Knowledge and Beliefs about Herb/Supplement Consumption and Herb/Supplement–Drug Interactions among the General Population, including Healthcare Professionals and Pharmacists: A Systematic Review and Guidelines for a Smart Decision System
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
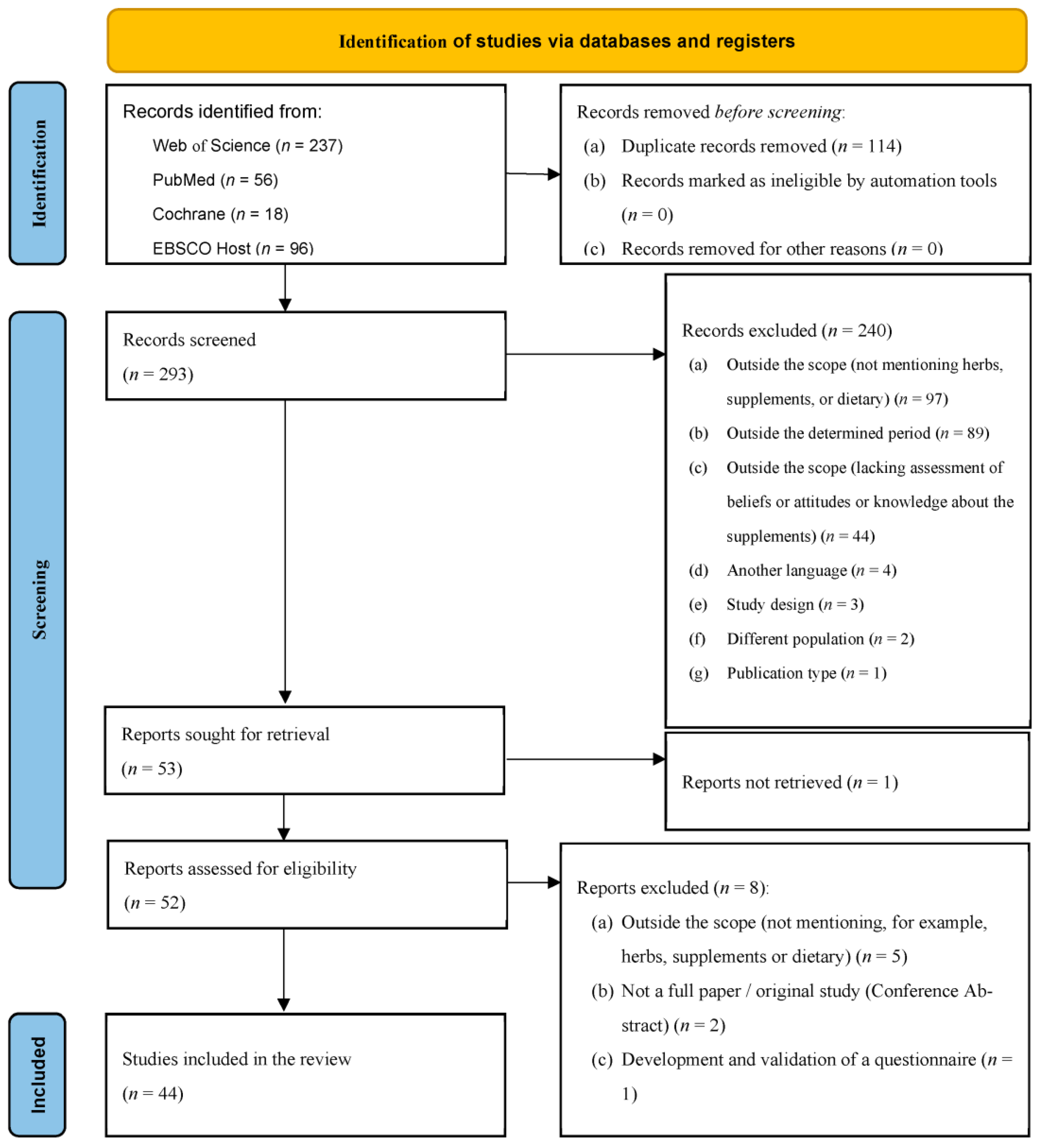
3. Results
3.1. Study Characteristics
3.2. Instruments of Data Collection
3.3. Procedures for Data Collection
3.4. Main Results
3.4.1. Herb/Supplement Consumption
3.4.2. Interaction between Herbs/Supplements and Prescription Drugs
3.4.3. Effects and Risks of Herb/Supplement Use
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hughes, G.D.; Aboyade, O.M.; Clark, B.L.; Puoane, T.R. The prevalence of traditional herbal medicine use among hypertensives living in South African communities. BMC Complement. Altern. Med. 2013, 13, 38. [Google Scholar] [CrossRef]
- Jaber, D.; Al-Zeidaneen, S. Women’s opinions, beliefs, and practices towards using different medicinal plants for postpartum health problems care. Jordan J. Pharm. Sci. 2021, 14, 309–322. [Google Scholar]
- John, L.J.; Shantakumari, N. Herbal Medicines Use during Pregnancy: A Review from the Middle East. Oman Med. J. 2015, 30, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Laelago, T.; Yohannes, T.; Lemango, F. Prevalence of herbal medicine use and associated factors among pregnant women attending antenatal care at public health facilities in Hossana Town, Southern Ethiopia: Facility based cross sectional study. Arch. Public Health 2016, 74, 7. [Google Scholar] [CrossRef]
- Bailey, R.L.; Gahche, J.J.; Lentino, C.V.; Dwyer, J.T.; Engel, J.S.; Thomas, P.R.; Betz, J.M.; Sempos, C.T.; Picciano, M.F. Dietary Supplement Use in the United States, 2003–2006. J. Nutr. 2011, 141, 261–266. [Google Scholar] [CrossRef]
- Bailey, R.L.; Gahche, J.J.; Miller, P.E.; Thomas, P.R.; Dwyer, J.T. Why US Adults Use Dietary Supplements. JAMA Intern. Med. 2013, 173, 355. [Google Scholar] [CrossRef] [PubMed]
- Ceremuga, T.; Ayala, M.; Henson, D.; Chicoine, R.; Celestino, J.; Chun, S.; DeGroot, J.; Glynn, A.; Randall, S.; Stanley, L.; et al. Knowledge Assessment of Military Personnel, Veterans, and Family Taking Dietary Supplements. AANA J. 2020, 88, 191–202. [Google Scholar] [PubMed]
- Rautiainen, S.; Manson, J.E.; Lichtenstein, A.H.; Sesso, H.D. Dietary supplements and disease prevention—A global overview. Nat. Rev. Endocrinol. 2016, 12, 407–420. [Google Scholar] [CrossRef]
- Rovira, M.-A.; Grau, M.; Castañer, O.; Covas, M.-I.; Schröder, H. Dietary Supplement Use and Health-Related Behaviors in a Mediterranean Population. J. Nutr. Educ. Behav. 2013, 45, 386–391. [Google Scholar] [CrossRef]
- Niveditha, A.S.; Geetha, R.V. Knowledge and awareness of natural anticarcinogenic herbs among the general population. Drug Invent. Today 2020, 14, 1245–1247. [Google Scholar]
- U.S. Food and Drug Administration. Dietary Supplement Products & Ingredients | FDA 2022. Available online: https://www.fda.gov/food/dietary-supplements/dietary-supplement-products-ingredients (accessed on 25 August 2022).
