Testing the Anticancer Effect of Matcha Using Zebrafish as an Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Matcha Extract Preparation
2.2. Animal Care and Handling
2.3. Zebrafish Maintenance and Breeding
2.4. Developmental Toxicity: Survival Rate and Hatching Rate Analyses
2.5. Cardiotoxicity Assessment: Live Imaging of Zebrafish
2.6. Neuromuscular Toxicity: Behavioral and Locomotion Assay
2.7. Establish a Xenograft Zebrafish Model
2.7.1. Cell Culture
2.7.2. Fluorescent Labeling of Breast Cancer Cells Prior to Xenotransplantation
2.7.3. Preparation of Zebrafish for Microinjection
2.7.4. Xenotransplantation of RFP-Labeled Human TNBC Cells in Zebrafish Larvae
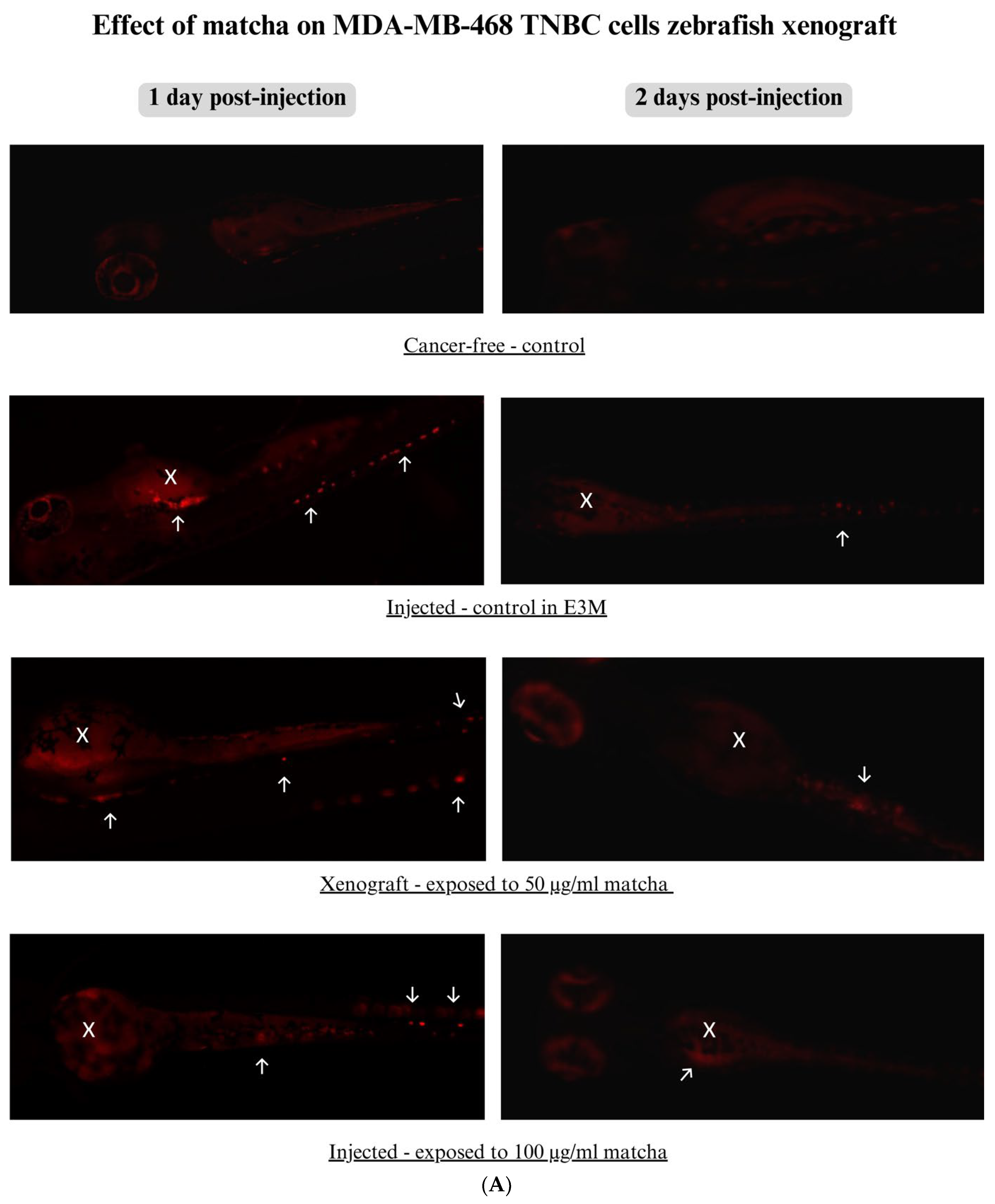
2.7.5. Imaging Breast Cancer Cell Progression in Zebrafish Larvae
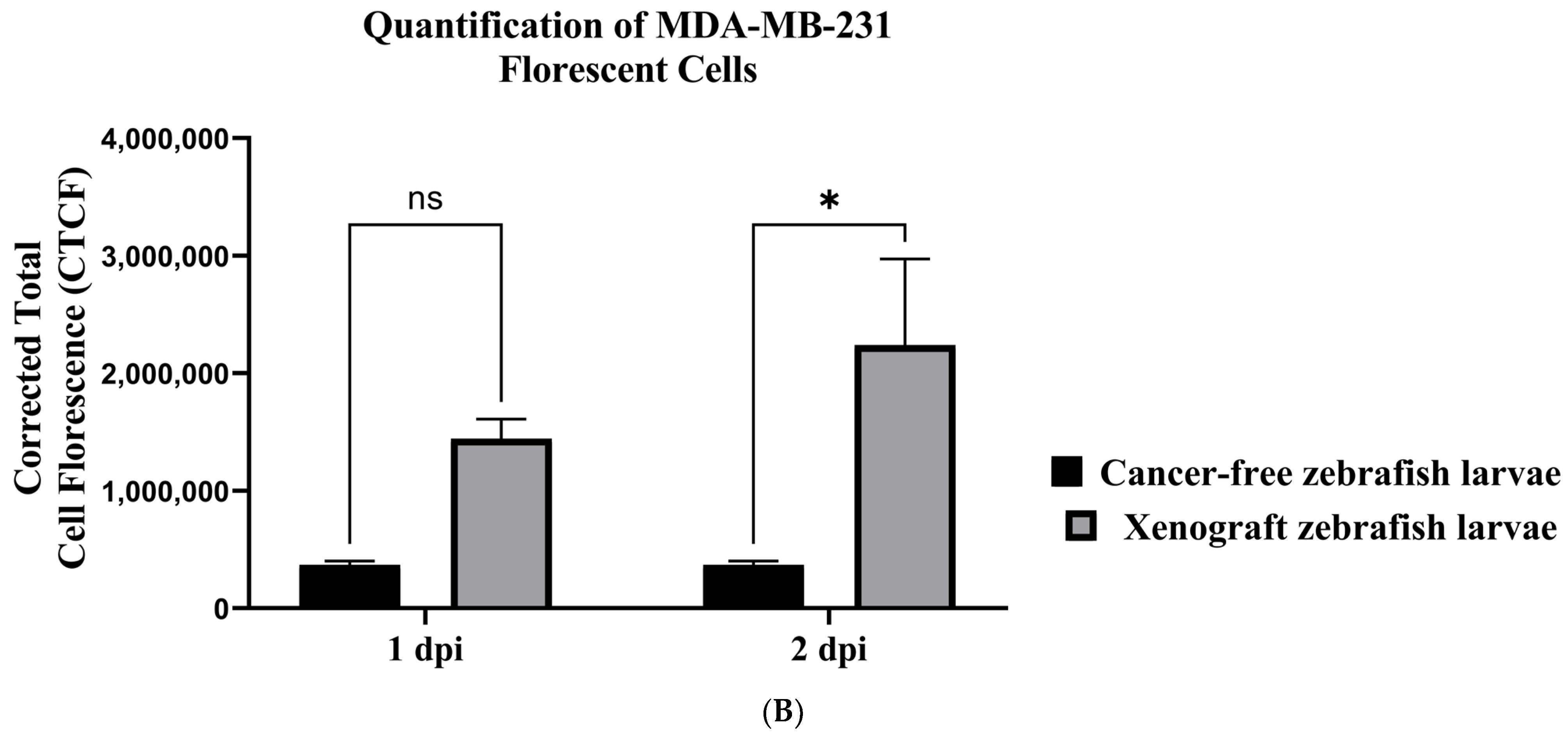
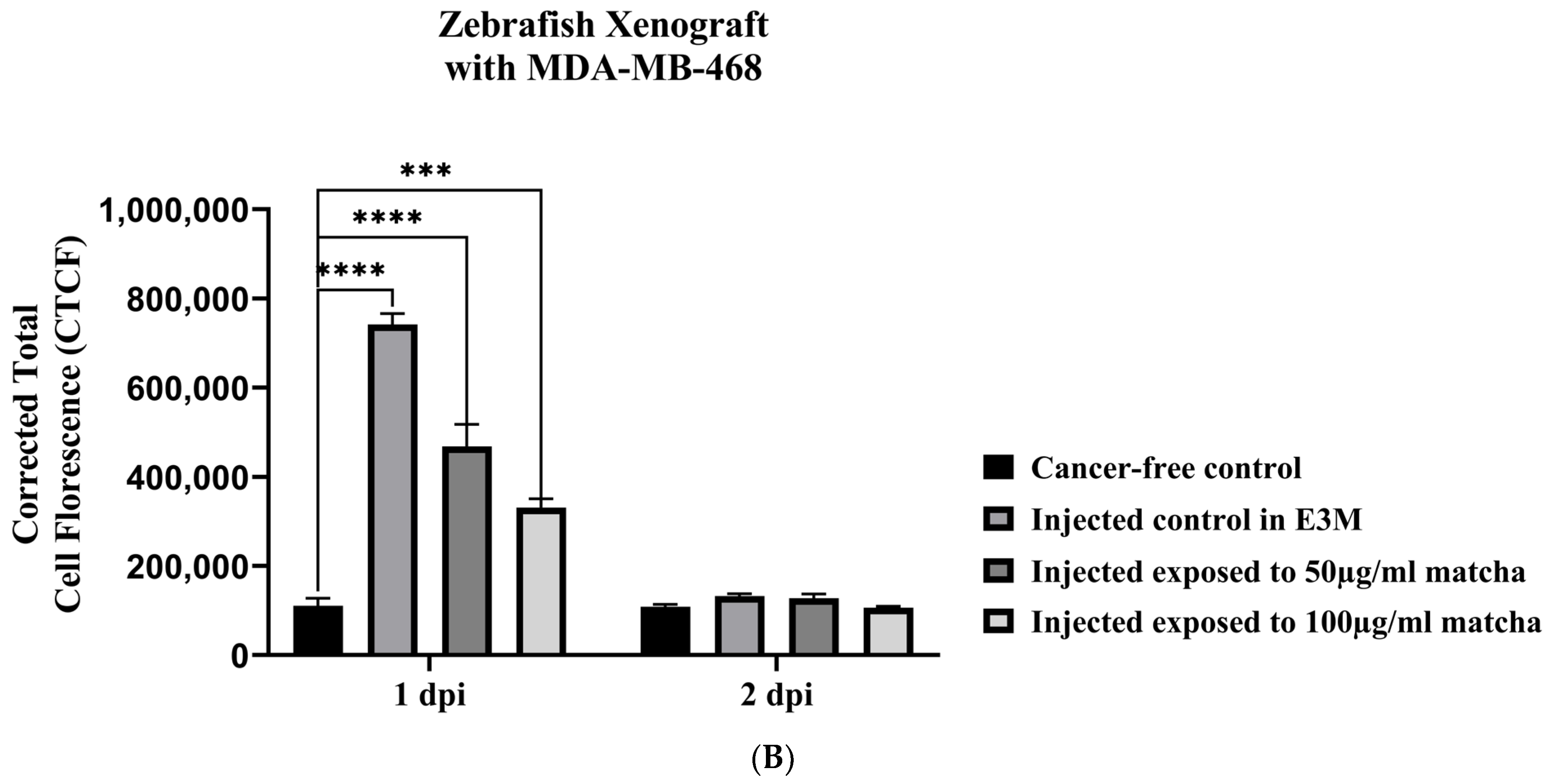
2.7.6. Quantification of Breast Cancer Cell Fluorescence
2.8. Statistical Analysis
3. Results
3.1. Developmental Toxicity of Matcha in Zebrafish Larvae
3.1.1. Survival Analysis
3.1.2. Hatching Rate Analysis
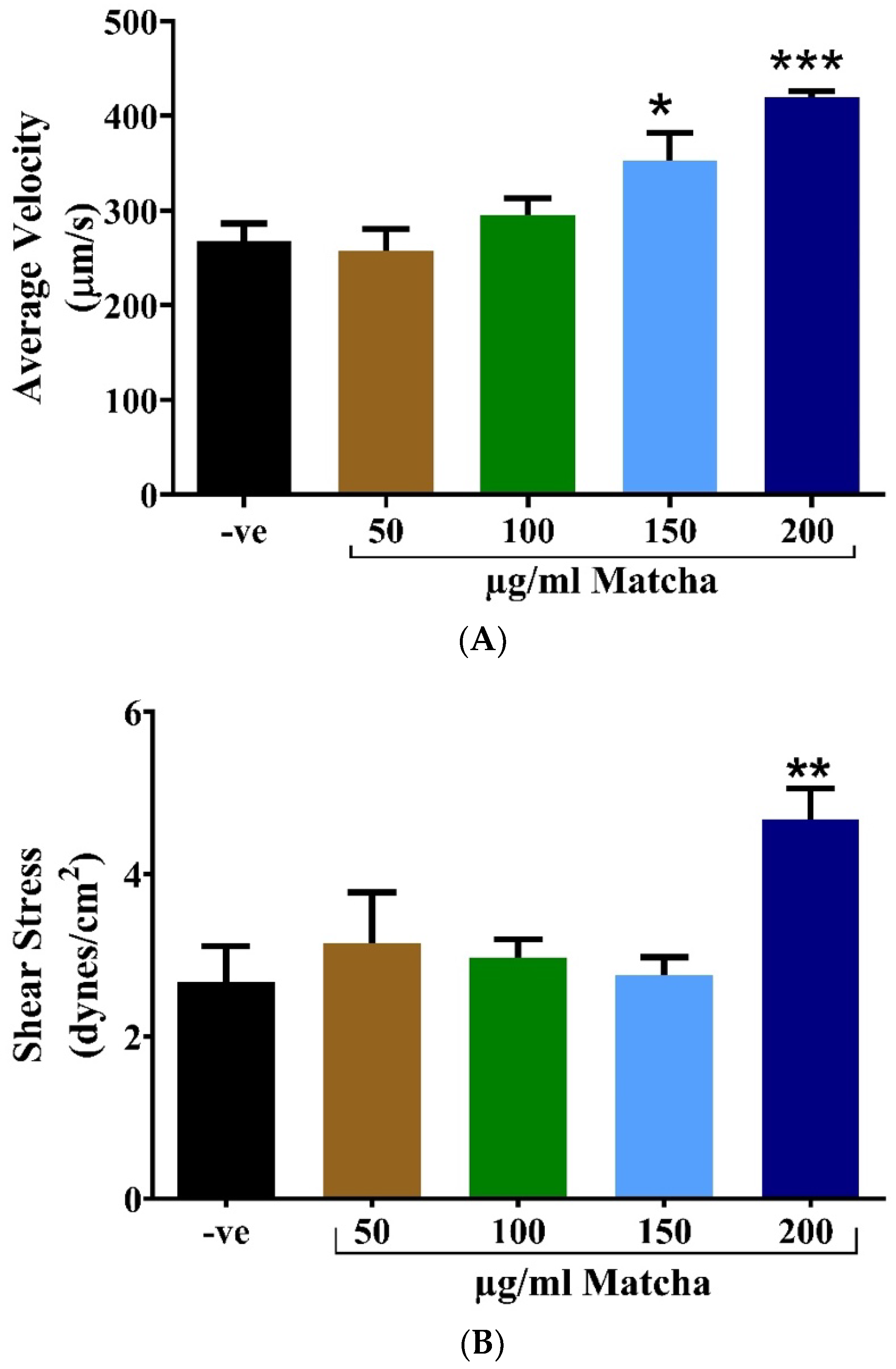
