Antioxidants and Sports Performance
Abstract
1. Introduction
2. Materials and Methods
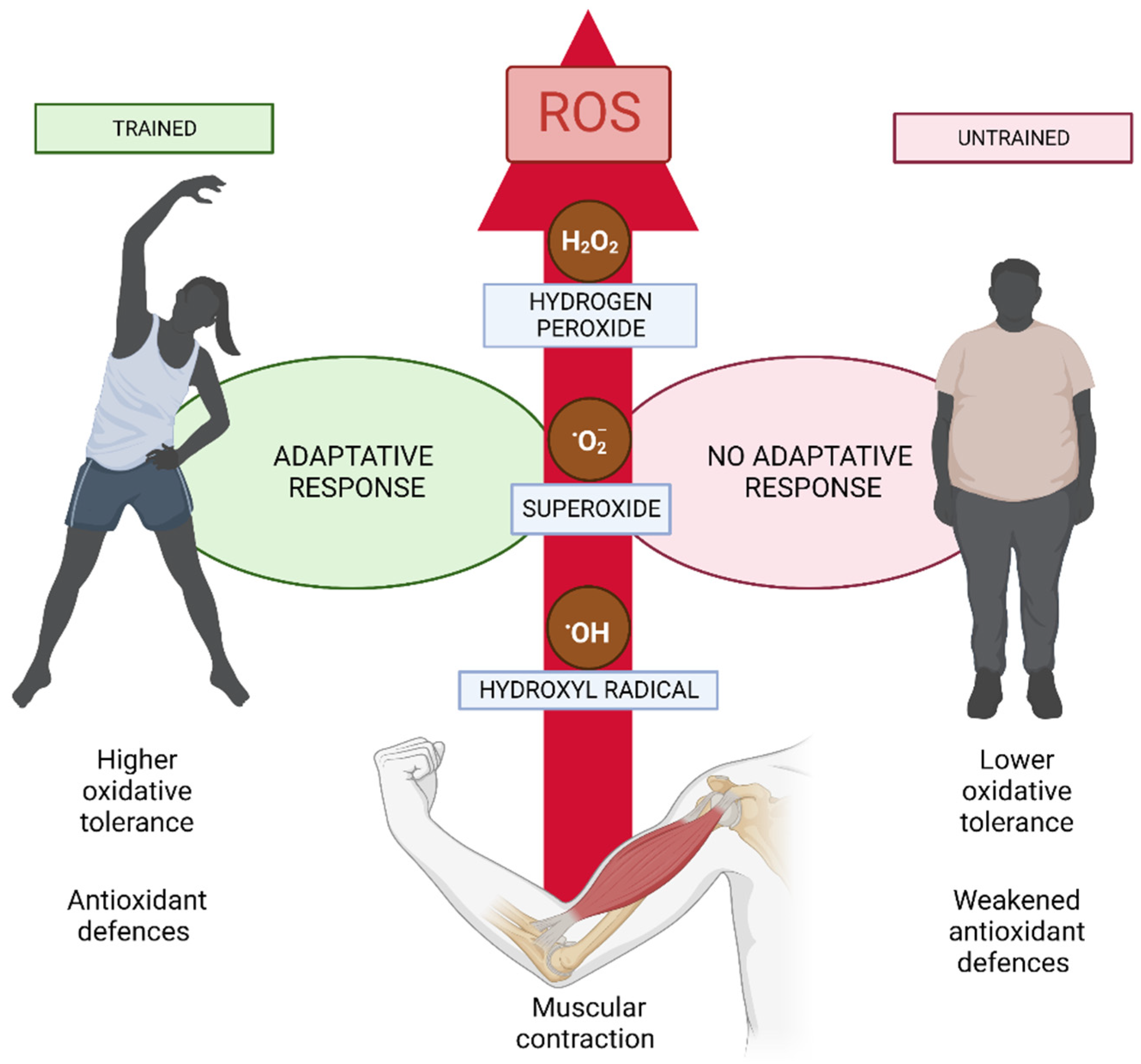
3. Physical Exercise and Reactive Oxygen Species Production
4. The Effect of Reactive Oxygen Species on Sports Performance
5. Training Adaptation and Reactive Oxygen Species
5.1. ROS in the Skeletal Muscle
5.2. ROS in Endurance Training
5.3. Role of Reactive Oxygen Species (ROS) in Resistance Training
6. Reactive Oxygen Species, Antioxidants, and Inflammation
6.1. The Effect of Antioxidants on Reactive Oxygen Species in Physical Activity
6.2. The Effect of Antioxidants on ROS and Inflammation in Sports
7. Antioxidant Capacity and the Microbiota
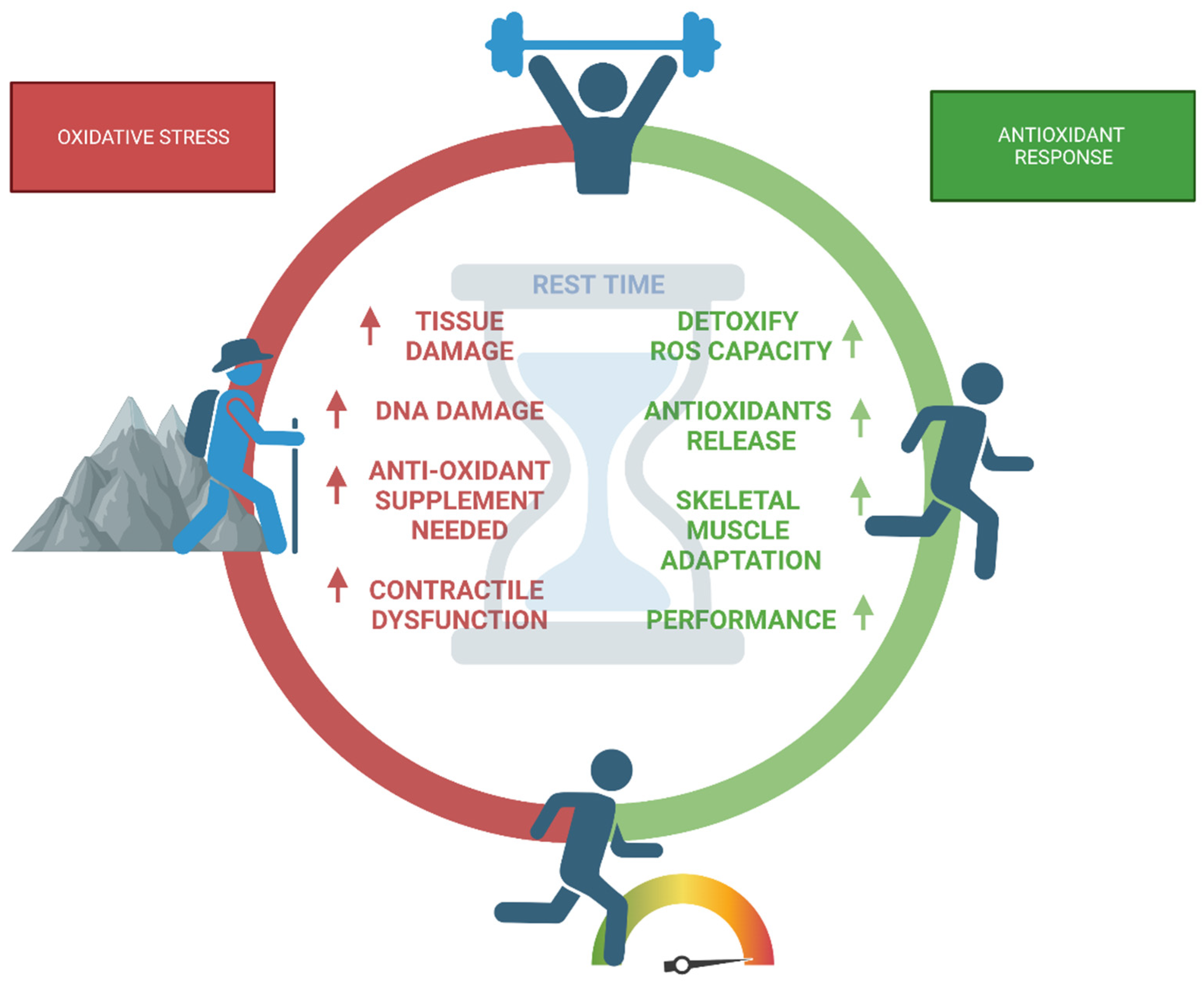
8. Antioxidants and Recovery from Training
9. The Effect of an Antioxidant Diet on Sports Performance
10. The Effect of Antioxidant Supplementation on Sports Performance
11. Key Points and Strategies for Antioxidant Supplementation in Sports Competitions
11.1. Vitamin C
11.2. Vitamin E
11.3. Resveratrol
11.4. Coenzyme Q10
11.5. Selenium
11.6. Curcumin
11.7. Omega-3
11.8. Zinc
11.9. Glutathione
12. Practical Applications
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Pan, M.; Liu, K.; Yang, J.; Liu, S.; Wang, S.; Wang, S. Advances on Food-Derived Peptidic Antioxidants—A Review. Antioxidants 2020, 9, 799. [Google Scholar] [CrossRef]
- Clarkson, P.M. Antioxidants and Physical Performance. Crit. Rev. Food Sci. Nutr. 1995, 35, 131–141. [Google Scholar] [CrossRef]
- De Sousa, C.V.; Sales, M.M.; Rosa, T.S.; Lewis, J.E.; de Andrade, R.V.; Simões, H.G. The Antioxidant Effect of Exercise: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 277–293. [Google Scholar] [CrossRef]
- McClean, C.; Davison, G.W. Circadian Clocks, Redox Homeostasis, and Exercise: Time to Connect the Dots? Antioxidants 2022, 11, 256. [Google Scholar] [CrossRef]
- Li, S.; Fasipe, B.; Laher, I. Potential Harms of Supplementation with High Doses of Antioxidants in Athletes. J. Exerc. Sci. Fit. 2022, 20, 269–275. [Google Scholar] [CrossRef]
- Novel Training and Other Strategies for Sport Performance at the 2011 ACSM Annual Meeting. Available online: http://www.sportsci.org/2011/wghACSM.htm (accessed on 3 April 2023).