- Corazza, O.; Simonato, P.; Demetrovics, Z.; Mooney, R.; Van De Ven, K.; Roman-Urrestarazu, A.; Rácmolnár, L.; De Luca, I.; Cinosi, E.; Santacroce, R.; et al. The emergence of Exercise Addiction, Body Dysmorphic Disorder, and other image-related psychopathological correlates in fitness settings: A cross sectional study. PLoS ONE 2019, 14, e0213060. [Google Scholar] [CrossRef] [PubMed]
- Dores, A.R.; Carvalho, I.P.; Burkauskas, J.; Simonato, P.; De Luca, I.; Mooney, R.; Ioannidis, K.; Gómez-Martínez, M.Á.; Demetrovics, Z.; Ábel, K.E.; et al. Exercise and Use of Enhancement Drugs at the Time of the COVID-19 Pandemic: A Multicultural Study on Coping Strategies During Self-Isolation and Related Risks. Front. Psychiatry 2021, 12, 648501. [Google Scholar] [CrossRef] [PubMed]
- Rogge, M.; Kumar, P.; Grundmann, O. ACCP Public Policy Committee Front-Line Health Care Professionals Lack Critical Knowledge in Dietary Supplement and Nutraceutical Products: A Call to Action for Comprehensive Educational Opportunities. J. Clin. Pharmacol. 2022, 62, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Boullata, J. Natural Health Product Interactions with Medication. Nutr. Clin. Pract. 2005, 20, 33–51. [Google Scholar] [CrossRef]
- Sood, A.; Sood, R.; Brinker, F.J.; Mann, R.; Loehrer, L.L.; Wahner-Roedler, D.L. Potential for Interactions Between Dietary Supplements and Prescription Medications. Am. J. Med. 2008, 121, 207–211. [Google Scholar] [CrossRef]
- Tsai, H.-H.; Lin, H.-W.; Simon Pickard, A.; Tsai, H.-Y.; Mahady, G.B. Evaluation of documented drug interactions and contraindications associated with herbs and dietary supplements: A systematic literature review: Evidence evaluation of drugs with herbs and dietary supplements. Int. J. Clin. Pract. 2012, 66, 1056–1078. [Google Scholar] [CrossRef]
- McDonnell, A.M.; Dang, C.H. Basic Review of the Cytochrome P450 System. J. Adv. Pract. Oncol. 2013, 4, 263–268. [Google Scholar] [CrossRef]
- Guengerich, F.P. Human Cytochrome P450 Enzymes. In Cytochrome P450; Ortiz de Montellano, P.R., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 523–785. ISBN 978-3-319-12107-9. [Google Scholar]
- Husain, I.; Dale, O.R.; Martin, K.; Gurley, B.J.; Adams, S.J.; Avula, B.; Chittiboyina, A.G.; Khan, I.A.; Khan, S.I. Screening of medicinal plants for possible herb-drug interactions through modulating nuclear receptors, drug-metabolizing enzymes and transporters. J. Ethnopharmacol. 2023, 301, 115822. [Google Scholar] [CrossRef]
- Zuo, H.-L.; Huang, H.-Y.; Lin, Y.-C.-D.; Cai, X.-X.; Kong, X.-J.; Luo, D.-L.; Zhou, Y.-H.; Huang, H.-D. Enzyme Activity of Natural Products on Cytochrome P450. Molecules 2022, 27, 515. [Google Scholar] [CrossRef]
- Husain, I.; Dale, O.R.; Manda, V.; Ali, Z.; Gurley, B.J.; Chittiboyina, A.G.; Khan, I.A.; Khan, S.I. Bulbine natalensis (currently Bulbine latifolia) and select bulbine knipholones modulate the activity of AhR, CYP1A2, CYP2B6, and P-gp. Planta Med. 2022, 88, 975–984. [Google Scholar] [CrossRef]
- Lin, J.H.; Yamazaki, M. Role of P-Glycoprotein in Pharmacokinetics: Clinical Implications. Clin. Pharmacokinet. 2003, 42, 59–98. [Google Scholar] [CrossRef]
- Cho, H.-J.; Yoon, I.-S. Pharmacokinetic Interactions of Herbs with Cytochrome P450 and P-Glycoprotein. Evid. Based Complement. Alternat. Med. 2015, 2015, 736431. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lim, L.Y.; Chowbay, B. Herbal Modulation of P-Glycoprotein. Drug Metab. Rev. 2004, 36, 57–104. [Google Scholar] [CrossRef] [PubMed]
- Husain, I.; Bala, K.; Khan, I.A.; Khan, S.I. A review on phytochemicals, pharmacological activities, drug interactions, and associated toxicities of licorice (Glycyrrhiza sp.). Food Front. 2021, 2, 449–485. [Google Scholar] [CrossRef]
- Arayne, M.S.; Sultana, N.; Bibi, Z. Grape fruit juice-drug interactions. Pak. J. Pharm. Sci. 2005, 18, 45–57. [Google Scholar]
- Lee, J.W.; Morris, J.K.; Wald, N.J. Grapefruit Juice and Statins. Am. J. Med. 2016, 129, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Nicolussi, S.; Drewe, J.; Butterweck, V.; Meyer zu Schwabedissen, H.E. Clinical relevance of St. John’s wort drug interactions revisited. Br. J. Pharmacol. 2020, 177, 1212–1226. [Google Scholar] [CrossRef]
- Mahnashi, M.H. Knowledge, Attitude, Practice, and the Perceived Barriers with Respect to the Use of Herbal Medicines. Curr. Top. Nutraceutical Res. 2020, 19, 29–35. [Google Scholar] [CrossRef]
- Hemberg, N.; Huggins, D.; Michaels, N.; Moose, J. Innovative Community Pharmacy Practice Models in North Carolina. N. C. Med. J. 2017, 78, 198–201. [Google Scholar] [CrossRef]
- Ng, J.Y.; Tahir, U.; Dhaliwal, S. Barriers, knowledge, and training related to pharmacists’ counselling on dietary and herbal supplements: A systematic review of qualitative studies. BMC Health Serv. Res. 2021, 21, 499. [Google Scholar] [CrossRef] [PubMed]
- Mossialos, E.; Naci, H.; Courtin, E. Expanding the role of community pharmacists: Policymaking in the absence of policy-relevant evidence? Health Policy 2013, 111, 135–148. [Google Scholar] [CrossRef]
- Owens, C.; Toone, T. A Survey of Dietary Supplement Knowledge, Attitudes, and Use in a Rural Population. J. Nutr. Food Sci. 2014, 4, 304. [Google Scholar] [CrossRef]
