The Replacement of Only One Portion of Starchy Carbohydrates with Green Leafy Vegetables Regresses Mid and Advanced Stages of NAFLD: Results from a Prospective Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
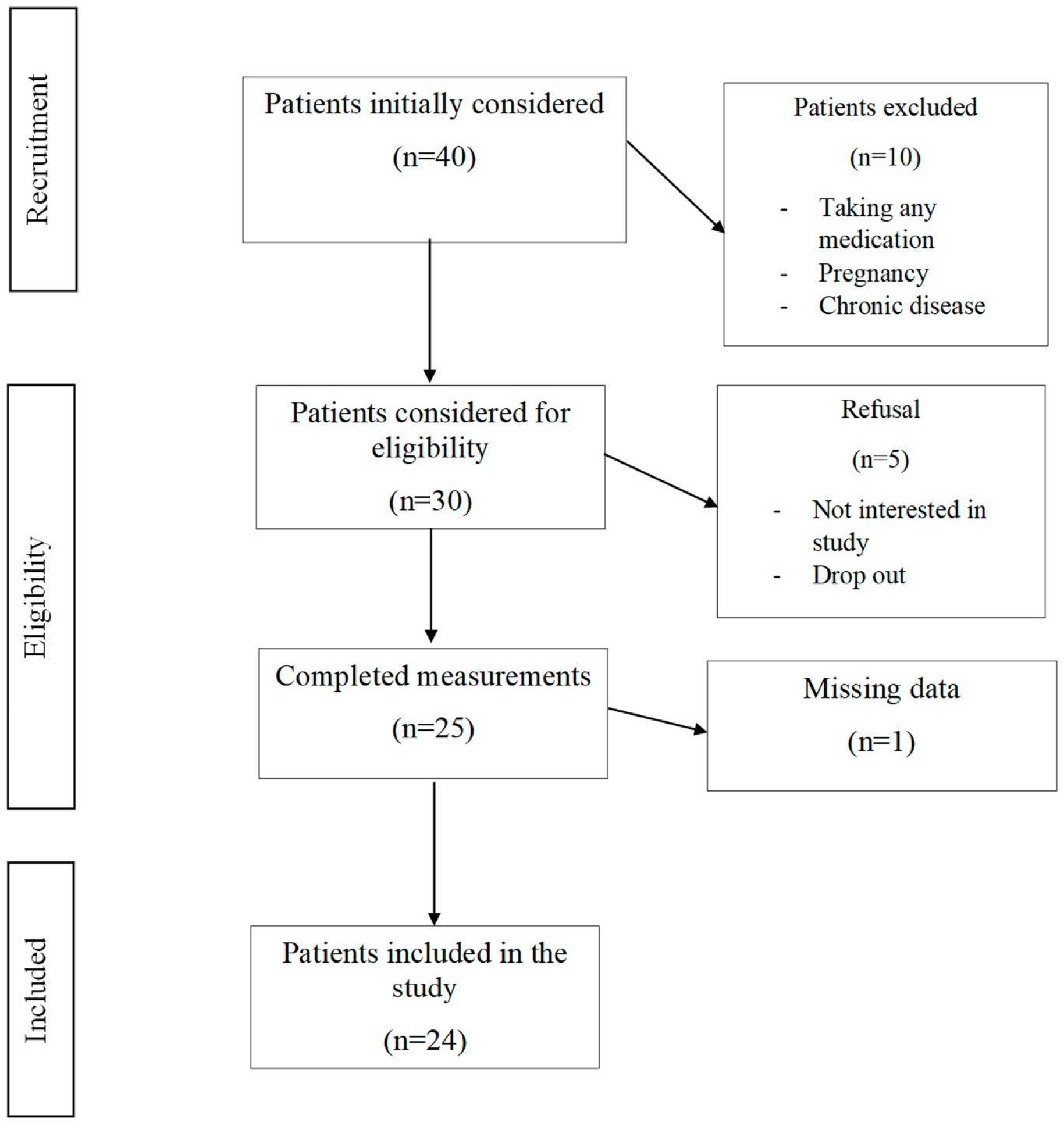
2.1. Study Design and Population
2.2. NAFLD Assessment
2.3. Anthropometric Parameters
2.4. Bioelectrical Impedance Analysis (BIA)
2.5. Biochemistry
2.6. Data Management and Statistical Methods
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2021, 20, 2809–2817.e28. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Younossi, Z.; Tacke, F.; Arrese, M.; Chander, S.B.; Mostafa, I.; Bugianesi, E.; Wai-Sun Wong, V.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Milic, N.; Di Renzo, L.; Preveden, T.; Medić-Stojanoska, M.; De Lorenzo, A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 7006–7016. [Google Scholar] [CrossRef]
- Powell, E.E.; Wai-Sun Wong, V.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Canbay, A.; Kachru, N.; Haas, J.S.; Sowa, J.-P.; Meise, D.; Ozbay, A.B. Patterns and predictors of mortality and disease progression among patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2020, 52, 1185–1194. [Google Scholar] [CrossRef]
- Marchesini, G.; Day, C.P.; Dufour, J.F.; Canbay, A.; Nobili, V.; Ratziu, V.; Tilg, H.; Roden, M.; Gastaldelli, A.; Yki-Jarvinen, H.; et al. EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Sofi, F.; Cesari, F.; Abbate, R.; Gensini, G.F.; Casini, A. Adherence to Mediterranean diet and health status: Meta-analysis. BMJ 2008, 337, a1344. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Lampignano, L.; De Pergola, G. Mediterranean Diet Pyramid: A Proposal for Italian People. A Systematic Review of Prospective Studies to Derive Serving Sizes. Nutrients 2019, 11, 1296. [Google Scholar] [CrossRef]
- Hattersley, J.G.; Pfeiffer, A.F.H.; Roden, M.; Petzke, K.; Hoffmann, D.; Rudovich, N.N.; Randeva, H.S.; Vatish, M.; Osterhoff, M.; Goegebakan, O.; et al. Modulation of Amino Acid Metabolic Signatures by Supplemented Isoenergetic Diets Differing in Protein and Cereal Fiber Content. J. Clin. Endocrinol. Metab. 2014, 99, E2599–E2609. [Google Scholar] [CrossRef] [PubMed]
- Plaz Torres, M.C.; Aghemo, A.; Lleo, A.; Bodini, G.; Furnari, M.; Marabotto, E.; Miele, L.; Giannini, E.G. Mediterranean Diet and NAFLD: What We Know and Questions That Still Need to Be Answered. Nutrients 2019, 11, 2971. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Raman, M.; Rioux, K.P.; Reimer, R.A. The potential role of prebiotic fibre for treatment and management of non-alcoholic fatty liver disease and associated obesity and insulin resistance. Liver Int. 2012, 32, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.; Errico, A.; Petruk, G.; Monti, D.M.; Barone, A.; Rigano, M.M. Bioactive Compounds in Brassicaceae Vegetables with a Role in the Prevention of Chronic Diseases. Molecules 2017, 23, 15. [Google Scholar] [CrossRef]
