Red Yeast Rice for the Improvement of Lipid Profiles in Mild-to-Moderate Hypercholesterolemia: A Narrative Review
Abstract
1. Introduction
2. Effect of RYR on LDL-C Plasma Level and Estimated Risk of ASCVD
2.1. Epidemiology and Natural History of Hypercholesterolemia and ASCVD
2.2. Purpose and Outcome of Treatment
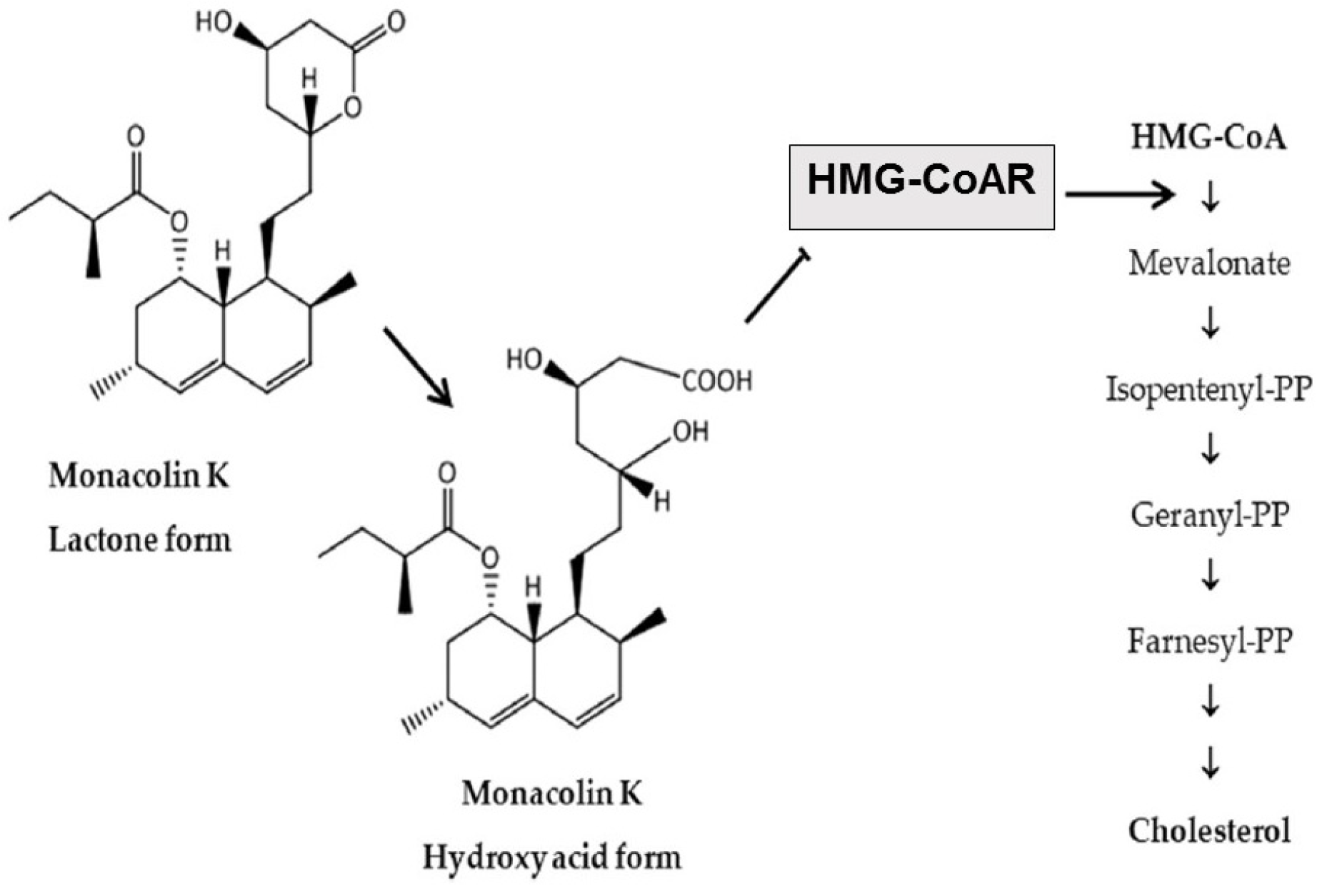
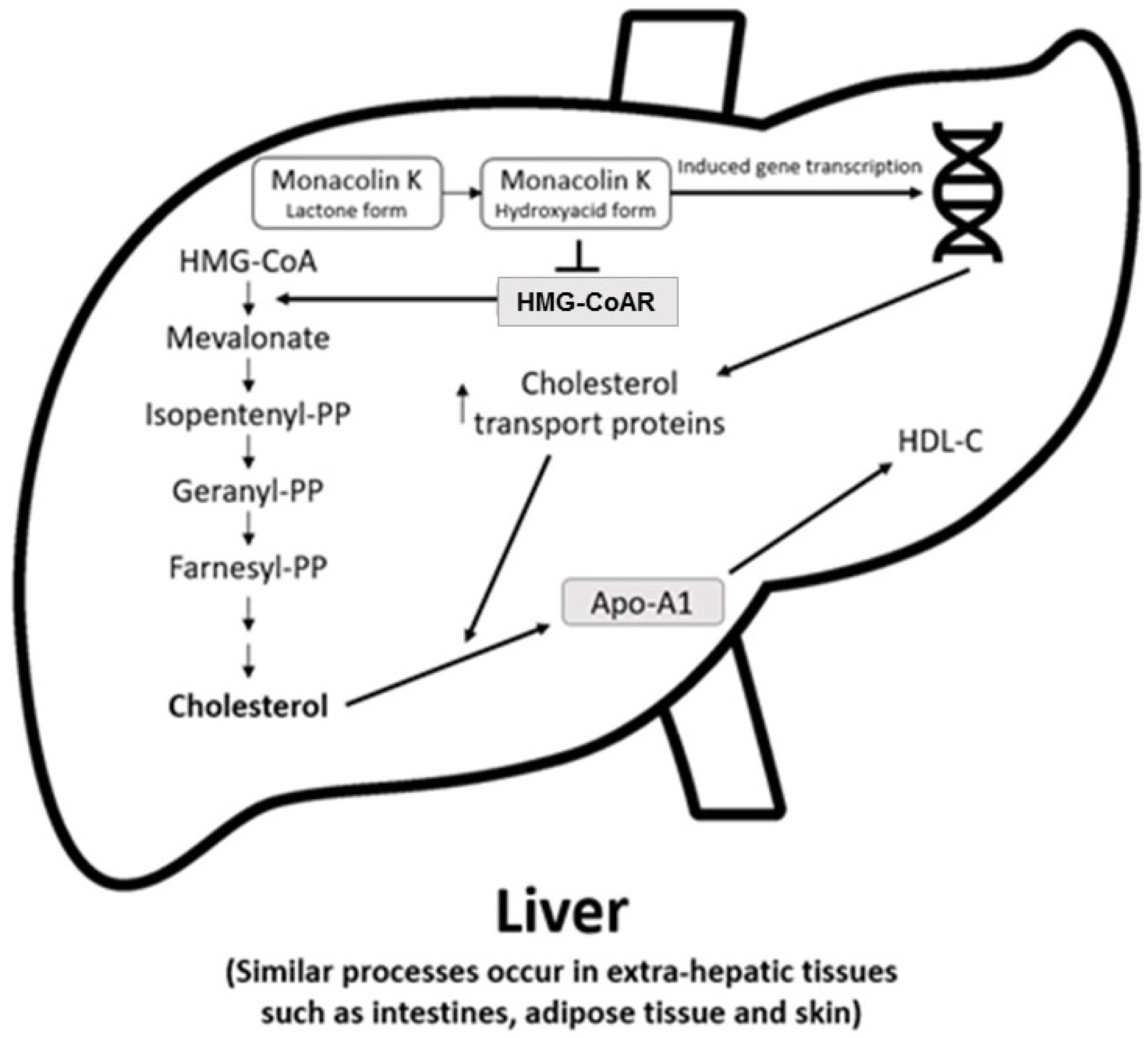
2.3. Metabolism of the Bioactive Components and Mechanism of Action of RYR
2.4. Effects of RYR on Lipids
2.4.1. RYR versus Placebo
2.4.2. RYR versus Other Statin Preparations
2.4.3. RYR versus Placebo and Other Statins Meta-Analyses
2.5. Effects of RYR on Inflammatory and Vascular Remodeling Biomarkers and Endothelial Function
2.6. Beneficial Effects of Exposure to RYR on ASCVD Risk and Events
2.6.1. RYR versus Placebo
2.6.2. RYR versus Other Statins
2.7. Beneficial Effects of RYR-Berberine Combinations
2.8. Convenience and Preference, and Health Economic Impact
3. Safety and Tolerability of RYR
3.1. Clinical Trials
3.2. Meta-Analyses
3.3. Real-World Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.G.; Fogacci, F.; Zambon, A. Red yeast rice for hypercholesterolemia: JACC focus seminar. J. Am. Coll. Cardiol. 2021, 77, 620–628. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Stoian, A.P.; Vrablik, M.; Al Rasadi, K.; Banach, M.; Toth, P.P.; Rizzo, M. Nutraceuticals in the management of dyslipidemia: Which, when, and for whom? Could nutraceuticals help low-risk individuals with non-optimal lipid levels? Curr. Atheroscler. Rep. 2021, 23, 57. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on the substantiation of health claims related to monacolin K from red yeast rice and maintenance of normal blood LDL-cholesterol concentrations (ID 1648, 1700) pursuant to article 13(1) of regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2304. [Google Scholar] [CrossRef]
- Fukami, H.; Higa, Y.; Hisano, T.; Asano, K.; Hirata, T.; Nishibe, S. A review of red yeast rice, a traditional fermented food in Japan and East Asia: Its characteristic ingredients and application in the maintenance and improvement of health in lipid metabolism and the circulatory system. Molecules 2021, 26, 1619. [Google Scholar] [CrossRef]
- Baumgartner, S.; Bruckert, E.; Gallo, A.; Plat, J. The position of functional foods and supplements with a serum LDL-C lowering effect in the spectrum ranging from universal to care-related CVD risk management. Atherosclerosis 2020, 311, 116–123. [Google Scholar] [CrossRef]
- Gerards, M.C.; Terlou, R.J.; Yu, H.; Koks, C.H.; Gerdes, V.E. Traditional Chinese lipid-lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—A systematic review and meta-analysis. Atherosclerosis 2015, 240, 415–423. [Google Scholar] [CrossRef]
- Li, P.; Wang, Q.; Chen, K.; Zou, S.; Shu, S.; Lu, C.; Wang, S.; Jiang, Y.; Fan, C.; Luo, Y. Red yeast rice for hyperlipidemia: A meta-analysis of 15 high-quality randomized controlled trials. Front. Pharmacol. 2021, 12, 819482. [Google Scholar] [CrossRef]
- Pirro, M.; Vetrani, C.; Bianchi, C.; Mannarino, M.R.; Bernini, F.; Rivellese, A.A. Joint position statement on “Nutraceuticals for the treatment of hypercholesterolemia” of the Italian Society of Diabetology (SID) and of the Italian Society for the Study of Arteriosclerosis (SISA). Nutr. Metab. Cardiovasc. Dis. 2017, 27, 2–17. [Google Scholar] [CrossRef]
- Banach, M.; Patti, A.M.; Giglio, R.V.; Cicero, A.F.G.; Atanasov, A.G.; Bajraktari, G.; Bruckert, E.; Descamps, O.; Djuric, D.M.; Ezhov, M.; et al. The role of nutraceuticals in statin intolerant patients. J. Am. Coll. Cardiol. 2018, 72, 96–118. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Colletti, A.; Bajraktari, G.; Descamps, O.; Djuric, D.M.; Ezhov, M.; Fras, Z.; Katsiki, N.; Langlois, M.; Latkovskis, G.; et al. Lipid lowering nutraceuticals in clinical practice: Position paper from an International Lipid Expert Panel. Arch. Med. Sci. 2017, 13, 965–1005. [Google Scholar] [CrossRef]
- Chinese Society of Cardiology Chinese guideline on the primary prevention of cardiovascular diseases. Cardiol. Discov. 2021, 1, 70–104. [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: Executive summary: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation 2019, 140, e563–e595. [Google Scholar] [CrossRef]
- Zhu, B.; Qi, F.; Wu, J.; Yin, G.; Hua, J.; Zhang, Q.; Qin, L. Red yeast rice: A systematic review of the traditional uses, chemistry, pharmacology, and quality control of an important Chinese folk medicine. Front. Pharmacol. 2019, 10, 1449. [Google Scholar] [CrossRef]
- World Health Organization. The Top 10 Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 9 August 2022).
- Carroll, M.D.; Fryar, C.D. Total and High-Density Lipoprotein Cholesterol in Adults: United States, 2015–2018. Centers for Disease Control and Prevention. 2020. Available online: https://www.cdc.gov/nchs/products/databriefs/db363.htm (accessed on 27 April 2023).
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Lin, C.-F.; Chang, Y.-H.; Chien, S.-C.; Lin, Y.-H. Epidemiology of dyslipidemia in the Asia Pacific region. Int. J. Gerontol. 2018, 12, 2–6. [Google Scholar] [CrossRef]
