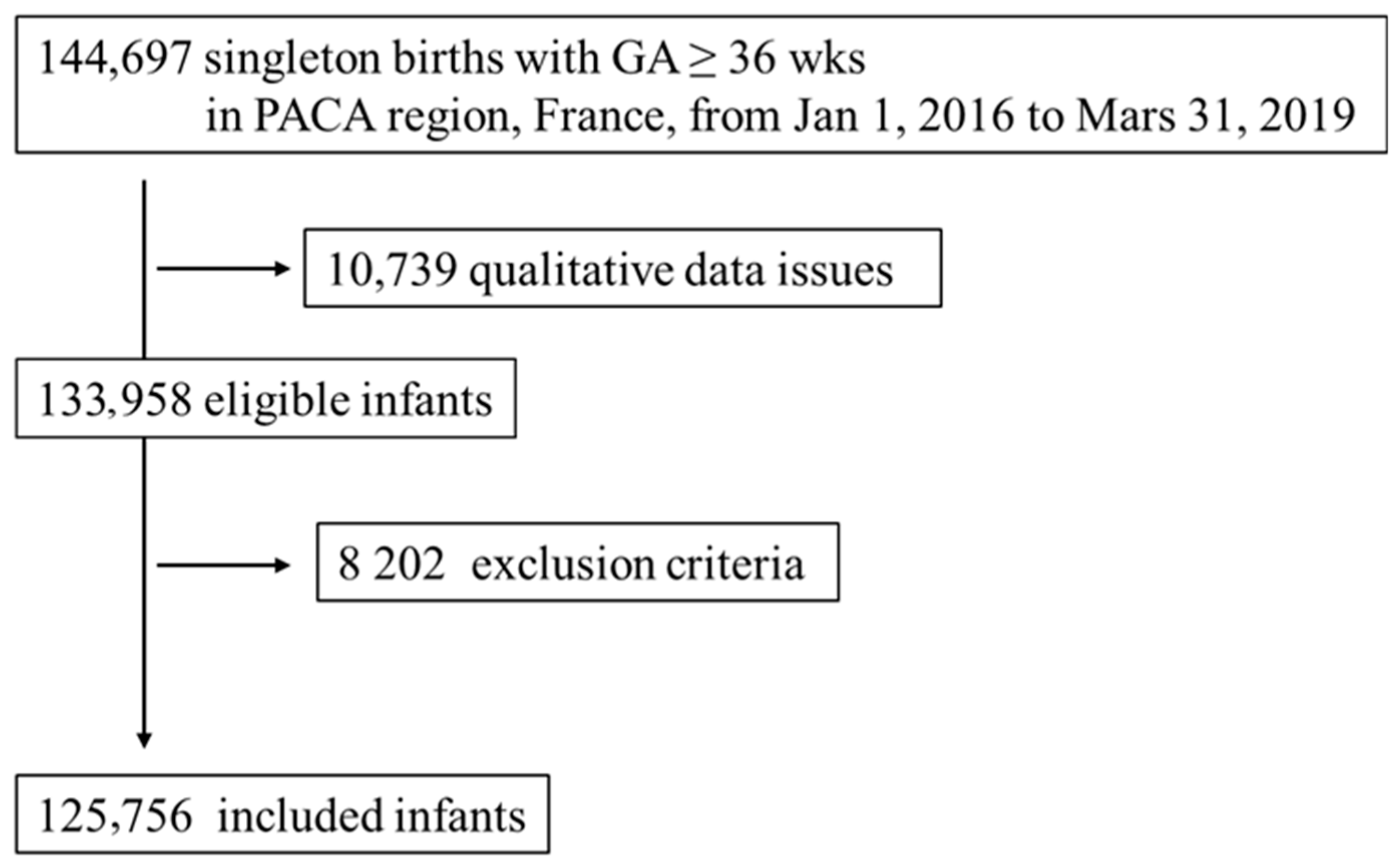
2.1. Study Design and Population
2.2. Primary Outcome
2.3. Maternal Gestational Vitamin D Supplementation
2.4. Maternal and Perinatal Data
2.5. Data Analysis
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Courbebaisse, M.; Souberbielle, J.-C.; Baptiste, A.; Taieb, J.; Tsatsaris, V.; Guibourdenche, J.; Senat, M.-V.; Haidar, H.; Jani, J.; Guizani, M.; et al. Vitamin D status during pregnancy and in cord blood in a large prospective French cohort. Clin. Nutr. 2019, 38, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.D.; Wagner, C.L.; Hulsey, T.C.; McNeil, R.B.; Ebeling, M.; Hollis, B.W. Vitamin D deficiency and insufficiency is common during pregnancy. Am. J. Perinatol. 2011, 28, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Kiely, M.; O’Donovan, S.M.; Kenny, L.C.; Hourihane, J.O.; Irvine, A.D.; Murray, D.M. Vitamin D metabolite concentrations in umbilical cord blood serum and associations with clinical characteristics in a large prospective mother-infant cohort in Ireland. J. Steroid. Biochem. Mol. Biol. 2017, 167, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Miliku, K.; Vinkhuyzen, A.; Blanken, L.M.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Hofman, A.; Tiemeier, H.; AP Steegers, E.; Gaillard, R.; et al. Maternal vitamin D concentrations during pregnancy, fetal growth patterns, and risks of adverse birth outcomes. Am. J. Clin. Nutr. 2016, 103, 1514–1522. [Google Scholar] [CrossRef]
- Sadeghian, M.; Asadi, M.; Rahmani, S.; Zanjani, M.A.; Sadeghi, O.; Hosseini, S.A.; Javid, A.Z. Circulating vitamin D and the risk of gestational diabetes: A systematic review and dose-response meta-analysis. Endocrine 2020, 70, 36–47. [Google Scholar] [CrossRef]
- Qin, L.L.; Lu, F.G.; Yang, S.H.; Xu, H.L.; Luo, B.A. Does maternal vitamin D deficiency increase the risk of preterm birth: A meta-analysis of observational studies. Nutrients 2016, 8, 301. [Google Scholar] [CrossRef]
- Karras, S.N.; Wagner, C.L.; Castracane, V.D. Understanding vitamin D metabolism in pregnancy: From physiology to pathophysiology and clinical outcomes. Metabolism 2018, 86, 112–123. [Google Scholar] [CrossRef]