- Younossi, Z.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Pugliese, N.; Plaz Torrez, M.C.; Petta, S.; Valenti, L.; Giannini, E.G.; Aghemo, A. Is there an ‘ideal’ diet for patients with NAFLD? Eur. J. Clin. Investig. 2022, 52, e13659. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- DiPietro, L.; Buchner, D.M.; Marquez, D.X.; Pate, R.R.; Pescatello, L.S.; Whitt-Glover, M.C. New scientific basis for the 2018 U.S. Physical Activity Guidelines. J. Sport Health Sci. 2019, 8, 197–200. [Google Scholar] [CrossRef]
- Babio, N.; Bulló, M.; Basora, J.; Martínez-González, M.A.; Fernández-Ballart, J.; Márquez-Sandoval, F.; Molina, C.; Salas-Salvadó, J. Adherence to the Mediterranean diet and risk of metabolic syndrome and its components. Nutr. Metab. Cardiovasc. Dis. 2016, 19, 563–570. [Google Scholar] [CrossRef]
- Bacil, G.P.; Cogliati, B.; Cardoso, D.R.; Barbisan, L.F.; Romualdo, G.R. Are isothiocyanates and polyphenols from Brassicaceae vegetables emerging as preventive/therapeutic strategies for NAFLD? The landscape of recent preclinical findings. Food Funct. 2022, 13, 8348–8362. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wai-Sun Wong, V.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.H.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef] [PubMed]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.; Wai-Sun Wong, V.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2022, 5, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tian, S.; Li, H.; Xu, Z.; Li, S.; Chen, Y.-L.; Liang, X.-Y.; Xiao, J.; Song, J.-Y.; She, R.-L.; et al. Population-specific cut-off points of fatty liver index: A study based on the National Health and Nutrition Examination Survey data. BMC Gastroenterol. 2022, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; Bruyère, O.; Geerinck, A.; Hajaoui, M.; Scafoglieri, A.; Perkisas, S.; Bautmans, I.; Gielen, E.; Reginster, J.-Y.; Buckinx, F. Equation models developed with bioelectric impedance analysis tools to assess muscle mass: A systematic review. Clin. Nutr. ESPEN 2020, 35, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.C. Bioelectrical impedance analysis for body composition assessment: Reflections on accuracy, clinical utility, and standardization. Eur. J. Clin. Nutr. 2019, 73, 194–199. [Google Scholar] [CrossRef]
- Warnick, G.R.; Knopp, R.H.; Fitzpatrick, V.; Branson, L. Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin. Chem. 1990, 36, 15–19. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; Song, P.; Xu, L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: Developments in research and prospects for the future. Drug Discov. Ther. 2015, 9, 380–385. [Google Scholar] [CrossRef]