- Bilitou, A.; Were, J.; Farrer, A.; Rabe, A.; Ming, S.W.Y.; Haq, I.; Dunton, K. Prevalence and patient outcomes of adult primary hypercholesterolemia and dyslipidemia in the UK: Longitudinal retrospective study using a primary care dataset from 2009 to 2019. Clinicoecon. Outcomes Res. 2022, 14, 189–203. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Yoo, W.; Alesh, I.; Mahajan, N.; Mirowska, K.K.; Mewada, A.; Kahn, J.; Afonso, L.; Williams, K.A.; Flack, J.M. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: A mendelian randomization analysis. J. Am. Coll. Cardiol. 2012, 60, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Majeed, F.; Penumetcha, R.; Flack, J.M.; Brook, R.D. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1l1, HMGCR, or both: A 2 × 2 factorial Mendelian randomization study. J. Am. Coll. Cardiol. 2015, 65, 1552–1561. [Google Scholar] [CrossRef]
- Penson, P.E.; Banach, M. Nutraceuticals for the control of dyslipidaemias in clinical practice. Nutrients 2021, 13, 2957. [Google Scholar] [CrossRef]
- Banach, M.; Bruckert, E.; Descamps, O.S.; Ellegård, L.; Ezhov, M.; Föger, B.; Fras, Z.; Kovanen, P.T.; Latkovskis, G.; März, W.; et al. The role of red yeast rice (RYR) supplementation in plasma cholesterol control: A review and expert opinion. Atheroscler. Suppl. 2019, 39, e1–e8. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific opinion on the safety of monacolins in red yeast rice. EFSA J. 2018, 16, e05368. [Google Scholar] [CrossRef]
- Song, J.; Luo, J.; Ma, Z.; Sun, Q.; Wu, C.; Li, X. Quality and authenticity control of functional red yeast rice-a review. Molecules 2019, 24, 1944. [Google Scholar] [CrossRef]
- Mannino, G.; Iovino, P.; Lauria, A.; Genova, T.; Asteggiano, A.; Notarbartolo, M.; Porcu, A.; Serio, G.; Chinigò, G.; Occhipinti, A.; et al. Bioactive triterpenes of protium heptaphyllum gum resin extract display cholesterol-lowering potential. Int. J. Mol. Sci. 2021, 22, 2664. [Google Scholar] [CrossRef]
- Martin, G.; Duez, H.; Blanquart, C.; Berezowski, V.; Poulain, P.; Fruchart, J.C.; Najib-Fruchart, J.; Glineur, C.; Staels, B. Statin-induced inhibition of the Rho-signaling pathway activates PPARalpha and induces HDL apoA-I. J. Clin. Investig. 2001, 107, 1423–1432. [Google Scholar] [CrossRef]
- Bjarnadottir, O.; Kimbung, S.; Johansson, I.; Veerla, S.; Jonsson, M.; Bendahl, P.O.; Grabau, D.; Hedenfalk, I.; Borgquist, S. Global transcriptional changes following statin treatment in breast cancer. Clin. Cancer. Res. 2015, 21, 3402–3411. [Google Scholar] [CrossRef]
- Dichtl, W.; Dulak, J.; Frick, M.; Alber, H.F.; Schwarzacher, S.P.; Ares, M.P.; Nilsson, J.; Pachinger, O.; Weidinger, F. HMG-CoA reductase inhibitors regulate inflammatory transcription factors in human endothelial and vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 58–63. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Ghorbanihaghjo, A.; Argani, H. The effect of statins on the organs: Similar or contradictory? J. Cardiovasc. Thorac. Res. 2017, 9, 64–70. [Google Scholar] [CrossRef]
- Chen, C.H.; Uang, Y.S.; Wang, S.T.; Yang, J.C.; Lin, C.J. Interaction between red yeast rice and CYP450 enzymes/P-glycoprotein and its implication for the clinical pharmacokinetics of lovastatin. Evid. Based Complement. Altern. Med. 2012, 2012, 127043. [Google Scholar] [CrossRef]
- Li, Z.; Seeram, N.P.; Lee, R.; Thames, G.; Minutti, C.; Wang, H.J.; Heber, D. Plasma clearance of lovastatin versus Chinese red yeast rice in healthy volunteers. J. Altern. Complement. Med. 2005, 11, 1031–1038. [Google Scholar] [CrossRef]
- Cohen, P.A.; Avula, B.; Khan, I.A. Variability in strength of red yeast rice supplements purchased from mainstream retailers. Eur. J. Prev. Cardiol. 2017, 24, 1431–1434. [Google Scholar] [CrossRef]
- Gordon, R.Y.; Cooperman, T.; Obermeyer, W.; Becker, D.J. Marked variability of monacolin levels in commercial red yeast rice products: Buyer beware! Arch. Intern. Med. 2010, 170, 1722–1727. [Google Scholar] [CrossRef]
- Marcheluzzo, S.; Faggian, M.; Zancato, M.; Peron, G. Analysis of monacolins and berberine in food supplements for lipid control: An overview of products sold on the Italian market. Molecules 2021, 26, 2222. [Google Scholar] [CrossRef]
- Tomlinson, B.; Chan, P.; Liu, Z.M. Statin intolerance-an Asian perspective. J. Atheroscler. Thromb. 2020, 27, 485–488. [Google Scholar] [CrossRef]
- Lee, E.; Ryan, S.; Birmingham, B.; Zalikowski, J.; March, R.; Ambrose, H.; Moore, R.; Lee, C.; Chen, Y.; Schneck, D. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin. Pharmacol. Ther. 2005, 78, 330–341. [Google Scholar] [CrossRef]
- Gandelman, K.; Fung, G.L.; Messig, M.; Laskey, R. Systemic exposure to atorvastatin between Asian and Caucasian subjects: A combined analysis of 22 studies. Am. J. Ther. 2012, 19, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Heber, D.; Yip, I.; Ashley, J.M.; Elashoff, D.A.; Elashoff, R.M.; Go, V.L. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am. J. Clin. Nutr. 1999, 69, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-P.; Liu, L.; Cheng, Y.-C.; Li, Y.-L. Effect of xuezhikang, a cholestin extract, on reflecting postprandial triglyceridemia after a high-fat meal in patients with coronary heart disease. Atherosclerosis 2003, 168, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.P.; Liu, L.; Cheng, Y.C.; Shishehbor, M.H.; Liu, M.H.; Peng, D.Q.; Li, Y.L. Xuezhikang, an extract of cholestin, protects endothelial function through antiinflammatory and lipid-lowering mechanisms in patients with coronary heart disease. Circulation 2004, 110, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Li, T.-C.; Lai, M.-M. Efficacy and safety of monascus purpureus went rice in subjects with hyperlipidemia. Eur. J. Endocrinol. 2005, 153, 679–686. [Google Scholar] [CrossRef]