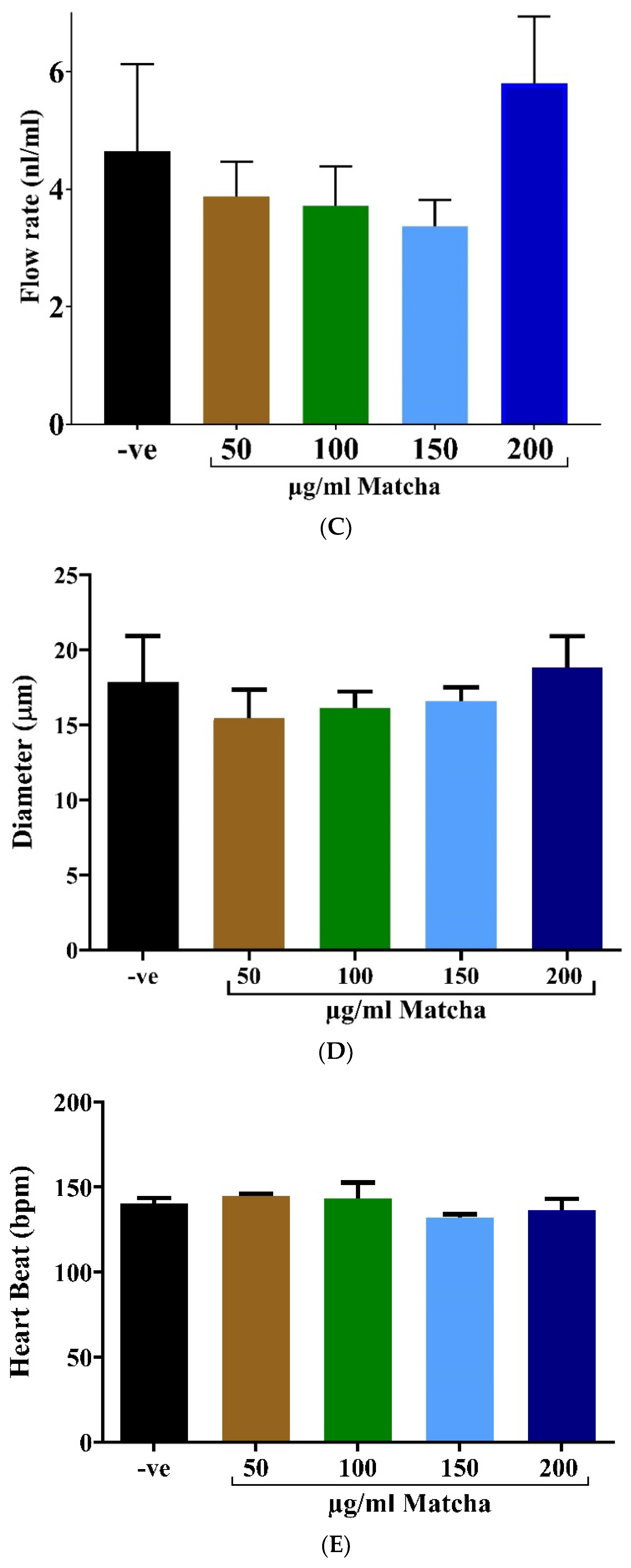
3.2. Cardiotoxicity Assessment
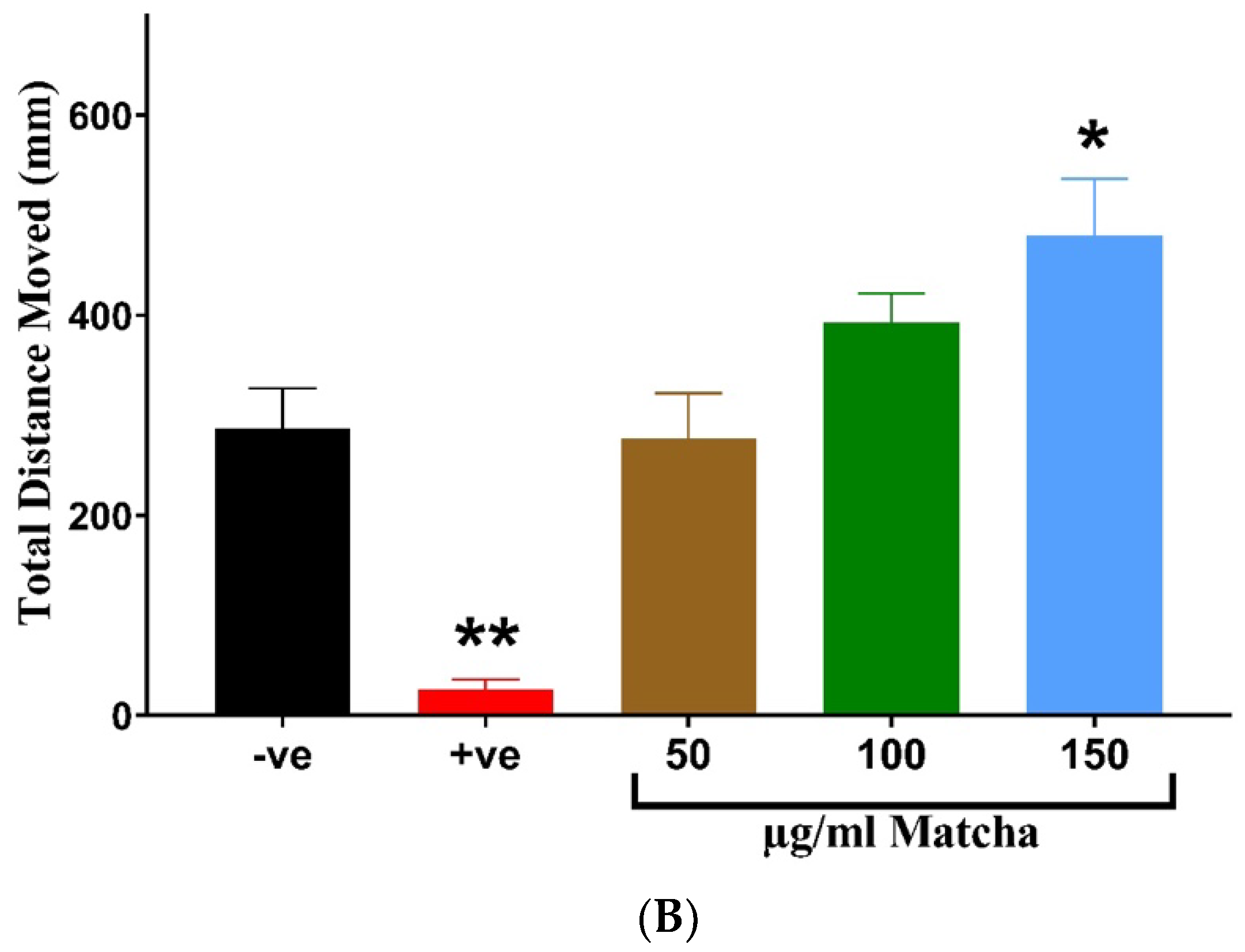
3.3. Neuromuscular Toxicity: Behavioral and Locomotion Assay
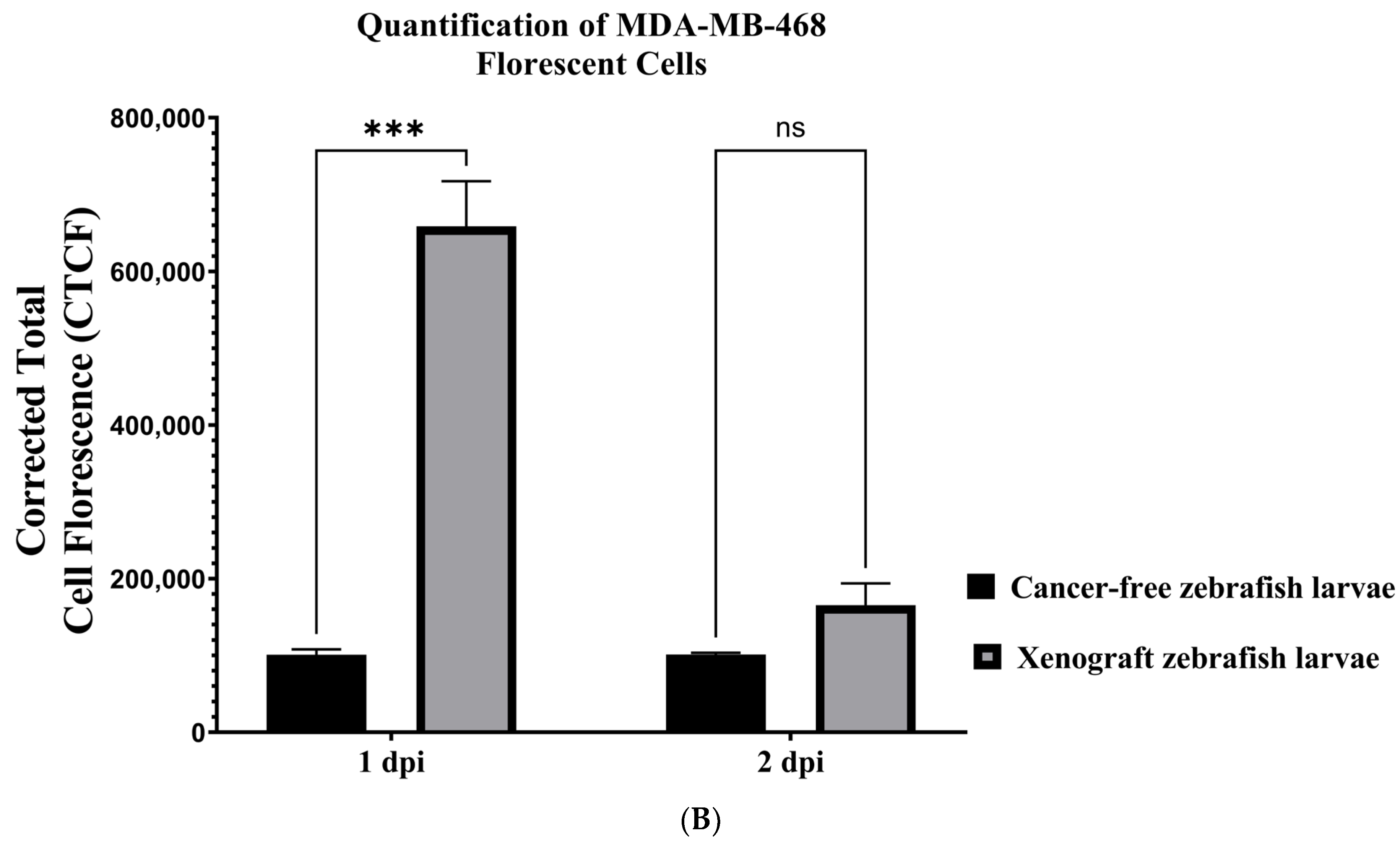
3.4. Establishing a Zebrafish Xenograft Model
3.5. Xenograft Zebrafish Model Exposed to Matcha
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Horn, G.; Moulton, K.; Oza, A.; Byler, S.; Kokolus, S.; Longacre, M. Cancer development, progression, and therapy: An epigenetic overview. Int. J. Mol. Sci. 2013, 14, 21087–21113. [Google Scholar] [CrossRef]
- Perue, C.; Sorlie, T.; Elsen, M.; van de Rijn, M.; Jeffrey, S.; Rees, C. Molecular portraits of human breast tumors. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Geyer, F.C.; Pareja, F.; Weigelt, B.; Rakha, E.; Ellis, I.O.; Schnitt, S.J.; Reis-Filho, J.S. The spectrum of triple-negative breast disease: High-and low-grade lesions. Am. J. Pathol. 2017, 187, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; El-Sayed, M.E.; Green, A.R.; Lee, A.H.; Robertson, J.F.; Ellis, I.O. Prognostic markers in triple-negative breast cancer. Cancer 2007, 109, 25–32. [Google Scholar] [CrossRef]