- Bustamante-Sanchez, A.; Villegas-Mora, B.E.; Martínez-Guardado, I.; Tornero-Aguilera, J.F.; Ardigò, L.P.; Nobari, H.; Clemente-Suárez, V.J. Physical Activity and Nutritional Pattern Related to Maturation and Development. Sustainability 2022, 14, 16958. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Paschalis, V.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Redox Basis of Exercise Physiology. Redox Biol. 2020, 35, 101499. [Google Scholar] [CrossRef]
- Williamson, J.; Davison, G. Targeted Antioxidants in Exercise-Induced Mitochondrial Oxidative Stress: Emphasis on DNA Damage. Antioxidants 2020, 9, 1142. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Hopkins, W.G. Impact of Dietary Antioxidants on Sport Performance: A Review. Sports Med. 2015, 45, 939–955. [Google Scholar] [CrossRef]
- Righi, N.C.; Schuch, F.B.; De Nardi, A.T.; Pippi, C.M.; de Almeida Righi, G.; Puntel, G.O.; da Silva, A.M.V.; Signori, L.U. Effects of Vitamin C on Oxidative Stress, Inflammation, Muscle Soreness, and Strength Following Acute Exercise: Meta-Analyses of Randomized Clinical Trials. Eur. J. Nutr. 2020, 59, 2827–2839. [Google Scholar] [CrossRef]
- Higgins, M.R.; Izadi, A.; Kaviani, M. Antioxidants and Exercise Performance: With a Focus on Vitamin E and C Supplementation. Int. J. Env. Res. Public. Health 2020, 17, 8452. [Google Scholar] [CrossRef]
- Elejalde, E.; Villarán, M.C.; Alonso, R.M. Grape Polyphenols Supplementation for Exercise-Induced Oxidative Stress. J. Int. Soc. Sports Nutr. 2021, 18, 3. [Google Scholar] [CrossRef]
- Harper, S.A.; Bassler, J.R.; Peramsetty, S.; Yang, Y.; Roberts, L.M.; Drummer, D.; Mankowski, R.T.; Leeuwenburgh, C.; Ricart, K.; Patel, R.P.; et al. Resveratrol and Exercise Combined to Treat Functional Limitations in Late Life: A Pilot Randomized Controlled Trial. Exp. Gerontol. 2021, 143, 111111. [Google Scholar] [CrossRef]
- Jones, L.; Bailey, S.J.; Rowland, S.N.; Alsharif, N.; Shannon, O.M.; Clifford, T. The Effect of Nitrate-Rich Beetroot Juice on Markers of Exercise-Induced Muscle Damage: A Systematic Review and Meta-Analysis of Human Intervention Trials. J. Diet. Suppl. 2022, 19, 749–771. [Google Scholar] [CrossRef]
- Antioxidant, Anti-Inflammatory and Immunomodulatory Effects of Spirulina in Exercise and Sport: A Systematic Review—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/36590230/ (accessed on 3 April 2023).
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant Supplements and Endurance Exercise: Current Evidence and Mechanistic Insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef]
- Steinbacher, P.; Eckl, P. Impact of Oxidative Stress on Exercising Skeletal Muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Vasilaki, A.; Palomero, J.; Kayani, A.; Zibrik, L.; McArdle, A.; Jackson, M.J. Studies of Mitochondrial and Nonmitochondrial Sources Implicate Nicotinamide Adenine Dinucleotide Phosphate Oxidase(s) in the Increased Skeletal Muscle Superoxide Generation That Occurs during Contractile Activity. Antioxid. Redox Signal. 2013, 18, 603–621. [Google Scholar] [CrossRef]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Close, G.L.; Kayani, A.; McArdle, A.; Viña, J.; Jackson, M.J. Effect of Xanthine Oxidase-Generated Extracellular Superoxide on Skeletal Muscle Force Generation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R2-8. [Google Scholar] [CrossRef]
- Russell, E.G.; Cotter, T.G. New Insight into the Role of Reactive Oxygen Species (ROS) in Cellular Signal-Transduction Processes. Int. Rev. Cell Mol. Biol. 2015, 319, 221–254. [Google Scholar] [CrossRef]
- Alfadda, A.A.; Sallam, R.M. Reactive Oxygen Species in Health and Disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Wiegman, C.H.; Michaeloudes, C.; Haji, G.; Narang, P.; Clarke, C.J.; Russell, K.E.; Bao, W.; Pavlidis, S.; Barnes, P.J.; Kanerva, J.; et al. Oxidative Stress-Induced Mitochondrial Dysfunction Drives Inflammation and Airway Smooth Muscle Remodeling in Patients with Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2015, 136, 769–780. [Google Scholar] [CrossRef]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef]
- Iatsenko, I.; Boquete, J.-P.; Lemaitre, B. Microbiota-Derived Lactate Activates Production of Reactive Oxygen Species by the Intestinal NADPH Oxidase Nox and Shortens Drosophila Lifespan. Immunity 2018, 49, 929–942.e5. [Google Scholar] [CrossRef]
- Specht, K.S.; Kant, S.; Addington, A.K.; McMillan, R.P.; Hulver, M.W.; Learnard, H.; Campbell, M.; Donnelly, S.R.; Caliz, A.D.; Pei, Y.; et al. Nox4 Mediates Skeletal Muscle Metabolic Responses to Exercise. Mol. Metab. 2021, 45, 101160. [Google Scholar] [CrossRef]
- Zuo, L.; Zhou, T.; Pannell, B.K.; Ziegler, A.C.; Best, T.M. Biological and Physiological Role of Reactive Oxygen Species--the Good, the Bad and the Ugly. Acta Physiol. 2015, 214, 329–348. [Google Scholar] [CrossRef]
- Hidalgo, C.; Sánchez, G.; Barrientos, G.; Aracena-Parks, P. A Transverse Tubule NADPH Oxidase Activity Stimulates Calcium Release from Isolated Triads via Ryanodine Receptor Type 1 S -Glutathionylation. J. Biol. Chem. 2006, 281, 26473–26482. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive Oxygen Species: Impact on Skeletal Muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [CrossRef]
- Powers, S.K.; Schrager, M. Redox Signaling Regulates Skeletal Muscle Remodeling in Response to Exercise and Prolonged Inactivity. Redox Biol. 2022, 54, 102374. [Google Scholar] [CrossRef]
- Bouviere, J.; Fortunato, R.S.; Dupuy, C.; Werneck-de-Castro, J.P.; Carvalho, D.P.; Louzada, R.A. Exercise-Stimulated ROS Sensitive Signaling Pathways in Skeletal Muscle. Antioxidants 2021, 10, 537. [Google Scholar] [CrossRef]
- Yavari, A.; Javadi, M.; Mirmiran, P.; Bahadoran, Z. Exercise-Induced Oxidative Stress and Dietary Antioxidants. Asian J. Sports Med. 2015, 6, e24898. [Google Scholar] [CrossRef]
- Muthusamy, V.R.; Kannan, S.; Sadhaasivam, K.; Gounder, S.S.; Davidson, C.J.; Boeheme, C.; Hoidal, J.R.; Wang, L.; Rajasekaran, N.S. Acute Exercise Stress Activates Nrf2/ARE Signaling and Promotes Antioxidant Mechanisms in the Myocardium. Free Radic. Biol. Med. 2012, 52, 366–376. [Google Scholar] [CrossRef]
- Akimoto, T.; Pohnert, S.C.; Li, P.; Zhang, M.; Gumbs, C.; Rosenberg, P.B.; Williams, R.S.; Yan, Z. Exercise Stimulates Pgc-1alpha Transcription in Skeletal Muscle through Activation of the P38 MAPK Pathway. J. Biol. Chem. 2005, 280, 19587–19593. [Google Scholar] [CrossRef]
- Jäger, S.; Handschin, C.; St-Pierre, J.; Spiegelman, B.M. AMP-Activated Protein Kinase (AMPK) Action in Skeletal Muscle via Direct Phosphorylation of PGC-1alpha. Proc. Natl. Acad. Sci. USA 2007, 104, 12017–12022. [Google Scholar] [CrossRef]
- Espinosa, A.; Leiva, A.; Peña, M.; Müller, M.; Debandi, A.; Hidalgo, C.; Carrasco, M.A.; Jaimovich, E. Myotube Depolarization Generates Reactive Oxygen Species through NAD(P)H Oxidase; ROS-Elicited Ca2+ Stimulates ERK, CREB, Early Genes. J. Cell. Physiol. 2006, 209, 379–388. [Google Scholar] [CrossRef]
- Ruas, J.L.; White, J.P.; Rao, R.R.; Kleiner, S.; Brannan, K.T.; Harrison, B.C.; Greene, N.P.; Wu, J.; Estall, J.L.; Irving, B.A.; et al. A PGC-1α Isoform Induced by Resistance Training Regulates Skeletal Muscle Hypertrophy. Cell 2012, 151, 1319–1331. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Calbet, J.A.L. Free Radicals and Sprint Exercise in Humans. Free Radic. Res. 2014, 48, 30–42. [Google Scholar] [CrossRef]