- Becker, D.J.; Gordon, R.Y.; Halbert, S.C.; French, B.; Morris, P.B.; Rader, D.J. Red yeast rice for dyslipidemia in statin-intolerant patients: A randomized trial. Ann. Intern. Med. 2009, 150, 830–839. [Google Scholar] [CrossRef]
- Bogsrud, M.P.; Ose, L.; Langslet, G.; Ottestad, I.; Strøm, E.C.; Hagve, T.A.; Retterstøl, K. HypoCol (red yeast rice) lowers plasma cholesterol—A randomized placebo controlled study. Scand. Cardiovasc. J. 2010, 44, 197–200. [Google Scholar] [CrossRef]
- Cicero, A.F.; Derosa, G.; Parini, A.; Maffioli, P.; D’Addato, S.; Reggi, A.; Giovannini, M.; Borghi, C. Red yeast rice improves lipid pattern, high-sensitivity C-reactive protein, and vascular remodeling parameters in moderately hypercholesterolemic Italian subjects. Nutr. Res. 2013, 33, 622–628. [Google Scholar] [CrossRef]
- Verhoeven, V.; Lopez Hartmann, M.; Remmen, R.; Wens, J.; Apers, S.; Van Royen, P. Red yeast rice lowers cholesterol in physicians—A double blind, placebo controlled randomized trial. BMC Complement. Altern. Med. 2013, 13, 178. [Google Scholar] [CrossRef]
- Moriarty, P.M.; Roth, E.M.; Karns, A.; Ye, P.; Zhao, S.P.; Liao, Y.; Capuzzi, D.M.; Bays, H.E.; Zhang, F.; Liu, S.; et al. Effects of Xuezhikang in patients with dyslipidemia: A multicenter, randomized, placebo-controlled study. J. Clin. Lipidol. 2014, 8, 568–575. [Google Scholar] [CrossRef]
- Heinz, T.; Schuchardt, J.P.; Möller, K.; Hadji, P.; Hahn, A. Low daily dose of 3 mg monacolin K from RYR reduces the concentration of LDL-C in a randomized, placebo-controlled intervention. Nutr. Res. 2016, 36, 1162–1170. [Google Scholar] [CrossRef]
- Wang, T.J.; Lien, A.S.; Chen, J.L.; Lin, C.H.; Yang, Y.S.; Yang, S.H. A randomized clinical efficacy trial of red yeast rice (monascus pilosus) against hyperlipidemia. Am. J. Chin. Med. 2019, 47, 323–335. [Google Scholar] [CrossRef]
- Minamizuka, T.; Koshizaka, M.; Shoji, M.; Yamaga, M.; Hayashi, A.; Ide, K.; Ide, S.; Kitamoto, T.; Sakamoto, K.; Hattori, A.; et al. Low dose red yeast rice with monacolin K lowers LDL cholesterol and blood pressure in japanese with mild dyslipidemia: A multicenter, randomized trial. Asia Pac. J. Clin. Nutr. 2021, 30, 424–435. [Google Scholar] [CrossRef]
- Kou, W.; Lu, Z.; Guo, J. Effect of xuezhikang on the treatment of primary hyperlipidemia. Zhonghua Nei Ke Za Zhi 1997, 36, 529–531. [Google Scholar]
- Chen, L.L.; Liu, J. The effects of Xuezhikang on hypercholesterolemia. Her. Med. 2002, 21, 31–32. [Google Scholar]
- Cui, F.; Zhang, Y.; Wei, Q.; Liu, C.; Wang, J.; Zhang, M. A novel medical treatment for lipid control in patients with unstable angina pectoris and statin-induced liver dysfunction. Acta Cardiol. Sin. 2015, 31, 66–71. [Google Scholar] [CrossRef]
- Ruscica, M.; Gomaraschi, M.; Mombelli, G.; Macchi, C.; Bosisio, R.; Pazzucconi, F.; Pavanello, C.; Calabresi, L.; Arnoldi, A.; Sirtori, C.R.; et al. Nutraceutical approach to moderate cardiometabolic risk: Results of a randomized, double-blind and crossover study with armolipid plus. J. Clin. Lipidol. 2014, 8, 61–68. [Google Scholar] [CrossRef]
- Marazzi, G.; Campolongo, G.; Pelliccia, F.; Quattrino, S.; Vitale, C.; Cacciotti, L.; Massaro, R.; Volterrani, M.; Rosano, G. Comparison of low-dose statin versus low-dose statin + armolipid plus in high-intensity statin-intolerant patients with a previous coronary event and percutaneous coronary intervention (ADHERENCE trial). Am. J. Cardiol. 2017, 120, 893–897. [Google Scholar] [CrossRef]
- Gheith, O.; Sheashaa, H.; Abdelsalam, M.; Shoeir, Z.; Sobh, M. Efficacy and safety of monascus purpureus went rice in subjects with secondary hyperlipidemia. Clin. Exp. Nephrol. 2008, 12, 189–194. [Google Scholar] [CrossRef]
- Shang, X. Clinical observation of xuezhikang and atorvastatin on dyslipidemia and hemorheology in patients with coronary heart disease. Guangxi Med. 2007, 8, 1158–1159. [Google Scholar] [CrossRef]
- Halbert, S.C.; French, B.; Gordon, R.Y.; Farrar, J.T.; Schmitz, K.; Morris, P.B.; Thompson, P.D.; Rader, D.J.; Becker, D.J. Tolerability of red yeast rice (2400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am. J. Cardiol. 2010, 105, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Tao, L.; Wu, S.; Wang, G.; Qian, L.; Li, J.; Liao, L.; Tang, J.; Ji, K. Red yeast rice induces less muscle fatigue symptom than simvastatin in dyslipidemic patients: A single center randomized pilot trial. BMC Cardiovasc. Disord. 2017, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Hu, S.-Y.; Wu, X.; Xu, H.-L.; Zhang, H.-M.; Wang, L. Anti-oxidant and anti-inflammatory effects of xuezhikang capsule on patients with coronary heart diseases. Prog. Mod. Biomed. 2011, 12, 2289–2291. [Google Scholar]
- Liu, L.T.; Wu, M.; Wang, H.X. Clinical study on the treatment of abnormal blood lipids complicated with carotid atherosclerosis with lipid-reducing red rice minute powder: A randomized controlled trial. Zhongguo Zhong Xi Yi Jie He Za Zhi 2011, 31, 1196–1200. [Google Scholar] [PubMed]
- Xu, G.; Lin, M.; Dai, X.; Hu, J. Comparing the effectiveness of Chinese patent medicines containing red yeast rice on hyperlipidaemia: A network meta-analysis of randomized controlled trials. Endocrinol. Diabetes Metab. 2022, 5, e00314. [Google Scholar] [CrossRef]
- Kandelouei, T.; Abbasifard, M.; Imani, D.; Aslani, S.; Razi, B.; Fasihi, M.; Shafiekhani, S.; Mohammadi, K.; Jamialahmadi, T.; Reiner, Z.; et al. Effect of statins on serum level of hs-CRP and CRP in patients with cardiovascular diseases: A systematic review and meta-analysis of randomized controlled trials. Mediat. Inflamm. 2022, 2022, 8732360. [Google Scholar] [CrossRef]