- Farooq, S.; Sehgal, A. Antioxidant Activity of Different Forms of Green Tea: Loose Leaf, Bagged and Matcha. Curr. Res. Nutr. Food Sci. 2018, 6. [Google Scholar] [CrossRef]
- Horie, H.; Ema, K.; Sumikawa, O. Chemical Components of Matcha and Powdered Green Tea. J. Cook. Sci. Jpn. 2017, 50, 182–188. [Google Scholar] [CrossRef]
- Goto, T.; Nagashima, H.; Yoshida, Y.; Kiso, M. Contents of individual tea catechins and caffeine in Japanese green tea. Chagyo Kenkyu Hokoku Tea Res. J. 1996, 1996, 21–28. [Google Scholar] [CrossRef]
- Ikegaya, K.; Takayanagi, H.; Anan, T. Chemical composition of matcha. Chagyo Kenkyu Hokoku Tea Res. J. 1984, 1984, 79–81. [Google Scholar] [CrossRef]
- Fujioka, K.; Iwamoto, T.; Shima, H.; Tomaru, K.; Saito, H.; Ohtsuka, M.; Yoshidome, A.; Kawamura, Y.; Manome, Y. The Powdering Process with a Set of Ceramic Mills for Green Tea Promoted Catechin Extraction and the ROS Inhibition Effect. Molecules 2016, 21, 474. [Google Scholar] [CrossRef] [PubMed]
