Citrin Deficiency: Clinical and Nutritional Features
Abstract
1. Introduction
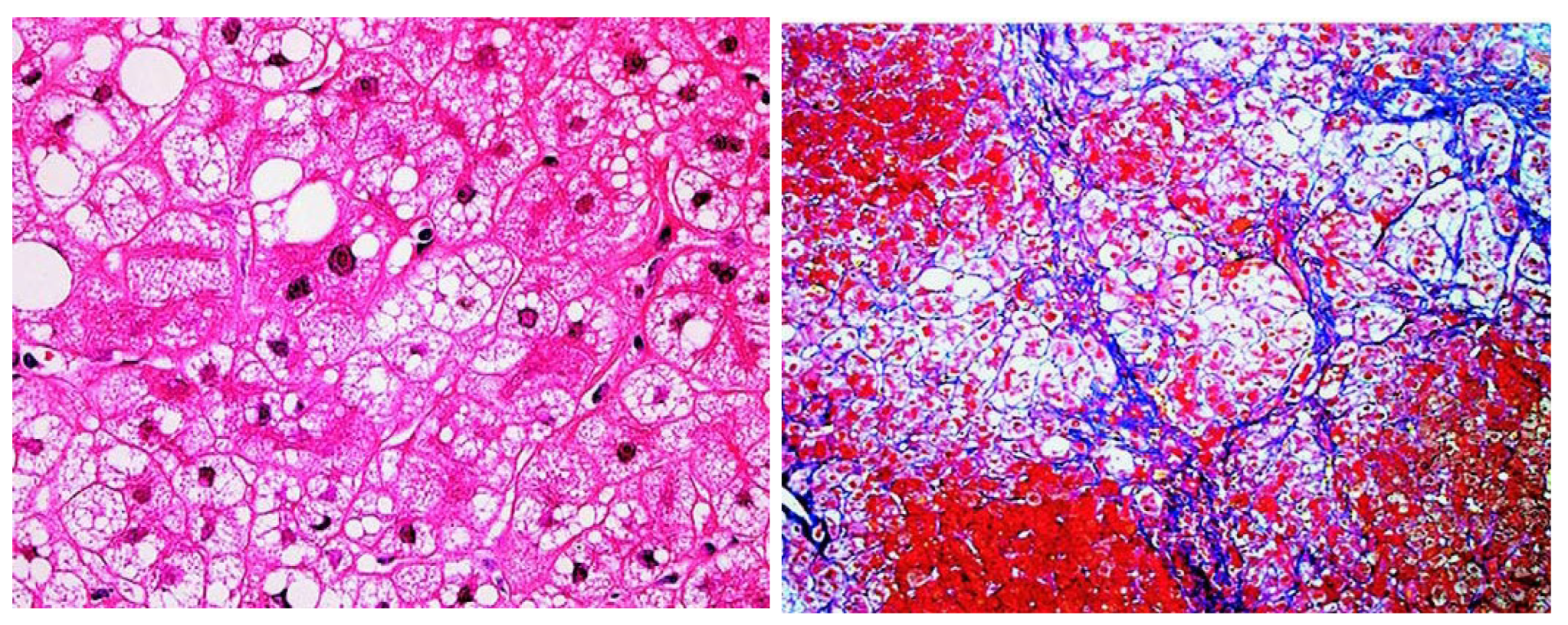
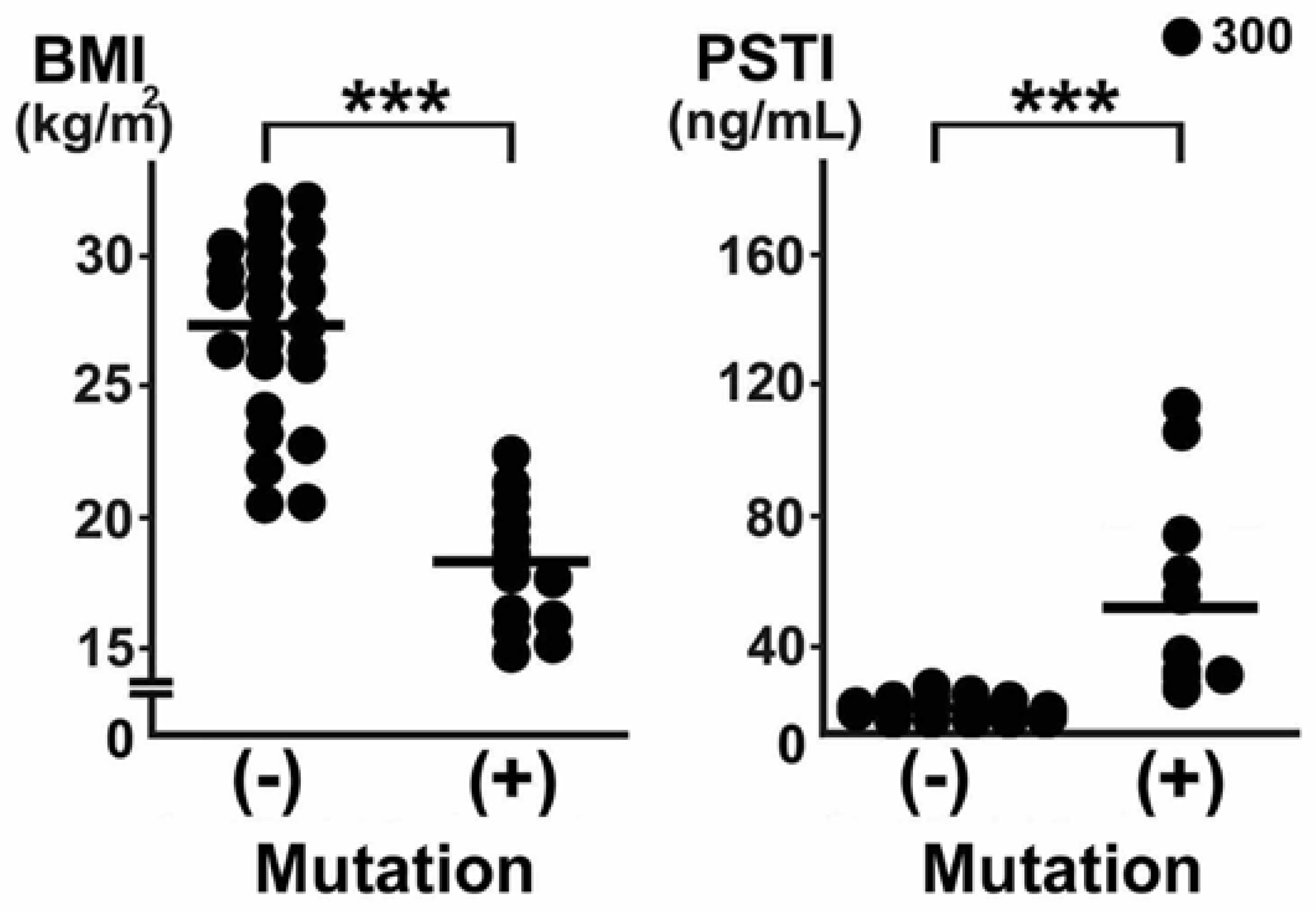
2. Clinical Characteristics of Fatty Liver Due to CD
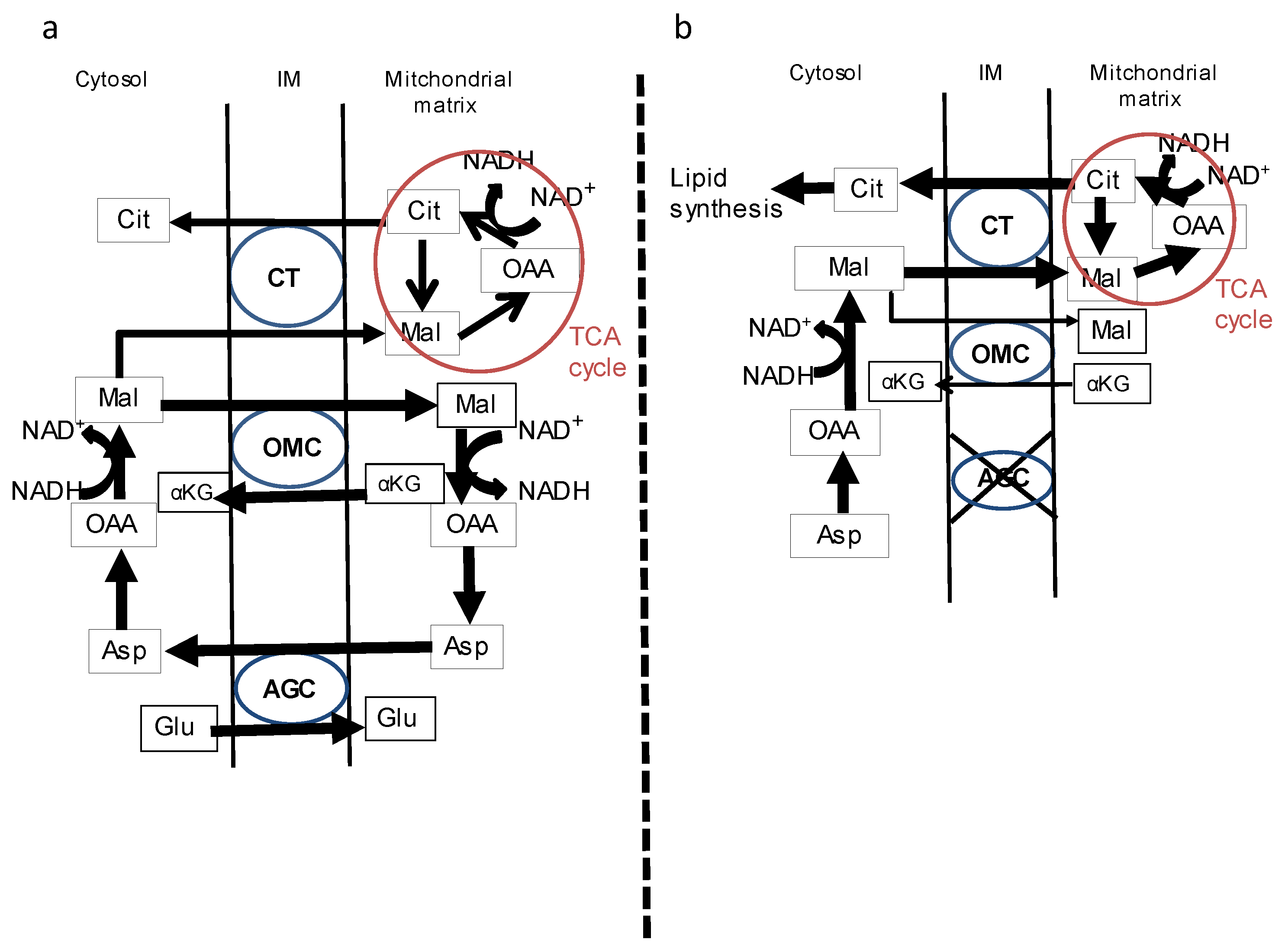
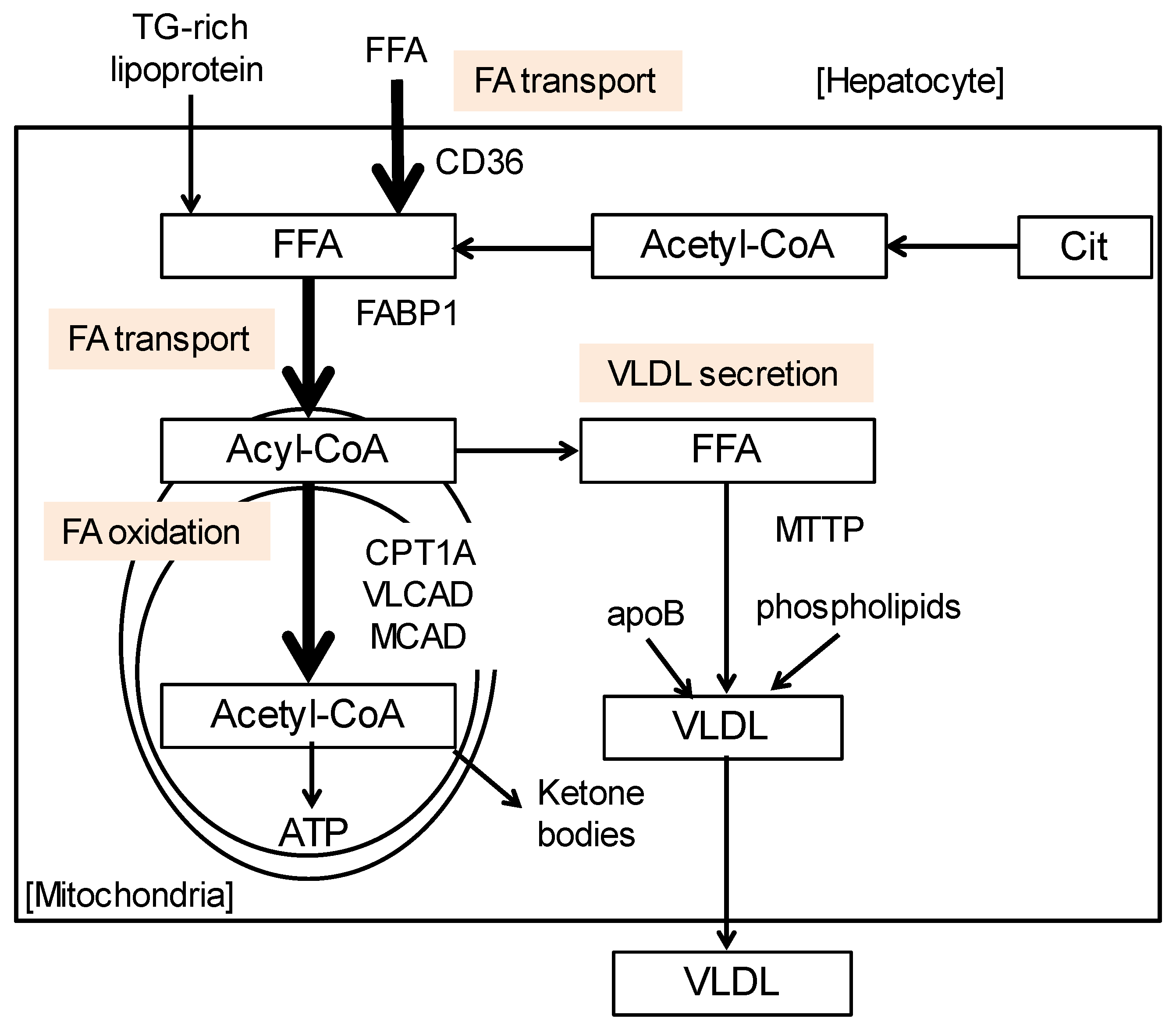
3. Possible Mechanism of Steatogenesis Due to CD
4. Treatment for CD
| Treatment | Target Disease | Concept | References |
|---|---|---|---|
| Low-carbohydrate diet | NICCD CTLN2 | Basal therapy | [18,47,48] |
| MCT + lactose-restricted formula | NICCD CTLN2 | Energy supply Correction of metabolic disorder | [53,54,55,56,57,58] |
| Sodium pyruvate | CTLN2 | Energy supply Correction of metabolic disorder | [59,60,61,62] |
| Ursodeoxycholic acid | NICCD | Hepatoprotection Anti-cholestasis | [18,63,64,65] |
| Mannitol | NICCD CTLN2 | Osmotic agent for treating brain edema due to hyperammonemia | [51] |
| Liver transplantation * | NICCD CTLN2 | Ultimate fundamental therapy | [16,66,67,68,69] |
5. Concluding Remarks: Suggestions for Clinicians and Dietitians
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Palmieri, L.; Pardo, B.; Lasorsa, F.M.; del Arco, A.; Kobayashi, K.; Iijima, M.; Runswick, M.J.; Walker, J.E.; Saheki, T.; Satrústegui, J.; et al. Citrin and aralar1 are Ca2+-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001, 20, 5060–5069. [Google Scholar] [CrossRef] [PubMed]