- Kang, C.; O’Moore, K.M.; Dickman, J.R.; Ji, L.L. Exercise Activation of Muscle Peroxisome Proliferator-Activated Receptor-Gamma Coactivator-1alpha Signaling Is Redox Sensitive. Free Radic. Biol. Med. 2009, 47, 1394–1400. [Google Scholar] [CrossRef]
- Low, I.C.C.; Loh, T.; Huang, Y.; Virshup, D.M.; Pervaiz, S. Ser70 Phosphorylation of Bcl-2 by Selective Tyrosine Nitration of PP2A-B56δ Stabilizes Its Antiapoptotic Activity. Blood 2014, 124, 2223–2234. [Google Scholar] [CrossRef]
- Thirupathi, A.; Wang, M.; Lin, J.K.; Fekete, G.; István, B.; Baker, J.S.; Gu, Y. Effect of Different Exercise Modalities on Oxidative Stress: A Systematic Review. Biomed. Res. Int. 2021, 2021, 1947928. [Google Scholar] [CrossRef]
- Orlando, P.; Silvestri, S.; Galeazzi, R.; Antonicelli, R.; Marcheggiani, F.; Cirilli, I.; Bacchetti, T.; Tiano, L. Effect of Ubiquinol Supplementation on Biochemical and Oxidative Stress Indexes after Intense Exercise in Young Athletes. Redox Rep. 2018, 23, 136–145. [Google Scholar] [CrossRef]
- Nikolaidis, M.G.; Jamurtas, A.Z.; Paschalis, V.; Fatouros, I.G.; Koutedakis, Y.; Kouretas, D. The Effect of Muscle-Damaging Exercise on Blood and Skeletal Muscle Oxidative Stress: Magnitude and Time-Course Considerations. Sports Med. 2008, 38, 579–606. [Google Scholar] [CrossRef]
- Di Meo, S.; Napolitano, G.; Venditti, P. Mediators of Physical Activity Protection against ROS-Linked Skeletal Muscle Damage. Int. J. Mol. Sci. 2019, 20, 3024. [Google Scholar] [CrossRef]
- Sawada, Y.; Ichikawa, H.; Ebine, N.; Minamiyama, Y.; Alharbi, A.A.D.; Iwamoto, N.; Fukuoka, Y. Effects of High-Intensity Anaerobic Exercise on the Scavenging Activity of Various Reactive Oxygen Species and Free Radicals in Athletes. Nutrients 2023, 15, 222. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C.; Saran, M.; Lengfelder, E. The Involvement of Oxygen Radicals during the Autoxidation of Adrenalin. Biochim. Biophys. Acta 1978, 540, 162–172. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Oxidative Stress and Redox Regulation: Adaptation, Damage, Repair, Senescence, and Death. In Free Radicals in Biology and Medicine; Halliwell, B., Gutteridge, J.M.C., Eds.; Oxford University Press: Oxford, UK, 2015; ISBN 978-0-19-871747-8. [Google Scholar]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-Induced Oxidative Stress: Friend or Foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Henríquez-Olguín, C.; Renani, L.B.; Arab-Ceschia, L.; Raun, S.H.; Bhatia, A.; Li, Z.; Knudsen, J.R.; Holmdahl, R.; Jensen, T.E. Adaptations to High-Intensity Interval Training in Skeletal Muscle Require NADPH Oxidase 2. Redox Biol. 2019, 24, 101188. [Google Scholar] [CrossRef]
- Radak, Z.; Suzuki, K.; Higuchi, M.; Balogh, L.; Boldogh, I.; Koltai, E. Physical Exercise, Reactive Oxygen Species and Neuroprotection. Free Radic. Biol. Med. 2016, 98, 187–196. [Google Scholar] [CrossRef]
- Miyata, M.; Kasai, H.; Kawai, K.; Yamada, N.; Tokudome, M.; Ichikawa, H.; Goto, C.; Tokudome, Y.; Kuriki, K.; Hoshino, H.; et al. Changes of Urinary 8-Hydroxydeoxyguanosine Levels during a Two-Day Ultramarathon Race Period in Japanese Non-Professional Runners. Int. J. Sports Med. 2008, 29, 27–33. [Google Scholar] [CrossRef]
- Azizbeigi, K.; Stannard, S.R.; Atashak, S.; Mosalman Haghighi, M. Antioxidant Enzymes and Oxidative Stress Adaptation to Exercise Training: Comparison of Endurance, Resistance, and Concurrent Training in Untrained Males. J. Exerc. Sci. Fit. 2014, 12, 1–6. [Google Scholar] [CrossRef]
- Hattori, N.; Hayashi, T.; Nakachi, K.; Ichikawa, H.; Goto, C.; Tokudome, Y.; Kuriki, K.; Hoshino, H.; Shibata, K.; Yamada, N.; et al. Changes of ROS during a Two-Day Ultra-Marathon Race. Int. J. Sports Med. 2009, 30, 426–429. [Google Scholar] [CrossRef]
- Belinchón-Demiguel, P.; Clemente-Suárez, V.J. Nutrition, hydration and ergogenic aids strategies in ultraendurance mountain events. J. Sports Med. Phys. Fit. 2019, 59, 791–797. [Google Scholar] [CrossRef]
- Close, G.L.; Ashton, T.; Cable, T.; Doran, D.; MacLaren, D.P.M. Eccentric Exercise, Isokinetic Muscle Torque and Delayed Onset Muscle Soreness: The Role of Reactive Oxygen Species. Eur. J. Appl. Physiol. 2004, 91, 615–621. [Google Scholar] [CrossRef]
- Miller, L.E.; McGinnis, G.R.; Kliszczewicz, B.; Slivka, D.; Hailes, W.; Cuddy, J.; Dumke, C.; Ruby, B.; Quindry, J.C. Blood Oxidative-Stress Markers during a High-Altitude Trek. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 65–72. [Google Scholar] [CrossRef]
- Siques, P.; Brito, J.; Pena, E. Reactive Oxygen Species and Pulmonary Vasculature During Hypobaric Hypoxia. Front. Physiol. 2018, 9, 865. [Google Scholar] [CrossRef]
- Gaur, P.; Prasad, S.; Kumar, B.; Sharma, S.K.; Vats, P. High-Altitude Hypoxia Induced Reactive Oxygen Species Generation, Signaling, and Mitigation Approaches. Int. J. Biometeorol. 2021, 65, 601–615. [Google Scholar] [CrossRef]
- Jackson, M.J.; Pye, D.; Palomero, J. The Production of Reactive Oxygen and Nitrogen Species by Skeletal Muscle. J. Appl. Physiol. 2007, 102, 1664–1670. [Google Scholar] [CrossRef]
- Urso, M.L.; Clarkson, P.M. Oxidative Stress, Exercise, and Antioxidant Supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.-P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; St-Pierre, J.; Jones, R.G. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Affourtit, C.; Esteves, T.C.; Green, K.; Lambert, A.J.; Miwa, S.; Pakay, J.L.; Parker, N. Mitochondrial Superoxide: Production, Biological Effects, and Activation of Uncoupling Proteins. Free. Radic. Biol. Med. 2004, 37, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, S.; Venditti, P. Mitochondria in Exercise-Induced Oxidative Stress. Biol. Signals Recept. 2001, 10, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.V.; Szalay, L.; Umar, F.; Kropik, K.; Staniek, K.; Niedermüller, H.; Bahrami, S.; Nohl, H. Skeletal Muscles, Heart, and Lung Are the Main Sources of Oxygen Radicals in Old Rats. Biochim. Biophys. Acta 2005, 1740, 382–389. [Google Scholar] [CrossRef]
- Fittipaldi, S.; Dimauro, I.; Mercatelli, N.; Caporossi, D. Role of Exercise-Induced Reactive Oxygen Species in the Modulation of Heat Shock Protein Response. Free. Radic. Res. 2014, 48, 52–70. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.; Bloomer, R.J. Acute Exercise and Oxidative Stress: A 30 Year History. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef]
- Powers, S.K.; Nelson, W.B.; Hudson, M.B. Exercise-Induced Oxidative Stress in Humans: Cause and Consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.-C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral Administration of Vitamin C Decreases Muscle Mitochondrial Biogenesis and Hampers Training-Induced Adaptations in Endurance Performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Klöting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Blüher, M. Antioxidants Prevent Health-Promoting Effects of Physical Exercise in Humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Yfanti, C.; Akerström, T.; Nielsen, S.; Nielsen, A.R.; Mounier, R.; Mortensen, O.H.; Lykkesfeldt, J.; Rose, A.J.; Fischer, C.P.; Pedersen, B.K. Antioxidant Supplementation Does Not Alter Endurance Training Adaptation. Med. Sci. Sports Exerc. 2010, 42, 1388–1395. [Google Scholar] [CrossRef]
- Effect of Antioxidant Supplementation on Insulin Sensitivity in Response to Endurance Exercise Training—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21325105/ (accessed on 3 April 2023).
