Gender and Liver Steatosis Discriminate Different Physiological Patterns in Obese Patients Undergoing Bariatric Surgery: Obesity Center Cohort
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
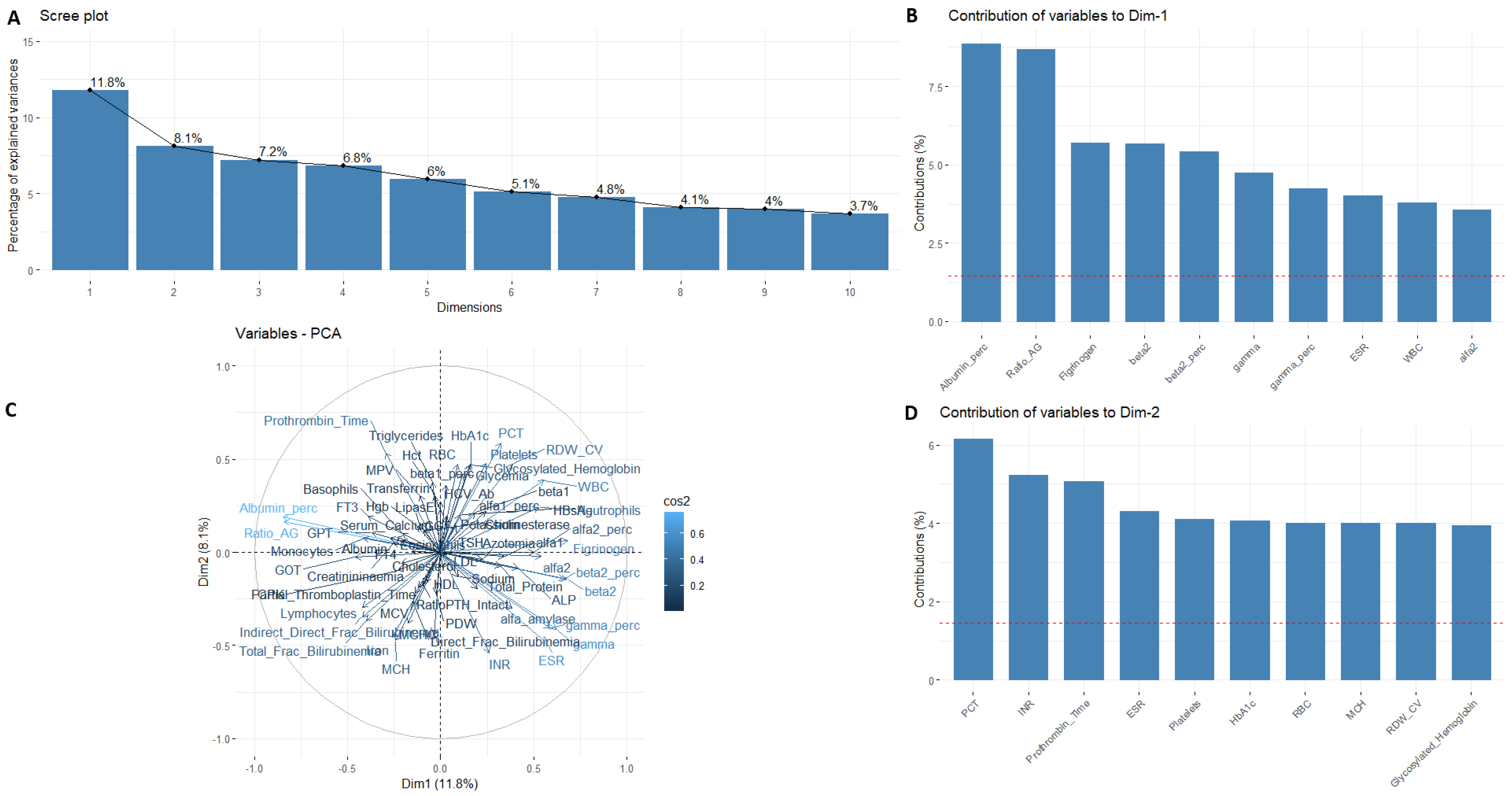
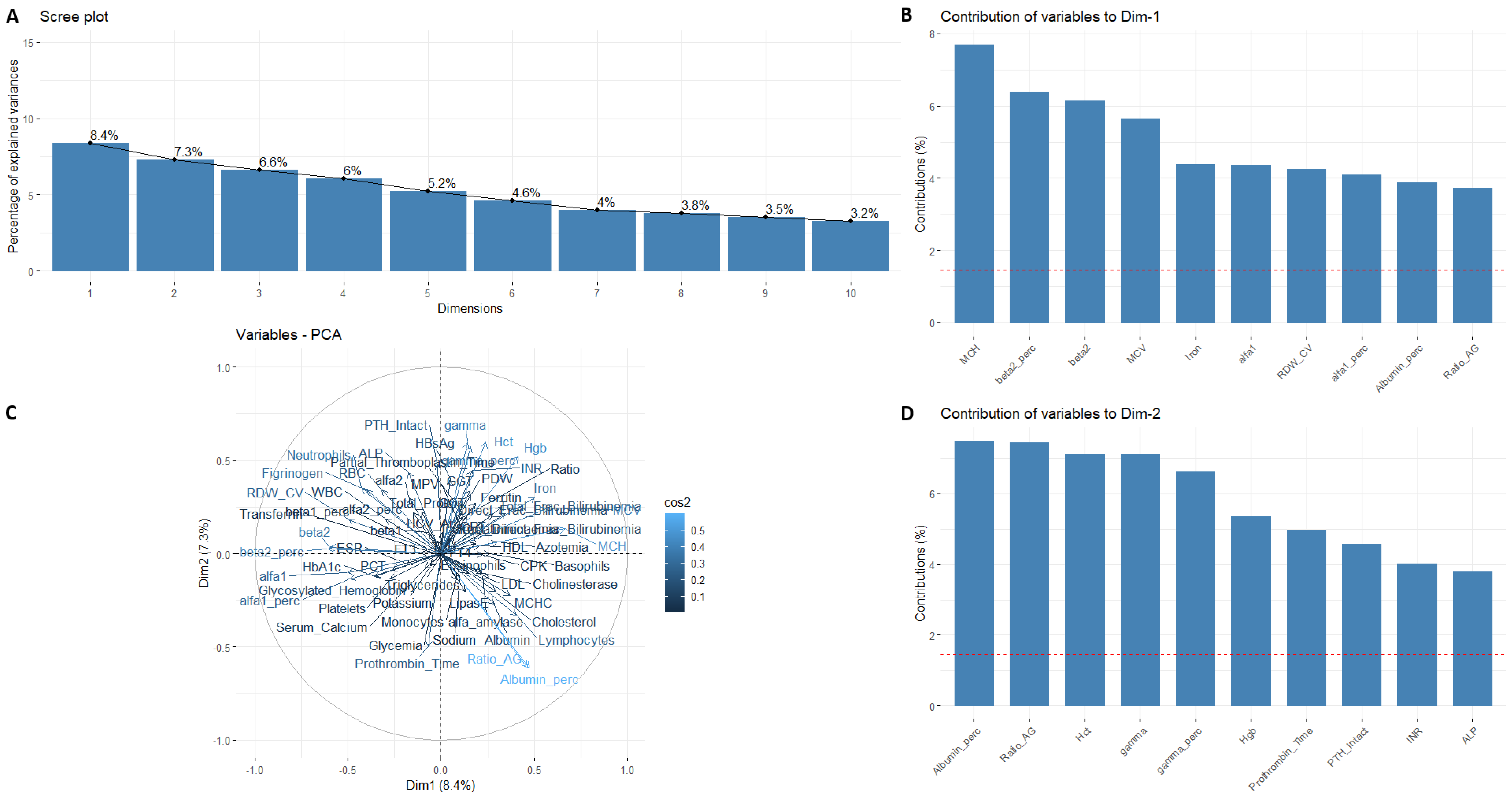
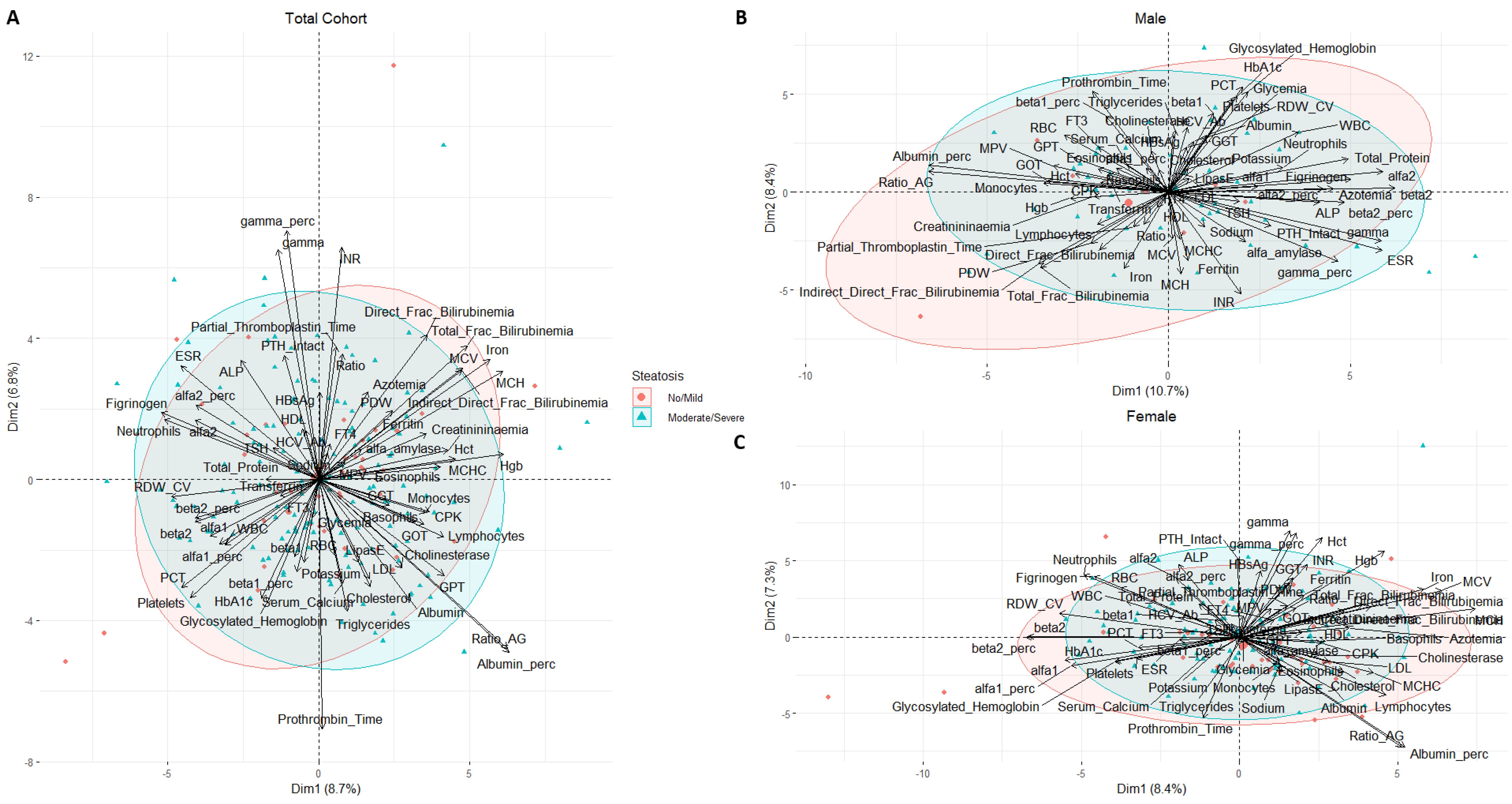
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayoral, L.P.; Andrade, G.M.; Mayoral, E.P.; Huerta, T.H.; Canseco, S.P.; Rodal Canales, F.J.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Pérez Santiago, A.D.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21. [Google Scholar] [PubMed]
- D’Errico, M.; Pavlova, M.; Spandonaro, F. The economic burden of obesity in Italy: A cost-of-illness study. Eur. J. Health Econ. 2022, 23, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Tchernof, A.; Despés, J.P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care. 2016, 22, s176–s185. [Google Scholar] [PubMed]
- Foschi, D.; Moroni, R.; De Luca, M.; Sarro, G.; Bernante, P.; Zappa, M.A.; Moroni, R.; Navarra, G.; Foletto, M.; Ceriani, V.; et al. Linee guida di chirurgia dell’obesità. In Società Italiana di Chirurgia Dell’Obesità e Delle Malattie (SICOB); SICOB: Rome, Italy, 2016. [Google Scholar]
- D’Angela, D.; Cambiano, C.; Glorioso, V. PariSanità-Osservatorio per l’equità di accesso alle prestazioni. In Assobiomedica C.R.E.A. Sanità; 2020; Available online: https://www.creasanita.it/index.php/it/il-progetto (accessed on 19 March 2023).