- Scripture, C.D.; Figg, W.D. Drug interactions in cancer therapy. Nat. Rev. Cancer 2006, 6, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Karny-Rahkovich, O.; Blatt, A.; Elbaz-Greener, G.A.; Ziv-Baran, T.; Golik, A.; Berkovitch, M. Dietary supplement consumption among cardiac patients admitted to internal medicine and cardiac wards. Cardiol. J. 2015, 22, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Péter, S.; Navis, G.; de Borst, M.H.; von Schacky, C.; van Orten-Luiten, A.C.B.; Zhernakova, A.; Witkamp, R.F.; Janse, A.; Weber, P.; Bakker, S.J.L.; et al. Public health relevance of drug–nutrition interactions. Eur. J. Nutr. 2017, 56, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Altieri, B.; Polese, B.; De Conno, B.; Muscogiuri, G.; Colao, A.; Savastano, S.; Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) Group. Nutritionist and obesity: Brief overview on efficacy, safety, and drug interactions of the main weight-loss dietary supplements. Int. J. Obes. Suppl. 2019, 9, 32–49. [Google Scholar] [CrossRef]
- Cordeiro De Oliveira, R.; Barão, F.; Ferreira, E.; Oliveira, A. A farmacoterapia no tratamento da obesidade. Rev. Bras. Obes. Nutr. e Emagrecimento 2009, 3, 375–388. [Google Scholar]
- Touger-Decker, R. Vitamin and Mineral Supplements: What is the dentist to do? J. Am. Dent. Assoc. 2007, 138, 1222–1226. [Google Scholar] [CrossRef]
- Loya, A.M.; González-Stuart, A.; Rivera, J.O. Prevalence of Polypharmacy, Polyherbacy, Nutritional Supplement Use and Potential Product Interactions among Older Adults Living on the United States-Mexico Border: A Descriptive, Questionnaire-Based Study. Drugs Aging 2009, 26, 423–436. [Google Scholar] [CrossRef]
- Rashrash, M.; Schommer, J.C.; Brown, L.M. Prevalence and Predictors of Herbal Medicine Use among Adults in the United States. J. Patient Exp. 2017, 4, 108–113. [Google Scholar] [CrossRef]
- Homer, P.M.; Mukherjee, S. Lay theories and consumer perceptions of dietary supplements. J. Consum. Behav. 2019, 18, 363–377. [Google Scholar] [CrossRef]
- Moscovici, S. Notes towards a description of Social Representations. Eur. J. Soc. Psychol. 1988, 18, 211–250. [Google Scholar] [CrossRef]
- Höijer, B. Social Representations Theory: A New Theory for Media Research. Nord. Rev. 2011, 32, 3–16. [Google Scholar] [CrossRef]
- Klein, J.D.; Wilson, K.M.; Sesselberg, T.S.; Gray, N.J.; Yussman, S.; West, J. Adolescents’ knowledge of and beliefs about herbs and dietary supplements: A qualitative study. J. Adolesc. Health 2005, 37, 409.e1–409.e7. [Google Scholar] [CrossRef]
- McLay, J.S.; Stewart, D.; George, J.; Rore, C.; Heys, S.D. Complementary and alternative medicines use by Scottish women with breast cancer. What, why and the potential for drug interactions? Eur. J. Clin. Pharmacol. 2012, 68, 811–819. [Google Scholar] [CrossRef]
- Sekhri, K.; Bhanwra, S.; Nandha, R. Herbal products: A survey of students perception and knowledge about their medicinal use. Int. J. Basic Clin. Pharmacol. 2013, 2, 71. [Google Scholar] [CrossRef]
- Vickers, K.A.; Jolly, K.B.; Greenfield, S.M. Herbal medicine: Women’s views, knowledge and interaction with doctors: A qualitative study. BMC Complement. Altern. Med. 2006, 6, 40. [Google Scholar] [CrossRef]
- Hoay Tan, C.L.; Yong Gan, V.B.; Saleem, F.; Hassali, M.A.A. My Opinions Matter! How Perspectives, Knowledge and Expectations Matter in Moderating the Success of New Pharmaceutical Services Implementation in Malaysia. Indian J. Pharm. Educ. Res. 2018, 52, 558–574. [Google Scholar] [CrossRef]
- Wilson, G.A.; Perepelkin, J.; Zhang, D.D. Improving pharmacy performance through market orientation and the implementation of expanded pharmacy services. Health Mark. Q. 2022, 39, 280–296. [Google Scholar] [CrossRef]
- Nichols-English, G.; Poirier, S. Optimizing adherence to pharmaceutical care plans. J. Am. Pharm. Assoc. 2000, 40, 475–485. [Google Scholar] [CrossRef]
- Cnudde, A.; Watrin, P.; Souard, F. HDI Highlighter, The First Intelligent Tool to Screen the Literature on Herb–Drug Interactions. Clin. Pharmacokinet. 2022, 61, 761–788. [Google Scholar] [CrossRef]
- Fasinu, P.S.; Bouic, P.J.; Rosenkranz, B. An Overview of the Evidence and Mechanisms of Herb–Drug Interactions. Front. Pharmacol. 2012, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Maia, E.; Praca, I. Herb–Drug Interactions: A Holistic Decision Support System in Healthcare. In Proceedings of the 2022 IEEE International Conference on E-health Networking, Application & Services (HealthCom), Genoa, Italy, 17–19 October 2022; pp. 1–6. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159. [Google Scholar] [CrossRef]
- Montero, I.; León, O.G. A guide for naming research studies in psychology. Int. J. Clin. Health Psychol. 2007, 7, 847–862. [Google Scholar]
- Makkaoui, N.; Halaoui, A.; Atoui, Z.; Siblini, H.; Habib, S.; Awada, H.; Zgheib, N.K. Knowledge, attitudes, and practices regarding drug interactions among community pharmacists. J. Public Health 2021, 29, 1357–1363. [Google Scholar] [CrossRef]