- Baba, Y.; Kaneko, T.; Takihara, T. Matcha consumption maintains attentional function following a mild acute psychological stress without affecting a feeling of fatigue: A randomized placebo-controlled study in young adults. Nutr. Res. 2021, 88, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, Y.; Ding, L.; Xu, P.; Wang, Y. Matcha Green Tea Alleviates Non-Alcoholic Fatty Liver Disease in High-Fat Diet-Induced Obese Mice by Regulating Lipid Metabolism and Inflammatory Responses. Nutrients 2021, 13, 1950. [Google Scholar] [CrossRef] [PubMed]
- Bonuccelli, G.; Sotgia, F.; Lisanti, M.P. Matcha green tea (MGT) inhibits the propagation of cancer stem cells (CSCs), by targeting mitochondrial metabolism, glycolysis and multiple cell signalling pathways. Aging 2018, 10, 1867–1883. [Google Scholar] [CrossRef] [PubMed]
- Keckstein, S.; Tilgener, C.; Jeschke, U.; Hofmann, S.; Vilsmaier, T.; Kaltofen, T.; Heidegger, H.; Batz, F.; Mahner, S.; Schroder, L. Effects of matcha tea extract on cell viability and peroxisome proliferator-activated receptor gamma expression on T47D breast cancer cells. Arch. Gynecol. Obstet. 2022, 306, 451–459. [Google Scholar] [CrossRef]