- Saheki, T.; Kobayashi, K.; Iijima, M.; Moriyama, M.; Yazaki, M.; Takei, Y.; Ikeda, S. Metabolic derangements in deficiency of citrin, a liver-type mitochondrial aspartate-glutamate carrier. Hepatol. Res. 2005, 33, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Saheki, T.; Kobayashi, K. Mitochondrial aspartate glutamate carrier (citrin) deficiency as the cause of adult-onset type II citrullinemia (CTLN2) and idiopathic neonatal hepatitis. J. Hum. Genet. 2002, 47, 333–341. [Google Scholar] [CrossRef]
- Kobayashi, K.; Sinasac, D.S.; Iijima, M.; Boright, A.P.; Begum, L.; Lee, J.R.; Yasuda, T.; Ikeda, S.; Hirano, R.; Terazono, H.; et al. The gene mutated in adult-onset type II citrullinemia encodes a putative mitochondrial carrier protein. Nat. Genet. 1999, 22, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Kobayashi, K.; Yasuda, T.; Nishi, I.; Iijima, M.; Nakagawa, M.; Osame, M.; Kondo, I.; Saheki, T. Screening of SLC25A13 mutations in early and late onset patients with citrin deficiency and in the Japanese population: Identification of two novel mutations and establishment of multiple DNA diagnosis methods for nine mutations. Hum. Mutat. 2002, 19, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Z.; Zhang, Z.H.; Lin, W.X.; Zhao, X.J.; Deng, M.; Ma, Y.L.; Guo, L.; Chen, F.P.; Xiao-Ling Long, X.L.; He, X.L.; et al. SLC25A13 Gene Analysis in Citrin Deficiency: Sixteen Novel Mutations in East Asian Patients, and the Mutation Distribution in a Large Pediatric Cohort in China. PLoS ONE 2013, 8, e74544. [Google Scholar] [CrossRef]