- Al-Nadaf, A.H.; Awadallah, A. Evaluation for the level of knowledge about herbal medicine use within people and university students in Mutah region. Pharmacia 2020, 67, 397–403. [Google Scholar] [CrossRef]
- Eltom, E.; Alenezi, W.; Zeeni, N. Knowledge and awareness of doctors about herbal drugs in the Northern Border Region of Saudi Arabia. Ann. Clin. Anal. Med. 2021, 12, 54–59. [Google Scholar] [CrossRef]
- Agrawal, K.; Goel, D. Herbal Use amongst Patients in a Tertiary Care Hospital: Pattern and Perceptions. Adv. Hum. Biol. 2016, 6, 129. [Google Scholar] [CrossRef]
- Koshak, A.E. Attitudes and Beliefs towards Herbal Medicines in Patients with Allergic Diseases: A pilot survey study in Western Saudi Arabia. J. Herb. Med. 2021, 25, 100413. [Google Scholar] [CrossRef]
- Soós, S.Á.; Jeszenői, N.; Darvas, K.; Harsányi, L. Complementary and alternative medicine: Attitudes, knowledge and use among surgeons and anaesthesiologists in Hungary. BMC Complement. Altern. Med. 2016, 16, 443. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.T.; Barbo, A.; Lopez, G.; Melhem-Bertrandt, A.; Lin, H.; Olopade, O.I.; Curlin, F.A. National Survey of US Oncologists’ Knowledge, Attitudes, and Practice Patterns Regarding Herb and Supplement Use by Patients with Cancer. J. Clin. Oncol. 2014, 32, 4095–4101. [Google Scholar] [CrossRef]
- Murtaza, G. An evaluation of Pakistani pharmacy students knowledge of herbal medicines in Pakistan. Afr. J. Pharm. Pharmacol. 2012, 6, 221–224. [Google Scholar] [CrossRef]
- Santanello, C.; Carr, A. Pharmacists’ Knowledge, Perceptions, and Practices Regarding Herbal Medicine. Innov. Pharm. 2019, 10, 15. [Google Scholar] [CrossRef]
- Atavwoda, A.; Gabriel, A. Assessment of pharmacists knowledge, attitude and practices regarding herbal drug information services. J. Basic Clin. Pharm. 2012, 3, 317. [Google Scholar] [CrossRef]
- Pereira da Silva, A.; Geraldes, M.; Díaz-Lanza, A.M.; Kovacs, I.; Costa, M.C. Family medicine physicians’ perception and attitudes of herbal substances use in greater Lisbon region. Phytomedicine 2018, 47, 1–11. [Google Scholar] [CrossRef]
- Thiab, S.; Barakat, M.; Al-Qudah, R.; Abutaima, R.; Jamal, R.; Riby, P. The perception of Jordanian population towards concomitant administration of food, beverages and herbs with drugs and their possible interactions: A cross-sectional study. Int. J. Clin. Pract. 2021, 75, e13780. [Google Scholar] [CrossRef] [PubMed]
- Shraim, N.Y.; Shawahna, R.; Sorady, M.A.; Aiesh, B.M.; Alashqar, G.S.; Jitan, R.I.; Abu Hanieh, W.M.; Hotari, Y.B.; Sweileh, W.M.; Zyoud, S.H. Community pharmacists’ knowledge, practices and beliefs about complementary and alternative medicine in Palestine: A cross-sectional study. BMC Complement. Altern. Med. 2017, 17, 429. [Google Scholar] [CrossRef]
- Dayer, L.; Dunn, E.; Pace, A.; Flowers, S. Pharmacists’ perceived knowledge of and confidence in dispensing oral antineoplastic agents. J. Am. Pharm. Assoc. 2016, 56, 141–144.e2. [Google Scholar] [CrossRef]
- Ali, S.W.; Al-Arifi, M.N.; Al-Manie, N.K.; Al-Saker, F.M.; Babelgaith, S.D.; Sales, I.S.; Asiri, Y.A.A. Evaluation of knowledge of Health care professionals on warfarin interactions with drug and herbal medicine in Central Saudi Arabia. Pak. J. Med. Sci. 2016, 32, 229–233. [Google Scholar] [CrossRef]
- Nwose, E.; Onodu, B.; Anyasodor, A.; Sedowo, M.; Okuzor, J.; Culas, R. Ethnopharmacological values of cassava and its potential for diabetes and dyslipidaemia management: Knowledge survey and critical review of report. J. Intercult. Ethnopharmacol. 2017, 6, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Stanojević-Ristić, Z.; Stević, S.; Rašić, J.; Valjarević, D.; Dejanović, M.; Valjarević, A. Influence of pharmacological education on perceptions, attitudes and use of dietary supplements by medical students. BMC Complement. Altern. Med. 2017, 17, 527. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Kiss, N.; McKavanagh, D.; Isenring, E. Attitudes, beliefs and behaviours of Australia dietitians regarding dietary supplements: A cross-sectional survey. Complement. Ther. Clin. Pract. 2016, 25, 87–91. [Google Scholar] [CrossRef]
- Stanojević-Ristić, Z.; Mrkić, I.; Ćorac, A.; Dejanović, M.; Mitić, R.; Vitković, L.; Rašić, J.; Valjarević, D.; Valjarević, A. Healthcare Professionals’ Knowledge and Behaviors Regarding Drug–Dietary Supplement and Drug–Herbal Product Interactions. Int. J. Environ. Res. Public Health 2022, 19, 4290. [Google Scholar] [CrossRef]
- Oshikoya, K.A.; Oreagba, I.A.; Ogunleye, O.O.; Oluwa, R.; Senbanjo, I.O.; Olayemi, S.O. Herbal medicines supplied by community pharmacies in Lagos, Nigeria: Pharmacists’ knowledge. Pharm. Pract. Internet 2013, 11, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Alaaeddine, N.; Khayat, M.; Alawleh, H. Perceptions and Practices Regarding Herbal Medicine Prescriptions among Physicians in Greater Beirut. Leban. Med. J. 2014, 62, 130–136. [Google Scholar] [CrossRef]
- Jimam, N.; Joseph, B.; Agba, D. Pharmacists’ knowledge and perceptions about herbal medicines: A case study of Jos and environs. Med. J. Dr. Patil Univ. 2017, 10, 229. [Google Scholar] [CrossRef]