- Cross, E.; Dearlove, D.J.; Hodson, L. Nutritional regulation of hepatic de novo lipogenesis in humans. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 65–71. [Google Scholar] [CrossRef]
- Cohen, C.C.; Li, K.W.; Alazraki, A.L.; Beysen, C.; Carrier, C.A.; Cleeton, R.L.; Dandan, M.; Figueroa, J.; Knight-Scott, J.; Knott, C.J.; et al. Dietary sugar restriction reduces hepatic de novo lipogenesis in adolescent boys with fatty liver disease. J. Clin. Investig. 2021, 131, e150996. [Google Scholar] [CrossRef]
- Gao, Y.; Hua, R.; Hu, K.; Wang, Z. Carbohydrates deteriorate fatty liver by activating the inflammatory response. Nutr. Res. Rev. 2022, 35, 252–267. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Ye, M.; Zhang, S.; Zhang, Q.; Meng, G.; Liu, L.; Wu, H.; Gu, Y.; Wang, Y.; et al. Does a high intake of green leafy vegetables protect from NAFLD? Evidence from a large population study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1691–1701. [Google Scholar] [CrossRef]
- Kabisch, S.; Bäther, S.; Dambeck, U.; Kemper, M.; Gerbracht, C.; Honsek, C.; Sachno, A.; Pfeiffer, A.F.H. Liver Fat Scores Moderately Reflect Interventional Changes in Liver Fat Content by a Low-Fat Diet but Not by a Low-Carb Diet. Nutrients 2018, 10, 157. [Google Scholar] [CrossRef]
- Keating, S.E.; Parker, H.M.; Hickman, I.J.; Gomersall, S.R.; Wallen, M.P.; Coombes, J.S.; Macdonald, G.A.; George, J.; Johnson, N.A. NAFLD in clinical practice: Can simple blood and anthropometric markers be used to detect change in liver fat measured by 1H-MRS? Liver Int. 2017, 37, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Du, L.-J.; He, Z.-Y.; Gu, X.; Hu, X.; Zhang, X.X.; Yang, L.-J.; Li, J.; Pan, L.-Y.; Li, Y.-Q.; Yang, B.; et al. Inverse Association of Fruit and Vegetable Consumption with Nonalcoholic Fatty Liver Disease in Chinese Patients with Type 2 Diabetes Mellitus. Nutrients 2022, 14, 4559. [Google Scholar] [CrossRef]
- Zhao, H.; Yang, A.; Mao, L.; Quan, Y.; Cui, J.; Sun, Y. Association between Dietary Fiber Intake and Non-alcoholic Fatty Liver Disease in Adults. Front. Nutr. 2020, 7, 593735. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, C.; Liu, J.; Liu, Z.; Ling, W.; Chen, Y. Greater serum carotenoid levels associated with lower prevalence of nonalcoholic fatty liver disease in Chinese adults. Sci. Rep. 2015, 5, 12951. [Google Scholar] [CrossRef]
- Morais, J.B.S.; Severo, J.S.; de Alencar, G.R.R.; de Oliveira, A.R.S.; Cruz, K.J.C.; do Nascimento Marreiro, D.; de Almendra Freitas, S.; de Carvalho, C.M.R.; de Carvalho e Martins, M.D.C.; de Macedo Gonçalves Frota, K. Effect of magnesium supplementation on insulin resistance in humans: A systematic review. Nutrition 2017, 38, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, X.; Song, Y.; Fan, L.; Wu, L.; Kabagambe, E.K.; Hou, L.; Shrubsole, M.J.; Liu, J.; Dai, Q. Intakes of magnesium, calcium and risk of fatty liver disease and prediabetes. Public Health Nutr. 2018, 21, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Xie, H.; Bai, H.; Lin, G.; Dong, Y.; Shi, D.; Wang, J.; Zhang, Q.; Zhang, Y.; et al. Impact of a low-carbohydrate and high-fiber diet on nonalcoholic fatty liver disease. Asia Pac. J. Clin. Nutr. 2020, 29, 483–490. [Google Scholar] [CrossRef]
