Effect of Inhibiting Butyrylcholinesterase Activity Using Fractionated Coffee Extracts Digested In Vitro in Gastrointestinal Tract: Docking Simulation and Calorimetric and Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. In Vitro Digestion of Fractionated Coffee Extracts
2.3. Analysis of Chlorogenic Acids and Caffeine by LC-ESI-MS
2.4. Viability of Bacteria in the In Vitro Digestive System
2.5. Inhibition of BChE Activity Evaluated by Isothermal Titration Calorimetry
2.6. Molecular Modelling
2.7. Statistical Analysis
3. Results and Discussion
3.1. The Effect of Different Fractions from Coffee Extracts on the Viability of Probiotic Lactic Acid Bacteria during In Vitro Digestion
3.2. Profile of Chlorogenic Acids and Caffeine in Fractions from Coffee Extracts after In Vitro Digestion
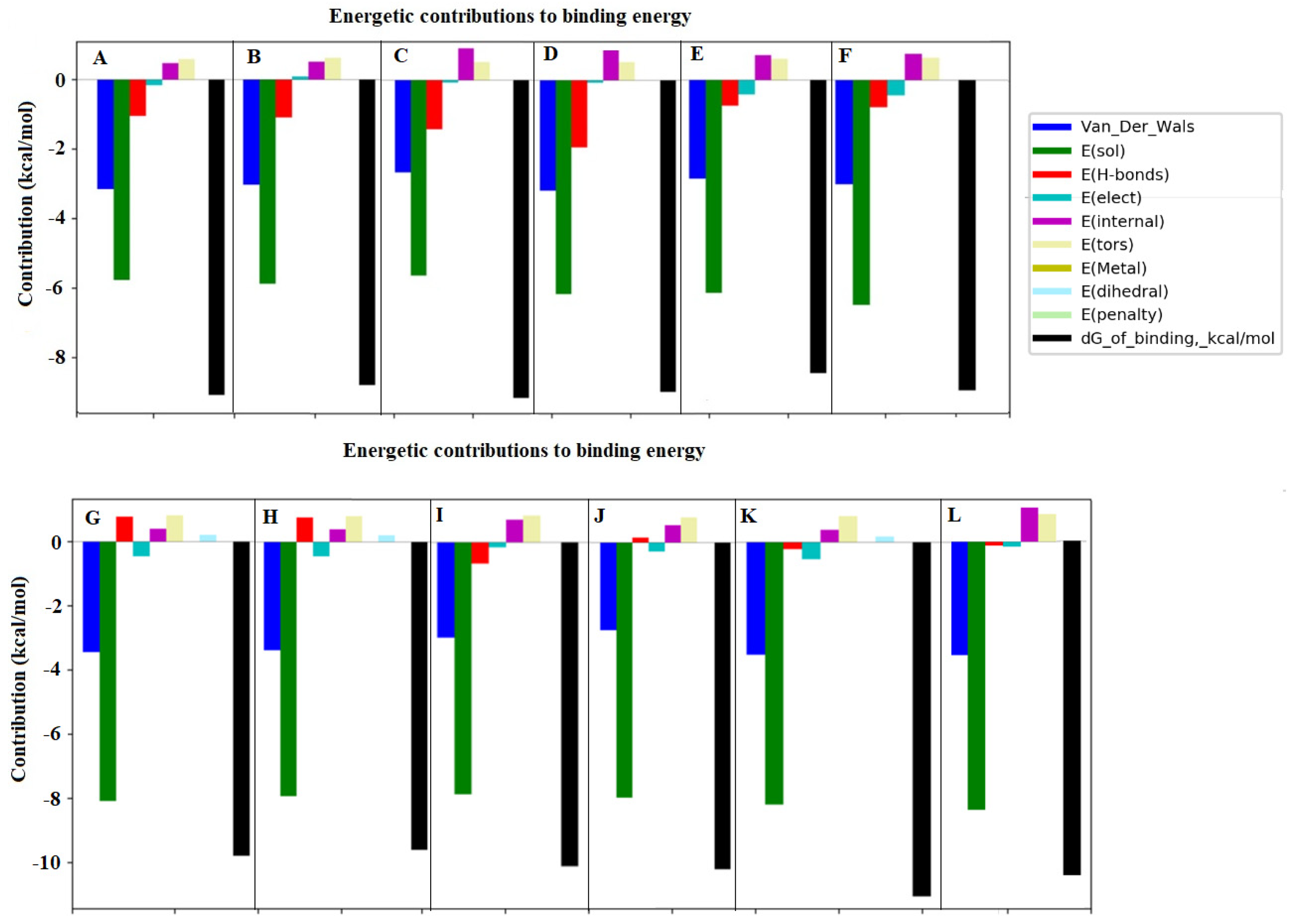
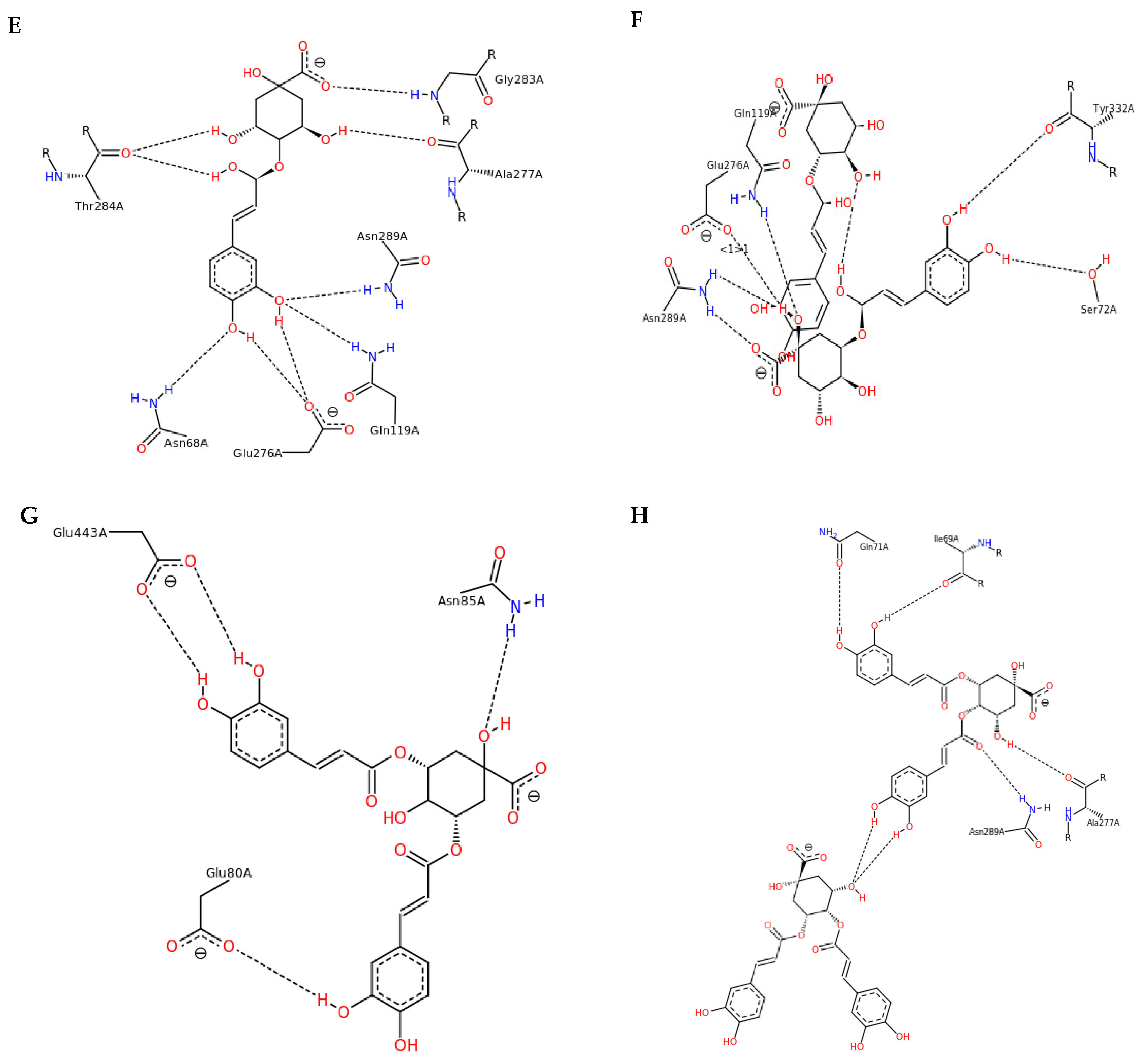
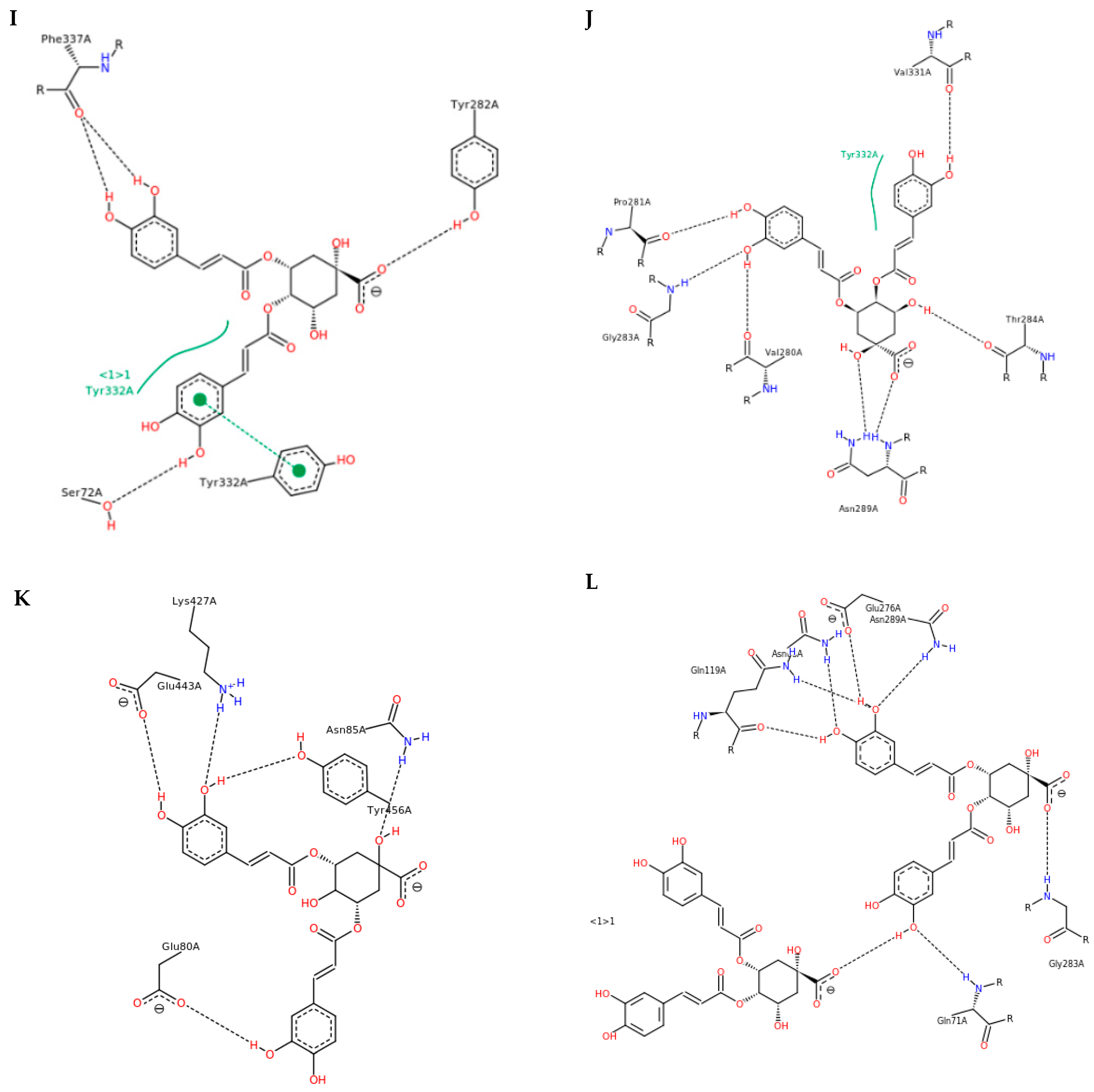
3.3. Interactions and Affinity of BChE and Fractionated Coffee Extracts Digested In Vitro
3.4. Simulation of Docking BChE and In Vitro Digested Coffee Fractions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Tian, T.; Liu, X.; Cui, Z. BCHE as a Prognostic Biomarker in Endometrial Cancer and Its Correlation with Immunity. J. Immunol. Res. 2022, 21, 6051092. [Google Scholar] [CrossRef] [PubMed]
- Severi, I.; Abbatelli, S.; Perugini, J.; Di Mercurio, E.; Senzacqua, M.; Giordano, A. Butyrylcholinesterase distribution in the mouse gastrointestinal tract: An immunohistochemical study. J. Anat. 2023, 242, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.K.L.; Filho, V.C.; de Souza, R.L.R.; Furtado-Alle, L. BChE inhibitors from marine organisms—A review. Chem. Biol. Interact. 2022, 367, 110136. [Google Scholar] [CrossRef]