- Tarn, D.M.; Barrientos, M.; Wang, A.Y.; Ramaprasad, A.; Fang, M.C.; Schwartz, J.B. Prevalence and Knowledge of Potential Interactions Between Over-the-Counter Products and Apixaban. J. Am. Geriatr. Soc. 2020, 68, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Hong, T.K.; Alshagga, M. Prevalence of herbal products use and perceptions on drug-herb interactions among university students in Klang Valley Malaysia—A cross sectional study. Bangladesh J. Med. Sci. 2021, 20, 361–367. [Google Scholar] [CrossRef]
- Sekhri, K.; Kaur, K. Public knowledge, use and attitude toward multivitamin supplementation: A cross-sectional study among general public. Int. J. Appl. Basic Med. Res. 2014, 4, 77. [Google Scholar] [CrossRef]
- Younis Younis, N.A.K. The Prevalence, Attitude and Awareness of Herbal Medicine Products Use among Pharmacy Practitioner in Jordan. Pharmacogn. J. 2019, 11, 1082–1087. [Google Scholar] [CrossRef]
- Bhat, B.B.; Udupa, N.; Ligade, V.S.; Khan, S.; Sreedhar, D. Assessment of knowledge and attitude of patients on herbal medicine use in Udupi region, Karnataka, India. Trop. J. Pharm. Res. 2019, 18, 117. [Google Scholar] [CrossRef]
- Sridhar, S.; Shariff, A.; Al Halabi, N.; Sarmini, R.; Harb, L. Assessment of perception, experience, and information-seeking behavior of the public of Ras Al-Khaimah, United Arab Emirates, toward usage and safety of complementary and alternative medicine. J. Pharm. Bioallied Sci. 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Taing, M.-W.; Clavarino, A.M.; McGuire, T.M. Australian community pharmacists’ knowledge of popular herbal/nutrient weight-loss complementary medicines. J. Pharm. Pract. Res. 2017, 47, 463–470. [Google Scholar] [CrossRef]
- Alsayari, A.; Almghaslah, D.; Khaled, A.; Annadurai, S.; Alkhairy, M.A.; Alqahtani, H.A.; Alsayed, B.A.; Alasiri, R.M.; Assiri, A.M. Community Pharmacists’ Knowledge, Attitudes, and Practice of Herbal Medicines in Asir Region, Kingdom of Saudi Arabia. Evid. Based Complement. Alternat. Med. 2018, 2018, 1568139. [Google Scholar] [CrossRef] [PubMed]
- Tank, M.; Franz, K.; Cereda, E.; Norman, K. Dietary supplement use in ambulatory cancer patients: A survey on prevalence, motivation and attitudes. J. Cancer Res. Clin. Oncol. 2021, 147, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Albright, C.L.; Schembre, S.M.; Steffen, A.D.; Wilkens, L.R.; Monroe, K.R.; Yonemori, K.M.; Murphy, S.P. Differences by Race/Ethnicity in Older Adults’ Beliefs about the Relative Importance of Dietary Supplements vs Prescription Medications: Results from the SURE Study. J. Acad. Nutr. Diet. 2012, 112, 1223–1229. [Google Scholar] [CrossRef]
- El Khoury, G.; Ramadan, W.; Zeeni, N. Herbal Products and Dietary Supplements: A Cross-Sectional Survey of Use, Attitudes, and Knowledge among the Lebanese Population. J. Community Health 2016, 41, 566–573. [Google Scholar] [CrossRef]
- Diaz-Cruz, E.S.; Bolten, B.C. An elective course to enhance students’ knowledge and confidence in natural products. Curr. Pharm. Teach. Learn. 2016, 8, 688–697. [Google Scholar] [CrossRef]
- Chikafu, H.; Mutero, I.; Chimbari, M. “If I Were to Suffer a Stroke Right Now, the First Place That I Should Be Taken to Is the Traditional Healer”: Community Beliefs and Health-Seeking Practices for Noncommunicable Diseases in Rural KwaZulu-Natal, South Africa. Qual. Rep. 2022, 27, 243–256. [Google Scholar] [CrossRef]
- Flower, A.; Winters, D.; Bishop, F.L.; Lewith, G. The challenges of treating women with recurrent urinary tract infections in primary care: A qualitative study of GPs’ experiences of conventional management and their attitudes towards possible herbal options. Prim. Health Care Res. Dev. 2015, 16, 597–606. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.A.; Alves, M.S.; Campos, T.S.P.; Nascimento, M.P. Therapeutic Group of the Elderly: Knowledge about Diabetes Mellitus and Arterial Hypertension. J. Health Sci. 2021, 22, 243–247. [Google Scholar] [CrossRef]
- De Oliveira Filho, L.M.; Fernandes da Silva Queiroz, J.; De Aguiar, M.I.; André da Silva Costa, E. Os saberes tradicionais e a utilização de plantas medicinais durante o período de pandemia da COVID-19. Perspect. Diálogo Rev. Educ. Soc. 2021, 8, 276–292. [Google Scholar] [CrossRef]
- Schiavo, M.; Schwambach, K.H.; Colet, C.d.F. Conhecimento sobre plantas medicinais e fitoterápicos de agentes comunitários de saúde de Ijuí/RS Knowledge on medicinal plants and herbal medicines by community health agents of Ijuí/RS. Rev. Pesqui. Cuid. Fundam. Online 2017, 9, 57–63. [Google Scholar] [CrossRef]
- Committee on Standards for Systematic Reviews of Comparative Effectiveness Research; Board on Health Care Services; Institute of Medicine. Finding What Works in Health Care: Standards for Systematic Reviews; Eden, J., Levit, L., Berg, A., Morton, S., Eds.; National Academies Press: Washington, DC, USA, 2011; p. 13059. ISBN 978-0-309-16425-2. [Google Scholar]
- Xiong, G.; Yang, Z.; Yi, J.; Wang, N.; Wang, L.; Zhu, H.; Wu, C.; Lu, A.; Chen, X.; Liu, S.; et al. DDInter: An online drug–drug interaction database towards improving clinical decision-making and patient safety. Nucleic Acids Res. 2022, 50, D1200–D1207. [Google Scholar] [CrossRef]