- Breznikar, B.; Dinevski, D. Bariatric surgery for morbid obesity: Pre-operative assessment, surgical techniques, and post-operative monitoring. J. Int. Med. Res. 2009, 37, 1632–1645. [Google Scholar] [CrossRef]
- Buchwald, H.; Oien, D.M. Metabolic/bariatric surgery worldwide 2008. Obes. Surg. 2009, 19, 1605–1611. [Google Scholar] [CrossRef]
- Reoch, J.; Mottillo, S.; Shimony, A.; Filion, K.B.; Christou, N.V.; Joseph, L.; Poirier, P.; Eisenberg, M.J. Safety of laparoscopic vs. open bariatric surgery: A systematic review and meta-analysis. Arch. Surg. 2011, 146, 1314–1322. [Google Scholar] [CrossRef]
- Kappor, N.; Arora, S.; Kalra, S. Gender disparities in people living with obesity—An unchartered territory. J. Midlife Health. 2021, 12, 103–107. [Google Scholar]
- Shekelle, P.G.; Newberry, S.; Maglione, M.; Maglione, M.; Li, Z.; Yermilov, I.; Hilton, L.; Suttorp, M.; Maggard, M.; Carter, J.; et al. Bariatric surgery in women of reproductive age: Special concerns for pregnancy. Evid. Rep. Technol. Assess. 2008, 169, 1–51. [Google Scholar]
- Dal Prà, C.; Fabris, R. Obesity and gender differences. Ital. J. Gender-Specific Med. 2020, 6, 3–14. [Google Scholar]
- Wang, D.Q.H.; Portincasa, P.; Brend, A.; Neuschwander-Tetri, B.A. Steatosis in the liver. Compr. Physiol. 2013, 3, 1493–1532. [Google Scholar] [PubMed]
- Denzer, C.; Thiere, D.; Muche, R.; Koening, W.; Mayer, H.; Kratzer, W.; Wabitsch, M. Gender-specific prevalences of fatty liver in obese children and adolescents: Roles of body fat distribution, sex steroids, and insulin resistance. J. Clin. Endocrinol. Metab. 2009, 94, 3872–3881. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Negro, F.; Hallaji, S.; Younossi, Y.; Lam, B.; Srishord, M. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine 2012, 91, 319–327. [Google Scholar] [CrossRef] [PubMed]
- SICOB. Indagine Conoscitiva Sulla Riduzione Dell’attività di Chirurgia Bariatrica COVID-19 Correlata. Available online: https://www.sicob.org/09_covid/chirurgia.html (accessed on 26 November 2022).
- Qayyum, A.; Chen, D.M.; Breiman, R.S.; Westphalen, A.C.; Yeh, B.M.; Jones, K.D.; Lu, Y.; Coakley, F.V.; Callen, P.W. Evaluation of diffuse liver steatosis by ultrasound, computed tomography, and magnetic resonance imaging: Which modality is best? Clin. Imaging 2009, 33, 110–115. [Google Scholar] [CrossRef]
- Kanter, R.; Caballero, B. Global gender disparities in obesity: A review. Adv. Nutr. 2012, 3, 491–498. [Google Scholar] [CrossRef]
- Pepino, M.Y.; Mennella, J.A. Cigarette smoking and obesity are associated with decreased fat perception in women. Comp. Study 2014, 22, 1050–1055. [Google Scholar] [CrossRef]
- Schwab, C.; Paar, M.; Fengler, V.H.; Ivastinovic, D.; Haas, A.; Seidel, G.; Glats, W.; Malle, E.M.; Weger, M.; Velikay-Parel, M.; et al. Gender differences in albumin and ascorbic acid in the vitreous antioxidant system. Free Radic. Biol Med. 2020, 146, 257–263. [Google Scholar] [CrossRef]
- Lufrano, D.; Trejo, S.A.; Llovera, R.E.; Salgueiro, M.; Fernandez, G.; Damonte, V.M.; Flecha, F.L.G.; Raingo, J.; Ermácora, M.R.; Perelló, M. Ghrelin binding to serum albumin and its biological impact. Mol. Cell. Endocrinol. 2016, 436, 130–140. [Google Scholar] [CrossRef]
- Fasano, M.; Curry, S.; Terreno, E.; Galliano, M.; Fanali, G.; Narciso, P.; Notari, S.; Ascenzi, P. The extraordinary ligand binding properties of human serum albumin. IUBMB Life 2005, 5712, 787–796. [Google Scholar] [CrossRef]
- Addai-Mensah, O.; Gyamfi, D.; Duneeh, R.V.; Danquah, K.O.; Annani-Akollor, M.E.E.; Boateng, L.; Owiredu, E.W.; Amponsah, F.A.; Afrihie, E.Y.; Asare, R.; et al. Determination of hematological reference ranges in healthy adults in three regions in Ghana. Biomed. Res Int. 2019, 2019, 7467512. [Google Scholar] [CrossRef]
- Indrayan, A.; Bhargava, M.; Shukla, S. Reference einterval with age-gender variation for 4 liver function parameters in an adult segment of the Indian population. Int. J. Med. Biochem. 2020, 3, 82–90. [Google Scholar]
- Wilson, A.M.; Kimura, E.; Harada, R.K.; Nair, N.; Narasimhan, B.; Meng, X.Y.; Zhang, F.; Beck, K.R.; Olin, J.W.; Fung, E.T.; et al. Beta2-microglobulin as a biomarker in peripheral arterial disease: Proteomic profiling and clinical studies. Circulation 2007, 116, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Clement, D.L. Hypertension and peripheral artery disease. J. Hypertens. 2020, 38, 2378–2379. [Google Scholar] [CrossRef] [PubMed]
- Alende-casto, V.; Alonso-Sampedro, M.; Vazquez-Temprano, N.; Tuñez, C.; Rey, D.; Garía-Iglesias, C.; Sopeña, B.; Gude, F.; Gonzalez-Quintela, A. Factors influencing erythrocyte sedimentation rate in adults: New evidence for an old test. Medicine 2019, 98, e16816. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Gender differences in glucose homeostasis and diabetes. Physiol. Behav. 2018, 187, 20–23. [Google Scholar] [CrossRef]
- Homma, H.; Kurachi, H.; Nishio, Y.; Takeda, T.; Yamamoto, T.; Adachi, K.; Morishige, K.; Ohmichi, M.; Matsuzawa, Y.; Murata, Y. Estrogen suppresses transcription of lipoprotein lipase gene. Existence of a unique estrogen response element on the lipoprotein lipase promoter. J. Biol. Chem. 2000, 275, 11404–11411. [Google Scholar] [CrossRef]
- Price, T.M.; O’Brien, S.N.; Welter, B.H.; George, R.; Anandjiwala, J.; Kilgore, M. Estrogen regulation of adipose tissue lipoprotein lipase-possible mechanism of body fat distribution. Am. J. Obstet. Gynecol. 1998, 178, 101–107. [Google Scholar] [CrossRef]
- Pedersen, S.B.; Kristensen, K.; Hermann, P.A.; Katzenellenbogen, J.A.; Richelsen, B. Estrogen controls lipolysis by up-regulating alpha2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor alpha. Implications for the female fat distribution. J. Clin. Endocrinol. Metab. 2004, 89, 1869–1878. [Google Scholar] [CrossRef]