- Gok, M.; Cicek, C.; Sari, S.; Bodur, E. Novel activity of human BChE: Lipid hydrolysis. Biochimie 2023, 204, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Grothe, M.J.; Ray, N.J.; Müller, M.L.T.; Teipel, S.J. Recent advances in cholinergic imaging and cognitive decline—Revisiting the cholinergic hypothesis of dementia. Curr. Geriatr. Rep. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Jaipea, S.; Saehlim, N.; Sutcharitruk, W.; Athipornchai, A.; Ingkaninan, K.; Saeeng, R. Synthesis of piperine analogues as AChE and BChE inhibitors for the treatment of Alzheimer’s disease. Phytochem. Lett. 2023, 53, 216–221. [Google Scholar] [CrossRef]
- Jabeen, F.; Ur Rehman, Z.; Shah, S.; Alharthy, R.D.; Jalil, S.; Khan, I.A.; Iqbal, J.; El-Latif, A.A.A. Deep learning-based prediction of inhibitors interaction with Butyrylcholinesterase for the treatment of Alzheimer’s disease. Comput. Electr. Eng. 2023, 105, 108475. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, M.; Guo, J.; Cao, D.; Luo, J.; Wan, Y.; Fang, Y.; Jin, Y.; Xie, S.-S.; Liu, J. Dual functional antioxidant and butyrylcholinesterase inhibitors for the treatment of Alzheimer’s disease: Design, synthesis and evaluation of novel melatonin-alkylbenzylamine hybrids. Bioorg. Med. Chem. 2023, 78, 117146. [Google Scholar] [CrossRef]
- Redondo-Puente, M.; Mateos, R.; Seguido, M.A.; García-Cordero, J.; González, S.; Tarradas, R.M.; Bravo-Clemente, L.; Sarriá, B. Appetite and Satiety Effects of the Acute and Regular Consumption of Green Coffee Phenols and Green Coffee Phenol/Oat β-Glucan Nutraceuticals in Subjects with Overweight and Obesity. Foods 2021, 10, 2511. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; Uranga, J.A.; del Castillo, M.D.; Abalo, R. Effects of Coffee and Its Components on the Gastrointestinal Tract and the Brain–Gut Axis. Nutrients 2021, 13, 88. [Google Scholar] [CrossRef]