- Parvez, M.K.; Rishi, V. Herb-Drug Interactions and Hepatotoxicity. Curr. Drug Metab. 2019, 20, 275–282. [Google Scholar] [CrossRef]
- Harnett, J.E.; Ung, C.O.L.; Hu, H.; Sultani, M.; Desselle, S.P. Advancing the pharmacist’s role in promoting the appropriate and safe use of dietary supplements. Complement. Ther. Med. 2019, 44, 174–181. [Google Scholar] [CrossRef]
- Trinh, K.; Pham, D.; Le, L. Semantic Relation Extraction for Herb-Drug Interactions from the Biomedical Literature Using an Unsupervised Learning Approach. In Proceedings of the 2018 IEEE 18th International Conference on Bioinformatics and Bioengineering (BIBE), Taichung, Taiwan, 29–31 October 2018; pp. 334–337. [Google Scholar]
- Lin, K.; Friedman, C.; Finkelstein, J. An automated system for retrieving herb-drug interaction related articles from MEDLINE. AMIA Jt. Summits Transl. Sci. Proc. 2016, 2016, 140–149. [Google Scholar]
- Wang, L.L.; Tafjord, O.; Cohan, A.; Jain, S.; Skjonsberg, S.; Schoenick, C.; Botner, N.; Ammar, W. SUPP.AI: Finding Evidence for Supplement-Drug Interactions. arXiv 2019, arXiv:1909.08135. [Google Scholar] [CrossRef]
- Zhou, L.; Sordo, M. Expert systems in medicine. In Artificial Intelligence in Medicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 75–100. ISBN 978-0-12-821259-2. [Google Scholar]
- Kinney, E.L. Expert system detection of drug interactions: Results in consecutive inpatients. Comput. Biomed. Res. 1986, 19, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Roach, J.; Lee, S.; Wilcke, J.; Ehrich, M. An expert system for information on pharmacology and drug interactions. Comput. Biol. Med. 1985, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, A. Design and Implementation of Rule_Base Expert System in Drug Interactions and Support Medical Decision Using Cat Swarm Optimization Algorithm. J. Adv. Res. Dyn. Control Syst. 2018, 10, 237–243. [Google Scholar]
- Ajazuddin; Saraf, S. Legal regulations of complementary and alternative medicines in different countries. Pharmacogn. Rev. 2012, 6, 154. [Google Scholar] [CrossRef]

| 1st Author (Year) | Domains/Dimensions Assessed in the Studies | Country | Design | Procedures for Data Collection | Sample (n) | Age (M ± SD) Min–Max | Gender |
|---|---|---|---|---|---|---|---|
| Makkaoui et al. (2021) [61] | Pharmacists’ attitudes, practices, resources and knowledge regarding drugs, food, and herb interactions. | Lebanon | Cross-sectional design | Questionnaires/surveys Individual interviews with a self-selected sample | Community pharmacists (89) | NR | NR |
| Al-Nadaf & Awadallah (2020) [62] | Knowledge and attitude about self-medicated use of herbal medicine and drug interactions. | Jordan | Cross-sectional design | Questionnaires/surveys | General population (926) | 27.96 ± 14.1 | F = 719 M = 207 |
| Eltom et al. (2021) [63] | Knowledge, awareness, and attitudes of physicians toward the use of medicinal herbs. | Saudi Arabia | Cross-sectional design | Questionnaires/surveys | Physicians (117) | NR | F = 76 M = 38 NR = 3 |
| Agrawal & Goel (2016) [64] | Use of herbal medicines; reasons for their use; source of information on herbal medicines; opinion on herbal medicines and their costs. Prevalence and perception of herbal drug usage in patients visiting the outpatient department (OPD) of a tertiary care hospital. | India | Cross-sectional design | Questionnaires/surveys | Patients (246) | 18–30 31–40 41–50 51–60 >60 | F = 119 M = 127 |
| Koshak (2021) [65] | Which herbal medicines; attitudes and beliefs toward the use of herbal medicines in patients with allergies. | Saudi Arabia | Cross-sectional design | Questionnaires/surveys | Patients with allergic disease (111) | 33 ± 18.0 | F = 78 M = 33 |
| Schiavo et al. (2017) [99] | Knowledge about medicinal plant herbal medicines. | Brazil | Cross-sectional and qualitative design | Interviews | Community health workers (13) | 32.7 ± 8.19 | F = 13 |
| Soós et al. (2016) [66] | Attitudes and knowledge of workers in perioperative care toward Herbal Medicine. | Hungary | Cross-sectional design | Questionnaires/surveys | Anesthesiologists and surgeons (258) | 39.9 ± 12.08 | F = 107 M = 151 |
| Lee et al. (2014) [67] | Prevalence of patients’ that use supplements; How oncologists communicated with patients regarding supplements. Oncologists’ knowledge, attitudes, and practice patterns regarding herbs and supplements use by their patients. | USA | Cross-sectional design | Questionnaires/surveys | Oncologists (392) | 48.4 ± 9.8 | F = 111 M = 277 NR = 4 |
| Murtaza et al. (2012) [68] | Students’ knowledge about herbal medicines; knowledge about interactions. | Pakistan | Cross-sectional design | Questionnaires/surveys | Students (2830) | 22 ± NR | F = 1265 M = 1515 NR = 50 |
| Filho et al. (2021) [98] | Knowledge about medicinal plants used for the treatment of disease symptoms. | Brazil | Quantitative and qualitative design | Questionnaires/surveys | Students (60) | 14–23 | F = 36 M = 22 NR = 2 |
| Chikafu et al. (2022) [95] | Awareness, beliefs, and health-seeking behavior about some diseases. | South Africa | Qualitative study | Focus group interview | General Public (76) | 18–40>40 | F = 41 M = 35 |