- Lu, Z.; Kou, W.; Du, B.; Wu, Y.; Zhao, S.; Brusco, O.A.; Morgan, J.M.; Capuzzi, D.M.; Li, S. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am. J. Cardiol. 2008, 101, 1689–1693. [Google Scholar] [CrossRef]
- Li, J.J.; Lu, Z.L.; Kou, W.R.; Chen, Z.; Wu, Y.F.; Yu, X.H.; Zhao, Y.C. Impact of Xuezhikang on coronary events in hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). Ann. Med. 2010, 42, 231–240. [Google Scholar] [CrossRef]
- Li, J.J.; Lu, Z.L.; Kou, W.R.; Chen, Z.; Wu, Y.F.; Yu, X.H.; Zhao, Y.C. Beneficial impact of Xuezhikang on cardiovascular events and mortality in elderly hypertensive patients with previous myocardial infarction from the China Coronary Secondary Prevention Study (CCSPS). J. Clin. Pharmacol. 2009, 49, 947–956. [Google Scholar] [CrossRef]
- Sungthong, B.; Yoothaekool, C.; Promphamorn, S.; Phimarn, W. Efficacy of red yeast rice extract on myocardial infarction patients with borderline hypercholesterolemia: A meta-analysis of randomized controlled trials. Sci. Rep. 2020, 10, 2769. [Google Scholar] [CrossRef]
- Yuan, R.; Yuan, Y.; Wang, L.; Xin, Q.; Wang, Y.; Shi, W.; Miao, Y.; Leng, S.X.; Chen, K.; Cong, W. Red yeast rice preparations reduce mortality, major cardiovascular adverse events, and risk factors for metabolic syndrome: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 744928. [Google Scholar] [CrossRef]
- Chang, C.-C.; Sun, M.-F.; Chou, Y.-C.; Yeh, C.-C.; Hu, C.-J.; Cherng, Y.-G.; Chen, T.-L.; Liao, C.-C. Decreased risk of stroke in people using red yeast rice prescriptions (LipoCol Forte®): A total population-based retrospective cohort study. Evid. Based Complement. Altern. Med. 2022, 2022, 8160425. [Google Scholar] [CrossRef]
- Banach, M.; Katsiki, N.; Latkovskis, G.; Rizzo, M.; Pella, D.; Penson, P.E.; Reiner, Z.; Cicero, A.F.G. Postmarketing nutrivigilance safety profile: A line of dietary food supplements containing red yeast rice for dyslipidemia. Arch. Med. Sci. 2021, 17, 856–863. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Kennedy, C.; Knežević, T.; Bove, M.; Georges, C.M.G.; Šatrauskienė, A.; Toth, P.P.; Fogacci, F. Efficacy and safety of armolipid plus®: An updated prisma compliant systematic review and meta-analysis of randomized controlled clinical trials. Nutrients 2021, 13, 638. [Google Scholar] [CrossRef]
- Pirro, M.; Mannarino, M.R.; Bianconi, V.; Simental-Mendía, L.E.; Bagaglia, F.; Mannarino, E.; Sahebkar, A. The effects of a nutraceutical combination on plasma lipids and glucose: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2016, 110, 76–88. [Google Scholar] [CrossRef]
- Castano, G.; Mas, R.; Fernandez, J.; Lopez, E.; Illnait, J.; Fernandez, L.; Mesa, M. Effects of policosanol on borderline to mildly elevated serum total cholesterol levels: A prospective, double-blind, placebo-controlled, parallel-group, comparative study. Curr. Ther. Res. Clin. Exp. 2003, 64, 522–537. [Google Scholar] [CrossRef]
- Cicero, A.F.; Morbini, M.; Parini, A.; Urso, R.; Rosticci, M.; Grandi, E.; Borghi, C. Effect of red yeast rice combined with antioxidants on lipid pattern, hs-CRP level, and endothelial function in moderately hypercholesterolemic subjects. Ther. Clin. Risk Manag. 2016, 12, 281–286. [Google Scholar] [CrossRef]
- Cicero, A.F.; Morbini, M.; Rosticci, M.; D’Addato, S.; Grandi, E.; Borghi, C. Middle-term dietary supplementation with red yeast rice plus Coenzyme Q10 improves lipid pattern, endothelial reactivity and arterial stiffness in moderately hypercholesterolemic subjects. Ann. Nutr. Metab. 2016, 68, 213–219. [Google Scholar] [CrossRef]
- Murphy, A.; Palafox, B.; O’Donnell, O.; Stuckler, D.; Perel, P.; AlHabib, K.F.; Avezum, A.; Bai, X.; Chifamba, J.; Chow, C.K.; et al. Inequalities in the use of secondary prevention of cardiovascular disease by socioeconomic status: Evidence from the PURE observational study. Lancet Glob. Health 2018, 6, e292–e301. [Google Scholar] [CrossRef]
- Yusuf, S.; Islam, S.; Chow, C.K.; Rangarajan, S.; Dagenais, G.; Diaz, R.; Gupta, R.; Kelishadi, R.; Iqbal, R.; Avezum, A.; et al. Use of secondary prevention drugs for cardiovascular disease in the community in high-income, middle-income, and low-income countries (the PURE study): A prospective epidemiological survey. Lancet 2011, 378, 1231–1243. [Google Scholar] [CrossRef]
- Toth, P.P.; Patti, A.M.; Giglio, R.V.; Nikolic, D.; Castellino, G.; Rizzo, M.; Banach, M. Management of statin intolerance in 2018: Still more questions than answers. Am. J. Cardiovasc. Drugs 2018, 18, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.; Santos, P. Determinants of non-adherence to the medications for dyslipidemia: A systematic review. Patient Prefer. Adherence 2021, 15, 1853–1871. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Derosa, G.; Parini, A.; Baronio, C.; Borghi, C. Factors associated with 2-year persistence in fully non reimbursed lipid-lowering treatments. Atherosclerosis 2014, 235, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, F.; Banach, M.; Mikhailidis, D.P.; Bruckert, E.; Toth, P.P.; Watts, G.F.; Reiner, Ž.; Mancini, J.; Rizzo, M.; Mitchenko, O.; et al. Safety of red yeast rice supplementation: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2019, 143, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Evans, M.A. Statin adverse effects: A review of the literature and evidence for a mitochondrial mechanism. Am. J. Cardiovasc. Drugs. 2008, 8, 373–418. [Google Scholar] [CrossRef]