- Ciaramelli, C.; Palmioli, A.; Ariroldi, C. Coffee variety, origin and extraction procedure: Implications for coffee beneficial effects on human health. Food Chem. 2019, 278, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Kakio, S.; Nakazawa, Y.; Kobata, K.; Funakoshi-Tago, M.; Suzuki, T.; Tamura, H. Roasted coffee reduces β-amyloid production by increasing proteasomal β-secretase degradation in human neuroblastoma SH-SY5Y cells. Mol. Nutr. Food Res. 2018, 62, 1800238. [Google Scholar] [CrossRef] [PubMed]
- Mancini, R.S.; Wang, Y.; Weaver, D.F. Phenylindanes in brewed coffee inhibit amyloid-beta and tau aggregation. Front. Neurosci. 2018, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Grzelczyk, J.; Szwajgier, D.; Baranowska-Wójcik, B.; Budryn, G.; Zakłos-Szyda, M.; Sosnowska, B. Bioaccessibility of coffee bean hydroxycinnamic acids during in vitro digestion influenced by the degree of roasting and activity of intestinal probiotic bacteria, and their activity in Caco-2 and HT29 cells. Food Chem. 2022, 392, 133328. [Google Scholar] [CrossRef]
- Szwajgier, D.; Paduch, R.; Kukuła-Koch, W.; Polak-Berecka, M.; Waśko, W. Study on biological activity of bread enriched with natural polyphenols in terms of growth inhibition of tumor intestine cells. J. Med. Food 2020, 23, 181–190. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Budryn, G.; Peña-García, J.; Szwajgier, D.; Gałązka-Czarnecka, I.; Oracz, J.; Pérez-Sánchez, H. Evaluation of the inhibition of monoamine oxidase A by bioactive coffee compounds protecting serotonin degradation. Food Chem. 2021, 348, 129108. [Google Scholar] [CrossRef]
- Budryn, G.; Grzelczyk, J.; Jaśkiewicz, A.; Żyżelewicz, D.; Pérez-Sánchez, H.; Cerón-Carrasco, J.P. Evaluation of butyrylcholinesterase inhibitory activity by chlorogenic acids and coffee extracts assed in ITC and docking simulation models. Food Res. Int. 2018, 109, 268–277. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pK(a) prediction and protonation state generation for drug-like molecules. J. Comput. Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef]