- Strobel, N.A.; Peake, J.M.; Matsumoto, A.; Marsh, S.A.; Coombes, J.S.; Wadley, G.D. Antioxidant Supplementation Reduces Skeletal Muscle Mitochondrial Biogenesis. Med. Sci. Sports Exerc. 2011, 43, 1017–1024. [Google Scholar] [CrossRef]
- Holloszy, J.O. Regulation by Exercise of Skeletal Muscle Content of Mitochondria and GLUT4. J. Physiol. Pharm. 2008, 59 (Suppl. S7), 5–18. [Google Scholar]
- Powers, S.K.; Talbert, E.E.; Adhihetty, P.J. Reactive Oxygen and Nitrogen Species as Intracellular Signals in Skeletal Muscle. J. Physiol. 2011, 589, 2129–2138. [Google Scholar] [CrossRef]
- Sahlin, K.; Shabalina, I.G.; Mattsson, C.M.; Bakkman, L.; Fernström, M.; Rozhdestvenskaya, Z.; Enqvist, J.K.; Nedergaard, J.; Ekblom, B.; Tonkonogi, M. Ultraendurance Exercise Increases the Production of Reactive Oxygen Species in Isolated Mitochondria from Human Skeletal Muscle. J. Appl. Physiol. 2010, 108, 780–787. [Google Scholar] [CrossRef]
- Powers, S.K.; Smuder, A.J.; Kavazis, A.N.; Hudson, M.B. Experimental Guidelines for Studies Designed to Investigate the Impact of Antioxidant Supplementation on Exercise Performance. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 2–14. [Google Scholar] [CrossRef]
- Hood, D.A.; Irrcher, I.; Ljubicic, V.; Joseph, A.-M. Coordination of Metabolic Plasticity in Skeletal Muscle. J. Exp. Biol. 2006, 209, 2265–2275. [Google Scholar] [CrossRef]
- Murgia, M.; Jensen, T.E.; Cusinato, M.; Garcia, M.; Richter, E.A.; Schiaffino, S. Multiple Signalling Pathways Redundantly Control Glucose Transporter GLUT4 Gene Transcription in Skeletal Muscle. J. Physiol. 2009, 587, 4319–4327. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do Antioxidant Supplements Interfere with Skeletal Muscle Adaptation to Exercise Training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef]
- Niki, E. Oxidative Stress and Antioxidants: Distress or Eustress? Arch. Biochem. Biophys. 2016, 595, 19–24. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox Regulation of Cell Survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: A Concept in Redox Biology and Medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Moylan, J.S.; Reid, M.B. Oxidative Stress, Chronic Disease, and Muscle Wasting. Muscle Nerve 2007, 35, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age-Related Diseases and Cancer. Recent. Pat. Inflamm. Allergy Drug. Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- García-López, D.; Häkkinen, K.; Cuevas, M.J.; Lima, E.; Kauhanen, A.; Mattila, M.; Sillanpää, E.; Ahtiainen, J.P.; Karavirta, L.; Almar, M.; et al. Effects of Strength and Endurance Training on Antioxidant Enzyme Gene Expression and Activity in Middle-Aged Men. Scand. J. Med. Sci. Sports 2007, 17, 595–604. [Google Scholar] [CrossRef]
- Cakir-Atabek, H.; Demir, S.; PinarbaŞili, R.D.; Gündüz, N. Effects of Different Resistance Training Intensity on Indices of Oxidative Stress. J. Strength. Cond. Res. 2010, 24, 2491–2497. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kwak, Y.-S. Impact of Aerobic and Anaerobic Exercise Training on Oxidative Stress and Antioxidant Defense in Athletes. J. Exerc. Rehabil. 2016, 12, 113–117. [Google Scholar] [CrossRef]
- Parise, G.; Phillips, S.M.; Kaczor, J.J.; Tarnopolsky, M.A. Antioxidant Enzyme Activity Is Up-Regulated after Unilateral Resistance Exercise Training in Older Adults. Free Radic. Biol. Med. 2005, 39, 289–295. [Google Scholar] [CrossRef]
- Vincent, K.R.; Vincent, H.K.; Braith, R.W.; Lennon, S.L.; Lowenthal, D.T. Resistance Exercise Training Attenuates Exercise-Induced Lipid Peroxidation in the Elderly. Eur. J. Appl. Physiol. 2002, 87, 416–423. [Google Scholar] [CrossRef]
- Padilha, C.S.; Ribeiro, A.S.; Fleck, S.J.; Nascimento, M.A.; Pina, F.L.C.; Okino, A.M.; Venturini, D.; Barbosa, D.S.; Mayhew, J.L.; Cyrino, E.S. Effect of Resistance Training with Different Frequencies and Detraining on Muscular Strength and Oxidative Stress Biomarkers in Older Women. Age 2015, 37, 104. [Google Scholar] [CrossRef]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef]
- Pingitore, A.; Lima, G.P.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and Oxidative Stress: Potential Effects of Antioxidant Dietary Strategies in Sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef]
- Brisswalter, J.; Louis, J. Vitamin Supplementation Benefits in Master Athletes. Sports Med. 2014, 44, 311–318. [Google Scholar] [CrossRef]
- Milan, S.J.; Hart, A.; Wilkinson, M. Vitamin C for Asthma and Exercise-Induced Bronchoconstriction. Cochrane Database Syst. Rev. 2013, 2013, CD010391. [Google Scholar] [CrossRef]
- Myburgh, K.H. Polyphenol Supplementation: Benefits for Exercise Performance or Oxidative Stress? Sports Med. 2014, 44, S57–S70. [Google Scholar] [CrossRef]
- Dato, S.; Crocco, P.; D’Aquila, P.; de Rango, F.; Bellizzi, D.; Rose, G.; Passarino, G. Exploring the Role of Genetic Variability and Lifestyle in Oxidative Stress Response for Healthy Aging and Longevity. Int. J. Mol. Sci. 2013, 14, 16443–16472. [Google Scholar] [CrossRef]
- Cureton, K.J.; Tomporowski, P.D.; Singhal, A.; Pasley, J.D.; Bigelman, K.A.; Lambourne, K.; Trilk, J.L.; McCully, K.K.; Arnaud, M.J.; Zhao, Q. Dietary Quercetin Supplementation Is Not Ergogenic in Untrained Men. J. Appl. Physiol. 2009, 107, 1095–1104. [Google Scholar] [CrossRef]
- Nanri, A.; Yoshida, D.; Yamaji, T.; Mizoue, T.; Takayanagi, R.; Kono, S. Dietary Patterns and C-Reactive Protein in Japanese Men and Women. Am. J. Clin. Nutr. 2008, 87, 1488–1496. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Garcia-Arellano, A.; Estruch, R.; Marquez-Sandoval, F.; Corella, D.; Fiol, M.; Gómez-Gracia, E.; Viñoles, E.; Arós, F.; Herrera, C.; et al. Components of the Mediterranean-Type Food Pattern and Serum Inflammatory Markers among Patients at High Risk for Cardiovascular Disease. Eur. J. Clin. Nutr. 2008, 62, 651–659. [Google Scholar] [CrossRef]
- Holt, E.M.; Steffen, L.M.; Moran, A.; Basu, S.; Steinberger, J.; Ross, J.A.; Hong, C.-P.; Sinaiko, A.R. Fruit and Vegetable Consumption and Its Relation to Markers of Inflammation and Oxidative Stress in Adolescents. J. Am. Diet. Assoc. 2009, 109, 414–421. [Google Scholar] [CrossRef]
- Karlsen, A.; Retterstøl, L.; Laake, P.; Paur, I.; Bøhn, S.K.; Sandvik, L.; Blomhoff, R. Anthocyanins Inhibit Nuclear Factor-KappaB Activation in Monocytes and Reduce Plasma Concentrations of pro-Inflammatory Mediators in Healthy Adults. J. Nutr. 2007, 137, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.E.; Olsen, T.; Paur, I.; Paulsen, G.; Bastani, N.E.; Garthe, I.; Raastad, T.; Matthews, J.; Blomhoff, R.; Bøhn, S.K. Effects of Antioxidant-Rich Foods on Altitude-Induced Oxidative Stress and Inflammation in Elite Endurance Athletes: A Randomized Controlled Trial. PLoS ONE 2019, 14, e0217895. [Google Scholar] [CrossRef] [PubMed]
- Knab, A.M.; Nieman, D.C.; Gillitt, N.D.; Shanely, R.A.; Cialdella-Kam, L.; Henson, D.A.; Sha, W. Effects of a Flavonoid-Rich Juice on Inflammation, Oxidative Stress, and Immunity in Elite Swimmers: A Metabolomics-Based Approach. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Shanely, R.A.; Nieman, D.C.; Perkins-Veazie, P.; Henson, D.A.; Meaney, M.P.; Knab, A.M.; Cialdell-Kam, L. Comparison of Watermelon and Carbohydrate Beverage on Exercise-Induced Alterations in Systemic Inflammation, Immune Dysfunction, and Plasma Antioxidant Capacity. Nutrients 2016, 8, 518. [Google Scholar] [CrossRef] [PubMed]
- Ammar, A.; Turki, M.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Kallel, C.; Abdelkarim, O.; Hoekelmann, A.; Bouaziz, M.; Ayadi, F.; et al. Pomegranate Supplementation Accelerates Recovery of Muscle Damage and Soreness and Inflammatory Markers after a Weightlifting Training Session. PLoS ONE 2016, 11, e0160305. [Google Scholar] [CrossRef]
- Bailey, D.M.; Williams, C.; Betts, J.A.; Thompson, D.; Hurst, T.L. Oxidative Stress, Inflammation and Recovery of Muscle Function after Damaging Exercise: Effect of 6-Week Mixed Antioxidant Supplementation. Eur. J. Appl. Physiol. 2011, 111, 925–936. [Google Scholar] [CrossRef]
- Mastaloudis, A.; Morrow, J.D.; Hopkins, D.W.; Devaraj, S.; Traber, M.G. Antioxidant Supplementation Prevents Exercise-Induced Lipid Peroxidation, but Not Inflammation, in Ultramarathon Runners. Free Radic. Biol. Med. 2004, 36, 1329–1341. [Google Scholar] [CrossRef]
- Teixeira, V.H.; Valente, H.F.; Casal, S.I.; Marques, A.F.; Moreira, P.A. Antioxidants Do Not Prevent Postexercise Peroxidation and May Delay Muscle Recovery. Med. Sci. Sports Exerc. 2009, 41, 1752–1760. [Google Scholar] [CrossRef]
- Cani, P.D.; Van Hul, M.; Lefort, C.; Depommier, C.; Rastelli, M.; Everard, A. Microbial Regulation of Organismal Energy Homeostasis. Nat. Metab. 2019, 1, 34–46. [Google Scholar] [CrossRef]