- Schroder, L.; Marahrens, P.; Koch, J.G.; Heidegger, H.; Vilsmeier, T.; Phan-Brehm, T.; Hofmann, S.; Mahner, S.; Jeschke, U.; Richter, D.U. Effects of green tea, matcha tea and their components epigallocatechin gallate and quercetin on MCF7 and MDA-MB-231 breast carcinoma cells. Oncol. Rep. 2019, 41, 387–396. [Google Scholar] [CrossRef]
- Vittori, M.; Motaln, H.; Turnšek, T.L. The study of glioma by xenotransplantation in zebrafish early life stages. J. Histochem. Cytochem. 2015, 63, 749–761. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Teame, T.; Zhang, Z.; Ran, C.; Zhang, H.; Yang, Y.; Ding, Q.; Xie, M.; Gao, C.; Ye, Y.; Duan, M. The use of zebrafish (Danio rerio) as biomedical models. Anim. Front. 2019, 9, 68–77. [Google Scholar] [CrossRef]
- Lam, S.; Chua, H.; Gong, Z.; Lam, T.; Sin, Y. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef]
- Parng, C.; Seng, W.L.; Semino, C.; McGrath, P. Zebrafish: A preclinical model for drug screening. Assay Drug Dev. Technol. 2002, 1, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wehmas, L.C.; Tanguay, R.L.; Punnoose, A.; Greenwood, J.A. Developing a novel embryo–larval Zebrafish xenograft assay to prioritize human glioblastoma therapeutics. Zebrafish 2016, 13, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Zampedri, C.; Martínez-Flores, W.A.; Melendez-Zajgla, J. The Use of Zebrafish Xenotransplant Assays to Analyze the Role of lncRNAs in Breast Cancer. Front. Oncol. 2021, 11, 687594. [Google Scholar] [CrossRef]
- Zhang, Q.; Gao, Q.; Zhao, L.; Li, X.; Wang, X.; Wang, Y.; Chen, D. Evaluation of the effect of green tea and its constituents on embryo development in a zebrafish model. J. Appl. Toxicol. 2022, 43, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Q.; Peng, X.; Xu, J.; Zhang, Y.; Zhu, J.; Wang, Y.; An, Y.; Chen, D. The effects of five types of tea solutions on epiboly process, neural and cardiovascular development, and locomotor capacity of zebrafish. Cell Biol. Toxicol. 2019, 35, 205–217. [Google Scholar] [CrossRef]
- Reed, B.; Jennings, M. Guidance on the Housing and Care of Zebrafish, Danio rerio; RSPCA: Horsham, UK, 2011. [Google Scholar]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish. 2000. Available online: http://zfin.org/zf_info/zfbook/zfbk.html (accessed on 15 January 2023).