- Roos, K.; Wu, C.; Damm, W.; Reboul, M.; Stevenson, J.M.; Lu, C.; Dahlgren, M.K.; Mondal, S.; Chen, W.; Wang, L.; et al. OPLS3e: Extending force field coverage for drug-like small molecules. J. Chem. Theory Comput. 2019, 15, 1863–1874. [Google Scholar] [CrossRef]
- Stroganov, O.V.; Novikov, F.N.; Stroylov, V.S.; Kulkov, V.; Chilov, G.G. Lead finder: An approach to improve accuracy of protein-ligand docking, binding energy estimation, and virtual screening. J. Chem. Inf. Model. 2008, 48, 2371–2385. [Google Scholar] [CrossRef] [PubMed]
- Stierand, K.; Rarey, M. From modeling to medicinal chemistry: Automatic generation of two-dimensional complex diagrams. ChemMedChem 2007, 2, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.; Tzounis, X.; Oruna-Concha, M.; Mottram, D.; Gibson, G.; Spencer, J. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Freeman, S.; Corey, M.; German, J.B.; Barile, D. Chemical characterization of potentially prebiotic oligosaccharides in brewed coffee and spent coffee grounds. J. Agric. Food Chem. 2017, 65, 2784–2792. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef]
- Sales, A.L.; de Paula, J.; Silva, C.M.; Cruz, A.; Miguel, M.A.L.; Farah, A. Effects of regular and decaffeinated roasted coffee (Coffea Arabica and Coffea canephora) extracts and bioactive compounds on in vitro probiotic bacterial growth. Food Funct. 2020, 11, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Salazar, N.; Ruiz-Saavedra, S.; Gómez-Martín, M.; de Los Reyes-Gavilán, C.G.; Gueimonde, M. Long-term coffee consumption is associated with fecal microbial composition in humans. Nutrients 2020, 12, 1287. [Google Scholar] [CrossRef]