- Cataldi, S.; Bonavolontà, V.; Poli, L.; Clemente, F.M.; De Candia, M.; Carvutto, R.; Silva, A.F.; Badicu, G.; Greco, G.; Fischetti, F. The Relationship between Physical Activity, Physical Exercise, and Human Gut Microbiota in Healthy and Unhealthy Subjects: A Systematic Review. Biology 2022, 11, 479. [Google Scholar] [CrossRef]
- Gazerani, P. Migraine and Diet. Nutrients 2020, 12, 1658. [Google Scholar] [CrossRef]
- Cerdá, B.; Pérez, M.; Pérez-Santiago, J.D.; Tornero-Aguilera, J.F.; González-Soltero, R.; Larrosa, M. Gut Microbiota Modification: Another Piece in the Puzzle of the Benefits of Physical Exercise in Health? Front. Physiol. 2016, 7, 51. [Google Scholar] [CrossRef]
- Kanter, M.M. Nutritional Antioxidants and Physical Activity. In Nutrition in Exercise and Sport, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Arias Calvo, A.; Feijoo Costa, G.; Moreira Vilar, M.T. Exploring the Potential of Antioxidants from Fruits and Vegetables and Strategies for Their Recovery. Innov. Food Sci. Emerg. Technol. 2022, 77, 102974. [Google Scholar] [CrossRef]
- Álvarez, J.; Fernández Real, J.M.; Guarner, F.; Gueimonde, M.; Rodríguez, J.M.; Saenz de Pipaon, M.; Sanz, Y. Gut Microbes and Health. Gastroenterol. Hepatol. 2021, 44, 519–535. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and Human Health. Pharm. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef]
- Ekpe, L.; Inaku, K.; Ekpe, V. Antioxidant Effects of Astaxanthin in Various Diseasesa Review. J. Mol. Pathophysiol. 2018, 7, 1. [Google Scholar] [CrossRef]
- Wu, L.; Lu, P.; Guo, X.; Song, K.; Lyu, Y.; Bothwell, J.; Wu, J.; Hawkins, O.; Clarke, S.L.; Lucas, E.A.; et al. β-Carotene Oxygenase 2 Deficiency-Triggered Mitochondrial Oxidative Stress Promotes Low-Grade Inflammation and Metabolic Dysfunction. Free Radic. Biol. Med. 2021, 164, 271–284. [Google Scholar] [CrossRef]
- Honarbakhsh, M.; Malta, K.; Ericsson, A.; Holloway, C.; Kim, Y.-K.; Hammerling, U.; Quadro, L. β-Carotene Improves Fecal Dysbiosis and Intestinal Dysfunctions in a Mouse Model of Vitamin A Deficiency. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2022, 1867, 159122. [Google Scholar] [CrossRef]
- Deis, L.; Quiroga, A.M.; De Rosas, M.I. Coloured Compounds in Fruits and Vegetables and Health. In Psychiatry and Neuroscience Update: From Epistemology to Clinical Psychiatry—Vol. IV.; Gargiulo, P.Á., Mesones Arroyo, H.L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 343–358. ISBN 978-3-030-61721-9. [Google Scholar]
- Hollman, P.C.H.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The Biological Relevance of Direct Antioxidant Effects of Polyphenols for Cardiovascular Health in Humans Is Not Established. J. Nutr. 2011, 141, 989S–1009S. [Google Scholar] [CrossRef] [PubMed]
- Pallauf, K.; Giller, K.; Huebbe, P.; Rimbach, G. Nutrition and Healthy Ageing: Calorie Restriction or Polyphenol-Rich “MediterrAsian” Diet? Oxidative Med. Cell. Longev. 2013, 2013, e707421. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S. Food Flavonols: Nutraceuticals with Complex Health Benefits and Functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- Flavonoids: Biosynthesis, Metabolism, Mechanism of Antioxidation and Clinical Implications: A Review. Available online: https://arccjournals.com/journal/agricultural-reviews/R-1922 (accessed on 3 April 2023).
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Gomes, J.V.P.; Rigolon, T.C.B.; da Silveira Souza, M.S.; Alvarez-Leite, J.I.; Della Lucia, C.M.; Martino, H.S.D.; Rosa, C.D.O.B. Antiobesity Effects of Anthocyanins on Mitochondrial Biogenesis, Inflammation, and Oxidative Stress: A Systematic Review. Nutrition 2019, 66, 192–202. [Google Scholar] [CrossRef]
- Chan, S.W.; Chu, T.T.W.; Choi, S.W.; Benzie, I.F.F.; Tomlinson, B. Impact of Short-Term Bilberry Supplementation on Glycemic Control, Cardiovascular Disease Risk Factors, and Antioxidant Status in Chinese Patients with Type 2 Diabetes. Phytother. Res. 2021, 35, 3236–3245. [Google Scholar] [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of Gut Microbiota, Microbial Metabolites and Interaction with Polyphenol in Host Immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef]
- Otten, A.T.; Bourgonje, A.R.; Peters, V.; Alizadeh, B.Z.; Dijkstra, G.; Harmsen, H.J.M. Vitamin C Supplementation in Healthy Individuals Leads to Shifts of Bacterial Populations in the Gut-A Pilot Study. Antioxidants 2021, 10, 1278. [Google Scholar] [CrossRef]
- Pawlowska, E.; Szczepanska, J.; Blasiak, J. Pro- and Antioxidant Effects of Vitamin C in Cancer in Correspondence to Its Dietary and Pharmacological Concentrations. Oxid. Med. Cell. Longev. 2019, 2019, 7286737. [Google Scholar] [CrossRef]
- Blaner, W.S.; Shmarakov, I.O.; Traber, M.G. Vitamin A and Vitamin E: Will the Real Antioxidant Please Stand Up? Annu. Rev. Nutr. 2021, 41, 105–131. [Google Scholar] [CrossRef]
- Orsavová, J.; Hlaváčová, I.; Mlček, J.; Snopek, L.; Mišurcová, L. Contribution of Phenolic Compounds, Ascorbic Acid and Vitamin E to Antioxidant Activity of Currant (Ribes L.) and Gooseberry (Ribes Uva-Crispa L.) Fruits. Food Chem. 2019, 284, 323–333. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Kim, S.; Lee, J.; Ha, J.; Oh, H.; Lee, Y.; Kim, Y.; Yoon, Y. Vitamin E (α-Tocopherol) Consumption Influences Gut Microbiota Composition. Int. J. Food Sci. Nutr. 2020, 71, 221–225. [Google Scholar] [CrossRef]
- Prasad, A.S.; Bao, B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: Clinical Therapeutic Implications. Antioxidants 2019, 8, 164. [Google Scholar] [CrossRef]
- Barma, M.D.; Muthupandiyan, I.; Samuel, S.R.; Amaechi, B.T. Inhibition of Streptococcus Mutans, Antioxidant Property and Cytotoxicity of Novel Nano-Zinc Oxide Varnish. Arch. Oral. Biol. 2021, 126, 105132. [Google Scholar] [CrossRef]
- Chinni, V.; El-Khoury, J.; Perera, M.; Bellomo, R.; Jones, D.; Bolton, D.; Ischia, J.; Patel, O. Zinc Supplementation as an Adjunct Therapy for COVID-19: Challenges and Opportunities. Br. J. Clin. Pharm. 2021, 87, 3737–3746. [Google Scholar] [CrossRef]
- Full Article: Use of Biological Nano Zinc as a Feed Additive in Quail Nutrition: Biosynthesis, Antimicrobial Activity and Its Effect on Growth, Feed Utilisation, Blood Metabolites and Intestinal Microbiota. Available online: https://www.tandfonline.com/doi/full/10.1080/1828051X.2021.1886001 (accessed on 3 April 2023).
- Skalny, A.V.; Aschner, M.; Lei, X.G.; Gritsenko, V.A.; Santamaria, A.; Alekseenko, S.I.; Prakash, N.T.; Chang, J.-S.; Sizova, E.A.; Chao, J.C.J.; et al. Gut Microbiota as a Mediator of Essential and Toxic Effects of Zinc in the Intestines and Other Tissues. Int. J. Mol. Sci. 2021, 22, 13074. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, C.; Luo, R.; Cao, X.; Liu, M.; Gu, Q.; Li, F.; Li, J.; Wu, X.; Yang, Z.; et al. Integrative Analysis of Multiomics Data Identifies Selenium-Related Gene ALAD Associating with Keshan Disease. Free Radic. Biol. Med. 2022, 193, 702–719. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, S.; Li, T.; Huang, X.; Dong, Y.; Wang, P.; Huang, J. Advances in the Study of the Mechanism by Which Selenium and Selenoproteins Boost Immunity to Prevent Food Allergies. Nutrients 2022, 14, 3133. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef]
- Mushtaq, M.; Fatima, M.; Hussain Shah, S.Z.; Khan, N.; Naveed, S.; Khan, M. Effects of Sodium Selenite, Selenium Methionine, and Selenium Yeast on Growth Performance, Carcass Composition, Blood Biochemistry, and Antioxidant Status of Intensively Reared Hypophthalmichthys Molitrix. Aquac. Rep. 2022, 24, 101182. [Google Scholar] [CrossRef]
- Yu, M.; Yue, J.; Hui, N.; Zhi, Y.; Hayat, K.; Yang, X.; Zhang, D.; Chu, S.; Zhou, P. Anti-Hyperlipidemia and Gut Microbiota Community Regulation Effects of Selenium-Rich Cordyceps Militaris Polysaccharides on the High-Fat Diet-Fed Mice Model. Foods 2021, 10, 2252. [Google Scholar] [CrossRef] [PubMed]
- Naliyadhara, N.; Kumar, A.; Kumar Gangwar, S.; Nair Devanarayanan, T.; Hegde, M.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A. Interplay of Dietary Antioxidants and Gut Microbiome in Human Health: What Has Been Learnt Thus Far? J. Funct. Foods 2023, 100, 105365. [Google Scholar] [CrossRef]
- Li, Z.; Dong, Y.; Chen, S.; Jia, X.; Jiang, X.; Che, L.; Lin, Y.; Li, J.; Feng, B.; Fang, Z.; et al. Organic Selenium Increased Gilts Antioxidant Capacity, Immune Function, and Changed Intestinal Microbiota. Front. Microbiol. 2021, 12, 723190. [Google Scholar] [CrossRef]
- Effect of Different Dietary Selenium Sources on Growth Performance, Antioxidant Capacity, Gut Microbiota, and Molecular Responses in Pacific White Shrimp Litopenaeus Vannamei. Available online: https://www.hindawi.com/journals/anu/2022/5738008/ (accessed on 3 April 2023).