- Pascot, A.; Lemieux, I.; Bergeron, J.; Tremblay, A.; Nadeau, A.; Prud’homme, D.; Couillard, C.; Lamarche, B.; Després, J.P. HDL particle size:a marker of the gender difference in the metabolic risk profile. Atherosclerosis 2002, 160, 339–406. [Google Scholar] [CrossRef]
- Bredella, M.A. Sex differences in body composition. Adv. Exp. Med. Biol. 2017, 1043, 9–27. [Google Scholar]
- Deng, W.; Tan, X.; Zhou, Q.; Ai, Z.; Liu, W.; Chen, W.; Yu, X.; Yang, Q. Gender-related differences in clinicopathological characteristics and renal outcomes of Chinese patients with IgA nephropathy. BMC Nephrol. 2018, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Ramamani, A.; Aruldhas, M.M.; Govindarajulu, P. Impact of testosterone and estradiol on region specificity of skeletal muscle-ATP, creatine phosphokinase and myokines in male and female Wistar rats. Acta Physiol. Cand. 1999, 166, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.H.; Wang, K.T.; Lee, P.P.; Liu, C.C.; Hsieh, Y.C.; Kuo, J.K.; Bulwer, B.E.; Hung, C.L.; Chang, S.C.; Shih, S.C.; et al. Gender-differences in the associations between circulating creatinine kinase, blood pressure, body mass and non-alcoholic fatty liver disease in asymptomatic asians. PLoS ONE 2017, 12, e0179898. [Google Scholar] [CrossRef] [PubMed]
- Skurtveit, S.; Tverdal, A. Sex differences in gamma-glutamyl transferase in people aged 40-42 years in two Norwegian countries. Drug Alcohol Depend. 2022, 67, 95–98. [Google Scholar] [CrossRef]
- Krishnamurthy, H.A. The serum gamma glutamyl transpeptidase-A noninvasive diagnostic bio marker of chronic anicteric nonalcoholic liver disease. J. Clin. Diagn. Res. 2013, 7, 691–694. [Google Scholar]
- Rushton, D.H.; Barth, J.H. What is the evidence for gender differences in ferritin and haemoglobin? Crit. Rev. Oncol. Hematol. 2010, 73, 1–9. [Google Scholar] [CrossRef]
- Arbiol-Roca, A.; Imperiali, C.E.; Montserrat, M.M.; Cerro, A.S.; Bosch de Basea, A.C.; Navarro, L.S.; Batch, D.D.; Politi, J.V. Reference intervals for a complete blood count on an automated haematology analyser Sysmex XN in healthy adults from the southern metropolitan area of Barcelona. EJIFCC 2018, 29, 48–54. [Google Scholar]
- Shaheen, N.A.; Rehan, H.; Moghairi, A.; Gmati, G.; Damlaj, M.; Salama, H.; Rather, M.; Mendoza, M.A.; Alanazi, A.; Al Ahmari, B.; et al. Hematological indices in the adult Saudi population: Reference intervals by gender, age, and region. Front. Med. 2022, 9, 901937. [Google Scholar] [CrossRef]
- Grau, M.; Cremer, J.M.; Schmeichel, S.; Kunkel, M.; Bloch, W. Comparisons of blood parameters, red blood cell deformability and circulating nutric oxide between males and female considering hormonal contraception: A longitudinal gender study. Front. Physiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Strich, D.; Karavani, G.; Edri, S.; Chay, C.; Gillis, D. FT3 is higher in males than in females and decreases over the lifespan. Endocr. Pract. 2017, 23, 803–807. [Google Scholar] [CrossRef]
- Miyashita, K.; Murakami, M.; Iriuchijima, T.; Takeuchi, T.; Mori, M. Regulation of rat liver type 1 iodothyronine deiodinase mRNA levels by testosterone. Mol. Cell Endocrinol. 1995, 115, 161–167. [Google Scholar] [CrossRef]
- Suzuki, S.; Nischio, S.; Takeda, T.; Komatsu, M. Gender-specific regulation of response to thyroid hormone in aging. Throid. Res. 2012, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Stárka, L.; Pospisilová, H.; Hill, M. Free testosterone and free dihydrotestosterone throughout the life span of men. J. Steroid. Biochem. Mol. Biol. 2009, 116, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Burra, P.; Bizzaro, D.; Gonta, A.; Shalaby, S.; Gambato, M.; Morelli, M.C.; Trapani, S.; Floreani, A.; Marra, F.; Brunetto, M.R.; et al. Clinical impact of sexual dimorphism in non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH). Liver Int. 2021, 41, 1713–1733. [Google Scholar] [CrossRef]
- Chang, Y.; Jung, H.S.; Yun, K.E.; Cho, J.; Ahn, J.; Chung, E.C.; Shin, H.; Ryu, S. Metabolically healthy obesity is associated with an increased risk of diabetes independently of nonalcoholic fatty liver disease. Obesity 2016, 24, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.M.; Yankowitz, J.; Widness, J.A.; Strauss, R.G. Etiology of differences in hematocrit between males and females: Sequence-based polymorphism in erythropoietin and its receptor. J. Gend. Specif. Med. 2001, 4, 35–40. [Google Scholar] [PubMed]
- Meffert, C.; Gerdes, N. Program adherence and effectiveness of a commercial nutrition program: The metabolic balance study. J. Nutr. Metab. 2010, 2010, 197656. [Google Scholar] [CrossRef]
- Lonardo, A.; Mantovani, A.; Lugari, S.; Targher, G. Epidemiology and pathophysiology of the association between NAFLD and metabolically healthy or metabolically unhealthy obesity. Ann. Hepatol. 2020, 19, 359–366. [Google Scholar] [CrossRef]
- Schiavo, L.; Pierro, R.; Asteria, C.; Calabrese, P.; Di Biasio, A.; Coluzzi, I.; Severino, L.; Giovanelli, A.; Pilone, V.; Silecchia, G. Low-calorie ketogenic diet with continuous positive airway pressure to alleviate severe obstructive sleep apnea syndrome in patients with obesity scheduled for bariatric/metabolic surgery: A pilot, prospective, randomized multicenter comparative study. Obes. Surg. 2022, 32, 634–642. [Google Scholar] [CrossRef]
- Ringel, M.M.; Ditto, P.H. The moralization of obesity. Soc. Sci. Med. 2019, 237, 112399. [Google Scholar] [CrossRef] [PubMed]
| Parameters * | Total Cohort (n = 250) | Males (n = 69) | Females (n = 181) | p ^ |
|---|---|---|---|---|
| Age (y) | 43.5 ± 11.9 | 42.4 ± 11.9 | 44.0 ± 11.9 | 0.45 |
| Age Classes (%) | 0.12 ψ | |||
| Young Adult (≤25 y) | 23 (9.2) | 10 (14.5) | 13 (7.2) | |
| Adult (26–44 y) | 106 (42.4) | 26 (37.7) | 80 (44.2) | |
| Middle Aged (45–59 y) | 99 (39.6) | 30 (43.5) | 69 (38.1) | |
| Elderly (≥60 y) | 22 (8.8) | 3 (4.3) | 19 (10.5) | |
| Level of Education (%) | 0.64 ψ | |||