- Castaldo, L.; Izzo, L.; Narváez, A.; Rodríguez-Carrasco, Y.; Grosso, M.; Ritieni, A. Colon Bioaccessibility under In Vitro Gastrointestinal Digestion of Different Coffee Brews Chemically Profiled through UHPLC-Q-Orbitrap HRMS. Foods 2021, 17, 179. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Podsędek, A.; Żyżelewicz, D.; Materska, M.; Jankowski, S.; Janda, B. Effect of different extraction methods on the recovery of chlorogenic acids, caffeine and Maillard reaction products in coffee beans. Eur. Food Res. Technol. 2009, 228, 913–922. [Google Scholar] [CrossRef]
- Teodorowicz, M.; van Neerven, J.; Savelkoul, H. Food Processing: The influence of the maillard reaction on immunogenicity and allergenicity of food proteins. Nutrients 2017, 9, 835. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Jasiecki, J.; Szczoczarz, A.; Cysewski, D.; Lewandowski, K.; Skowron, P.; Waleron, K.; Wasąg, B. Butyrylcholinesterase-protein interactions in human serum. Int. J. Mol. Sci. 2021, 22, 10662. [Google Scholar] [CrossRef]
- Bajda, M.; Więckowska, A.; Hebda, M.; Guzior, N.; Sotriffer, C.A.; Malawska, B. Structure-based search for new inhibitors of cholinesterases. Int. J. Mol. Sci. 2013, 14, 5608–5632. [Google Scholar] [CrossRef] [PubMed]
- Atay, M.S.; Sari, S.; Bodur, E. Molecular and Computational Analysis Identify Statins as Selective Inhibitors of Human Butyrylcholinesterase. Protein J. 2023, 42, 104–111. [Google Scholar] [CrossRef]
- Murakawa, T.; Matsushita, Y.; Suzuki, T.; Khan, M.T.H.; Kurita, N. Ab initio molecular simulations for proposing potent inhibitors to butyrylcholinesterases. J. Mol. Graph. Model. 2014, 54, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Movahhedin, N.; Zengin, G.; Bahadori, M.B.; Sarikurkcu, C.; Bahadori, S.; Dinparast, L. Ajuga chamaecistus subsp. scoparia (Boiss.) Rech.f.: A new source of phytochemicals for antidiabetic, skin-care, and neuroprotective uses. Ind. Crops Prod. 2016, 94, 89–96. [Google Scholar] [CrossRef]