- Reid, M.B. Redox Interventions to Increase Exercise Performance. J. Physiol. 2016, 594, 5125–5133. [Google Scholar] [CrossRef]
- Slattery, K.; Bentley, D.; Coutts, A.J. The Role of Oxidative, Inflammatory and Neuroendocrinological Systems during Exercise Stress in Athletes: Implications of Antioxidant Supplementation on Physiological Adaptation during Intensified Physical Training. Sports Med. 2015, 45, 453–471. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Cobley, J.N.; Paschalis, V.; Veskoukis, A.S.; Theodorou, A.A.; Kyparos, A.; Nikolaidis, M.G. Principles for Integrating Reactive Species into in Vivo Biological Processes: Examples from Exercise Physiology. Cell. Signal. 2016, 28, 256–271. [Google Scholar] [CrossRef]
- Tsai, K.; Hsu, T.G.; Hsu, K.M.; Cheng, H.; Liu, T.Y.; Hsu, C.F.; Kong, C.W. Oxidative DNA Damage in Human Peripheral Leukocytes Induced by Massive Aerobic Exercise. Free Radic. Biol. Med. 2001, 31, 1465–1472. [Google Scholar] [CrossRef]
- Torre, M.F.; Martinez-Ferran, M.; Vallecillo, N.; Jiménez, S.L.; Romero-Morales, C.; Pareja-Galeano, H. Supplementation with Vitamins C and E and Exercise-Induced Delayed-Onset Muscle Soreness: A Systematic Review. Antioxidants 2021, 10, 279. [Google Scholar] [CrossRef]
- Canals-Garzón, C.; Guisado-Barrilao, R.; Martínez-García, D.; Chirosa-Ríos, I.J.; Jerez-Mayorga, D.; Guisado-Requena, I.M. Effect of Antioxidant Supplementation on Markers of Oxidative Stress and Muscle Damage after Strength Exercise: A Systematic Review. Int. J. Env. Res. Public. Health 2022, 19, 1803. [Google Scholar] [CrossRef]
- Costello, J.T.; Baker, P.R.A.; Minett, G.M.; Bieuzen, F.; Stewart, I.B.; Bleakley, C. Whole-Body Cryotherapy (Extreme Cold Air Exposure) for Preventing and Treating Muscle Soreness after Exercise in Adults. Cochrane Database Syst. Rev. 2015, 2015, CD010789. [Google Scholar] [CrossRef]
- Ranchordas, M.K.; Rogerson, D.; Soltani, H.; Costello, J.T. Antioxidants for Preventing and Reducing Muscle Soreness after Exercise: A Cochrane Systematic Review. Br. J. Sports Med. 2020, 54, 74–78. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of Tart Cherry Juice on Indices of Recovery Following Marathon Running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef]
- Leonardo-Mendonça, R.C.; Ocaña-Wilhelmi, J.; de Haro, T.; de Teresa-Galván, C.; Guerra-Hernández, E.; Rusanova, I.; Fernández-Ortiz, M.; Sayed, R.K.A.; Escames, G.; Acuña-Castroviejo, D. The Benefit of a Supplement with the Antioxidant Melatonin on Redox Status and Muscle Damage in Resistance-Trained Athletes. Appl. Physiol. Nutr. Metab. 2017, 42, 700–707. [Google Scholar] [CrossRef]
- Ortiz-Franco, M.; Planells, E.; Quintero, B.; Acuña-Castroviejo, D.; Rusanova, I.; Escames, G.; Molina-López, J. Effect of Melatonin Supplementation on Antioxidant Status and DNA Damage in High Intensity Trained Athletes. Int. J. Sports Med. 2017, 38, 1117–1125. [Google Scholar] [CrossRef]
- Stefan, M.; Sharp, M.; Gheith, R.; Lowery, R.; Ottinger, C.; Wilson, J.; Durkee, S.; Bellamine, A. L-Carnitine Tartrate Supplementation for 5 Weeks Improves Exercise Recovery in Men and Women: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2021, 13, 3432. [Google Scholar] [CrossRef]
- Silva, L.A.; Pinho, C.A.; Silveira, P.C.L.; Tuon, T.; De Souza, C.T.; Dal-Pizzol, F.; Pinho, R.A. Vitamin E Supplementation Decreases Muscular and Oxidative Damage but Not Inflammatory Response Induced by Eccentric Contraction. J. Physiol. Sci. 2010, 60, 51–57. [Google Scholar] [CrossRef]
- De Oliveira, D.C.X.; Rosa, F.T.; Simões-Ambrósio, L.; Jordao, A.A.; Deminice, R. Antioxidant Vitamin Supplementation Prevents Oxidative Stress but Does Not Enhance Performance in Young Football Athletes. Nutrition 2019, 63–64, 29–35. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, T.; Zhang, Y.; Liu, T.; Gagnon, G.; Ebrahim, J.; Johnson, J.; Chu, Y.-F.; Ji, L.L. Avenanthramide Supplementation Reduces Eccentric Exercise-Induced Inflammation in Young Men and Women. J. Int. Soc. Sports Nutr. 2020, 17, 41. [Google Scholar] [CrossRef]
- Drobnic, F.; Riera, J.; Appendino, G.; Togni, S.; Franceschi, F.; Valle, X.; Pons, A.; Tur, J. Reduction of Delayed Onset Muscle Soreness by a Novel Curcumin Delivery System (Meriva®): A Randomised, Placebo-Controlled Trial. J. Int. Soc. Sports Nutr. 2014, 11, 31. [Google Scholar] [CrossRef]
- He, F.; Hockemeyer, J.A.K.; Sedlock, D. Does Combined Antioxidant Vitamin Supplementation Blunt Repeated Bout Effect? Int. J. Sports Med. 2015, 36, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Ashton, T.; Cable, T.; Doran, D.; Holloway, C.; McArdle, F.; MacLaren, D.P.M. Ascorbic Acid Supplementation Does Not Attenuate Post-Exercise Muscle Soreness Following Muscle-Damaging Exercise but May Delay the Recovery Process. Br. J. Nutr. 2006, 95, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Kashef, M. Effect of Vitamin E Supplementation on Delayed Onset Muscle Soreness in Young Men. J. Phys. Act. Horm. 2018, 2, 15–28. [Google Scholar]
- McGinley, C.; Shafat, A.; Donnelly, A.E. Does Antioxidant Vitamin Supplementation Protect against Muscle Damage? Sports Med. 2009, 39, 1011–1032. [Google Scholar] [CrossRef]
- Peternelj, T.-T.; Coombes, J.S. Antioxidant Supplementation during Exercise Training: Beneficial or Detrimental? Sports Med. 2011, 41, 1043–1069. [Google Scholar] [CrossRef]
- Fitó, M.; Guxens, M.; Corella, D.; Sáez, G.; Estruch, R.; de la Torre, R.; Francés, F.; Cabezas, C.; López-Sabater, M.D.C.; Marrugat, J.; et al. Effect of a Traditional Mediterranean Diet on Lipoprotein Oxidation: A Randomized Controlled Trial. Arch. Intern. Med. 2007, 167, 1195–1203. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Hopkins, W.G.; Lowe, T.E. Effects of Dietary Antioxidants on Training and Performance in Female Runners. Eur. J. Sport Sci. 2014, 14, 160–168. [Google Scholar] [CrossRef]
- Aguiló, A.; Tauler, P.; Sureda, A.; Cases, N.; Tur, J.; Pons, A. Antioxidant Diet Supplementation Enhances Aerobic Performance in Amateur Sportsmen. J. Sports Sci. 2007, 25, 1203–1210. [Google Scholar] [CrossRef]
- Paulsen, G.; Cumming, K.T.; Holden, G.; Hallén, J.; Rønnestad, B.R.; Sveen, O.; Skaug, A.; Paur, I.; Bastani, N.E.; Østgaard, H.N.; et al. Vitamin C and E Supplementation Hampers Cellular Adaptation to Endurance Training in Humans: A Double-Blind, Randomised, Controlled Trial. J. Physiol. 2014, 592, 1887–1901. [Google Scholar] [CrossRef]
- Daneshvar, P.; Hariri, M.; Ghiasvand, R.; Askari, G.; Darvishi, L.; Mashhadi, N.S.; Khosravi-Boroujeni, H. Effect of Eight Weeks of Quercetin Supplementation on Exercise Performance, Muscle Damage and Body Muscle in Male Badminton Players. Int. J. Prev. Med. 2013, 4, S53–S57. [Google Scholar]
- MacRae, H.S.H.; Mefferd, K.M. Dietary Antioxidant Supplementation Combined with Quercetin Improves Cycling Time Trial Performance. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 405–419. [Google Scholar] [CrossRef]
- Darvishi, L.; Ghiasvand, R.; Hariri, M.; Askari, G.; Rezai, P.; Aghaie, M.; Iraj, B.; Khosravi-Boroujeni, H.; Mashhadi, N.S. Quercetin Supplementation Does Not Attenuate Exercise Performance and Body Composition in Young Female Swimmers. Int. J. Prev. Med. 2013, 4, S43–S47. [Google Scholar]
- Ganio, M.S.; Armstrong, L.E.; Johnson, E.C.; Klau, J.F.; Ballard, K.D.; Michniak-Kohn, B.; Kaushik, D.; Maresh, C.M. Effect of Quercetin Supplementation on Maximal Oxygen Uptake in Men and Women. J. Sports Sci. 2010, 28, 201–208. [Google Scholar] [CrossRef]
- Gliemann, L.; Schmidt, J.F.; Olesen, J.; Biensø, R.S.; Peronard, S.L.; Grandjean, S.U.; Mortensen, S.P.; Nyberg, M.; Bangsbo, J.; Pilegaard, H.; et al. Resveratrol Blunts the Positive Effects of Exercise Training on Cardiovascular Health in Aged Men. J. Physiol. 2013, 591, 5047–5059. [Google Scholar] [CrossRef]
- Wylie, L.J.; Kelly, J.; Bailey, S.J.; Blackwell, J.R.; Skiba, P.F.; Winyard, P.G.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Beetroot Juice and Exercise: Pharmacodynamic and Dose-Response Relationships. J. Appl. Physiol. 2013, 115, 325–336. [Google Scholar] [CrossRef]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute Dietary Nitrate Supplementation Improves Cycling Time Trial Performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef]