- Shin, J.T.; Pomerantsev, E.V.; Mably, J.D.; MacRae, C.A. High-resolution cardiovascular function confirms functional orthology of myocardial contractility pathways in zebrafish. Physiol. Genom. 2010, 42, 300–309. [Google Scholar] [CrossRef]
- Yalcin, H.C.; Amindari, A.; Butcher, J.T.; Althani, A.; Yacoub, M. Heart function and hemodynamic analysis for zebrafish embryos. Dev. Dyn. 2017, 246, 868–880. [Google Scholar] [CrossRef]
- Benslimane, F.M.; Zakaria, Z.Z.; Shurbaji, S.; Abdelrasool, M.K.A.; Al-Badr, M.A.H.; Al Absi, E.S.K.; Yalcin, H.C. Cardiac function and blood flow hemodynamics assessment of zebrafish (Danio rerio) using high-speed video microscopy. Micron 2020, 136, 102876. [Google Scholar] [CrossRef]
- Yalcin, H.; Abuhabib, U.; Kitaz, N.; Mohamed, A.; Zakaria, Z. Generating an imagingbased approach for enhanced structural and functional analysis of zebrafish cardiovascular systems. Qatar Found. Annu. Res. Conf. Proc. 2018, 2018, HBPD769. [Google Scholar]
- Baba, Y.; Takihara, T.; Sagesaka, Y.; Kaneko, T. Effects of a daily intake of matcha on cognitive function in middle-aged and older subjects -A placebo-controlled, randomized, double-blind, parallel-group study. Jpn. Pharmacol. Ther. 2019, 47, 1689–1702. [Google Scholar]
- Unno, K.; Furushima, D.; Hamamoto, S.; Iguchi, K.; Yamada, H.; Morita, A.; Pervin, M.; Nakamura, Y. Stress-reducing effect of cookies containing matcha green tea: Essential ratio among theanine, arginine, caffeine and epigallocatechin gallate. Heliyon 2019, 5, e01653. [Google Scholar] [CrossRef] [PubMed]
- Ashihara, H.; Suzuki, T. Distribution and biosynthesis of caffeine in plants. Front. Biosci. 2004, 9, 1864–1876. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Chen, F.; Li, S.; Zhang, H. (-)-Epigallocatechin-3-gallate (EGCG) prevents aminoglycosides-induced ototoxicity via anti-oxidative and anti-apoptotic pathways. Int. J. Pediatr. Otorhinolaryngol. 2021, 150, 110920. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Jia, X.; Feng, H.; Tang, C.; Huang, Y.; Zhao, Z.; Hao, J.; Li, H.; Du, J.; Liu, Y.; et al. Nutrient combinations exhibit universal antianxiety, antioxidant, neuro-protecting, and memory-improving activities. Front. Nutr. 2022, 9, 996692. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, C.; Jin, C.; Bao, Z.; Yang, G.; Jin, Y. Catechin from green tea had the potential to decrease the chlorpyrifos induced oxidative stress in larval zebrafish (Danio rerio). Pestic. Biochem. Physiol. 2022, 182, 105028. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, X.; Yan, B.; Yuan, Q.; Dong, X.; Du, Q.; Zhang, J.; Shan, L.; Ding, Z.; Zhou, L.; et al. Green tea-derived theabrownin induces cellular senescence and apoptosis of hepatocellular carcinoma through p53 signaling activation and bypassed JNK signaling suppression. Cancer Cell. Int. 2022, 22, 39. [Google Scholar] [CrossRef]
- Kochman, J.; Jakubczyk, K.; Antoniewicz, J.; Mruk, H.; Janda, K. Health Benefits and Chemical Composition of Matcha Green Tea: A Review. Molecules 2020, 26, 85. [Google Scholar] [CrossRef]
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish Embryos and Larvae as Alternative Animal Models for Toxicity Testing. Int. J. Mol. Sci. 2021, 22, 13417. [Google Scholar] [CrossRef]
- Brown, D.R.; Samsa, L.A.; Qian, L.; Liu, J. Advances in the study of heart development and disease using zebrafish. J. Cardiovasc. Dev. Dis. 2016, 3, 13. [Google Scholar] [CrossRef]
- Salman, H.E.; Yalcin, H.C. Advanced blood flow assessment in Zebrafish via experimental digital particle image velocimetry and computational fluid dynamics modeling. Micron 2020, 130, 102801. [Google Scholar] [CrossRef]
- Eisa-Beygi, S.; Benslimane, F.M.; El-Rass, S.; Prabhudesai, S.; Abdelrasoul, M.K.A.; Simpson, P.M.; Yalcin, H.C.; Burrows, P.E.; Ramchandran, R. Characterization of endothelial cilia distribution during cerebral-vascular development in zebrafish (Danio rerio). Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2806–2818. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, P.; Saint-Amant, L.; Buss, R.R.; Chong, M.; McDearmid, J.R.; Brustein, E. Development of the locomotor network in zebrafish. Progress. Neurobiol. 2002, 68, 85–111. [Google Scholar] [CrossRef] [PubMed]
- Clayman, C.L.; Connaughton, V.P. Neurochemical and Behavioral Consequences of Ethanol and/or Caffeine Exposure: Effects in Zebrafish and Rodents. Curr. Neuropharmacol. 2022, 20, 560–578. [Google Scholar] [PubMed]
- Smit, H.J.; Rogers, P.J. Effects of low doses of caffeine on cognitive performance, mood and thirst in low and higher caffeine consumers. Psychopharmacology 2000, 152, 167–173. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar]