- Lykkedegn, S.; Sorensen, G.L.; Beck-Nielsen, S.S.; Christesen, H.T. The impact of vitamin D on fetal and neonatal lung maturation. A systematic review. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L587–L602. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Wang, P.; Tan, Z.-X.; Fu, L.; Fan, Y.-J.; Luo, B.; Zhang, Z.-H.; Xu, S.; Chen, Y.-H.; Zhao, H.; Xu, D.-X. Gestational vitamin D deficiency impairs fetal lung development through suppressing type II pneumocyte differentiation. Reprod. Toxicol. 2020, 94, 40–47. [Google Scholar] [CrossRef]
- Thorsteinsdottir, F.; Cardoso, I.; Keller, A.; Stougaard, M.; Frederiksen, P.; Cohen, A.S.; Maslova, E.; Jacobsen, R.; Backer, V.; Heitmann, B.L. Neonatal Vitamin D Status and Risk of Asthma in Childhood: Results from the D-Tect Study. Nutrients 2020, 12, 842. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-González, R.M.; García-Marcos, L.; Morales, E. Prenatal vitamin D status and respiratory and allergic outcomes in childhood: A meta-analysis of observational studies. Pediatr. Allergy Immunol. 2018, 29, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Lucas, R.M.; Walsh, J.P.; Zosky, G.R.; Whitehouse, A.J.; Zhu, K.; Allen, K.L.; Kusel, M.M.; Anderson, D.; Mountain, J.A. Vitamin D in fetal development: Findings from a birth cohort study. Pediatrics 2015, 135, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-H.; Liao, S.-L.; Tsai, M.-H.; Hua, M.-C.; Chiu, C.-Y.; Yeh, K.-W.; Yao, T.-C.; Huang, J.-L. Low cord-serum 25-hydroxyvitamin D levels are associated with poor lung function performance and increased respiratory infection in infancy. PLoS ONE 2017, 12, e0173268. [Google Scholar] [CrossRef] [PubMed]
- Zosky, G.R.; Hart, P.H.; Whitehouse, A.J.O.; Kusel, M.M.; Ang, W.; Foong, R.E.; Chen, L.; Holt, P.G.; Sly, P.D.; Hall, G.L. Vitamin D deficiency at 16 to 20 weeks’ gestation is associated with impaired lung function and asthma at 6 years of age. Ann. Am. Thorac. Soc. 2014, 1, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Papalia, H.; Samonini, A.; Buffat, C.; Gras, E.; Robert, C.D.; Landrier, J.-F.; Pauly, V.; Boubred, F. Low Vitamin D Levels at Birth and Early Respiratory Outcome in Infants with Gestational Age Less Than 29 Weeks. Front. Pediatr. 2022, 9, 790839. [Google Scholar] [CrossRef]
- Litonjua, A.A.; Carey, V.J.; Laranjo, N.; Harshfield, B.J.; McElrath, T.F.; O’Connor, G.T.; Sandel, M.; Iverson, R.E.; Lee-Paritz, A.; Strunk, R.C.; et al. Effect of Prenatal Supplementation with Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA 2016, 315, 362–370. [Google Scholar] [CrossRef]