- Gholami, A.; Minai-Tehrani, D.; Eriksson, L.A. In silico and in vitro studies confirm Ondansetron as a novel acetylcholinesterase and butyrylcholinesterase inhibitor. Sci. Rep. 2023, 13, 643. [Google Scholar] [CrossRef]

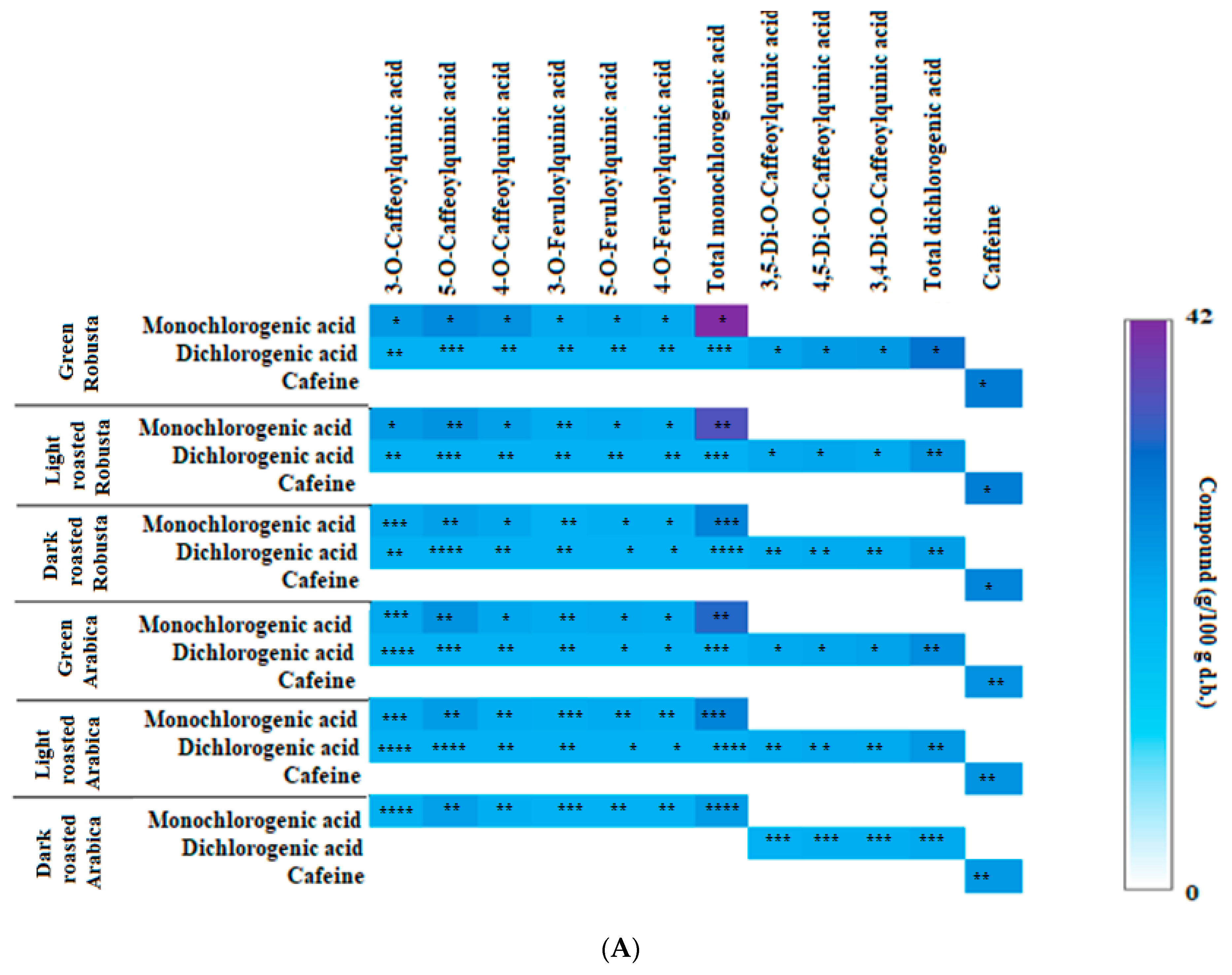

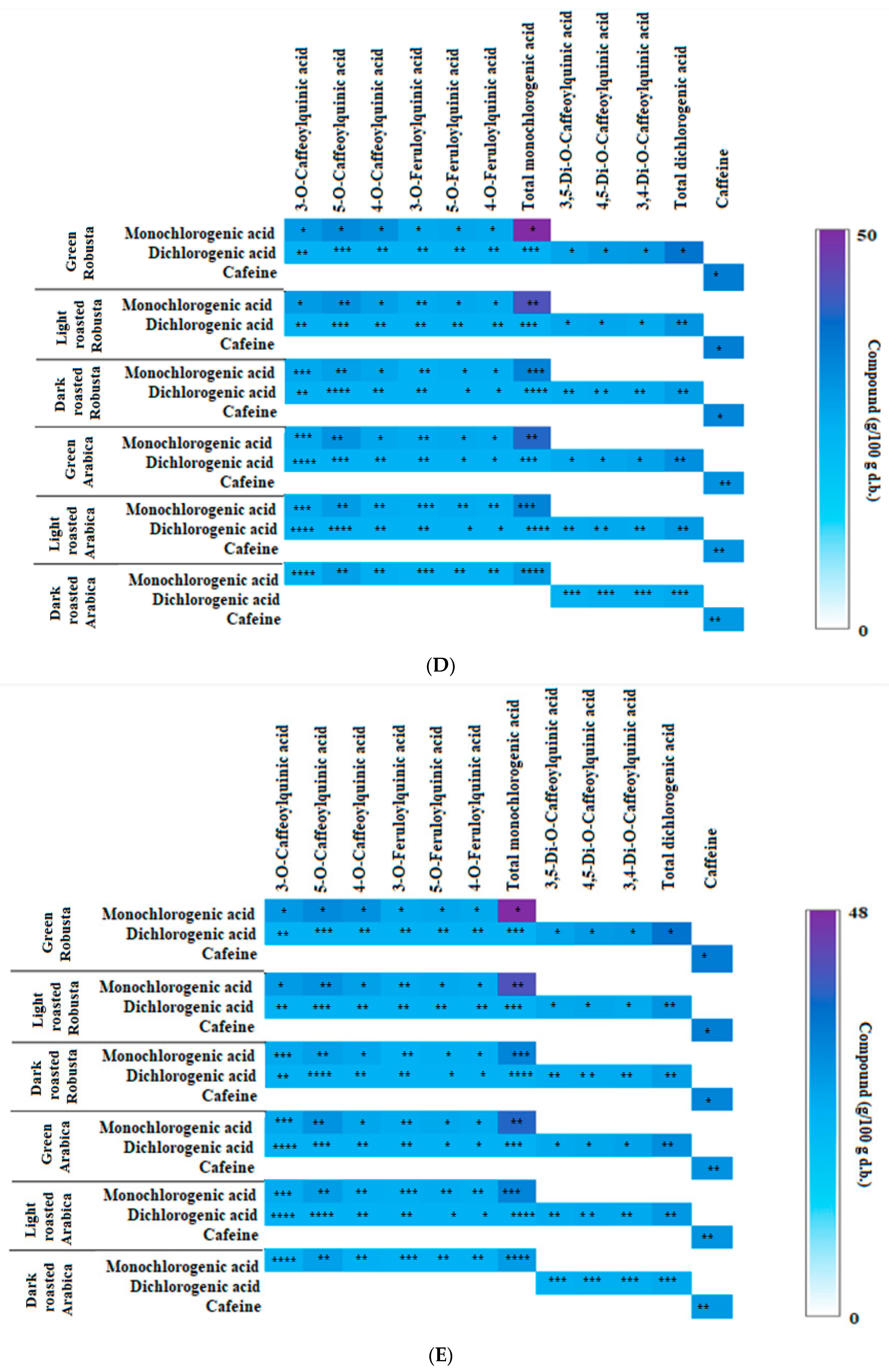
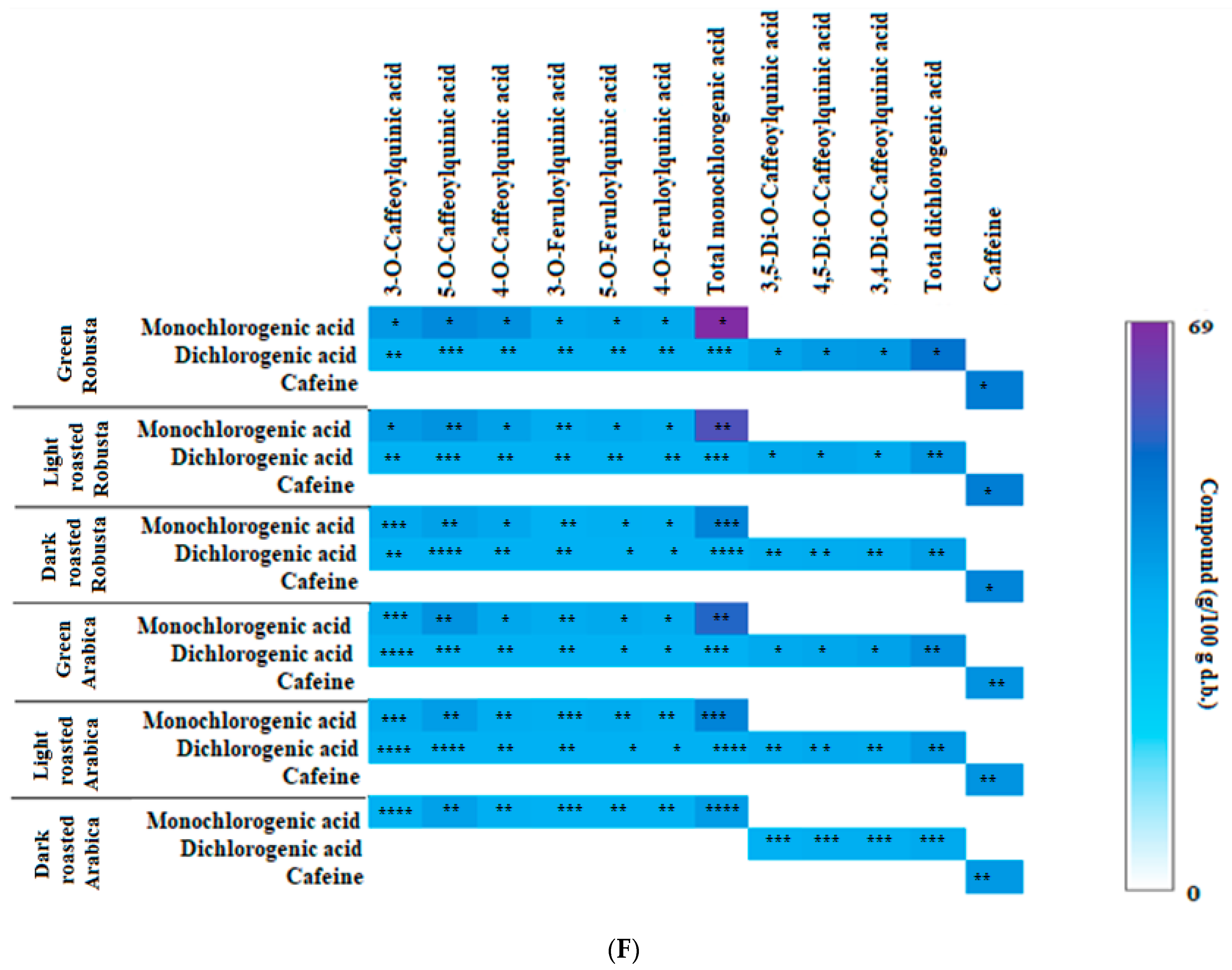



| Fraction from Coffee Extract | KA × 103 (L/mol) | ∆H (kJ/mol) | ∆G (kJ/mol) | ∆S (J/mol × K) | Inhibitory Activity (%) | μmol/μmol Enzyme IC50 | Ki (μmol/L) KM ACh 35.9 |
|---|---|---|---|---|---|---|---|
| Green Arabica | |||||||
| Monochlorogenic acid | 14.06 ± 0.95 a | −0.70 ± 0.03 a | −18.67 ± 0.03 a | 62.65 ± 0.01 a | 51.77 ± 0.03 a | 1.25 ± 0.01 b | 0.24 ± 0.02 a |
| Dichlorogenic acid | 12.35 ± 2.35 a | −0.75± 0.02 a | −30.23 ± 0.02 b | 95.36 ± 0.02 b | 86.37 ± 0.10 b | 0.86 ± 0.00 a | 0.32 ± 0.01 a |
| Caffeine | 40.58 ± 6.98 b | −0.31 ± 0.01 b | −15.28 ± 0.02 a | 48.42 ± 0.02 c | 53.20 ± 0.02 a | 1.20 ± 0.01 b | 0.56 ± 0.03 b |
| Light roasted Arabica | |||||||
| Monochlorogenic acid | 1.92 ± 0.20 a | −1.17 ± 0.09 c | −25.41 ± 0.03 c | 78.41 ± 0.05 d | 54.83 ± 0.02 a | 1.59 ± 0.01 c | 0.34 ± 0.01 a |
| Dichlorogenic acid | 47.15 ± 1.35 b | −0.54 ± 0.03 a | −21.81 ± 0.02 c | 60.80 ± 0.05 a | 74.99 ± 0.06 b | 1.35 ± 0.01 b | 0.62 ± 0.02 b |
| Caffeine | 41.48 ± 2.25 b | −0.11 ± 0.02 d | −20.48 ± 0.02 c | 65.89 ± 0.02 a | 73.03 ± 0.04 b | 1.51 ± 0.01 c | 0.57 ± 0.02 b |
| Dark roasted Arabica | |||||||
| Monochlorogenic acid | 27.10 ± 1.10 a | −0.39 ± 0.02 b | −22.39 ± 0.04 c | 71.16 ± 0.02 d | 71.44 ± 0.10 b | 1.91 ± 0.01 d | 0.13 ± 0.04 c |
| Dichlorogenic acid | 58.41 ± 3.25 b | −0.05 ± 0.04 d | −20.05 ± 0.02 c | 64.69 ± 0.05 a | 95.40 ± 0.12 c | 1.48 ± 0.00 b | 0.25 ± 0.01 a |
| Caffeine | 23.90 ± 1.45 a | −0.31 ± 0.01 b | −21.31 ± 0.01 c | 67.96 ± 0.04 a | 75.56 ± 0.06 b | 2.29 ± 0.01 d | 0.67 ± 0.03 b |
| Green Robusta | |||||||