| None | 5 (2.2) | 1 (1.5) | 4 (2.4) | |
| Elementary School | 9 (3.9) | 4 (6.0) | 5 (3.1) | |
| Secondary school | 173 (75.2) | 51 (76.1) | 122 (74.8) | |
| High school | 41 (17.8) | 10 (14.9) | 31 (19.0) | |
| Degree | 2 (0.9) | 1 (1.5) | 1 (0.6) | |
| Marital Status (%) | 0.47 ψ | |||
| Unmarried | 44 (27.3) | 13 (27.1) | 31 (27.4) | |
| Married | 103 (64.0) | 33 (68.7) | 70 (61.9) | |
| Separated | 7 (4.3) | 0 (0.0) | 7 (6.2) | |
| Divorced | 4 (2.5) | 1 (2.1) | 3 (2.6) | |
| Widow/er | 3 (1.9) | 1 (2.1) | 2 (1.8) | |
| Smoking habit (%) | 0.10 ψ | |||
| No | 121 (51.3) | 31 (47.0) | 90 (52.9) | |
| Ex | 47 (19.9) | 19 (28.8) | 28 (16.5) | |
| Yes | 68 (28.8) | 16 (24.2) | 52 (30.6) | |
| BMI (kg/m2) | 46.6 ± 8.4 | 47.9 ± 10.2 | 46.1 ± 7.5 | 0.54 |
| Alcohol Consumption (die) (%) | 0.62 ψ | |||
| None | 148 (74.4) | 43 (76.8) | 105 (73.4) | |
| Occasional | 51 (25.6) | 13 (23.2) | 38 (26.6) | |
| Physical Activity (%) | 0.95 ψ | |||
| Poor | 161 (85.2) | 47 (85.4) | 114 (85.1) | |
| Occasional | 28 (14.8) | 8 (14.5) | 20 (14.9) | |
| Employment (Yes) (%) | 85 (59.7) | 32 (82.0) | 53 (51.5) | 0.001 |
| Diet (%) | 0.30 ψ | |||
| Ketogenic | 164 (69.2) | 49 (74.2) | 115 (67.2) | |
| Low-Carb | 56 (23.6) | 15 (22.7) | 41 (24.0) | |
| Hypocaloric | 17 (7.2) | 2 (3.0) | 15 (8.8) | |
| Surgery (%) | 0.39 ψ | |||
| Sleeve | 117 (47.8) | 37 (55.2) | 80 (44.9) | |
| Bypass | 113 (46.1) | 29 (43.3) | 84 (47.2) | |
| LAGB | 2 (0.8) | 0 (0.0) | 2 (1.1) | |
| SAGI | 4 (1.6) | 1 (1.5) | 3 (1.7) | |
| Redo-Bypass | 8 (3.3) | 0 (0.0) | 8 (4.5) | |
| Redo-Sleeve | 1 (0.4) | 0 (0.0) | 1 (0.6) | |
| Hospitalization (days) | 3.7 ± 2.7 | 3.7 ± 2.0 | 3.7 ± 3.0 | 0.65 |
| Blood tests | ||||
| Total Protein (g/dL) | 6.6 ± 0.6 | 6.6 ± 0.7 | 6.6 ± 0.6 | 0.85 |
| Albumin (%) | 53.3 ± 4.3 | 54.1 ± 4.6 | 53.0 ± 4.1 | 0.03 |
| Alpha-1 (%) | 2.7 ± 1.7 | 2.5 ± 0.4 | 2.9 ± 2.0 | 0.09 |
| Alpha-2 (%) | 12.2 ± 1.9 | 11.8 ± 1.9 | 12.4 ± 1.9 | 0.08 |
| Beta-1 (%) | 10.1 ± 2.0 | 9.9 ± 1.6 | 10.2 ± 2.2 | 0.27 |
| Gamma (%) | 14.7 ± 3.8 | 14.6 ± 3.4 | 14.8 ± 3.9 | 0.35 |
| Ratio A/G | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.2 | 0.03 |
| Albumin (g/dL) | 3.5 ± 0.4 | 3.6 ± 0.5 | 3.5 ± 0.4 | 0.19 |
| Alpha-1 (g/dL) | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.49 |
| Alpha-2 (g/dL) | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.1 | 0.20 |
| Beta-1 (g/dL) | 0.7 ± 0.2 | 0.7 ± 0.1 | 0.7 ± 0.2 | 0.52 |
| Gamma (g/dL) | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.33 |
| Beta-2 (%) | 6.9 ± 1.8 | 7.2 ± 1.4 | 6.7 ± 1.9 | 0.03 |
| Beta-2 (g/dL) | 0.5 ± 0.1 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.03 |
| Glycemia (mg/dL) | 96.4 ± 14.1 | 100.6 ± 16.1 | 94.7 ± 12.9 | 0.02 |
| Triglycerides (mg/dL) | 115.1 ± 49.7 | 127.2 ± 61.3 | 110.3 ± 43.5 | 0.04 |
| Cholesterol (mg/mL) | 182.8 ± 35.3 | 175.5 ± 28.7 | 185.7 ± 37.2 | 0.10 |
| Total Fractional Bilirubinemia (mg/dL) | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.10 |
| Direct Fractional Bilirubinemia (mg/dL) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.93 |
| Indirect Fractional Bilirubinemia (mg/dL) | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.06 |
| Azotemia (mg/dL) | 35.1 ± 10.2 | 36.2 ± 9.3 | 34.7 ± 10.6 | 0.23 |
| Creatininemia (mg/dL) | 0.8 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | <0.0001 |
| CPK (U/L) | 115.9 ± 69.3 | 146.0 ± 82.2 | 103.8 ± 59.5 | 0.0002 |
| GOT (U/L) | 23.2 ± 8.0 | 24.7 ± 8.7 | 22.6 ± 7.6 | 0.09 |
| GGT (U/I) | 22.7 ± 16.9 | 27.5 ± 19.5 | 20.7 ± 15.4 | 0.001 |
| ALP (mU/mL) | 157.7 ± 62.9 | 154.8 ± 60.5 | 158.9 ± 64.0 | 0.70 |
| Iron (mg/dL) | 59.3 ± 26.4 | 65.0 ± 23.7 | 57.0 ± 27.2 | 0.009 |
| Ferritin (ng/mL) | 82.4 ± 81.0 | 127.9 ± 77.3 | 64.3 ± 75.2 | <0.0001 |
| Transferrin (mg/dL) | 319.3 ± 105.6 | 285.6 ± 55.3 | 332.8 ± 117.4 | 0.003 |
| α—Amylase (UI/L) | 54.8 ± 31.9 | 61.2 ± 53.0 | 52.3 ± 17.2 | 0.83 |
| Sodium (mEq/L) | 138.7 ± 2.4 | 138.5 ± 2.3 | 138.8 ± 2.5 | 0.35 |
| Potassium (mEq/L) | 4.1 ± 0.5 | 4.2 ± 0.4 | 4.1 ± 0.6 | 0.44 |
| Serum Calcium (mg/dL) | 8.9 ± 0.6 | 9.0 ± 0.7 | 8.9 ± 0.5 | 0.57 |
| Cholinesterase (U/L) | 10135.8 ± 2044.1 | 10632.0 ± 2290.6 | 9937.3 ± 1909.0 | 0.08 |
| GPT (U/L) | 32.1 ± 16.8 | 39.5 ± 20.1 | 29.2 ± 14.3 | 0.0003 |
| Hepatitis C (IgG) | 0.2 ± 1.1 | 0.1 ± 0.3 | 0.2 ± 1.3 | 0.30 |
| HBsAg | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.76 |
| TSH (mUI/mL) | 2.1 ± 1.7 | 1.6 ± 0.8 | 2.2 ± 1.9 | 0.008 |
| FT3 (pg/mL) | 3.3 ± 0.7 | 3.5 ± 0.5 | 3.3 ± 0.8 | 0.001 |
| FT4 (ng/mL) | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.14 |
| Lipase (U/L) | 36.5 ± 12.0 | 37.1 ± 12.7 | 36.2 ± 11.8 | 0.46 |
| PTH Intact (pg/mL) | 77.8 ± 36.6 | 70.2 ± 36.0 | 80.9 ± 36.5 | 0.03 |
| LDL (mg/dL) | 116.0 ± 32.4 | 113.3 ± 28.4 | 117.0 ± 33.9 | 0.70 |
| HDL (mg/dL) | 45.2 ± 11.8 | 39.0 ± 7.7 | 47.7 ± 12.2 | <0.0001 |
| Prothrombin Time—P (%) | 108.3 ± 19.3 | 108.6 ± 16.1 | 108.2 ± 20.5 | 0.83 |
| I.N.R. | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.2 | 0.77 |
| Partial Thromboplastin Time (sec) | 27.8 ± 8.8 | 26.4 ± 5.0 | 28.4 ± 9.9 | 0.17 |
| Ratio | 1.0 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.3 | 0.24 |
| Fibrinogen-P (mg/dL) | 383.2 ± 82.0 | 379.1 ± 109.4 | 384.9 ± 68.5 | 0.19 |
| WBC (K/mcL) | 7.2 ± 1.8 | 7.7 ± 2.1 | 7.0 ± 1.7 | 0.10 |
| RBC (M/mcL) | 4.9 ± 0.4 | 5.1 ± 0.4 | 4.8 ± 0.4 | <0.0001 |
| Hgb (g/dL) | 13.7 ± 1.2 | 14.8 ± 0.9 | 13.3 ± 1.0 | <0.0001 |
| HCT (%) | 41.6 ± 3.8 | 44.6 ± 3.2 | 40.5 ± 3.3 | <0.0001 |
| MCV (fL) | 85.4 ± 5.8 | 86.7 ± 4.4 | 84.9 ± 6.2 | 0.13 |
| MCH (pg) | 28.2 ± 2.1 | 28.9 ± 1.4 | 27.9 ± 2.2 | 0.006 |
| MCHC (g/dL) | 33.0 ± 1.1 | 33.3 ± 1.1 | 32.8 ± 1.1 | 0.009 |
| Platelets (K/mcL) | 246.7 ± 55.9 | 233.3 ± 56.2 | 252.0 ± 55.1 | 0.03 |
| RDW-CV (%) | 14.3 ± 1.1 | 14.2 ± 1.1 | 14.4 ± 1.1 | 0.20 |
| MPV (fL) | 9.2 ± 1.0 | 9.1 ± 1.1 | 9.3 ± 0.9 | 0.35 |