- Lin, W.X.; Zeng, H.S.; Zhang, Z.H.; Mao, M.; Zheng, Q.Q.; Zhao, S.T.; Cheng, Y.; Chen, F.P.; Wang-Rong Wen, W.R.; Song, Y.Z. Molecular diagnosis of pediatric patients with citrin deficiency in China: SLC25A13 mutation spectrum and the geographic distribution. Sci. Rep. 2016, 6, 29732. [Google Scholar] [CrossRef]
- Kobayashi, K.; Bang Lu, Y.; Xian Li, M.; Nishi, I.; Hsiao, K.J.; Choeh, K.; Yang, Y.; Hwu, W.L.; Reichardt, J.K.; Palmieri, F.; et al. Screening of nine SLC25A13 mutations: Their frequency in patients with citrin deficiency and high carrier rates in Asian populations. Mol. Genet. Metab. 2003, 80, 356–359. [Google Scholar] [CrossRef]
- Lu, Y.B.; Kobayashi, K.; Ushikai, M.; Tabata, A.; Iijima, M.; Li, M.X.; Lei, L.; Kawabe, K.; Taura, S.; Yang, Y.; et al. Frequency and distribution in East Asia of 12 mutations identified in the SLC25A13 gene of Japanese patients with citrin deficiency. J. Hum. Genet. 2005, 50, 338–346. [Google Scholar] [CrossRef]
- Zeng, Q.; Yang, Y.; Luo, J.; Xu, J.; Deng, C.; Yang, Y.; Tan, S.; Sun, S.; Li, Y.; Ou, T. Rapid Genetic Diagnosis of Citrin Deficiency by Multicolor Melting Curve Analysis. Front. Pediatr. 2021, 9, 654527. [Google Scholar] [CrossRef]
- Lin, W.X.; Yaqub, M.R.; Zhang, Z.H.; Mao, M.; Zeng, H.S.; Chen, F.P.; Li, W.M.; Cai, W.Z.; Li, Y.Q.; Tan, Z.Y.; et al. Molecular epidemiologic study of citrin deficiency by screening for four reported pathogenic SLC25A13 variants in the Shaanxi and Guangdong provinces, China. Transl. Pediatr. 