- Goldring, S.T.; Griffiths, C.J.; Martineau, A.R.; Robinson, S.; Yu, C.; Poulton, S.; Kirkby, J.C.; Stocks, J.; Hooper, R.; Shaheen, S.O.; et al. Prenatal vitamin d supplementation and child respiratory health: A randomised controlled trial. PLoS ONE 2013, 8, e66627. [Google Scholar] [CrossRef]
- Grant, C.C.; Crane, J.; Mitchell, E.A.; Sinclair, J.; Stewart, A.; Milne, T.; Knight, J.; Gilchrist, C.; Camargo, C.A., Jr. Vitamin D supplementation during pregnancy and infancy reduces aeroallergen sensitization: A randomized controlled trial. Allergy 2016, 71, 1325–1334. [Google Scholar] [CrossRef]
- Chawes, B.L.; Bønnelykke, K.; Stokholm, J.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.M.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Effect of Vitamin D3 Supplementation During Pregnancy on Risk of Persistent Wheeze in the Offspring: A Randomized Clinical Trial. JAMA 2016, 315, 353–361. [Google Scholar] [CrossRef]
- McAllister, D.A.; Liu, L.; Shi, T.; Chu, Y.; Reed, C.; Burrows, J.; Adeloye, D.; Rudan, I.; Black, R.E.; Campbell, H.; et al. Global, regional, and national estimates of pneumonia morbidity and mortality in children younger than 5 years between 2000 and 2015: A systematic analysis. Lancet Glob. Health 2019, 7, e47–e57. [Google Scholar] [CrossRef] [PubMed]
- van Meel, E.R.; Mensink-Bout, S.M.; Dekker, H.T.D.; Ahluwalia, T.S.; Annesi-Maesano, I.; Arshad, S.H.; Baïz, N.; Barros, H.; von Berg, A.; Bisgaard, H.; et al. Early-life respiratory tract infections and the risk of school-age lower lung function and asthma: A meta-analysis of 150 000 European children. Eur. Respir. J. 2022, 60, 2102395. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.A.L.; Upton, M.N.; Henderson, A.J.; Dedman, D.; McCarthy, A.; Smith, G.D.; Ben-Shlomo, Y. Lower respiratory tract infection in the first year of life is associated with worse lung function in adult life: Prospective results from the Barry Caerphilly Growth study. Ann. Epidemiol. 2013, 23, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Galobardes, B.; McCarron, P.; Jeffreys, M.; Davey Smith, G. Association between early life history of respiratory disease and morbidity and mortality in adulthood. Thorax 2008, 63, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Documentation du SNDS. Available online: https://documentation-snds.health-data-hub.fr/ (accessed on 12 January 2022).