| PCT (%) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.009 |
| PDW (%) | 49.9 ± 6.2 | 51.1 ± 5.1 | 49.4 ± 6.5 | 0.02 |
| Neutrophils (%) | 62.8 ± 6.9 | 62.7 ± 6.6 | 62.8 ± 7.0 | 0.76 |
| Lymphocytes (%) | 27.3 ± 6.2 | 26.6 ± 5.8 | 27.5 ± 6.4 | 0.32 |
| Monocytes (%) | 5.6 ± 1.2 | 6.0 ± 1.2 | 5.4 ± 1.2 | 0.001 |
| Eosinophils (%) | 2.1 ± 1.2 | 2.3 ± 1.1 | 2.1 ± 1.2 | 0.03 |
| Basophils (%) | 0.5 ± 0.2 | 0.5 ± 0.3 | 0.5 ± 0.2 | 0.72 |
| ESR | 19.2 ± 12.9 | 12.1 ± 9.4 | 22.1 ± 12.9 | <0.0001 |
| HbA1c (%) | 5.6 ± 0.7 | 5.7 ± 1.0 | 5.6 ± 0.6 | 0.18 |
| Glycosylated Hemoglobin (mmol/mol) | 38.5 ± 7.0 | 40.0 ± 7.5 | 37.9 ± 6.7 | 0.13 |
| Blood Group (%) | 0.25 ψ | |||
| 0 | 106 (44.5) | 32 (48.5) | 74 (43.0) | |
| A | 95 (39.9) | 28 (42.4) | 67 (38.9) | |
| AB | 11 (4.6) | 3 (4.5) | 8 (4.6) | |
| B | 26 (10.9) | 3 (4.5) | 23 (13.4) | |
| Rh Factor (%) | 0.63 ψ | |||
| Negative | 25 (10.4) | 8 (11.9) | 17 (9.8) | |
| Positive | 215 (89.6) | 59 (88.1) | 156 (90.2) | |
| Concomitant diseases | ||||
| Allergy (Yes) (%) | 89 (36.6) | 18 (27.3) | 71 (40.1) | 0.06 ψ |
| Hypertension (Yes) (%) | 110 (44.0) | 38 (55.1) | 72 (39.8) | 0.03 ψ |
| Diabetes (Yes) (%) | 97 (38.8) | 33 (47.8) | 64 (35.4) | 0.07 ψ |
| Ultrasound Results | ||||
| Liver Volume (%) | 0.003 ψ | |||
| Normal | 89 (38.4) | 14 (22.6) | 75 (44.1) | |
| Increased | 143 (61.6) | 48 (77.4) | 95 (55.9) | |
| Liver Margins (%) | 0.23 ψ | |||
| Smooth | 197 (98.0) | 45 (95.7) | 152 (98.7) | |
| Irregular | 4 (2.0) | 2 (4.3) | 2 (1.3) | |
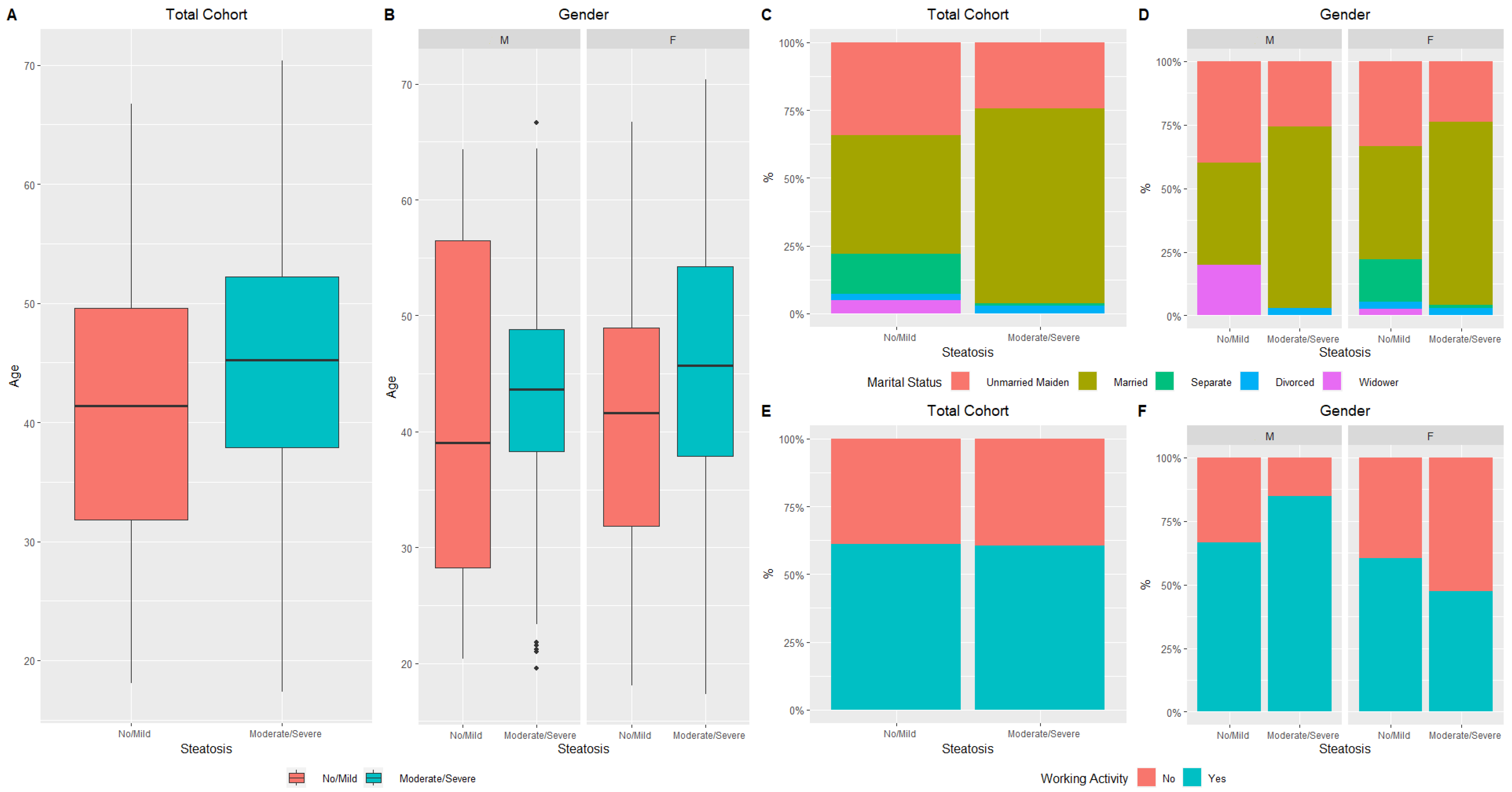
| Steatosis (%) | 0.006 ψ | |||
| No/Mild | 66 (28.4) | 9 (14.7) | 57 (33.3) | |
| Moderate/Severe | 166 (71.5) | 52 (85.2) | 114 (66.7) | |
| Gallbladder Volume (%) | 0.03 ψ | |||
| Ablated | 24 (10.7) | 3 (5.2) | 21 (12.6) | |
| Normal | 183 (81.3) | 47 (81.0) | 136 (81.4) | |
| Expanse | 13 (5.8) | 4 (6.9) | 9 (5.4) | |
| Contracted | 1 (0.4) | 1 (1.7) | 0 (0.0) | |
| Scleroatrophic | 4 (1.8) | 3 (1.7) | 1 (0.6) | |
| Gallbladder Stones (Yes) (%) | 35 (17.2) | 11 (19.0) | 24 (16.4) | 0.67 ψ |
| Spleen Volume (%) | 0.57 ψ | |||
| Normal | 214 (92.6) | 56 (94.9) | 158 (91.9) | |
| Increased | 17 (7.4) | 3 (5.1) | 14 (8.1) |
| Parameters * | Total Cohort (n = 250) | p ^ | Males (n = 69) | p ^ | Females (n = 181) | p ^ | p ¥ | p † | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No/Mild Steatosis (n = 66) | Moderate/ Severe Steatosis (n = 166) | No/Mild Steatosis (n = 9) | Moderate/ Severe Steatosis (n = 52) | No/Mild Steatosis (n = 57) | Moderate/ Severe Steatosis (n = 114) | ||||||
| Age (y) | 40.8 ± 12.0 | 44.6 ± 11.9 | 0.03 | 40.9 ± 16.3 | 42.7 ± 11.5 | 0.62 | 40.8 ± 11.3 | 45.5 ± 12.0 | 0.01 | 0.99 | 0.18 |
| Age Classes (%) | 0.50 ψ | 0.52 ψ | 0.32 ψ | 0.32 α | 0.22 β | ||||||
| Young Adult (≤25 y) | 7 (10.6) | 15 (9.0) | 2 (22.2) | 7 (13.5) | 5 (8.8) | 8 (7.0) | |||||
| Adult (26–44 y) | 32 (48.5) | 65 (39.2) | 3 (33.3) | 20 (38.5) | 29 (50.9) | 45 (39.5) | |||||
| Middle Age (45–59 y) | 23 (34.8) | 70 (42.2) | 3 (3.3) | 23 (44.2) | 20 (35.1) | 47 (41.2) | |||||
| Old Age (≥ 60 y) | 4 (6.1) | 16 (9.6) | 1 (11.1) | 2 (3.8) | 3 (5.3) | 14 (12.3) | |||||
| Degree of Education (%) | 0.65 ψ | 0.21 ψ | 0.71 ψ | 0.20 α | 0.66 β | ||||||
| No | 1 (1.7) | 2 (1.3) | 0 (0.0) | 0 (0.0) | 1 (2.0) | 2 (1.9) | |||||
| Elementary School | 3 (5.3) | 5 (3.2) | 2 (25.0) | 2 (3.9) | 1 (2.0) | 3 (2.9) | |||||
| Secondary school | 41 (71.9) | 121 (77.6) | 5 (62.5) | 40 (78.4) | 36 (73.5) | 81 (77.1) | |||||
| High school | 11 (19.3) | 27 (17.3) | 1 (12.5) | 8 (15.7) | 10 (20.4) | 19 (18.1) | |||||
| Short Degree | 1 (1.7) | 1 (0.6) | 0 (0.0) | 1 (2.0) | 1 (2.0) | 0 (0.0) | |||||
| Marital Status (%) | <0.001 ψ | 0.15 ψ | 0.003 ψ | 0.47 α | 0.99 β | ||||||
| Unmarried maiden | 14 (34.1) | 26 (24.5) | 2 (40.0) | 9 (25.7) | 12 (33.3) | 17 (23.9) | |||||
| Married | 18 (43.9) | 76 (71.7) | 2 (40.0) | 25 (71.4) | 16 (44.4) | 51 (71.8) | |||||
| Separate | 6 (14.6) | 1 (0.9) | 0 (0.0) | 0 (0.0) | 6 (16.7) | 1 (1.4) | |||||
| Divorced | 1 (2.4) | 3 (2.8) | 0 (0.0) | 1 (2.9) | 1 (2.8) | 2 (2.8) | |||||
| Widower | 2 (4.9) | 0 (0.0) | 1 (20.0) | 0 (0.0) | 1 (2.8) | 0 (0.0) | |||||
| Smoke (%) | 0.47 ψ | 0.33 ψ | 0.53 ψ | 0.34 α | 0.10 β | ||||||
| No | 31 (51.7) | 82 (51.6) | 6 (75.0) | 22 (43.1) | 25 (48.1) | 60 (55.6) | |||||
| Ex | 9 (15.0) | 34 (21.4) | 1 (12.5) | 16 (31.4) | 8 (15.4) | 18 (16.7) | |||||
| Yes | 20 (33.3) | 43 (27.0) | 1 (12.5) | 13 (25.5) | 19 (36.5) | 30 (27.8) | |||||
| BMI (Kg/m2) | 44.1 ± 6.2 | 47.1 ± 8.4 | 0.02 | 46.4 ± 6.3 | 47.4 ± 9.9 | 0.93 | 43.7 ± 6.2 | 47.0 ± 7.7 | 0.008 ψ | 0.18 | 0.71 |
| Alcohol Consumption (die) (%) | 0.41 ψ | 0.66 ψ | 0.49 ψ | 0.99 α | 0.73 β | ||||||
| No | 41 (77.4) | 5 (71.4) | 6 (85.7) | 33 (73.3) | 35 (76.1) | 62 (70.4) | |||||
| Sporadic | 12 (22.6) | 38 (28.6) | 1 (14.3) | 12 (26.7) | 11 (23.9) | 26 (29.5) | |||||
| Physical Activity (%) | 0.16 ψ | 0.99 ψ | 0.10 ψ | 0.99 α | 0.51 β | ||||||
| Poor | 39 (78.0) | 109 (86.5) | 6 (85.7) | 36 (83.7) | 33 (76.7) | 73 (87.9) | |||||
| Occasional | 11 (22.0) | 17 (13.5) | 1 (14.3) | 7 (16.3) | 10 (23.3) | 10 (15.0) | |||||
| Working Activity (Yes) (%) | 25 (61.0) | 57 (60.6) | 0.97 ψ | 2 (66.7) | 28 (84.8) | 0.43 ψ | 23 (60.5) | 29 (47.5) | 0.21 ψ | 0.99 α | <0.001 β |
| Diet (%) | 0.05 ψ | 0.99 ψ | 0.03 ψ | 0.39 α | 0.65 β | ||||||
| Ketogenic | 36 (59.0) | 119 (75.3) | 6 (85.7) | 38 (74.5) | 30 (55.6) | 81 (75.7) | |||||
| Low-Carb | 19 (31.1) | 29 (18.3) | 1 (14.3) | 11 (21.6) | 18 (33.3) | 18 (16.8) | |||||
| Hypocaloric | 6 (9.8) | 10 (6.3) | 0 (0.0) | 2 (3.9) | 6 (11.1) | 8 (7.5) | |||||
| Surgery (%) | 0.009 ψ | 0.72 ψ | 0.01 ψ | 0.72 α | 0.58 β | ||||||
| Sleeve | 21 (32.8) | 87 (53.4) | 4 (50.0) | 29 (56.9) | 17 (30.4) | 58 (51.8) | |||||
| Bypass | 38 (59.4) | 68 (41.7) | 4 (50.0) | 22 (43.1) | 34 (60.7) | 46 (41.1) | |||||