- Jia, X.; Zhong, L.; Song, Y.; Hu, Y.; Wang, G.; Sun, S. Consumption of citrus and cruciferous vegetables with incident type 2 diabetes mellitus based on a meta-analysis of prospective study. Prim. Care Diabetes 2016, 10, 272–280. [Google Scholar] [CrossRef]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef] [PubMed]
- Juraschek, S.P.; Kovell, L.C.; Appel, L.J.; Miller, E.M.; Sacks, F.M.; Christenson, R.H.; Rebuck, H.; Chang, A.R.; Mukamal, K.J. Associations between Dietary Patterns and Subclinical Cardiac Injury: An Observational Analysis from the DASH Trial. Ann. Intern. Med. 2020, 172, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, S.; Dykun, I.; Balcer, B.; Totzeck, M.; Rassaf, T.; Mahabadi, A.A. Higher BNP/NT-pro BNP levels stratify prognosis equally well in patients with and without heart failure: A meta-analysis. ESC Heart Fail. 2022, 9, 3198–3209. [Google Scholar] [CrossRef]
- Sari, R.; Balci, M.K.; Altunbas, H.; Krayalcin, U. Clinical Endocrinology The effect of body weight and weight loss on thyroid volume and function in obese women. Clin. Endocrinol. 2003, 59, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Isa, A.; de Sousa, G.; Dieffenbach, R.; Andler, W. Thyroid hormones and their relation to weight status. Horm. Res. Paediatr. 2008, 70, 51–57. [Google Scholar] [CrossRef] [PubMed]
| Parameters * | Median (IQR) or Frequency (%) |
|---|---|
| Gender (F) (%) | 17 (70.8) |
| Age (years) | 47.5 (41.5–52.5) |
| Smoker (Yes) (%) | 4 (16.7) |
| IPAQ | 1032 (480–1701) |
| IPAQ (%) | |
| Inactive | 7 (29.2) |
| Sufficiently Active | 15 (62.5) |
| Active or Very Active | 2 (8.3) |
| Snoring (Yes) (%) | 17 (70.8) |
| PREDIMED score | 8 (7–8) |
| Parameters * | Vegetable Diet | p ^ | |
|---|---|---|---|
| Before | After | ||
| Antropometric | |||
| Weight (kg) | 95.0 (83.0–109.2) | 89.7 (75.2–103.0) | <0.0001 |
| BMI (kg/m2) | 35.3 (31.2–39.0) | 33.3 (28.6–37.3) | <0.0001 |
| Waist (cm) | 110.0 (103.0–124.0) | 106.5 (95.0–112.5) | <0.0001 |
| Neck (cm) | 39.5 (38.0–42.5) | 38.0 (35.0–41.5) | <0.0001 |
| Systolic Blood Pressure (mmHg) | 130 (130–137) | 127 (120–135) | 0.11 |
| Diastolic Blood Pressure (mmHg) | 85 (80–90) | 82 (75–90) | 0.24 |
| Blood | |||
| FPG (mg/dL) | 94 (89–98) | 90 (86–99) | 0.99 |
| Insulin (µUI/mL) | 14.0 (10.4–19.8) | 10.5 (8.6–14.7) | 0.06 |
| HOMA-IR (mg/dL) | 3.0 (2.5–4.3) | 2.5 (1.9–3.5) | 0.06 |
| HbA1c (mmol/mol) | 38.0 (34.0–40.5) | 36.0 (33.5–39.0) | 0.01 |
| Triglycerides (mg/dL) | 90 (64–132) | 72 (62–90) | 0.03 |