- Boudemaghe, T.; Belhadj, I. Data resource profile: The french national uniform hospital discharge data set database (PMSI). Int. J. Epidemiol. 2017, 46, 392. [Google Scholar] [CrossRef]
- Linard, M.; Blondel, B.; Estellat, C.; Deneux-Tharaux, C.; Luton, D.; Oury, J.; Schmitz, T.; Mandelbrot, L.; Azria, E.; The PreCARE Study Group. Association between inadequate antenatal care utilisation and severe perinatal and maternal morbidity: An analysis in the PreCARE cohort. BJOG 2018, 125, 587–595. [Google Scholar] [CrossRef]
- AUDIPOG. Available online: https://www.audipog.net/Courbes-morpho (accessed on 12 January 2022).
- Rey, G.; Jougla, E.; Fouillet, A.; Hémon, D. Ecological association between a deprivation index and mortality in France over the period 1997–2001: Variations with spatial scale, degree of urbanicity, age, gender and cause of death. BMC Public Health 2009, 9, 33. [Google Scholar] [CrossRef]
- Boubred, F.; Pauly, V.; Romain, F.; Fond, G.; Boyer, L. The role of neighbourhood socioeconomic status in large for gestational age. PLoS ONE 2020, 15, e0233416. [Google Scholar] [CrossRef]
- Kho, A.T.; Bhattacharya, S.; Tantisira, K.G.; Carey, V.J.; Gaedigk, R.; Leeder, J.S.; Kohane, I.S.; Weiss, S.T.; Mariani, T.J. Transcriptomic analysis of human lung development. Am. J. Respir. Crit. Care Med. 2010, 181, 54–63. [Google Scholar] [CrossRef]
- Phokela, S.S.; Peleg, S.; Moya, F.R.; Alcorn, J.L. Regulation of human pulmonary surfactant protein gene expression by 1α,25-dihydroxyvitamin D3. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L617–L626. [Google Scholar] [CrossRef]
- Foong, R.E.; Bosco, A.; Jones, A.C.; Gout, A.; Gorman, S.; Hart, P.H.; Zosky, G.R. The effects of in utero vitamin D deficiency on airway smooth muscle mass and lung function. Am. J. Respir. Cell Mol. Biol. 2015, 53, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Xun, P.; Pike, K.; Wills, A.K.; Chawes, B.L.; Bisgaard, H.; Cai, W.; Wan, Y.; He, K. In utero exposure to 25-hydroxyvitamin D and risk of childhood asthma, wheeze, and respiratory tract infections: A meta-analysis of birth cohort studies. J. Allergy Clin. Immunol. 2017, 139, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Lipińska-Opałka, A.; Tomaszewska, A.; Kubiak, J.Z.; Kalicki, B. Vitamin D and Immunological Patterns of Allergic Diseases in Children. Nutrients 2021, 13, 177. [Google Scholar] [CrossRef] [PubMed]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science 1983, 221, 1181–1183. [Google Scholar] [CrossRef]
- Farias, M.C.D.O.; Cavalcante, T.L.T.; Assunção, M.L.; Bueno, N.B. Association between maternal or cord blood concentrations of 25-hydroxycholecalciferol or vitamin D supplementation during pregnancy and the cytokines profile in the umbilical cord blood: Systematic literature review. J. Steroid Biochem. Mol. Biol. 2020, 203, 105739. [Google Scholar] [CrossRef]