| Monochlorogenic acid | 1.95 ± 0.20 a | −1.22 ± 0.01 c | −25.41 ± 0.12 c | 78.24 ± 0.03 d | 66.32 ± 0.03 a | 1.29 ± 0.00 b | 0.88 ± 0.01 d |
| Dichlorogenic acid | 53.05 ± 2.25 b | −1.10 ± 0.02 c | −22.15 ± 0.02 c | 68.09 ± 0.06 a | 75.40 ± 0.06 b | 0.70 ± 0.00 a | 0.72 ± 0.00 d |
| Caffeine | 55.56 ± 2.55 b | 5.44 ± 0.03 e | −21.01 ± 0.03 c | −50.36 ± 0.02 e | 64.85 ± 0.01 a | 1.30 ± 0.01 b | 0.32 ± 0.02 a |
| Light roasted Robusta | |||||||
| Monochlorogenic acid | 2.51 ± 0.15 a | −8.88 ± 1.15 f | −17.48 ± 0.02 a | 27.82 ± 0.06 f | 62.75 ± 0.02 a | 1.56 ± 0.00 c | 0.38 ± 0.03 a |
| Dichlorogenic acid | 10.26 ± 0.35 a | −16.54 ± 3.55 g | −30.19 ± 0.10 b | 44.15 ± 0.09 b | 98.44 ± 0.10 c | 1.30 ± 0.01 b | 0.23 ± 0.02 a |
| Caffeine | 43.57 ± 2.20 b | −0.62 ± 0.00 a | −21.60 ± 0.03 c | 67.86 ± 0.05 a | 73.72 ± 0.03 b | 1.11 ± 0.01 b | 0.43 ± 0.03 c |
| Dark roasted Robusta | |||||||
| Monochlorogenic acid | 2.24 ± 0.55 a | −1.20 ± 0.09 c | −27.98 ± 0.10 c | 86.62 ± 0.02 d | 80.77 ± 0.02 b | 2.21 ± 0.01 d | 0.53 ± 0.01 c |
| Dichlorogenic acid | 7.86 ± 1.15 a | −8.90 ± 0.04 f | −29.06 ± 0.12 c | 65.21 ± 0.03 a | 97.86 ± 0.10 c | 1.42 ± 0.01 b | 0.14 ± 0.08 a |
| Caffeine | 9.43 ± 2.35 a | −0.20 ± 0.01 d | −28.52 ± 0.11 c | 91.60 ± 0.05 b | 84.68 ± 0.02 b | 1.57 ± 0.01 c | 0.46 ± 0.04 b |
| Sequence | Energy (kJ/mol) | |||
|---|---|---|---|---|
| Step 1 | Step 2 | Step 3 | Average Step | |
| 3-CQA, 4-CQA, 5-CQA | −41.51 | −45.86 | −33.47 | −40.28 |
| 3-CQA, 5-CQA, 4-CQA | −41.51 | −38.07 | −35.15 | −38.24 |
| 4-CQA, 3-CQA, 5-CQA | −42.34 | −45.44 | −36.02 | −41.27 |
| 4-CQA, 5-CQA, 3-CQA | −42.34 | −40.58 | −38.99 | −40.64 |
| 5-CQA, 3-CQA, 4-CQA | −38.66 | −34.35 | −35.02 | −36.01 |
| 5-CQA, 4-CQA, 3-CQA | −38.66 | −40.92 | −37.99 | −39.19 |
| 3,4-DCQA, 3,5-DCQA, 4,5-DCQA | −50.75 | −42.47 | −37.53 | −43.58 |