- Cai, T.; Abel, L.; Langford, O.; Monaghan, G.; Aronson, J.K.; Stevens, R.J.; Lay-Flurrie, S.; Koshiaris, C.; McManus, R.J.; Hobbs, F.D.R.; et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: Systematic review with pairwise, network, and dose-response meta-analyses. BMJ 2021, 374, n1537. [Google Scholar] [CrossRef]
- Righetti, L.; Dall’Asta, C.; Bruni, R. Risk assessment of RYR food supplements: Perception vs. reality. Front. Nutr. 2021, 8, 792529. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on the risks for public and animal health related to the presence of cintrinin in food and feed. EFSA J. 2012, 10, 2605. [Google Scholar]
- López Sáncheza, P.; de Nijsa, M.; Spanjerb, M.; Pietric, A.; Bertuzzic, T.; Starski, A.; Postupolski, J.; Castellari, M.; Hortós, M. Generation of Occurrence Data on Citrinin in Food; EFSA Supporting Publication 2017:EN-1177; EFSA: Parma, Italy, 2017. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Moghadasian, M.H. Nutraceuticals and nutrition supplements: Challenges and opportunities. Nutrients 2020, 12, 1593. [Google Scholar] [CrossRef]
- Sartore, G.; Burlina, S.; Ragazzi, E.; Ferraresso, S.; Valentini, R.; Lapolla, A. Mediterranean diet and red yeast rice supplementation for the management of hyperlipidemia in statin-intolerant patients with or without type 2 diabetes. Evid. Based Complement. Altern. Med. 2013, 2013, 743473. [Google Scholar] [CrossRef]
- Li, D.Q.; Kim, R.B.; McArthur, E.; Fleet, J.L.; Hegele, R.A.; Shah, B.R.; Weir, M.A.; Molnar, A.O.; Dixon, S.; Tu, J.V.; et al. Statin safety in Chinese: A population-based study of older adults. PLoS ONE 2016, 11, e0150990. [Google Scholar] [CrossRef]
- Shang, Q.; Liu, Z.; Chen, K.; Xu, H.; Liu, J. A systematic review of Xuezhikang, an extract from red yeast rice, for coronary heart disease complicated by dyslipidemia. Evid. Based Complement. Altern. Med. 2012, 2012, 636547. [Google Scholar] [CrossRef]
- Mazzanti, G.; Moro, P.A.; Raschi, E.; Da Cas, R.; Menniti-Ippolito, F. Adverse reactions to dietary supplements containing red yeast rice: Assessment of cases from the Italian surveillance system. Br. J. Clin. Pharmacol. 2017, 83, 894–908. [Google Scholar] [CrossRef]
- Vrolijk, M.F.; van de Koppel, S.; van Hunsel, F. Red yeast rice (monascus purpureus) supplements: Case series assessment of spontaneously reported cases to The Netherlands Pharmacovigilance Centre Lareb. Br. J. Clin. Pharmacol. 2021, 87, 2146–2151. [Google Scholar] [CrossRef]
- Chen, T.L.; Lin, C.S.; Lin, J.A.; Yeh, C.C.; Sung, L.C.; Chang, Y.C.; Shih, C.C.; Liao, C.C. Evaluating risk of incident diabetes between patients who used lovastatin and red yeast rice prescriptions (LipoCol Forte): A retrospective cohort study based on a real-world database. Diabetes. Metab. Syndr. Obes. 2020, 13, 89–98. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Statin eligibility for primary prevention of cardiovascular disease according to 2021 European prevention guidelines compared with other international guidelines. JAMA Cardiol. 2022, 7, 836–843. [Google Scholar] [CrossRef]
- Osadnik, T.; Golawski, M.; Lewandowski, P.; Morze, J.; Osadnik, K.; Pawlas, N.; Lejawa, M.; Jakubiak, G.K.; Mazur, A.; Schwingschackl, L.; et al. A network meta-analysis on the comparative effect of nutraceuticals on lipid profile in adults. Pharmacol. Res. 2022, 183, 106402. [Google Scholar] [CrossRef]
- Cheeley, M.K.; Saseen, J.J.; Agarwala, A.; Ravilla, S.; Ciffone, N.; Jacobson, T.A.; Dixon, D.L.; Maki, K.C. NLA scientific statement on statin intolerance: A new definition and key considerations for ASCVD risk reduction in the statin intolerant patient. J. Clin. Lipidol. 2022, 16, 361–375. [Google Scholar] [CrossRef]
- Warden, B.A.; Guyton, J.R.; Kovacs, A.C.; Durham, J.A.; Jones, L.K.; Dixon, D.L.; Jacobson, T.A.; Duell, P.B. Assessment and management of statin-associated muscle symptoms (SAMS): A clinical perspective from the National Lipid Association. J. Clin. Lipidol. 2023, 17, 19–39. [Google Scholar] [CrossRef]
- Chen, G.; Chen, W.; Xu, J.; Ma, G.; Hu, X.; Chen, G. The current trend and challenges of developing red yeast rice-based food supplements for hypercholesterolemia. J. Future Foods 2023, 3, 312–329. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Untea, A.E.; Turcu, R.P.; Saracila, M.; Panaite, T.D.; Cornescu, G.M. Nutritional composition and bioactive compounds of basil, thyme and sage plant additives and their functionality on broiler thigh meat quality. Foods 2022, 11, 1105. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.C.; Lombardo-Cristina, V.; Marina, M.L. Multifunctional and collaborative protection of proteins, peptides, phenolic compounds, and other molecules against oxidation in apricot seeds extracts. Antioxidants 2022, 11, 2354. [Google Scholar] [CrossRef] [PubMed]
- Caruso, A.; Barbarossa, A.; Tassone, A.; Ceramella, J.; Carocci, A.; Catalano, A.; Basile, G.; Fazio, A.; Iacopetta, D.; Franchini, C.; et al. Pomegranate: Nutraceutical with promising benefits on human health. Appl. Sci. 2020, 10, 6915. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Pati, S.; Abdul Kari, Z.; Atan Edinur, H.; Chakraborty, R. Novel bioactive compounds from marine sources as a tool for functional food development. Front. Marine Sci. 2022, 9, 832957. [Google Scholar] [CrossRef]
| Component | Number Identified |
|---|---|
| Monacolins (including monacolins K, L, Q, R, and S) | 23 |
| Pigments | 25 |
| Organic acids and amino acids | 9 (including citrinin) |
| Sterols | 9 |
| Decalin derivatives | 7 |
| Flavonoids | 2 |
| Terpenoids | 5 |
| Lignans | 2 |
| Coumarin | 1 |
| Polysaccharides | 9 |