- Wylie, L.J.; Mohr, M.; Krustrup, P.; Jackman, S.R.; Ermιdis, G.; Kelly, J.; Black, M.I.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M. Dietary Nitrate Supplementation Improves Team Sport-Specific Intense Intermittent Exercise Performance. Eur. J. Appl. Physiol. 2013, 113, 1673–1684. [Google Scholar] [CrossRef]
- Hoon, M.W.; Johnson, N.A.; Chapman, P.G.; Burke, L.M. The Effect of Nitrate Supplementation on Exercise Performance in Healthy Individuals: A Systematic Review and Meta-Analysis. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 522–532. [Google Scholar] [CrossRef]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative Stress: Role of Physical Exercise and Antioxidant Nutraceuticals in Adulthood and Aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef]
- Shafat, A.; Butler, P.; Jensen, R.L.; Donnelly, A.E. Effects of Dietary Supplementation with Vitamins C and E on Muscle Function during and after Eccentric Contractions in Humans. Eur. J. Appl. Physiol. 2004, 93, 196–202. [Google Scholar] [CrossRef]
- Xie, D.; Hu, J.; Yang, Z.; Wu, T.; Xu, W.; Meng, Q.; Cao, K.; Luo, X. Vitamin Supplementation Protects against Nanomaterial-Induced Oxidative Stress and Inflammation Damages: A Meta-Analysis of In Vitro and In Vivo Studies. Nutrients 2022, 14, 2214. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.A.; Silva, E.T.; Caris, A.V.; Lira, F.S.; Tufik, S.; Dos Santos, R.V.T. Vitamin E Supplementation Inhibits Muscle Damage and Inflammation after Moderate Exercise in Hypoxia. J. Hum. Nutr. Diet. 2016, 29, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Sacheck, J.M.; Decker, E.A.; Clarkson, P.M. The Effect of Diet on Vitamin E Intake and Oxidative Stress in Response to Acute Exercise in Female Athletes. Eur. J. Appl. Physiol. 2000, 83, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Takanami, Y.; Iwane, H.; Kawai, Y.; Shimomitsu, T. Vitamin E Supplementation and Endurance Exercise. Sports Med. 2000, 29, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, A.; Saucier, C.; Bisbal, C.; Lambert, K. Grape Polyphenols in the Treatment of Human Skeletal Muscle Damage Due to Inflammation and Oxidative Stress during Obesity and Aging: Early Outcomes and Promises. Molecules 2022, 27, 6594. [Google Scholar] [CrossRef]
- Pugh, J.; Close, G. Nutritional Antioxidants for Sports Performance. In Oxidative Eustress in Exercise Physiology; CRC Press: Boca Raton, FL, USA, 2022; pp. 115–122. ISBN 978-1-00-305161-9. [Google Scholar]
- Kan, N.-W.; Ho, C.-S.; Chiu, Y.-S.; Huang, W.-C.; Chen, P.-Y.; Tung, Y.-T.; Huang, C.-C. Effects of Resveratrol Supplementation and Exercise Training on Exercise Performance in Middle-Aged Mice. Molecules 2016, 21, 661. [Google Scholar] [CrossRef]
- Huang, C.-C.; Liu, C.-C.; Tsao, J.-P.; Hsu, C.-L.; Cheng, I.-S. Effects of Oral Resveratrol Supplementation on Glycogen Replenishment and Mitochondria Biogenesis in Exercised Human Skeletal Muscle. Nutrients 2020, 12, 3721. [Google Scholar] [CrossRef]
- Scribbans, T.D.; Ma, J.K.; Edgett, B.A.; Vorobej, K.A.; Mitchell, A.S.; Zelt, J.G.E.; Simpson, C.A.; Quadrilatero, J.; Gurd, B.J. Resveratrol Supplementation Does Not Augment Performance Adaptations or Fibre-Type-Specific Responses to High-Intensity Interval Training in Humans. Appl. Physiol. Nutr. Metab. 2014, 39, 1305–1313. [Google Scholar] [CrossRef]
- Marx, W.; Kelly, J.; Marshall, S.; Cutajar, J.; Annois, B.; Pipingas, A.; Tierney, A.; Itsiopoulos, C. Effect of Resveratrol Supplementation on Cognitive Performance and Mood in Adults: A Systematic Literature Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Rev. 2018, 76, 432–443. [Google Scholar] [CrossRef]
- Ostman, B.; Sjödin, A.; Michaëlsson, K.; Byberg, L. Coenzyme Q10 Supplementation and Exercise-Induced Oxidative Stress in Humans. Nutrition 2012, 28, 403–417. [Google Scholar] [CrossRef]
- Cooke, M.; Iosia, M.; Buford, T.; Shelmadine, B.; Hudson, G.; Kerksick, C.; Rasmussen, C.; Greenwood, M.; Leutholtz, B.; Willoughby, D.; et al. Effects of Acute and 14-Day Coenzyme Q10 Supplementation on Exercise Performance in Both Trained and Untrained Individuals. J. Int. Soc. Sports Nutr. 2008, 5, 8. [Google Scholar] [CrossRef]
- Leelarungrayub, D.; Sawattikanon, N.; Klaphajone, J.; Pothongsunan, P.; Bloomer, R.J. Coenzyme Q10 Supplementation Decreases Oxidative Stress and Improves Physical Performance in Young Swimmers: A Pilot Study. Open Sports Med. J. 2010, 4, 1–8. [Google Scholar] [CrossRef]
- Emami, A.; Tofighi, A.; Asri-Rezaei, S.; Bazargani-Gilani, B. The Effect of Short-Term Coenzyme Q10 Supplementation and Pre-Cooling Strategy on Cardiac Damage Markers in Elite Swimmers. Br. J. Nutr. 2018, 119, 381–390. [Google Scholar] [CrossRef]
- Astani, K.; Bashiri, J.; Pourrazi, H.; Nourazar, M.A. Effect of High-Intensity Interval Training and Coenzyme Q10 Supplementation on Cardiac Apoptosis in Obese Male Rats. ARYA Atheroscler. 2022, 18, 1–9. [Google Scholar] [CrossRef]
- Ghiasi, S.; Bashiri, J.; Pourrazi, H.; Jadidi, R. The Effect of High-Intensity Interval Training and CoQ10 Administration on Hepatic CEACAM1 and PDGFA Proteins in Diet-Induced Obese Rats. Sport Sci. Health 2022, 1–8. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Navascués, L.J.; Córdova Martínez, A.; Seco-Calvo, J. The Role of Selenium Mineral Trace Element in Exercise: Antioxidant Defense System, Muscle Performance, Hormone Response, and Athletic Performance. A Systematic Review. Nutrients 2020, 12, 1790. [Google Scholar] [CrossRef]
- Rayman, M.P.; Stranges, S.; Griffin, B.A.; Pastor-Barriuso, R.; Guallar, E. Effect of Supplementation with High-Selenium Yeast on Plasma Lipids: A Randomized Trial. Ann. Intern. Med. 2011, 154, 656–665. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Seco Calvo, J.; Córdova Martínez, A.; Caballero García, A.; Fernandez-Lazaro, C.I. Modulation of Exercise-Induced Muscle Damage, Inflammation, and Oxidative Markers by Curcumin Supplementation in a Physically Active Population: A Systematic Review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef]
- Nicol, L.M.; Rowlands, D.S.; Fazakerly, R.; Kellett, J. Curcumin Supplementation Likely Attenuates Delayed Onset Muscle Soreness (DOMS). Eur. J. Appl. Physiol. 2015, 115, 1769–1777. [Google Scholar] [CrossRef]
- Tanabe, Y.; Maeda, S.; Akazawa, N.; Zempo-Miyaki, A.; Choi, Y.; Ra, S.-G.; Imaizumi, A.; Otsuka, Y.; Nosaka, K. Attenuation of Indirect Markers of Eccentric Exercise-Induced Muscle Damage by Curcumin. Eur. J. Appl. Physiol. 2015, 115, 1949–1957. [Google Scholar] [CrossRef]
- Criswell, D.; Powers, S.; Dodd, S.; Lawler, J.; Edwards, W.; Renshler, K.; Grinton, S. High Intensity Training-Induced Changes in Skeletal Muscle Antioxidant Enzyme Activity. Med. Sci. Sports Exerc. 1993, 25, 1135–1140. [Google Scholar] [CrossRef]
- Beaton, L.J.; Allan, D.A.; Tarnopolsky, M.A.; Tiidus, P.M.; Phillips, S.M. Contraction-Induced Muscle Damage Is Unaffected by Vitamin E Supplementation. Med. Sci. Sports Exerc. 2002, 34, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Tsao, J.-P.; Liu, C.-C.; Wang, H.-F.; Bernard, J.R.; Huang, C.-C.; Cheng, I.-S. Oral Resveratrol Supplementation Attenuates Exercise-Induced Interleukin-6 but Not Oxidative Stress after a High Intensity Cycling Challenge in Adults. Int. J. Med. Sci. 2021, 18, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Novelle, M.G.; Wahl, D.; Diéguez, C.; Bernier, M.; de Cabo, R. Resveratrol Supplementation: Where Are We Now and Where Should We Go? Ageing Res. Rev. 2015, 21, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Drobnic, F.; Lizarraga, M.A.; Caballero-García, A.; Cordova, A. Coenzyme Q10 Supplementation and Its Impact on Exercise and Sport Performance in Humans: A Recovery or a Performance-Enhancing Molecule? Nutrients 2022, 14, 1811. [Google Scholar] [CrossRef]
- Kon, M.; Tanabe, K.; Akimoto, T.; Kimura, F.; Tanimura, Y.; Shimizu, K.; Okamoto, T.; Kono, I. Reducing Exercise-Induced Muscular Injury in Kendo Athletes with Supplementation of Coenzyme Q10. Br. J. Nutr. 2008, 100, 903–909. [Google Scholar] [CrossRef]