| LDL (mg/dL) | 112 (97–135) | 110 (96–123) | 0.99 |
| HDL (mg/dL) | 51 (43–58) | 54 (49–61) | 0.40 |
| Total Cholesterol (mg/dL) | 193 (161–216) | 181 (158–197) | 0.29 |
| TSH (µUI/mL) | 1.8 (1.4–2.3) | 1.5 (1.2–2.0) | 0.02 |
| FT3 (pg/mL) | 3.3 (3.0–3.6) | 3.3 (2.8–3.5) | 0.84 |
| FT4 (ng/mL) | 1.1 (1.0–1.3) | 1.2 (1.1–1.2) | 0.31 |
| Vitamin D (ng/mL) | 22.1 (19.5–26.2) | 23.8 (16.9–28.9) | 0.54 |
| Uric acid (mg/dL) | 4.9 (4.1–6.2) | 5.0 (4.1–5.9) | 0.82 |
| Creatinine (mg/dL) | 0.7 (0.7–0.9) | 0.8 (0.7–0.9) | 0.38 |
| GFR (mL/min/1.73 m2) | 89 (73–90) | 86 (73–90) | 0.18 |
| AST (U/L) | 18 (15–27) | 17 (14–19) | 0.01 |
| ALT (U/L) | 19 (13–30) | 17 (12–22) | 0.06 |
| GGT (U/L) | 16 (14–27) | 16 (13–20) | 0.02 |
| Ferritin (ng/mL) | 52.7 (29.3–151.5) | 63.5 (40.9–139.0) | 0.54 |
| PTH (pg/mL) | 56.3 (46.8–56.3) | 53.0 (44.8–53.0) | 0.06 |
| NT proBNP (pg/mL) | 47 (34–52) | 46 (34–46) | 0.03 |
| Albumin (g/dL) | 4.2 (4.2–4.2) | 4.3 (4.3–4.4) | 0.0005 |
| Liver | |||
| Fibroscan CAP (dB/m) | 278 (253–354) | 278 (200–331) | 0.84 |
| Fibroscan CAP (%) | 0.69 ¥ | ||
| ≤300 dB/m | 14 (58.33) | 16 (66.67) | |
| >300 dB/m | 10 (41.67) | 8 (33.33) | |
| Fibroscan E (kPA) | 5.3 (4.1–6.7) | 4.9 (4.3–5.9) | 0.68 |
| FM (kg) | 37.9 (27.7–43.5) | 32.3 (23.4–40.7) | <0.0001 |
| FFM (kg) | 54.3 (50.0–68.4) | 53.0 (48.5–66.3) | 0.31 |
| TBW (L) | 40.0 (37.3–50.5) | 39.1 (35.4–48.7) | 0.15 |
| ECW (L) | 18.3 (15.9–22.7) | 17.3 (15.2–20.8) | 0.03 |
| SMM (kg) | 29.7 (23.4–36.7) | 30.3 (24.0–35.6) | 0.99 |
| FLI | 85 (54–95) | 73 (33–89) | <0.0001 |
| FAST | 0.05 (0.02–0.15) | 0.03 (0.02–0.09) | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Nucci, S.; Rinaldi, R.; Di Chito, M.; Donghia, R.; Giannuzzi, V.; Shahini, E.; Cozzolongo, R.; Pesole, P.L.; Coletta, S.; De Pergola, G.; et al. The Replacement of Only One Portion of Starchy Carbohydrates with Green Leafy Vegetables Regresses Mid and Advanced Stages of NAFLD: Results from a Prospective Pilot Study. Nutrients 2023, 15, 2289. https://doi.org/10.3390/nu15102289
De Nucci S, Rinaldi R, Di Chito M, Donghia R, Giannuzzi V, Shahini E, Cozzolongo R, Pesole PL, Coletta S, De Pergola G, et al. The Replacement of Only One Portion of Starchy Carbohydrates with Green Leafy Vegetables Regresses Mid and Advanced Stages of NAFLD: Results from a Prospective Pilot Study. Nutrients. 2023; 15(10):2289. https://doi.org/10.3390/nu15102289
Chicago/Turabian StyleDe Nucci, Sara, Roberta Rinaldi, Martina Di Chito, Rossella Donghia, Vito Giannuzzi, Endrit Shahini, Raffaele Cozzolongo, Pasqua Letizia Pesole, Sergio Coletta, Giovanni De Pergola, and et al. 2023. "The Replacement of Only One Portion of Starchy Carbohydrates with Green Leafy Vegetables Regresses Mid and Advanced Stages of NAFLD: Results from a Prospective Pilot Study" Nutrients 15, no. 10: 2289. https://doi.org/10.3390/nu15102289