| Name | Mechanisms of Action | Main Lipid-Lowering Component(s) | Effects on Lipids | Safety and Tolerability |
|---|---|---|---|---|
| Artichoke leaf extract | Inhibition of liver cholesterol synthesis via action on HMG-CoAR; effects on sterol regulatory element binding protein and acyl-CoA acyl transferase (ACAT) | Luteolin | Up to 10% reduction in LDL-C; small reduction in TG | Transient minor GI effects |
| Bergamot | Inhibition of liver cholesterol synthesis via inhibition of HMG-CoAR and ACAT; may also increase fecal cholesterol excretion and reduce intestinal cholesterol absorption of bile acids | Brutieridin, melitidin, neoeriocitrin | Up to 15% reduction in LDL-C; small reduction in TG | |
| Rice bran oil | Inhibition of liver cholesterol synthesis via inhibition of HMG-CoAR; reduction in intestinal cholesterol absorption | Gamma-oryzanol | 0.18 mmol/L (7 mg/dL) reduction in LDL-C across 11 RCTs (p < 0.001) | No known side effects |
| Garlic | Inhibition of liver cholesterol synthesis via inhibition of HMG-CoAR, squalene mono-oxygenase, and acetyl-CoA synthetase; may also promote bile acid excretion | Allicin | Up to 5% reduction in LDL-C | Minimal, mostly GI side effects |
| Green tea extracts | Antioxidant effects; may also interfere with cholesterol absorption and inhibitcholesterol synthesis via inhibition of HMG-CoAR | Catechins, including epigallocatechin-3-gallate | Up to 5% reduction in LDL-C | Potential iron and folate deficiency with high doses; rare GI side effects, rash, transient elevation of BP |
| Vitamin B5 derivatives | Inhibition of fatty acid and cholesterol synthesis | Pantethine | Up to 11% reduction in LDL-C; smaller reductions in TG and total cholesterol | Well tolerated |
| Omega-3 | Reduced VLDL and TG synthesis; increased fatty acid oxidation | Docosahexaenoic acid; eicosapentaenoic acid | 25–30% reduction in TG levels; variable effects on LDL-C depending on components | Well tolerated, rare abdominal discomfort; fishy aftertaste |
| Coptis, Hydrastis, and Berberis spp. | Increased LDL-C excretion via increased expression of hepatic LDL receptors via inhibition of PCSK9 | Berberine | ~15% reduction in LDL-C; smallreduction in TG | Mild-to-moderate GI effects |
| Lupin | Unclear; multiple proposed mechanisms | Bioactive peptides; isoflavones | 12% reduction in LDL-C; increase in HDL-C in some studies | Well tolerated; minor GI events |
| Soy | Unclear; multiple proposed mechanisms | Bioactive peptides; isoflavones | Up to 5% reduction in LDL-C; small reduction in TG and increase in HDL-C | Long-term use of high doses may disrupt fertility and thyroid function; may reduce absorption of calcium and other minerals |
| Turmeric | Unclear; multiple proposed mechanisms | Curcumin | Inconsistent effects reported; some studies report significant improvements in LDL-C, TG, and HDL-C | Well tolerated |
| Study | Population | Primary/Secondary Prevention | Interventions | Study |
|---|---|---|---|---|
| Placebo comparator | ||||
| Heber, 1999 [44] | Dyslipidemia n = 88 | Primary | RYR 2400 mg/d (MK 4.8 mg/d) vs. PBO 12 weeks | CFB Wk 12: RYR −1.01; PBO −0.13 p < 0.001 vs. PBO for LDL-C concentrations at Wk 12 |
| Zhao, 2003 [45] Zhao, 2004 [46] | CHD n = 50 | Secondary | XZK 600 mg BID vs. PBO 6 weeks | XZK BL 3.32, Wk 6 2.38; %CFB −34% p < 0.001 vs. BL PBO BL 3.35, Wk 6 3.26; p = NS vs. BL |
| Lin, 2005 [47] | Dyslipidemia n = 79 | Primary | RYR 600 mg BID vs. PBO 8 weeks | %CFB, RYR −27.7%; PBO −1.5% p < 0.001 vs. BL and PBO |
| Becker, 2009 [48] | Dyslipidemia, statin intolerance n = 62 | Primary | RYR 1800 mg BID (MK 3.06 mg BD) vs. PBO 24 weeks | %CFB Wk 24: RYR −21.3%; PBO −8.7% p = 0.011 vs. PBO for LDL-C concentrations at Wk 24 |
| Bogsrud, 2010 [49] | Dyslipidemia, DM2 n = 42 | NR | RYR 4 capsules/d (MK 7.2 mg/d) vs. PBO 16 weeks | %CFB, RYR vs. PBO: −23.0%; p < 0.001 |
| Cicero, 2013 [50] | Mild dyslipidemia n = 25 | Primary | MK 10 mg/d vs. PBO 4 weeks | %CFB, RYR vs. PBO: −22.0%; p < 0.01 |
| Verhoeven, 2013 a [51] | Dyslipidemia n = 54 | NR | RYR 2 capsules QD (MK 10 mg/d) vs. PBO 8 weeks | %CFB, RYR −22.2%; PBO +1.65%; p < 0.001 |
| Moriarty, 2014 [52] | Dyslipidemia n = 116 | Primary | XZK 600 mg BID or XZK 1200 mg BID vs. PBO 12 weeks | %CFB Wk 12: XZK 1200 mg −26.4%; p < 0.001 vs. BL and PBO %CFB Wk 12: XZK 2400 mg −27.0%; p < 0.001 vs. BL and PBO PBO +0.5% p = NS vs. BL |
| Heinz, 2016 [53] | Dyslipidemia n = 151 | Primary | RYR 200 mg/d (MK 3 mg/d) vs. PBO 12 weeks | %CFB, RYR −14.8%; p < 0.001 vs. PBO %CFB, PBO −2.7%; p = NS vs. BL |
| Wang, 2019 [54] | Dyslipidemia n = 69 | NR | GABA-rich RYR 250 mg capsules BID (RYR 335 mg/d; GABA 0.14 mg/d) MK-rich RYR 250 mg capsules BID (RYR 400 mg/d; MK 8 mg/d) vs. PBO 3 months | Median (mg/dL): RYR MK BL 153, 3 m 122; p < 0.05 vs. BL, RYR GABA, and PBO RYR GABA BL 151, 3 m 156; p = 0.009 vs. BL; p = NS vs. PBO PBO BL 154, 3 m 152; p = NS vs. BL |
| Minamizuka, 2021 [55] | Mild dyslipidemia n = 19 | Primary | RYR 200 mg/d (MK 2 mg/d) + dietary therapy vs. dietary therapy alone 8 weeks | Median CFB: RYR −0.96; control −0.20; p = 0.030 vs. control |
| Statin comparator | ||||
| Xiaobin, 2007 [62] | CHD with dyslipidemia n = 130 | Secondary | XZK (dose NA) vs. ATV (dose NA) 2 months | %CFB: XZK NA; ATV NA p < 0.01 for both vs. BL |
| Gheith, 2008 [61] | Nephrotic syndrome with dyslipidemia n = 72 | NR | RYR 1.2 g/d for 1 month then 600 mg/d vs. FLV 20 mg/d or PBO 1 year | NR |