- Mandell, E.; Powers, K.N.; Harral, J.W.; Seedorf, G.J.; Hunter, K.S.; Abman, S.H.; Dodson, R.B. Intrauterine endotoxin-induced impairs pulmonary vascular function and right ventricular performance in infant rats and improvement with early vitamin D therapy. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 309, L1438–L1446. [Google Scholar] [CrossRef]
- Yurt, M.; Liu, J.; Sakurai, R.; Gong, M.; Husain, S.M.; Siddiqui, M.A.; Husain, M.; Villarreal, P.; Akcay, F.; Torday, J.S.; et al. Vitamin D supplementation blocks pulmonary structural and functional changes in a rat model of perinatal vitamin D deficiency. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L859–L867. [Google Scholar] [CrossRef]
- Wolsk, H.M.; Chawes, B.L.; Litonjua, A.A.; Hollis, B.W.; Waage, J.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Weiss, S.T. Prenatal vitamin D supplementation reduces risk of asthma/recurrent wheeze in early childhood: A combined analysis of two randomized controlled trials. PLoS ONE 2017, 12, e0186657. [Google Scholar] [CrossRef]
- Roth, D.E.; Al Mahmud, A.; Raqib, R.; Black, R.E.; Baqui, A.H. Pharmacokinetics of a single oral dose of vitamin D3 (70,000 IU) in pregnant and non-pregnant women. Nutr. J. 2012, 11, 114. [Google Scholar] [CrossRef]
- Knihtilä, H.M.; Stubbs, B.J.; Carey, V.J.; Laranjo, N.; Chu, S.H.; Kelly, R.S.; Zeiger, R.S.; Bacharier, L.B.; O’Connor, G.T.; Lasky-Su, J.; et al. Low gestational vitamin D level and childhood asthma are related to impaired lung function in high-risk children. J. Allergy Clin. Immunol. 2021, 148, 110–119. [Google Scholar] [CrossRef]
- Ong, T.; Schechter, M.; Yang, J.; Peng, L.; Emerson, J.; Gibson, R.L.; Morgan, W.; Rosenfeld, M.; EPIC Study Group. Socioeconomic Status, Smoke Exposure, and Health Outcomes in Young Children with Cystic Fibrosis. Pediatrics 2017, 139, e20162730. [Google Scholar] [CrossRef] [PubMed]
- Food Supplements for Pregnant Women. Available online: https://www.anses.fr/fr/content/complements-alimentaires-destinés-aux-femmes-enceintes (accessed on 18 December 2022).
| Characteristics | Overall (n = 125,756) | Respiratory Illness (n = 46,142 [37%]) | No Respiratory Illness (n = 79,614 [63%]) |
|---|---|---|---|
| Maternal age (years) | |||
| <20 | 1612 (1%) | 550 (1%) | 1062 (1%) |
| 20–29 | 53,920 (43%) | 19,914 (43%) | 34,006 (43%) |
| 30–39 | 64,709 (51%) | 23,834 (52%) | 40,875 (51%) |
| >40 | 5515 (4%) | 1844 (4%) | 3671 (5%) |
| Pregnancy follow-up | |||
| Inadequate | 9319 (7%) | 2910 (6%) | 6409 (8%) |
| Intermediate | 20,824 (17%) | 7251 (16%) | 13,573 (17%) |
| Adequate | 95,613 (76%) | 35,981 (75%) | 59,632 (75%) |
| CHCI | 30,524 (24%) | 11,217 (24%) | 19,307 (24%) |
| NDI | |||
| Deprived | 20,751 (16%) | 7222 (16%) | 13,529 (17%) |
| Medium | 75,764 (60%) | 28,161 (61%) | 47,603 (60%) |
| Affluant | 28,828 (23%) | 10,609 (23%) | 18,219 (23%) |
| Obstetrical pathology | 19,766 (16%) | 7314 (16%) | 12,452 (15%) |