- Wrobel, J.K.; Power, R.; Toborek, M. Biological Activity of Selenium: Revisited. IUBMB Life 2016, 68, 97–105. [Google Scholar] [CrossRef]
- Bai, K.-Y.; Liu, G.-H.; Fan, C.-H.; Kuo, L.-T.; Hsu, W.-H.; Yu, P.-A.; Chen, C.-L. 12-Week Curcumin Supplementation May Relieve Postexercise Muscle Fatigue in Adolescent Athletes. Front. Nutr. 2022, 9, 1078108. [Google Scholar] [CrossRef]
- Salehi, M.; Mashhadi, N.S.; Esfahani, P.S.; Feizi, A.; Hadi, A.; Askari, G. The Effects of Curcumin Supplementation on Muscle Damage, Oxidative Stress, and Inflammatory Markers in Healthy Females with Moderate Physical Activity: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Int. J. Prev. Med. 2021, 12, 94. [Google Scholar] [CrossRef]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef]
- McGlory, C.; Wardle, S.L.; Macnaughton, L.S.; Witard, O.C.; Scott, F.; Dick, J.; Bell, J.G.; Phillips, S.M.; Galloway, S.D.R.; Hamilton, D.L.; et al. Fish Oil Supplementation Suppresses Resistance Exercise and Feeding-Induced Increases in Anabolic Signaling without Affecting Myofibrillar Protein Synthesis in Young Men. Physiol. Rep. 2016, 4, e12715. [Google Scholar] [CrossRef]
- Kara, E.; Gunay, M.; Cicioglu, I.; Ozal, M.; Kilic, M.; Mogulkoc, R.; Baltaci, A.K. Effect of Zinc Supplementation on Antioxidant Activity in Young Wrestlers. Biol. Trace Elem. Res. 2010, 134, 55–63. [Google Scholar] [CrossRef]
- Lukaski, H.C. Vitamin and Mineral Status: Effects on Physical Performance. Nutrition 2004, 20, 632–644. [Google Scholar] [CrossRef]
- Cinar, V.; Akbulut, T.; Kilic, Y.; Özdal, M.; Sarikaya, M. The Effect of 6-Week Zinc Supplement and Weight Training on the Blood Lipids of the Sedentaries and Athletes. Cell. Mol. Biol. 2018, 64, 1–5. [Google Scholar] [CrossRef]
- Schmitt, B.; Vicenzi, M.; Garrel, C.; Denis, F.M. Effects of N-Acetylcysteine, Oral Glutathione (GSH) and a Novel Sublingual Form of GSH on Oxidative Stress Markers: A Comparative Crossover Study. Redox Biol. 2015, 6, 198–205. [Google Scholar] [CrossRef]
- Saitoh, T.; Satoh, H.; Nobuhara, M.; Machii, M.; Tanaka, T.; Ohtani, H.; Saotome, M.; Urushida, T.; Katoh, H.; Hayashi, H. Intravenous Glutathione Prevents Renal Oxidative Stress after Coronary Angiography More Effectively than Oral N-Acetylcysteine. Heart Vessel. 2011, 26, 465–472. [Google Scholar] [CrossRef]
- Søndergård, S.D.; Cintin, I.; Kuhlman, A.B.; Morville, T.H.; Bergmann, M.L.; Kjær, L.K.; Poulsen, H.E.; Giustarini, D.; Rossi, R.; Dela, F.; et al. The Effects of 3 Weeks of Oral Glutathione Supplementation on Whole Body Insulin Sensitivity in Obese Males with and without Type 2 Diabetes: A Randomized Trial. Appl. Physiol. Nutr. Metab. 2021, 46, 1133–1142. [Google Scholar] [CrossRef]
- Rhodes, K.; Braakhuis, A. Performance and Side Effects of Supplementation with N-Acetylcysteine: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 1619–1636. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Mielgo-Ayuso, J.; Quiles, J.L.; Varela-Lopez, A.; Aranda, P. Effect of α-tocopherol megadoses on hematologic parameters and antioxidant capacity of rats in an ultraendurance probe. Physiol. Int. 2017, 104, 291–300. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; Ruisoto, P.; Navarro-Jiménez, E.; Ramos-Campo, D.J.; Tornero-Aguilera, J.F. Metabolic Health, Mitochondrial Fitness, Physical Activity, and Cancer. Cancers 2023, 15, 814. [Google Scholar] [CrossRef]
- Carreira-Míguez, M.; Ramos-Campo, D.J.; Clemente-Suárez, V.J. Differences in Nutritional and Psychological Habits in Hypertension Patients. BioMed Res. Int. 2022, 2022, 1920996. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Rubio-Zarapuz, A.; Martínez-Guardado, I.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F. Nutritional and Exercise Interventions in Cancer-Related Cachexia: An Extensive Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 4604. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suárez, V.J.; Ramos-Campo, D.J.; Mielgo-Ayuso, J.; Dalamitros, A.A.; Nikolaidis, P.A.; Hormeño-Holgado, A.; Tornero-Aguilera, J.F. Nutrition in the Actual COVID-19 Pandemic. A Narrative Review. Nutrients 2021, 13, 1924. [Google Scholar] [CrossRef] [PubMed]
| Antioxidant | Physiological Function | Effect on Sports Performance | Scientific Evidence |
|---|---|---|---|
| Vitamin C | Helps protect cells from oxidative damage and improves iron absorption | Possible improvement in aerobic capacity | Small |
| Vitamin E | Protects cell membranes from oxidative damage | Possible improvement in aerobic capacity | Small |
| Quercetin | Anti-inflammatory, antidiabetic, antioxidant, anti-infective, and cardioprotective effects | Improvement in exercise capacity (VO2 max and endurance exercise performance) | Small |
| Resveratrol | Neuroprotective and cardioprotective effects | May improve endurance capacity and skeletal muscle strength | Small |
| Beetroot juice (nitric oxide) | Hemodynamic (vasodilator) and metabolic functions (promotes oxygen transfer in the muscle) | Improves cardiorespiratory performance at the anaerobic threshold and VO2 max intensities | Medium |
| Coenzyme Q10 | Cellular energy production, antioxidant protection, regulation of the immune system, and improvement of cardiovascular and cognitive function | Possible improvement in aerobic capacity | Small |
| Spirulina | Strengthening the immune system, lowering cholesterol, and regulating blood sugar | Possible improvement in oxygen uptake and exercise tolerance at submaximal intensities | Small |
| Polyphenols | Enhances the production of vasodilating factors and blood flow | Enhancement of vascular function, muscle perfusion, and oxygen extraction during exercise | Small |
| Vitamin E (mg/100 g) | Vitamin C (mg/100 g) | Vitamin A (mcg/100 g) | Zinc (mg/100 g) | Selenium (mcg/100 g) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Sunflower oil | 55 | Kiwi | 500 | Animal vetches | 5800 | Cooked oysters | 37 | Brazil nuts | 1917 |
| Maize oil | 31 | Guava | 480 | Sorrel | 2100 | Wheat germ | 12.2 | Pork kidneys | 311 |
| Wheat germ | 30 | Red pepper | 204 | Carrots | 2000 | Hemp seeds | 9.9 | Lamb | 218 |
| Hazelnuts | 26 | Red currant | 200 | Spinach (cooked) | 1000 | Roast beef | 8.5 | Eggs | 192 |
| Almonds | 25 | Parsley | 150 | Parsley | 1160 | Almonds | 5 | Oysters | 154 |
| Coconut | 17 | Persimmon | 130 | Butter | 970 | Peanuts | 4.8 | Veal | 141 |
| Corn germ | 16 | Brussels sprouts | 100 | Sweet potatoes | 670 | Cooked veal liver | 4.5 | Turkey | 144 |
| Soybean oil | 14 | Lemon | 80 | Soybean oil | 583 | Cooked turkey | 4.5 | Mustard seed | 208 |
| Soya bean sprouts | 13 | Cauliflower | 70 | Fresh and frozen tuna and bonito | 450 | Cooked veal | 4.4 | Sunflower seeds | 78.2 |
| Olive oil | 12 | Spinach | 60 | Cheese | 240 | Cooked chicken liver | 4.3 | Garlic | 14.2 |
| Margarine | 10 | Strawberry | 60 | Eggs | 220 | Cooked crab | 4.3 | ||
| Peanuts | 9 | Orange | 50 | Other vegetables | 130 | Cooked lamb | 4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clemente-Suárez, V.J.; Bustamante-Sanchez, Á.; Mielgo-Ayuso, J.; Martínez-Guardado, I.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Antioxidants and Sports Performance. Nutrients 2023, 15, 2371. https://doi.org/10.3390/nu15102371
Clemente-Suárez VJ, Bustamante-Sanchez Á, Mielgo-Ayuso J, Martínez-Guardado I, Martín-Rodríguez A, Tornero-Aguilera JF. Antioxidants and Sports Performance. Nutrients. 2023; 15(10):2371. https://doi.org/10.3390/nu15102371
Chicago/Turabian StyleClemente-Suárez, Vicente Javier, Álvaro Bustamante-Sanchez, Juan Mielgo-Ayuso, Ismael Martínez-Guardado, Alexandra Martín-Rodríguez, and José Francisco Tornero-Aguilera. 2023. "Antioxidants and Sports Performance" Nutrients 15, no. 10: 2371. https://doi.org/10.3390/nu15102371
APA StyleClemente-Suárez, V. J., Bustamante-Sanchez, Á., Mielgo-Ayuso, J., Martínez-Guardado, I., Martín-Rodríguez, A., & Tornero-Aguilera, J. F. (2023). Antioxidants and Sports Performance. Nutrients, 15(10), 2371. https://doi.org/10.3390/nu15102371