| LAGB | 2 (3.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.6) | 0 (0.0) | |||||
| SAGI | 0 (0.0) | 2 (1.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.8) | |||||
| Redo-Bypass | 3 (4.7) | 5 (3.1) | 0 (0.0) | 0 (0.0) | 3 (5.4) | 5 (4.5) | |||||
| Redo-Sleeve | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.9) | |||||
| Hospitalization (days) | 3.3 ± 2.3 | 3.8 ± 2.9 | 0.001 | 3.1 ± 0.8 | 3.7 ± 2.2 | 0.60 | 3.3 ± 2.5 | 3.8 ± 3.2 | 0.002 | 0.55 | 0.62 |
| Blood tests | |||||||||||
| Total Protein (g/dL) | 6.5 ± 0.7 | 6.7 ± 0.6 | 0.50 | 6.1 ± 1.2 | 6.7 ± 0.7 | 0.14 | 6.6 ± 0.7 | 6.7 ± 0.5 | 0.92 | 0.28 | 0.53 |
| Albumin (%) | 54.0 ± 4.1 | 53.1 ± 4.2 | 0.27 | 56.0 ± 3.5 | 54.2 ± 4.1 | 0.44 | 53.7 ± 4.1 | 52.6 ± 4.1 | 0.17 | 0.19 | 0.01 |
| Alpha-1 (%) | 3.3 ± 3.2 | 2.6 ± 0.8 | 0.45 | 2.5 ± 0.4 | 2.4 ± 0.3 | 0.90 | 3.4 ± 3.4 | 2.6 ± 0.9 | 0.67 | 0.46 | 0.10 |
| Alpha-2 (%) | 11.8 ± 1.6 | 12.3 ± 2.0 | 0.04 | 11.5 ± 2.2 | 11.8 ± 1.9 | 0.82 | 11.9 ± 1.6 | 12.6 ± 2.0 | 0.007 | 0.78 | 0.02 |
| Beta-1 (%) | 10.0 ± 2.1 | 10.2 ± 2.1 | 0.45 | 10.5 ± 3.3 | 9.9 ± 1.3 | 0.81 | 10.0 ± 2.0 | 10.3 ± 2.3 | 0.30 | 0.84 | 0.31 |
| Gamma (%) | 14.0 ± 4.4 | 14.9 ± 3.6 | 0.57 | 12.4 ± 1.8 | 14.6 ± 3.5 | 0.11 | 14.2 ± 4.6 | 15.1 ± 3.6 | 0.80 | 0.05 | 0.35 |
| Ratio A/G | 1.2 ± 0.2 | 1.1 ± 0.2 | 0.27 | 1.3 ± 0.2 | 1.2 ± 0.2 | 0.42 | 1.2 ± 0.2 | 1.1 ± 0.2 | 0.16 | 0.17 | 0.01 |
| Albumin (g/dL) | 3.5 ± 0.4 | 3.6 ± 0.4 | 0.60 | 3.3 ± 0.5 | 3.6 ± 0.4 | 0.15 | 3.5 ± 0.4 | 3.5 ± 0.4 | 0.73 | 0.42 | 0.04 |
| Alpha-1 (g/dL) | 0.2 ± 0.2 | 0.9 ± 0.1 | 0.72 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.02 | 0.2 ± 0.2 | 0.2 ± 0.1 | 0.68 | 0.03 | 0.97 |
| Alpha-2 (g/dL) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.03 | 0.7 ± 0.2 | 0.8 ± 0.1 | 0.50 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.01 | 0.72 | 0.10 |
| Beta-1 (g/dL) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.67 | 0.6 ± 0.2 | 0.7 ± 0.1 | 0.94 | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.60 | 0.96 | 0.72 |
| Gamma (g/dL) | 0.9 ± 0.3 | 1.0 ± 0.3 | 0.28 | 0.8 ± 0.2 | 1.0 ± 0.3 | 0.09 | 0.9 ± 0.3 | 1.0 ± 0.2 | 0.43 | 0.09 | 0.29 |
| Beta-2 (%) | 6.8 ± 2.7 | 6.9 ± 1.3 | 0.22 | 6.9 ± 1.2 | 7.0 ± 1.2 | 0.72 | 6.8 ± 2.9 | 6.8 ± 1.3 | 0.41 | 0.55 | 0.16 |
| Beta-2 (g/dL) | 0.4 ± 0.2 | 0.5 ± 0.1 | 0.14 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.25 | 0.4 ± 0.2 | 0.4 ± 0.1 | 0.44 | 0.87 | 0.05 |
| Glycemia (mg/dL) | 93.7 ± 9.8 | 97.5 ± 15.3 | 0.14 | 92.4 ± 5.5 | 102.8 ± 16.9 | 0.09 | 93.9 ± 10.3 | 0.9 ± 13.8 | 0.71 | 0.90 | 0.007 |
| Triglycerides (mg/dL) | 94.4 ± 28.1 | 122.2 ± 54.0 | 0.0005 | 91.1 ± 25.2 | 132.7 ± 66.0 | 0.08 | 95.0 ± 28.8 | 117.4 ± 47.0 | 0.006 | 0.92 | 0.14 |
| Cholesterol (mg/mL) | 185.1 ± 39.9 | 182.2 ± 34.5 | 0.88 | 184.3 ± 34.6 | 174.4 ± 28.0 | 0.29 | 185.2 ± 41.0 | 185.8 ± 36.7 | 0.62 | 0.87 | 0.08 |
| Total Fractional Bilirubinemia (mg/dL) | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.08 | 0.9 ± 0.4 | 0.7 ± 0.3 | 0.04 | 0.7 ± 0.3 | 0.6 ± 0.3 | 0.21 | 0.07 | 0.30 |
| Direct Fractional Bilirubinemia (mg/dL) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.26 | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.35 | 0.2 ± 0.1 | 0.2 ± 0.2 | 0.44 | 0.75 | 0.60 |
| Indirect Fractional Bilirubinemia (mg/dL) | 0.5 ± 0.3 | 0.5 ± 0.2 | 0.13 | 0.7 ± 0.4 | 0.5 ± 0.2 | 0.03 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.26 | 0.04 | 0.21 |
| Azotemia (mg/dL) | 36.1 ± 12.5 | 34.8 ± 9.4 | 0.81 | 34.0 ± 8.6 | 36.8 ± 9.3 | 0.39 | 36.5 ± 13.0 | 33.9 ± 9.4 | 0.40 | 0.71 | 0.07 |
| Creatininaemia (mg/dL) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.40 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.73 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.10 | 0.008 | <0.0001 |
| CPK (U/L) | 101.0 ± 50.7 | 122.3 ± 73.7 | 0.15 | 122.7 ± 46.2 | 152.6 ± 84.4 | 0.45 | 97.4 ± 51.1 | 108.2 ± 63.8 | 0.51 | 0.09 | 0.001 |
| GOT (U/L) | 20.9 ± 6.2 | 23.9 ± 8.2 | 0.02 | 19.9 ± 4.9 | 25.4 ± 8.9 | 0.11 | 21.0 ± 6.4 | 23.2 ± 7.9 | 0.12 | 0.82 | 0.18 |
| GGT (U/I) | 18.2 ± 13.6 | 23.6 ± 17.8 | 0.01 | 19.9 ± 10.8 | 28.1 ± 20.5 | 0.27 | 17.9 ± 14.1 | 21.5 ± 16.1 | 0.09 | 0.44 | 0.007 |
| ALP (mU/mL) | 151.3 ± 69.7 | 160.6 ± 61.6 | 0.29 | 134.1 ± 77.2 | 155.1 ± 60.9 | 0.53 | 154.1 ± 68.9 | 163.1 ± 62.1 | 0.29 | 0.61 | 0.42 |
| Iron (mg/dL) | 59.3 ± 22.8 | 59.4 ± 28.2 | 0.85 | 75.7 ± 19.3 | 64.3 ± 24.6 | 0.09 | 56.7 ± 22.4 | 57.1 ± 29.6 | 0.82 | 0.03 | 0.04 |
| Ferritin (ng/mL) | 59.4 ± 72.4 | 86.4 ± 80.1 | 0.001 | 177.9 ± 114.7 | 112.8 ± 61.2 | 0.18 | 40.1 ± 38.9 | 74.1 ± 85.1 | 0.001 | 0.0002 | <0.0001 |
| Transferrin (mg/dL) | 348.0 ± 162.6 | 312.5 ± 80.6 | 0.14 | 280.4 ± 61.6 | 286.5 ± 56.7 | 0.99 | 359.0 ± 171.5 | 324.6 ± 87.2 | 0.29 | 0.09 | 0.02 |
| α—Amylase (UI/L) | 50.0 ± 17.1 | 53.2 ± 19.6 | 0.44 | 53.7 ± 12.9 | 54.3 ± 24.9 | 0.32 | 49.4 ± 17.7 | 52.7 ± 16.7 | 0.19 | 0.35 | 0.38 |
| Sodium (mEq/L) | 139.0 ± 2.5 | 138.7 ± 2.4 | 0.63 | 138.7 ± 1.6 | 138.5 ± 2.5 | 0.63 | 139.0 ± 2.6 | 138.8 ± 2.4 | 0.87 | 0.94 | 0.35 |
| Potassium (mEq/L) | 4.3 ± 0.8 | 4.1 ± 0.4 | 0.41 | 4.5 ± 0.5 | 4.1 ± 0.4 | 0.10 | 4.2 ± 0.8 | 4.1 ± 0.4 | 0.80 | 0.04 | 0.83 |
| Serum Calcium (mg/dL) | 8.9 ± 0.5 | 8.9 ± 0.6 | 0.66 | 9.2 ± 0.6 | 9.0 ± 0.8 | 0.35 | 8.9 ± 0.5 | 8.9 ± 0.5 | 0.79 | 0.24 | 0.57 |
| Cholinesterase (U/L) | 9425.4 ± 2117.7 | 10,368.4 ± 2022.8 | 0.01 | 9557.1 ± 2671.4 | 10,763.0 ± 2308.5 | 0.31 | 9403.9 ± 2051.7 | 10,184.7 ± 1859.1 | 0.04 | 0.97 | 0.26 |
| GPT (U/L) | 25.9 ± 14.4 | 33.4 ± 16.1 | 0.0004 | 25.3 ± 6.9 | 40.2 ± 19.6 | 0.04 | 26.0 ± 15.3 | 30.2 ± 13.2 | 0.01 | 0.45 | 0.002 |
| Hepatitis C (IgG) | 0.3 ± 1.5 | 0.2 ± 0.9 | 0.43 | 0.0 ± 0.0 | 0.1 ± 0.3 | 0.10 | 0.3 ± 1.7 | 0.2 ± 1.1 | 0.77 | 0.08 | 0.61 |
| HBsAg | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.96 | 0.1 ± 0.2 | 0.1 ± 0.0 | 0.22 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.56 | 0.19 | 0.76 |