| Maternal Vitamin D3 | 54,696 (43%) | 19,938 (43%) | 34,758 (44%) |
| Caesarean section | 15,858 (13%) | 5961 (13%) | 9897 (12%) |
| Birth season | |||
| Summer–Autumn | 50,999 (40%) | 19,130 (41%) | 31,869 (40%) |
| GA (weeks) | |||
| 36 | 2258 (2%) | 939 (2%) | 1319 (2%) |
| 37 | 6293 (5%) | 2500 (5%) | 3793 (5%) |
| 38 | 18,469 (15%) | 7240 (16%) | 11,229 (14%) |
| 39 | 37,689 (30%) | 13,999 (30%) | 23,690 (30%) |
| >40 | 61,047 (48%) | 21,464 (46%) | 39,583 (50%) |
| Female sex | 62,012 (49%) | 20,118 (44%) | 41,894 (53%) |
| Birth weight | |||
| AGA | 102,274 (82%) | 37,675 (82%) | 65,049 (82%) |
| SGA | 11,479 (9%) | 4104 (9%) | 7375 (9%) |
| LGA | 11,553 (9%) | 4363 (10%) | 7190 (9%) |
| Neonatal pathology | 15,217 (12%) | 5607 (12%) | 9610 (12%) |
| Characteristics | Adjusted OR (95%CI) | p |
|---|---|---|
| Maternal age (years) | ||
| <20 | 0.931 (0.84–1.03) | 0.18 |
| 20–29 | Reference [1] | 1 |
| 30–39 | 0.98 (0.96–1.01) | 0.21 |
| >40 | 0.84 (0.79–0.89) | <0.001 |
| Pregnancy follow-up | ||
| Inadequate | Reference [1] | |
| Intermediate | 1.12 (1.05–1.19) | <0.0001 |
| Adequate | 1.32 (1.25–1.40) | <0.0001 |
| CHCI | 1.02 (0.99–1.05) | 0.12 |
| NDI (Affluent) | 1.00 (0.97–1.03) | 0.98 |
| Obstetrical pathology | 0.99 (0.96–1.30) | 0.85 |
| Maternal Vitamin D3 supplementation | 0.97 (0.95–0.99) | 0.01 |
| Caesarean section | 0.98 (0.95–1.02) | 0.38 |
| Birth season | ||
| Summer–Autumn | 1.06 (1.03–1.08) | <0.001 |
| GA (weeks) | ||
| 36 | 1.26 (1.16–1.38) | <0.0001 |
| 37 | 1.16 (1.10–1.23) | <0.0001 |
| 38 | 1.11 (1.07–1.15) | <0.0001 |
| 39 | Reference [1] | 1 |
| >40 | 0.85 (0.80–0.90) | <0.0001 |
| Female sex | 0.70 (0.68–0.71) | <0.0001 |
| Birth weight | ||
| AGA | Reference [1] | |
| SGA | 0.97 (0.93–1.01) | 0.13 |
| LGA | 1.05 (1.01–1.09) | 0.01 |
| Neonatal pathology | 1.02 (0.99–1.06) | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loddo, F.; Nauleau, S.; Lapalus, D.; Tardieu, S.; Bernard, O.; Boubred, F. Association of Maternal Gestational Vitamin D Supplementation with Respiratory Health of Young Children. Nutrients 2023, 15, 2380. https://doi.org/10.3390/nu15102380
Loddo F, Nauleau S, Lapalus D, Tardieu S, Bernard O, Boubred F. Association of Maternal Gestational Vitamin D Supplementation with Respiratory Health of Young Children. Nutrients. 2023; 15(10):2380. https://doi.org/10.3390/nu15102380
Chicago/Turabian StyleLoddo, Fanny, Steve Nauleau, David Lapalus, Sophie Tardieu, Olivier Bernard, and Farid Boubred. 2023. "Association of Maternal Gestational Vitamin D Supplementation with Respiratory Health of Young Children" Nutrients 15, no. 10: 2380. https://doi.org/10.3390/nu15102380
APA StyleLoddo, F., Nauleau, S., Lapalus, D., Tardieu, S., Bernard, O., & Boubred, F. (2023). Association of Maternal Gestational Vitamin D Supplementation with Respiratory Health of Young Children. Nutrients, 15(10), 2380. https://doi.org/10.3390/nu15102380