- Welsh, J. Chapter 40—Animal Models for Studying Prevention and Treatment of Breast Cancer. In Animal Models for the Study of Human Disease; Conn, P.M., Ed.; Academic Press: Boston, MA, USA, 2013; pp. 997–1018. [Google Scholar] [CrossRef]
- Liu, H.; Zang, C.; Fenner, M.; Possinger, K.; Elstner, E. PPARγ ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res. Treat. 2003, 79, 63–74. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35. [Google Scholar] [CrossRef]
- Xiao, J.; McGill, J.R.; Nasir, A.; Lekan, A.; Johnson, B.; Wilkins, D.J.; Pearson, G.W.; Tanner, K.; Goodarzi, H.; Glasgow, E.; et al. Zebrafish xenografts to isolate unique human breast cancer metastatic cell populations. bioRxiv 2021. [Google Scholar] [CrossRef]
- Amaro, A.; Angelini, G.; Mirisola, V.; Esposito, A.I.; Reverberi, D.; Matis, S.; Maffei, M.; Giaretti, W.; Viale, M.; Gangemi, R. A highly invasive subpopulation of MDA-MB-231 breast cancer cells shows accelerated growth, differential chemoresistance, features of apocrine tumors and reduced tumorigenicity in vivo. Oncotarget 2016, 7, 68803. [Google Scholar] [CrossRef]
- Rebelo de Almeida, C.; Mendes, R.V.; Pezzarossa, A.; Gago, J.; Carvalho, C.; Alves, A.; Nunes, V.; Brito, M.J.; Cardoso, M.J.; Ribeiro, J.; et al. Zebrafish xenografts as a fast screening platform for bevacizumab cancer therapy. Commun. Biol. 2020, 3, 299. [Google Scholar] [CrossRef]
- Berens, E.B.; Sharif, G.M.; Wellstein, A.; Glasgow, E. Testing the Vascular Invasive Ability of Cancer Cells in Zebrafish (Danio rerio). J. Vis. Exp. 2016, 117, e55007. [Google Scholar] [CrossRef]
- Shahi Thakuri, P.; Gupta, M.; Singh, S.; Joshi, R.; Glasgow, E.; Lekan, A.; Agarwal, S.; Luker, G.D.; Tavana, H. Phytochemicals inhibit migration of triple negative breast cancer cells by targeting kinase signaling. BMC Cancer 2020, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Schwarz-Cruz y Celis, A.; Ceballos-Cancino, G.; Vazquez-Santillan, K.; Espinosa, M.; Zampedri, C.; Bahena, I.; Ruiz, V.; Maldonado, V.; Melendez-Zajgla, J. Basal-Type Breast Cancer Stem Cells Over-Express Chromosomal Passenger Complex Proteins. Cells 2020, 9, 709. [Google Scholar] [CrossRef]
- Xiao, J.; McGill, J.R.; Nasir, A.; Lekan, A.; Johnson, B.; Wilkins, D.J.; Pearson, G.W.; Tanner, K.; Goodarzi, H.; Glasgow, E.; et al. Identifying drivers of breast cancer metastasis in progressively invasive subpopulations of zebrafish-xenografted MDA-MB-231. Mol. Biomed. 2022, 3, 16. [Google Scholar] [CrossRef]
- Fan, R.-Y.; Wu, J.-Q.; Liu, Y.-Y.; Liu, X.-Y.; Qian, S.-T.; Li, C.-Y.; Wei, P.; Song, Z.; He, M.-F. Zebrafish xenograft model for studying mechanism and treatment of non-small cell lung cancer brain metastasis. J. Exp. Clin. Cancer Res. 2021, 40, 371. [Google Scholar] [CrossRef]
- Guo, Y.; Fan, Y.; Pei, X. Fangjihuangqi Decoction inhibits MDA-MB-231 cell invasion in vitro and decreases tumor growth and metastasis in triple-negative breast cancer xenografts tumor zebrafish model. Cancer Med. 2020, 9, 2564–2578. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yan, B.; Zhang, L.; Zhou, L.; Zhang, J.; Yu, W.; Dong, X.; Yao, L.; Shan, L. Theabrownin induces apoptosis and tumor inhibition of hepatocellular carcinoma Huh7 cells through ASK1-JNK-c-Jun pathway. OncoTargets Ther. 2020, 13, 8977. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhou, L.; Yan, B.; Yan, L.; Liu, F.; Tong, P.; Yu, W.; Dong, X.; Xie, L.; Zhang, J. Theabrownin triggers DNA damage to suppress human osteosarcoma U2 OS cells by activating p53 signalling pathway. J. Cell. Mol. Med. 2018, 22, 4423–4436. [Google Scholar] [CrossRef]
- Andrade, W.; Seabrook, T.J.; Johnston, M.G.; Hay, J.B. The use of the lipophilic fluorochrome CM-DiI for tracking the migration of lymphocytes. J. Immunol. Methods 1996, 194, 181–189. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokary, S.; Zakaria, Z.; Bawadi, H.; Al-Asmakh, M. Testing the Anticancer Effect of Matcha Using Zebrafish as an Animal Model. Nutrients 2023, 15, 2369. https://doi.org/10.3390/nu15102369
Sokary S, Zakaria Z, Bawadi H, Al-Asmakh M. Testing the Anticancer Effect of Matcha Using Zebrafish as an Animal Model. Nutrients. 2023; 15(10):2369. https://doi.org/10.3390/nu15102369
Chicago/Turabian StyleSokary, Sara, Zain Zakaria, Hiba Bawadi, and Maha Al-Asmakh. 2023. "Testing the Anticancer Effect of Matcha Using Zebrafish as an Animal Model" Nutrients 15, no. 10: 2369. https://doi.org/10.3390/nu15102369
APA StyleSokary, S., Zakaria, Z., Bawadi, H., & Al-Asmakh, M. (2023). Testing the Anticancer Effect of Matcha Using Zebrafish as an Animal Model. Nutrients, 15(10), 2369. https://doi.org/10.3390/nu15102369