| TSH (mUI/mL) | 2.4 ± 2.5 | 2.0 ± 1.4 | 0.12 | 1.9 ± 1.0 | 1.6 ± 0.8 | 0.62 | 2.5 ± 2.6 | 2.1 ± 1.6 | 0.30 | 0.35 | 0.008 |
| FT3 (pg/mL) | 3.4 ± 0.9 | 3.4 ± 0.7 | 0.99 | 3.4 ± 0.5 | 3.5 ± 0.5 | 0.47 | 3.4 ± 1.0 | 3.3 ± 0.7 | 0.39 | 0.59 | 0.001 |
| FT4 (ng/mL) | 1.2 ± 0.3 | 1.1 ± 0.2 | 0.48 | 1.2 ± 0.2 | 1.1 ± 0.2 | 0.49 | 1.2 ± 0.3 | 1.1 ± 0.2 | 0.78 | 0.79 | 0.14 |
| Lipase (U/L) | 35.9 ± 14.3 | 36.9 ± 11.5 | 0.26 | 33.9 ± 4.7 | 38.8 ± 13.5 | 0.33 | 36.2 ± 15.3 | 36.1 ± 10.3 | 0.61 | 0.82 | 0.46 |
| PTH Intact (pg/mL) | 69.9 ± 33.5 | 82.4 ± 37.3 | 0.02 | 56.3 ± 25.5 | 74.2 ± 37.1 | 0.27 | 72.1 ± 34.3 | 86.2 ± 37.0 | 0.01 | 0.11 | 0.03 |
| LDL (mg/dL) | 114.4 ± 37.7 | 116.1 ± 31.1 | 0.52 | 109.0 ± 46.8 | 111.9 ± 24.3 | 0.75 | 115.2 ± 36.6 | 118.1 ± 33.8 | 0.36 | 0.73 | 0.70 |
| HDL (mg/dL) | 48.0 ± 10.2 | 44.2 ± 11.7 | 0.02 | 42.7 ± 6.9 | 38.9 ± 7.9 | 0.21 | 48.8 ± 10.5 | 46.7 ± 12.3 | 0.22 | 0.14 | <0.0001 |
| Prothrombin Time—P (%) | 105.8 ± 18.5 | 108.4 ± 19.4 | 0.41 | 111.2 ± 13.8 | 108.4 ± 17.2 | 0.70 | 105.0 ± 19.1 | 108.4 ± 20.5 | 0.30 | 0.72 | 0.83 |
| I.N.R. | 1.0 ± 0.4 | 1.0 ± 0.1 | 0.42 | 0.9 ± 0.0 | 1.0 ± 0.1 | 0.66 | 1.0 ± 0.4 | 1.0 ± 0.1 | 0.29 | 0.69 | 0.77 |
| Partial Thromboplastin Time (sec) | 27.9 ± 5.5 | 28.2 ± 10.0 | 0.26 | 25.2 ± 2.6 | 27.1 ± 5.3 | 0.35 | 28.3 ± 5.7 | 28.7 ± 11.5 | 0.17 | 0.06 | 0.17 |
| Ratio | 1.0 ± 0.2 | 1.0 ± 0.3 | 0.20 | 0.9 ± 0.1 | 0.1 ± 0.2 | 0.55 | 1.0 ± 0.2 | 1.0 ± 0.4 | 0.13 | 0.13 | 0.24 |
| Fibrinogen-P (mg/dL) | 371.5 ± 65.8 | 383.9 ± 79.2 | 0.32 | 367.2 ± 64.1 | 367.3 ± 95.3 | 0.79 | 372.2 ± 66.7 | 391.6 ± 69.7 | 0.10 | 0.97 | 0.19 |
| WBC (K/mcL) | 6.6 ± 1.9 | 7.4 ± 1.7 | 0.01 | 8.3 ± 2.7 | 7.4 ± 1.8 | 0.56 | 6.3 ± 1.7 | 7.3 ± 1.7 | 0.004 | 0.08 | 0.10 |
| RBC (M/mcL) | 4.8 ± 0.4 | 4.9 ± 0.4 | 0.02 | 5.0 ± 0.3 | 5.1 ± 0.3 | 0.60 | 4.7 ± 0.4 | 4.8 ± 0.4 | 0.19 | 0.04 | <0.0001 |
| Hgb (g/dL) | 13.4 ± 1.0 | 13.8 ± 1.2 | 0.05 | 14.9 ± 0.8 | 14.8 ± 1.0 | 0.82 | 13.2 ± 0.9 | 13.4 ± 1.1 | 0.48 | 0.0004 | <0.0001 |
| HCT (%) | 40.8 ± 3.43 | 41.9 ± 3.8 | 0.03 | 45.1 ± 4.0 | 44.3 ± 3.1 | 0.76 | 40.1 ± 2.8 | 40.7 ± 3.5 | 0.23 | 0.002 | <0.0001 |
| MCV (fL) | 85.4 ± 5.7 | 85.5 ± 6.0 | 0.92 | 89.2 ± 4.6 | 86.7 ± 4.4 | 0.20 | 84.8 ± 5.6 | 95.0 ± 6.6 | 0.79 | 0.05 | 0.13 |
| MCH (pg) | 28.1 ± 2.1 | 28.2 ± 2.1 | 0.90 | 29.4 ± 1.3 | 28.9 ± 1.4 | 0.43 | 27.9 ± 2.2 | 27.8 ± 2.3 | 0.84 | 0.07 | 0.006 |
| MCHC (g/dL) | 32.9 ± 1.1 | 32.9 ± 1.1 | 0.98 | 33.0 ± 1.3 | 33.4 ± 1.1 | 0.79 | 32.9 ± 1.1 | 32.7 ± 1.1 | 0.50 | 0.38 | 0.009 |
| Platelets (K/mcL) | 249.6 ± 58.6 | 246.5 ± 56.4 | 0.99 | 250.7 ± 68.1 | 229.7 ± 57.2 | 0.54 | 249.4 ± 57.8 | 254.3 ± 54.6 | 0.51 | 0.89 | 0.03 |
| RDW-CV (%) | 14.3 ± 1.1 | 14.3 ± 1.1 | 0.76 | 14.6 ± 1.6 | 14.0 ± 1.0 | 0.37 | 14.3 ± 1.0 | 14.4 ± 1.2 | 0.74 | 0.94 | 0.20 |
| MPV (fL) | 9.2 ± 1.0 | 9.2 ± 1.0 | 0.65 | 9.6 ± 1.1 | 9.0 ± 1.1 | 0.37 | 9.1 ± 1.0 | 9.3 ± 0.9 | 0.29 | 0.35 | 0.35 |
| PCT (%) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.83 | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.15 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.39 | 0.73 | 0.009 |
| PDW (%) | 47.8 ± 5.4 | 50.5 ± 6.2 | 0.01 | 50.3 ± 4.4 | 51.5 ± 5.1 | 0.51 | 47.4 ± 5.5 | 50.1 ± 6.7 | 0.04 | 0.12 | 0.002 |
| Neutrophils (%) | 61.6 ± 6.9 | 63.0 ± 6.9 | 0.13 | 61.5 ± 4.3 | 62.0 ± 6.5 | 0.87 | 61.6 ± 7.2 | 63.4 ± 7.0 | 0.09 | 0.91 | 0.76 |
| Lymphocytes (%) | 28.5 ± 5.9 | 27.0 ± 6.3 | 0.05 | 28.1 ± 4.1 | 27.0 ± 5.9 | 0.49 | 28.6 ± 6.2 | 27.0 ± 6.6 | 0.08 | 0.61 | 0.32 |
| Monocytes (%) | 5.6 ± 1.1 | 5.6 ± 1.2 | 0.59 | 6.1 ± 0.5 | 6.0 ± 1.3 | 0.74 | 5.6 ± 1.2 | 5.4 ± 1.1 | 0.32 | 0.17 | 0.001 |
| Eosinophils (%) | 2.1 ± 1.2 | 2.2 ± 1.2 | 0.76 | 2.1 ± 0.6 | 2.4 ± 1.1 | 0.66 | 2.1 ± 1.3 | 2.0 ± 1.2 | 0.76 | 0.62 | 0.03 |
| Basophils (%) | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.39 | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.99 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.31 | 0.83 | 0.72 |
| ESR | 20.0 ± 13.6 | 19.0 ± 12.6 | 0.63 | 8.5 ± 7.3 | 11.8 ± 8.7 | 0.25 | 21.7 ± 13.5 | 22.3 ± 12.8 | 0.69 | 0.01 | <0.0001 |
| HbA1c (%) | 5.5 ± 0.7 | 5.7 ± 0.8 | 0.04 | 5.3 ± 0.4 | 5.8 ± 1.1 | 0.01 | 5.6 ± 0.7 | 5.6 ± 0.6 | 0.38 | 0.14 | 0.18 |
| Glycosylated Hemoglobin (mmol/mol) | 37.0 ± 7.3 | 39.0 ± 6.9 | 0.02 | 34.1 ± 4.8 | 41.2 ± 7.4 | 0.009 | 37.5 ± 7.7 | 38.1 ± 6.4 | 0.31 | 0.15 | 0.13 |
| Blood Group (%) | 0.15 ψ | 0.64 ψ | 0.04 ψ | 0.71 α | 0.25 β | ||||||
| 0 | 24 (37.5) | 72 (45.9) | 4 (44.4) | 24 (49.0) | 20 (36.4) | 48 (44.4) | |||||
| A | 34 (53.1) | 58 (36.9) | 4 (44.4) | 22 (44.9) | 30 (54.5) | 36 (33.3) | |||||
| AB | 2 (3.1) | 7 (4.5) | 0 (0.0) | 1 (2.0) | 2 (3.6) | 6 (5.6) | |||||
| B | 4 (6.2) | 20 (12.7) | 1 (11.1) | 2 (4.1) | 3 (5.4) | 18 (16.7) | |||||
| Rh Factor (%) | 0.88 ψ | 0.99 ψ | 0.84 ψ | 0.99 α | 0.63 β | ||||||
| Negative | 6 (9.4) | 16 (10.1) | 1 (11.1) | 5 (10.0) | 5 (9.1) | 11 (10.1) | |||||
| Positive | 58 (9.6) | 143 (89.9) | 8 (88.9) | 45 (90.0) | 50 (90.9) | 98 (89.9) | |||||
| Pathologies | |||||||||||
| Allergy (Yes) (%) | 21 (33.3) | 60 (37.0) | 0.60 ψ | 2 (25.0) | 11 (22.0) | 0.99 ψ | 19 (34.5) | 49 (43.7) | 0.25 ψ | 0.71 α | 0.06 β |
| Hypertension (Yes) (%) | 29 (43.9) | 73 (44.0) | 0.99 ψ | 5 (55.5) | 29 (55.8) | 0.99 ψ | 24 (42.1) | 44 (38.6) | 0.66 ψ | 0.49 α | 0.03 β |
| Diabetes (Yes) (%) | 26 (39.4) | 61 (36.7) | 0.71 ψ | 4 (44.4) | 26 (50.0) | 0.99 ψ | 22 (38.6) | 35 (30.7) | 0.31 ψ | 0.73 α | 0.07 β |
| Ultrasound Results | |||||||||||
| Liver Volume (%) | <0.001 ψ | <0.001 ψ | <0.001 ψ | 0.09 α | 0.003 β | ||||||
| Normal | 61 (93.8) | 27 (16.5) | 7 (77.8) | 6 (11.8) | 54 (96.4) | 21 (18.6) | |||||
| Increased | 4 (6.1) | 137 (83.5) | 2 (22.2) | 45 (88.2) | 2 (3.6) | 92 (81.4) | |||||
| Liver Margins (%) | 0.99 ψ | 0.35 ψ | 0.55 ψ | 0.14 α | 0.23 β | ||||||
| Smooth | 61 (98.4) | 136 (97.8) | 8 (88.9) | 37 (97.4) | 53 (100.0) | 99 (98.0) | |||||