2021, 10, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Fiermonte, G.; Parisi, G.; Martinelli, D.; De Leonardis, F.; Torre, G.; Pierri, C.L.; Saccari, A.; Lasorsa, F.M.; Vozza, A.; Palmieri, F.; et al. A new Caucasian case of neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD): A clinical, molecular, and functional study. Mol. Genet. Metab. 2011, 104, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Tabata, A.; Sheng, J.S.; Ushikai, M.; Song, Y.Z.; Gao, H.Z.; Lu, Y.B.; Okumura, F.; Iijima, M.; Mutoh, K.; Kishida, S.; et al. Identification of 13 novel mutations including a retrotransposal insertion in SLC25A13 gene and frequency of 30 mutations found in patients with citrin deficiency. J. Hum. Genet. 2008, 53, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Dimmock, D.; Maranda, B.; Dionisi-Vici, C.; Wang, J.; Kleppe, S.; Fiermonte, G.; Bai, R.; Hainline, B.; Hamosh, A.; O’Brien, W.E.; et al. Citrin deficiency, a perplexing global disorder. Mol. Genet. Metab. 2009, 96, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Z.; Guo, L.; Yang, Y.L.; Han, L.S.; Kobayashi, K.; Saheki, T. Failure to thrive and dyslipidemia caused by citrin deficiency: A novel clinical phenotype. Zhongguo Dang Dai Er. Ke Za Zhi 2009, 11, 328–332. [Google Scholar]
- Tamamori, A.; Okano, Y.; Ozaki, H.; Fujimoto, A.; Kajiwara, M.; Fukuda, K.; Kobayashi, K.; Saheki, T.; Tagami, Y.; Yamano, T. Neonatal intrahepatic cholestasis caused by citrin deficiency: Severe hepatic dysfunction in an infant requiring liver transplantation. Eur. J. Pediatr. 2002, 161, 609–613. [Google Scholar] [CrossRef]