| Liu, 2011 [66] | Hyperlipidemia, carotid atherosclerosis n = 60 | Secondary | LRRMP 350 mg/d vs. XZK 1.2 g/d vs. LOV 20 mg/d 6 months | NA p = NS intergroup comparison |
| Li, 2011 [65] | CHD and dyslipidemia n = 64 | Secondary | XZK 1.2 g/d vs. LOV 40 mg/d 8 weeks | Lowered vs. BL p < 0.05 vs. BL; p = NS vs. LOV |
| Halbert, 2010 [63] | Dyslipidemia, statin intolerance n = 43 | Primary and secondary | RYR 2400 mg BID (9.96 mg MK/d) vs. PRV 20 mg BID 12 weeks | %CFB: RYR −30.2%; PRV −27.0% ΔLDL-C (CFB RYR vs. PRV) a: −10.7 mg/dL; p = NS |
| Ruscica, 2014 [59] | Dyslipidemia and metabolic syndrome n = 30 | Primary | RYR 200 mg/d (Armolipid Plus® [MK 3 mg/d]) vs. PRV 10 mg/d 8 weeks | %CFB: RYR −21.1%; PRV −22.6% both p < 0.0001 vs. BL; p = NS Armolipid Plus® vs. PRV |
| Marazzi, 2017 [60] | CAD with PCI in preceding 12 months, HDS intolerant, poor response with LDS n = 100 | Secondary | RYR 200 mg/d (Armolipid Plus® [MK 3 mg/d]) plus LDS vs. LDS (ATV 5–10 mg/d, RSV 5 mg/d, or SMV 10–20 mg/d) 3 months | %CFB: RYR + LDS −26.8%; LDS −4.3% p < 0.0001 Armolipid Plus® + LDS vs. LDS |
| Kou, 1997 [56] | Hyperlipidemia n = 108 | Primary | XZK 1.2 g/d vs. SMV 10 mg/d 8 weeks | %CFB: XZK −28.0%; SMV −29.5% p < 0.001 for both vs. BL |
| Chen, 2002 [57] | Hypercholesterolemia n = 65 | Primary | XZK 1.2 g/d vs. SMV 10 mg/d 4 weeks | %CFB: XZK −28.2%; SMV −22.7%; p = NA |
| Xue, 2017 [64] | Dyslipidemia n = 65 | Primary | RYR 1.2 g/d vs. SMV 20 mg/d 4 weeks | %CFB: RYR −33.4%; SMV −30.9% p < 0.001 vs. BL for both p = NS for RYR vs. SMV |
| Cui, 2015 [58] | Unstable angina pectoris, statin intolerance b n = 90 | Secondary | XZK 600 mg BID vs. SMV 20 mg QD vs. SMV stopped and restarted at 20 mg QD c 8 weeks | LDL-C (mg/dL): XZK BL 152, Wk 8 119; p < 0.05 vs. BL SMV BL 151, Wk 8 118; p < 0.05 vs. BL |
| Study | Population | Interventions | Incidence of CV Outcomes | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Revasculariz-Ation | Fatal MI | Non-Fatal MI | Stroke | CV Events a | CV Mortality | All-Cause Mortality | |||
| Clinical trials | |||||||||
| Cui, 2015 [58] | Unstable angina pectoris, statin intolerance n = 90 | XZK 600 mg BID vs. SMV 20 mg QD vs. SMV stopped and restarted at 20 mg QD Duration = 8 weeks | – | – | – | – | XZK 3.3% SMV 3.3% SMV stopped 13.3% (p < 0.05 for SMV stopped vs. XZK and SMV) | – | – |
| Lu, 2008 [69] | Previous MI, average LDL-C levels n = ~5000 | XZK 600 mg BID vs. PBO Duration = 4.5 years | 33% reduction | – | – | – | XZK 5.7% PBO 10.4% b 45% reduction | 30% reduction | 33% reduction |
| Li, 2010 [70] | Subgroup: previous MI, hypertensive n = 2704 | XZK 600 mg BID vs. PBO Duration = 4.5 years | – | XZK 1.0% PBO 1.3% (p = NS) 29% reduction | XZK 2.2% PBO 5.4% (p < 0.001) 60% reduction | XZK 3.5% PBO 5.1% (p = 0.06) 32% reduction | XZK 6.7% PBO 11.9% (p = 0.0214) 43% reduction | XZK 4.5% PBO 6.5% (p = 0.001) 30% reduction | XZK 5.9% PBO 9.3% (p = 0.001) 36% reduction |
| Li, 2009 [71] | Subgroup: previous MI, elderly, hypertensive, average LDL-C levels n = 1530 | XZK 600 mg BID vs. PBO Duration = 4.5 years | – | – | – | XZK 8.8% PBO 14.3% 38% reduction | XZK 6.4% PBO 9.0% 29% reduction | ||
| Meta-analyses | |||||||||
| Sungthong, 2020 [72] | Previous MI, borderline hypercholesterolemia n = 10,699 | RYR 600 mg BID vs. PBO Duration = 4 weeks to 4.5 years | RR 0.58 (95% CI 0.48, 0.71) p < 0.00001 42% reduction | RR 0.78 (95% CI 0.55, 1.10) p = 0.16 22% reduction | RR 0.42 (95% CI 0.34, 0.52) p < 0.00001 58% reduction | – | – | – | – |
| Yuan, 2022 [73] | Metabolic syndrome n = 5440 | XZK vs. control (PBO or routine treatment) Duration = 4 weeks to 4.5 years | – | – | – | – | MACE: RR 0.54 (95% CI 0.43, 0.66); p < 0.00001 46% reduction | – | RR 0.62 (95% CI 0.49, 0.78); p < 0.0001 38% reduction |
| Real-world retrospective cohort study | |||||||||
| Chang, 2022 [74] | No history of stroke n = 69,446 | RYR vs. LOV Duration = NR | – | – | – | – RYR 3.97/1000 PYs LOV 6.99/100 PYs 35% reduction | – | – | – |
| Study | Number of Subjects | Type of AE | n (%) | ||||
|---|---|---|---|---|---|---|---|
| N (RYR Dose; MK [Lovastatin] Dose) | Control | RYR | Control | ||||
| Placebo comparator | |||||||
| Heber, 1999 [44] | 42 (2.4 g/d; 4.8 mg/d) | 41 | Serious Total | 0 (0) 1 (2) | 0 (0) 3 (7) | ||
| Musculoskeletal chest pain | 1 (2) | 0 (0) | |||||
| Headache | 0 (0) | 1 (2) | |||||
| Pneumonia | 0 (0) | 1 (2) | |||||
| Rash/pruritus/skin allergy | 0 (0) | 1 (2) | |||||
| Lin, 2005 [47] | 37 (1.2 g/d; 11.4 mg/d) | 38 | Serious Breast carcinoma (not related to RYR) Total | 1 (3) 1 (3) 21 (57) | 0 (0) 28 (74) | ||
| Increased CPK | 1 (3) | 0 (0) | |||||
| Increased ALT | 1 (3) | 0 (0) | |||||
| Diarrhea | 0 (0) | 1 (3) | |||||
| Nausea | 0 (0) | 1 (3) | |||||
| Leukopenia | 0 (0) | 1 (3) | |||||
| LDH increase | 1 (3) | 0 (0) | |||||
| Becker, 2009 [48] a | 31 (3.6 g/d; 6 mg/d) | 31 | Total | NR | NR | ||
| Serious | NR | NR | |||||
| Myalgia | 2 (6) | 1 (3) | |||||
| Loose stools | 1 (3) | 0 (0) | |||||
| Dizziness | 1 (3) | 0 (0) | |||||
| Bogsrud, 2010 [49] | 22 (4 capsules; 7.2 mg/d) | 20 | Serious Total | 0 (0) 8 (36) | 0 (0) 1 (5) | ||
| Back pain | 1 (5) | 0 (0) | |||||
| Increased CPK | 1 (5) | 0 (0) | |||||
| Diarrhea | 2 (9) | 0 (0) | |||||
| Flatulence | 1 (5) | 1 (5) | |||||
| Crohn’s disease | 1 (5) | 0 (0) | |||||
| General discomfort | 1 (5) | 0 (0) | |||||
| Influenza | 1 (5) | 0 (0) | |||||
| Verhoeven, 2013 [51] | 31 (2 capsules/day; 10.05 mg/d) | 21 | Serious Total | NR NR | NR NR | ||
| Muscle stiffness | 2 (6) | 0 (0) | |||||
| Muscle cramps | 3 (10) | 1 (5) | |||||
| Myalgia | 4 (13) | 2 (10) | |||||