| 3,4-DCQA, 4,5-DCQA, 3,5-DCQA | −50.75 | −37.99 | −40.96 | −43.23 |
| 3,5-DCQA, 3,4-DCQA, 4,5-DCQA | −50.42 | −41.17 | −41.59 | −44.39 |
| 3,5-DCQA, 4,5-DCQA, 3,4-DCQA | −50.42 | −42.05 | −41.76 | −44.74 |
| 4,5-DCQA, 3,4-DCQA, 3,5-DCQA | −49.16 | −45.56 | −46.40 | −47.04 |
| 4,5-DCQA, 3,5-DCQA, 3,4-DCQA | −49.12 | −45.52 | −41.04 | −45.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzelczyk, J.; Szwajgier, D.; Baranowska-Wójcik, E.; Pérez-Sánchez, H.; Carmena-Bargueño, M.; Sosnowska, B.; Budryn, G. Effect of Inhibiting Butyrylcholinesterase Activity Using Fractionated Coffee Extracts Digested In Vitro in Gastrointestinal Tract: Docking Simulation and Calorimetric and Studies. Nutrients 2023, 15, 2366. https://doi.org/10.3390/nu15102366
Grzelczyk J, Szwajgier D, Baranowska-Wójcik E, Pérez-Sánchez H, Carmena-Bargueño M, Sosnowska B, Budryn G. Effect of Inhibiting Butyrylcholinesterase Activity Using Fractionated Coffee Extracts Digested In Vitro in Gastrointestinal Tract: Docking Simulation and Calorimetric and Studies. Nutrients. 2023; 15(10):2366. https://doi.org/10.3390/nu15102366
Chicago/Turabian StyleGrzelczyk, Joanna, Dominik Szwajgier, Ewa Baranowska-Wójcik, Horacio Pérez-Sánchez, Miguel Carmena-Bargueño, Bożena Sosnowska, and Grażyna Budryn. 2023. "Effect of Inhibiting Butyrylcholinesterase Activity Using Fractionated Coffee Extracts Digested In Vitro in Gastrointestinal Tract: Docking Simulation and Calorimetric and Studies" Nutrients 15, no. 10: 2366. https://doi.org/10.3390/nu15102366
APA StyleGrzelczyk, J., Szwajgier, D., Baranowska-Wójcik, E., Pérez-Sánchez, H., Carmena-Bargueño, M., Sosnowska, B., & Budryn, G. (2023). Effect of Inhibiting Butyrylcholinesterase Activity Using Fractionated Coffee Extracts Digested In Vitro in Gastrointestinal Tract: Docking Simulation and Calorimetric and Studies. Nutrients, 15(10), 2366. https://doi.org/10.3390/nu15102366