| Santanello & Carr (2019) [69] | Perceptions and practices regarding herbal medicine. | USA | Cross-sectional design | Questionnaires/surveys | Community pharmacists (127) | 20–64 | NR |
| Atavwoda & Gabriel (2012) [70] | Pharmacists’ knowledge, attitude, and practices regarding herbal drug information services. | Nigeria | Cross-sectional design | Questionnaires/surveys | Pharmacists (273) | 21–60 | F = 130 M = 143 |
| Pereira da Silva et al. (2018) [71] | Perception, knowledge, and attitudes of herbal medicine. | Portugal | Cross-sectional design | Questionnaires/surveys | Physicians (80) | 51.9 ± 10.0 | F = 57 M = 23 |
| Thiab et al. (2021) [72] | Community perception of interactions (food/drink/medicine); knowledge of interactions. | Jordan | Cross-sectional design | Questionnaires/surveys | General public (789) | <18 18–25 26–40 40–65 | F = 614 M = 175 |
| Shraim et al. (2017) [73] | Knowledge, practices, and beliefs about complementary and alternative medicine. | Palestine | Cross-sectional design | Questionnaires/surveys | Community pharmacists (281) | 20–29 30–39 40–49 50–59 >60 | F = 132 M = 149 |
| Dayer et al. (2016) [74] | How pharmacists maintain knowledge about the identification of the drug and/or herbal interactions and in the identification of adverse events. | USA | Cross-sectional design | Questionnaires/surveys | Pharmacists (246) | NR | NR |
| Al-Arifi et al. (2016) [75] | Knowledge about warfarin-herb interactions with drug and medicinal herbs. | Saudi Arabia | Cross-sectional design | Questionnaires/surveys | Physicians, pharmacists, and nurses (90) | 25–35 36–45 46–55 >55 | F = 37 M = 53 |
| Nwose et al. (2017) [76] | Knowledge about the practice of using cassava in health. | Nigeria | Cross-sectional design | Questionnaires/surveys | General public (101) | <25 26–35 36–45 46–55 >55 | F = 60 M = 41 |
| Stanojević-Ristić et al. (2017) [77] | Attitude of the dietetic supplements used; perceptions of the effectiveness of dietary supplements; attitudes toward potential harmful effects and interaction with medicines; perception of the risk of adverse reactions to dietary supplements. | Serbia | Cross-sectional design | Questionnaires/surveys | Medical students (334) | 23 ± 2.0 | F= 188 M=146 |
| Marx et al. (2016) [78] | Attitudes, beliefs, and behaviors regarding dietary supplements. | Australia | Cross-sectional design | Questionnaires/surveys | Dietitians (231) | <30 31–40 41–50 51–60 >61 | NR |
| Stanojević-Ristić et al. (2022) [79] | Knowledge and behaviors regarding drug–dietary supplement and drug–herbal product interactions. | Serbia | Cross-sectional design | Questionnaires/surveys | General and specialist doctors, and nurses (346) | ≤29 30–39 40–49 ≥50 | F = 211 M =135 |
| Oshikoya et al. (2013) [80] | Knowledge about the type of herbal medicines and their indications; knowledge about the use, contraindication and potential drug-herb interactions. | Nigeria | Cross-sectional design | Questionnaires/surveys | Pharmacists (103) | 20–35 36–50 >65 | F = 29 M = 74 |
| Alaaeddine et al. (2014) [81] | Attitudes regarding the use of herbal medicines; knowledge about herbal medicines; general practices related to herbal medicine prescriptions. | Lebanon | Cross-sectional design | Questionnaires/surveys | Physicians (212) | 49.18 ± 9.38 | F = 107 M = 105 |
| Jimam et al. (2017) [82] | Knowledge on herbal medicines; sources of information on herbal medicines; perceptions on herbal medicine. | Nigeria | Cross-sectional design | Questionnaires/surveys | Pharmacists (177) | 34.0 ± NR | F = 48 M = 129 |
| Tarn et al. (2020) [83] | Knowledge and prevalence of potential interactions with Apixaban and dietary supplements. | USA | Cross-sectional design | Questionnaires/surveys | Patients (791) | 71 ± 11.8 | F = 315 M = 472 Other = 4 |
| Yan et al. (2021) [84] | Prevalence and preference of herbal products usage; perceptions of herbal products and awareness toward the drug-herb interactions. | Malaysia | Cross-sectional design | Questionnaires/surveys | University students (231) | 22.0 ± NR | F = 111 M = 120 |
| Sekhri & Kaur (2014) [85] | Attitude toward, and knowledge of multivitamin supplements, their consumption, and their effects. | General Public | Cross-sectional design | Questionnaires/surveys | General public (120) | F: 38.75 ± 12.87 M: 43.85 ± 15.44 | F = 54 M = 66 |
| Flower et al. (2015) [96] | Perceptions of herbal medicines; concerns about herbal medicines: knowledge, risk. | UK | Qualitative study | Interviews | General physicians (15) | 44 ± NR 34-59 | F = 7 M = 8 |
| Younis (2019) [86] | Attitude, prevalence, and awareness toward herbal medicine products; their safety, information sources, the need to consult a physician prior to their use. | Jordan | Cross-sectional design | Questionnaires/surveys | Pharmacists (230) | 35.4 ± 7.8 | F = 142 M = 88 |
| Santos et al. (2021) [97] | Behavior of consuming, concomitantly, boldo teas, cider grass, nuts skin, and lavender with traditional drugs. | Brazil | Qualitative study | Focus Group Interviews | Patients (12) | 64–83 | F = 9 M = 3 |