| Irregular | 1 (1.6) | 3 (2.2) | 1 (11.1) | 1 (2.6) | 0 (0.0) | 2 (2.0) | |||||
| Gallbladder Volume (%) | 0.98 ψ | 0.82 ψ | 0.63 ψ | 0.39 α | 0.03 β | ||||||
| Ablated | 7 (11.3) | 16 (10.1) | 0 (0.0) | 2 (4.3) | 7 (13.2) | 14 (12.5) | |||||
| Normal | 51 (82.3) | 132 (83.0) | 8 (88.8) | 39 (83.0) | 43 (81.1) | 93 (83.0) | |||||
| Expanse | 3 (4.8) | 7 (4.4) | 1 (11.1) | 2 (4.3) | 2 (3.8) | 5 (4.5) | |||||
| Contracted | 0 (0.0) | 1 (0.6) | 0 (0.0) | 1 (2.1) | 0 (0.0) | 0 (0.0) | |||||
| Scleroatrophic | 1 (1.6) | 3 (1.9) | 0 (0.0) | 3 (6.4) | 1 (1.9) | 0 (0.0) | |||||
| Gallbladder Stones (Yes) (%) | 5 (8.9) | 30 (20.7) | 0.05 ψ | 0 (0.0) | 11 (23.4) | 0.18 ψ | 5 (10.6) | 19 (19.4) | 0.18 ψ | 0.58 α | 0.67 β |
| Spleen Volume (%) | 0.55 ψ | 0.99 ψ | 0.44 ψ | 0.58 α | 0.57 β | ||||||
| Normal | 60 (90.9) | 151 (93.2) | 9 (100.0) | 46 (93.9) | 51 (89.5) | 105 (92.9) | |||||
| Increased | 6 (9.1) | 11 (6.8) | 0 (0.0) | 3 (6.1) | 6 (10.5) | 8 (7.1) | |||||
| Parameters * | Males | p ^ | Females | p ^ | ||
|---|---|---|---|---|---|---|
| Prehospitalization | Surgery | Prehospitalization | Surgery | |||
| BMI (Kg/m2) | 47.9 ± 10.2 | 49.1 ± 10.2 | 0.17 | 46.1 ± 7.5 | 45.1 ± 7.3 | 0.09 |
| Glycemia (mg/dL) | 100.6 ± 16.1 | 99.6 ± 23.2 | 0.79 | 94.7 ± 12.9 | 97.3 ± 18.9 | 0.11 |
| Fractional Total Bilirubinemia (mg/dL) | 0.7 ± 0.3 | 0.8 ± 0.3 | 0.09 | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.46 |
| Direct fractional bilirubinemia (mg/dL) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.19 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.86 |
| Indirect fractional bilirubinemia (mg/dL) | 0.5 ± 0.2 | 0.6 ± 0.3 | 0.18 | 0.5 ± 0.2 | 0.5 ± 0.3 | 0.74 |
| Azotemia (mg/dL) | 36.2 ± 9.3 | 31.8 ± 15.2 | 0.0002 | 34.7 ± 10.6 | 28.0 ± 11.4 | <0.0001 |
| Creatininaemia (mg/dL) | 0.9 ± 0.1 | 0.9 ± 0.2 | 0.009 | 0.8 ± 0.1 | 0.7 ± 0.2 | <0.0001 |
| GOT (U/L) | 24.7 ± 8.7 | 25.6 ± 9.3 | 0.79 | 22.6 ± 7.6 | 27.8 ± 18.1 | <0.0001 |
| GGT (U/I) | 27.5 ± 19.5 | 23.8 ± 18.4 | 0.002 | 20.3 ± 15.4 | 18.9 ± 19.8 | <0.0001 |
| ALP (mU/mL) | 154.8 ± 60.5 | 115.3 ± 55.8 | <0.0001 | 158.9 ± 64.0 | 115.5 ± 52.3 | <0.0001 |
| Sodium (mEq/L) | 138.5 ± 2.3 | 140.4 ± 3.5 | 0.02 | 138.8 ± 2.5 | 140.2 ± 4.2 | 0.001 |
| Potassium (mEq/L) | 4.2 ± 0.4 | 4.2 ± 0.4 | 0.08 | 4.1 ± 0.6 | 4.2 ± 0.5 | 0.99 |
| GPT (U/L) | 39.5 ± 20.1 | 39.1 ± 22.6 | 0.35 | 29.2 ± 14.3 | 34.7 ± 29.1 | 0.23 |
| Lipase (U/L) | 37.1 ± 12.7 | 30.9 ± 14.2 | 0.001 | 36.2 ± 11.8 | 30.0 ± 13.9 | <0.0001 |
| Prothrombin Time—P (%) | 108.6 ± 16.1 | 103.0 ± 11.6 | 0.34 | 108.2 ± 20.5 | 94.8 ± 18.4 | 0.0005 |
| I.N.R. | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.34 | 1.0 ± 0.2 | 1.0 ± 0.1 | 0.0003 |
| Partial Thromboplastin Time (sec) | 26.4 ± 5.0 | 25.7 ± 3.4 | 0.18 | 28.4 ± 9.9 | 28.5 ± 13.2 | 0.99 |
| Ratio | 1.0 ± 0.2 | 0.9 ± 0.1 | 0.99 | 1.0 ± 0.3 | 1.1 ± 0.5 | 0.14 |
| WBC (K/mcL) | 7.7 ± 2.1 | 10.6 ± 2.8 | <0.0001 | 7.0 ± 1.7 | 10.0 ± 2.8 | <0.0001 |
| RBC (M/mcL) | 5.1 ± 0.4 | 4.8 ± 0.5 | <0.0001 | 4.8 ± 0.4 | 4.4 ± 0.4 | <0.0001 |
| Hgb (g/dL) | 14.8 ± 0.9 | 13.8 ± 1.3 | <0.0001 | 13.3 ± 1.0 | 12.4 ± 1.1 | <0.0001 |
| HCT (%) | 44.6 ± 3.2 | 41.6 ± 4.6 | <0.0001 | 40.5 ± 3.3 | 37.6 ± 3.5 | <0.0001 |
| MCV (fL) | 86.7 ± 4.4 | 86.9 ± 6.4 | 0.05 | 84.9 ± 6.2 | 84.9 ± 6.5 | 0.03 |
| MCH (pg) | 28.9 ± 1.4 | 28.8 ± 1.8 | 0.36 | 27.9 ± 2.2 | 28.0 ± 2.3 | 0.80 |
| MCHC (g/dL) | 33.3 ± 1.1 | 33.2 ± 1.7 | 0.24 | 32.8 ± 1.1 | 33.0 ± 1.2 | 0.09 |
| Platelets (K/mcL) | 233.3 ± 56.2 | 210.8 ± 57.1 | <0.0001 | 252.0 ± 55.1 | 229.2 ± 53.9 | <0.0001 |
| RDW-CV (%) | 14.2 ± 1.1 | 13.9 ± 1.1 | 0.42 | 14.4 ± 1.1 | 14.2 ± 1.2 | 0.03 |
| MPV (fL) | 9.1 ± 1.1 | 9.4 ± 1.0 | 0.14 | 9.3 ± 0.9 | 9.3 ± 1.0 | 0.99 |
| PCT (%) | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.002 | 0.2 ± 0.1 | 0.2 ± 0.1 | <0.0001 |
| PDW (%) | 51.1 ± 5.1 | 51.2 ± 6.3 | 0.09 | 49.4 ± 6.5 | 49.3 ± 6.3 | 0.19 |
| Neutrophils (%) | 62.7 ± 6.6 | 78.8 ± 6.9 | <0.0001 | 62.8 ± 7.0 | 79.2 ± 7.2 | <0.0001 |
| Lymphocytes (%) | 26.6 ± 5.8 | 12.8 ± 5.0 | <0.0001 | 27.5 ± 6.4 | 13.6 ± 5.9 | <0.0001 |
| Monocytes (%) | 6.0 ± 1.2 | 6.2 ± 1.7 | 0.09 | 5.4 ± 1.2 | 5.4 ± 1.5 | 0.62 |
| Eosinophils (%) | 2.3 ± 1.1 | 0.7 ± 0.7 | <0.0001 | 2.1 ± 1.2 | 0.7 ± 0.6 | <0.0001 |
| Basophils (%) | 0.5 ± 0.3 | 0.2 ± 0.1 | <0.0001 | 0.5 ± 0.2 | 0.2 ± 0.2 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donghia, R.; Schiano Di Cola, R.; Cesaro, F.; Vitale, A.; Lippolis, G.; Lisco, T.; Isernia, R.; De Pergola, G.; De Nucci, S.; Rinaldi, R.; et al. Gender and Liver Steatosis Discriminate Different Physiological Patterns in Obese Patients Undergoing Bariatric Surgery: Obesity Center Cohort. Nutrients 2023, 15, 2381. https://doi.org/10.3390/nu15102381
Donghia R, Schiano Di Cola R, Cesaro F, Vitale A, Lippolis G, Lisco T, Isernia R, De Pergola G, De Nucci S, Rinaldi R, et al. Gender and Liver Steatosis Discriminate Different Physiological Patterns in Obese Patients Undergoing Bariatric Surgery: Obesity Center Cohort. Nutrients. 2023; 15(10):2381. https://doi.org/10.3390/nu15102381
Chicago/Turabian StyleDonghia, Rossella, Rita Schiano Di Cola, Filomena Cesaro, Andrea Vitale, Giuseppe Lippolis, Teresa Lisco, Roberta Isernia, Giovanni De Pergola, Sara De Nucci, Roberta Rinaldi, and et al. 2023. "Gender and Liver Steatosis Discriminate Different Physiological Patterns in Obese Patients Undergoing Bariatric Surgery: Obesity Center Cohort" Nutrients 15, no. 10: 2381. https://doi.org/10.3390/nu15102381
APA StyleDonghia, R., Schiano Di Cola, R., Cesaro, F., Vitale, A., Lippolis, G., Lisco, T., Isernia, R., De Pergola, G., De Nucci, S., Rinaldi, R., Liso, M., & Giardiello, C. (2023). Gender and Liver Steatosis Discriminate Different Physiological Patterns in Obese Patients Undergoing Bariatric Surgery: Obesity Center Cohort. Nutrients, 15(10), 2381. https://doi.org/10.3390/nu15102381