| Increased CPK | 5 (16) | 3 (14) | |||||
| Liver pain | 0 (0) | 1 (5) | |||||
| Belches | 1 (3) | 0 (0) | |||||
| Erectile dysfunction | 0 (0) | 1 (5) | |||||
| Insomnia | 1 (3) | 1 (5) | |||||
| Pruritus | 0 (0) | 1 (5) | |||||
| Moriarty, 2014 [52] b | XZK (1.2 g/d) n = 36 | XZK (2.4 g/d) n = 42 | 37 | Serious Fracture, extremity (not related to RYR) Pulmonary embolism (not related to RYR) Thyroid cancer (not related to RYR) Total | 1.2 g/d 0 (0) 0 (0) 1 (3) 17 (47) | 2.4 g/d 1 (2) 1 (2) 0 (0) 22 (52) | PBO 0 (0) 0 (0) 0 (0) 19 (51) |
| Musculoskeletal/connective tissue disorders | 4 (11) | 5 (12) | 1 (3) | ||||
| - Muscle spasm | 0 (0) | 2 (5) | 0 (0) | ||||
| - Myalgia | 1 (3) | 2 (5) | 0 (0) | ||||
| - Jaw pain | 1 (3) | 0 (0) | 0 (0) | ||||
| Investigations/laboratory abnormalities | 2 (6) | 1 (2) | 6 (16) | ||||
| - Increased CPK | 0 (0) | 0 (0) | 2 (5) | ||||
| - Increased ALT | 0 (0) | 0 (0) | 2 (5) | ||||
| - Increased AST - Increased leukocyte count | 0 (0) 2 (6) | 0 (0) 0 (0) | 2 (5) 0 (0) | ||||
| Gastrointestinal disorders | 5 (14) | 10 (24) | 10 (27) | ||||
| - Diarrhea | 2 (6) | 0 (0) | 1 (3) | ||||
| - Dyspepsia | 3 (8) | 1 (2) | 1 (3) | ||||
| - Nausea | 0 (0) | 2 (5) | 2 (5) | ||||
| - Abdominal discomfort | 0 (0) | 0 (0) | 2 (5) | ||||
| - Epigastric pain | 1 (3) | 0 (0) | 0 (0) | ||||
| Nervous system disorders | 1 (3) | 3 (7) | 3 (8) | ||||
| - Headache | 1 (3) | 2 (5) | 2 (5) | ||||
| Infections | 4 (11) | 5 (12) | 4 (11) | ||||
| - URTI | 0 (0) | 2 (5) | 3 (8) | ||||
| Rash | 1 (3) | 0 (0) | 0 (0) | ||||
| Skin flushing | 0 (0) | 0 (0) | 1 (3) | ||||
| Wang, 2019 [54] c | MK-RYR (400 mg/d; 8 mg/d) n = 23 | GABA-RYR (335 mg/d; NA) n = 23 | 23 | Serious Total Elevated creatinine Increased ALT Increased AST | 0 (0) 1 (4) 0 (0) 1 (4) 1 (4) | 0 (0) 1 (4) 0 (0) 1 (4) 1 (4) | 0 (0) 3 (13) 1 (4) 0 (0) 0 (0) |
| Poor general health | 0 (0) | 0 (0) | 1 (4) | ||||
| Anxiety | 1 (4) | 0 (0) | 0 (0) | ||||
| Skin allergy | 0 (0) | 0 (0) | 1 (4) | ||||
| Minamizuka, 2021 [55] | 10 (200 mg/d; 2 mg/d) | 8 | Serious | NR | NR | ||
| Skin rash | 0 (0) | 0 (0) | |||||
| Muscle pain | 0 (0) | 0 (0) | |||||
| Total | 0 (0) | 0 (0) | |||||
| Statin comparator | |||||||
| Xiaobin, 2007 [62] | XZK; NA | ATV; NA n = 130 overall | NA | NA | |||
| Gheith, 2008 [61] | RYR 1.2 g/d for 1 month then 600 mg/d n = 20 | FLV 20 mg/d n = 30 PBO n = 22 | NR | NR | |||
| Liu, 2011 [66] | LRRMP (350 mg/d; NA) n = 20; XZK (1.2 g/d; NA) n = 20 | LOV 20 mg/d n = 20 | Serious Total | 0 (0) NA | 1 (5) d NA | ||
| Li, 2011 [65] | XZK (1.2 g/d; NA) n = 32 | LOV 40 mg/d n = 32 | NA | NA | |||
| Halbert, 2010 [63] | RYR (4.8 g/d; 9.96 mg/d) n = 21 | PRV 40 mg/d n = 22 | Serious Total | NR NR | NR NR | ||
| Persistent myalgia only | |||||||
| - Generalized | 0 (0) | 3 (14) | |||||
| - Local | 2 (10) | 1 (5) | |||||
| - Local and generalized | 2 (10) | 4 (18) | |||||
| Persistent and intermittent myalgia | |||||||
| - Generalized | 1 (5) | 6 (27) | |||||
| - Local | 4 (19) | 3 (14) | |||||
| - Local and generalized | 5 (24) | 8 (36) | |||||
| Muscle weakness | 1 (5) | 1 (5) | |||||
| Abdominal gas, bloating | 2 (10) | 0 (0) | |||||
| Alopecia | 2 (10) | 0 (0) | |||||
| Arthralgia | 1 (5) | 1 (5) | |||||
| Back pain | 5 (24) | 6 (27) | |||||
| Diarrhea | 2 (10) | 0 (0) | |||||
| Dizziness | 0 (0) | 2 (9) | |||||
| Dyspepsia | 1 (5) | 0 | |||||
| Fatigue | 0 | 3 (14) | |||||
| Fracture, extremity | 1 (5) | 0 | |||||
| Headache | 2 (10) | 2 (9) | |||||
| Motor co-ordination decreased, left hand | 0 | 1 (5) | |||||
| Ruscica, 2014 [59] | RYR (200 mg/d; 3 mg/d) n = 30 | PRV 10 mg/d n = 30 | Serious Total | NR NR | NR NR | ||
| Marazzi, 2017 [60] | RYR (200 mg/d; 3 mg/d) n = 50 | SMV 10–20 mg/d or ATV 5–10 mg/d or RSV 5 mg/d n = 50 | Serious | NR | NR | ||
| Total | NR | NR | |||||
| Musculoskeletal discomfort | 3 (6) | 3 (6) | |||||
| Hepatobiliary disorders | 0 (0) | 0 (0) | |||||
| Gastrointestinal disorders | 2 (4) | 1 (2) | |||||
| Metabolic disorders | 0 (0) | 0 (0) | |||||
| Kou, 1997 [56] | XZK (1.2 g/d; NA) n = 53 | SMV 10 mg/d n = 55 | Severe Total | NA NA | NA NA | ||
| Chen, 2002 [57] | XZK (1.2 g/d; NA) n = NA e | SMV 10 mg/d n = NA e | Serious Total | 0 (0) 0 (0) | 0 (0) 0 (0) | ||
| Xue, 2017 [64] | RYR (1.2 g/d; NA) n = 27 | SMV 20 mg/d n = 33 | Serious Total | 0 (0) 0 (0) | 0 (0) 0 (0) | ||
| Cui, 2015 [58] | XZK 600 mg BID n = 30 | SMV 20 mg QD n = 30 SMV stopped and restarted at 20 mg QD n = 30 | NR | NR | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicero, A.F.G.; Fogacci, F.; Stoian, A.P.; Toth, P.P. Red Yeast Rice for the Improvement of Lipid Profiles in Mild-to-Moderate Hypercholesterolemia: A Narrative Review. Nutrients 2023, 15, 2288. https://doi.org/10.3390/nu15102288
Cicero AFG, Fogacci F, Stoian AP, Toth PP. Red Yeast Rice for the Improvement of Lipid Profiles in Mild-to-Moderate Hypercholesterolemia: A Narrative Review. Nutrients. 2023; 15(10):2288. https://doi.org/10.3390/nu15102288
Chicago/Turabian StyleCicero, Arrigo F. G., Federica Fogacci, Anca Pantea Stoian, and Peter P. Toth. 2023. "Red Yeast Rice for the Improvement of Lipid Profiles in Mild-to-Moderate Hypercholesterolemia: A Narrative Review" Nutrients 15, no. 10: 2288. https://doi.org/10.3390/nu15102288
APA StyleCicero, A. F. G., Fogacci, F., Stoian, A. P., & Toth, P. P. (2023). Red Yeast Rice for the Improvement of Lipid Profiles in Mild-to-Moderate Hypercholesterolemia: A Narrative Review. Nutrients, 15(10), 2288. https://doi.org/10.3390/nu15102288