| Jaber & Al-Zeidaneen (2021) [2] | Consumption of medicinal plants; the main indication of use for different postpartum health problems. | Jordan | Cross-sectional design | Interviews | Postpartum patients (300) | 18–45 | F = 300 |
| Bhat et al. (2019) [87] | Knowledge and attitude of patients on the usage of herbal medicines. | India | Cross-sectional design | Questionnaires/surveys | Patients (322) | 43.02 ± 14.33 | F = 147 M = 175 |
| Sridhar et al. (2017) [88] | Complementary and alternative medicine usage; perception, experience, and information-seeking behavior. | United Arab Emirates | Cross-sectional design | Questionnaires/surveys | General Public (403) | 18–28 29–39 40–50 51–60 >61 | F = 218 M = 185 |
| Taing et al. (2017) [89] | Knowledge about popular herbal/nutrient weight-loss complementary medicines, their efficacy, potential side effects, and drug interactions. | Australia | Cross-sectional design | Questionnaires/surveys | Pharmacists (99) | 33.5 ± 10.0 | F = 61 M = 39 |
| Alsayari et al. (2018) [90] | Knowledge, attitudes and practice regarding the indications, side effects, and contraindications of used herbal medicines. | Saudi Arabia | Cross-sectional design | Questionnaires/surveys | Pharmacists (233) | 20–49 | M = 233 |
| Tank et al. (2021) [91] | Prevalence, motivation, and attitudes in the use of dietary supplements. | Germany | Cross-sectional design | Questionnaires/surveys and interviews | Cancer patients (1217) | 67.6 ± 12.9 | F = 624 M = 593 |
| Albright et al. (2012) [92] | Reasons/motivations for taking dietary supplements versus prescription medications. | USA | Cross-sectional design | Questionnaires/surveys and focus group interviews | General public (396) | 67.5 ± 7.4 52–88 | F = 205 M = 191 |
| el Khoury et al. (2016) [93] | Attitude and knowledge about medicinal drugs and dietary supplements. | Lebanon | Cross-sectional design | Questionnaires/surveys | Patients (726) | 18–29 30–39 40–49 50–59 60–69 ≥70 | F = 434 M = 292 |
| Niveditha & Geetha (2020) [10] | Knowledge and awareness of natural anti-carcinogenic herbs and their uses. | General Public | Cross-sectional design | Questionnaires/surveys | General public (100) | NR | NR |
| Ceremuga et al. (2020) [7] | Knowledge about using herbal supplements; reasons for taking dietary supplements. | USA | Cross-sectional design | Questionnaires/surveys | Preoperative patients (2623) | 18–24 25–29 30–39 40–49 50–59 60–69 70–79 ≥80 | F = 1009 M = 1614 |
| Mahnashi (2021) [30] | Knowledge, attitude, and practice about the use of herbal drugs. | Saudi Arabia | Cross-sectional design | Questionnaires/surveys | Pharmacists (62) | 20–25 26–30 31–35 36–40 46–50 | M = 62 |
| 1st Author (Year) | Domains/Dimensions Assessed in the Studies | Country | Design | Procedures for Data Collection | Sample (n) | Age (M ± SD) Min–Max | Gender |
|---|---|---|---|---|---|---|---|
| Diaz-Cruz & Bolten (2016) [94] | Knowledge and level of confidence regarding complementary alternative medicine. | USA | Experimental design | Formative and summative assessments 1 | Pharmacy students (Phase 1–209) (Phase 2–38) (Phase 3–40) 2 | 23.8 ± NR | Phase 1 NR Phase 2 F = 30 M = 8 Phase 3 F = 32 M = 8 |
| Miles Homer et al. (2019) [43] | Perceptions of dietary supplements. | USA | Experimental design | Intervention–Food and Drug Administration | Students 1st study (251) 2nd study (231) | 1st study 23.5 ± NR 2nd study 22.8 ± NR | 1st study F = 133 M = 118 2nd study F = 129 M = 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dores, A.R.; Peixoto, M.; Castro, M.; Sá, C.; Carvalho, I.P.; Martins, A.; Maia, E.; Praça, I.; Marques, A. Knowledge and Beliefs about Herb/Supplement Consumption and Herb/Supplement–Drug Interactions among the General Population, including Healthcare Professionals and Pharmacists: A Systematic Review and Guidelines for a Smart Decision System. Nutrients 2023, 15, 2298. https://doi.org/10.3390/nu15102298
Dores AR, Peixoto M, Castro M, Sá C, Carvalho IP, Martins A, Maia E, Praça I, Marques A. Knowledge and Beliefs about Herb/Supplement Consumption and Herb/Supplement–Drug Interactions among the General Population, including Healthcare Professionals and Pharmacists: A Systematic Review and Guidelines for a Smart Decision System. Nutrients. 2023; 15(10):2298. https://doi.org/10.3390/nu15102298
Chicago/Turabian StyleDores, Artemisa R., Miguel Peixoto, Maria Castro, Catarina Sá, Irene P. Carvalho, Andreia Martins, Eva Maia, Isabel Praça, and António Marques. 2023. "Knowledge and Beliefs about Herb/Supplement Consumption and Herb/Supplement–Drug Interactions among the General Population, including Healthcare Professionals and Pharmacists: A Systematic Review and Guidelines for a Smart Decision System" Nutrients 15, no. 10: 2298. https://doi.org/10.3390/nu15102298
APA StyleDores, A. R., Peixoto, M., Castro, M., Sá, C., Carvalho, I. P., Martins, A., Maia, E., Praça, I., & Marques, A. (2023). Knowledge and Beliefs about Herb/Supplement Consumption and Herb/Supplement–Drug Interactions among the General Population, including Healthcare Professionals and Pharmacists: A Systematic Review and Guidelines for a Smart Decision System. Nutrients, 15(10), 2298. https://doi.org/10.3390/nu15102298