- Shigeta, T.; Kasahara, M.; Kimura, T.; Fukuda, A.; Sasaki, K.; Arai, K.; Nakagawa, A.; Nakagawa, S.; Kobayashi, K.; Soneda, S.; et al. Liver transplantation for an infant with neonatal intrahepatic cholestasis caused by citrin deficiency using heterozygote living donor. Pediatr. Transpl. 2010, 14, E86–E88. [Google Scholar] [CrossRef]
- Okano, Y.; Ohura, T.; Sakamoto, O.; Inui, A. Current treatment for citrin deficiency during NICCD and adaptation/compensation stages: Strategy to prevent CTLN2. Mol. Genet. Metab. 2019, 127, 175–183. [Google Scholar] [CrossRef]
- Ito, T.; Shiraki, K.; Sekoguchi, K.; Yamanaka, T.; Sugimoto, K.; Takase, K.; Tameda, Y.; Nakano, T. Hepatocellular carcinoma associated with adult-type citrullinemia. Dig. Dis. Sci. 2000, 45, 2203–2206. [Google Scholar] [CrossRef]
- Tsai, C.W.; Yang, C.C.; Chen, H.L.; Hwu, W.L.; Wu, M.Z.; Liu, K.L.; Wu, M.S. Homozygous SLC25A13 mutation in a Taiwanese patient with adult-onset citrullinemia complicated with steatosis and hepatocellular carcinoma. J. Formos. Med. Assoc. 2006, 105, 852–856. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhu, S.; Zhang, M.; Dong, Y.; Wang, F.S. A 6-Year-Old Child with Citrin Deficiency and Advanced Hepatocellular Carcinoma. Pediatrics 2019, 143, e20181931. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhang, J.; Li, X.; Wang, H.; Feng, C.; Fang, F.; Shu, S. A Case Report: Can Citrin Deficiency Lead to Hepatocellular Carcinoma in Children? Front. Pediatr. 2019, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Hagiwara, S.; Hashizume, H.; Kanda, D.; Sato, K.; Sohara, N.; Kakizaki, S.; Takahashi, H.; Mori, M.; Kaneko, H.; et al. Adult onset type II citrullinemia as a cause of non-alcoholic steatohepatitis. J. Hepatol. 2006, 44, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Yazaki, M.; Kobayashi, K. A lean man with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2007, 5, A32. [Google Scholar] [CrossRef]
- Yazaki, M.; Hashikura, Y.; Takei, Y.; Ikegami, T.; Miyagawa, S.; Yamamoto, K.; Tokuda, T.; Kobayashi, K.; Saheki, T.; Ikeda, S. Feasibility of auxiliary partial orthotopic liver transplantation from living donors for patients with adult-onset type II citrullinemia. Liver Transpl. 2004, 10, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Yazaki, M.; Tanaka, N.; Sano, K.; Hashimoto, E.; Takei, Y.; Song, Y.Z.; Tanaka, E.; Kiyosawa, K.; Saheki, T.; et al. Citrin deficiency as a cause of chronic liver disorder mimicking non-alcoholic fatty liver disease. J. Hepatol. 2008, 49, 810–820. [Google Scholar] [CrossRef]
- Hirota, M.; Ohmuraya, M.; Baba, H. Genetic background of pancreatitis. Postgrad. Med. J. 2006, 82, 775–778. [Google Scholar] [CrossRef]
- Ikeda, S.; Kawa, S.; Takei, Y.; Yamamoto, K.; Shimojo, H.; Tabata, K.; Kobayashi, K.; Saheki, T. Chronic pancreatitis associated with adult-onset type II citrullinemia: Clinical and pathologic findings. Ann. Intern. Med. 2004, 141, W109–W110. [Google Scholar] [CrossRef]
- Tanaka, N.; Horiuchi, A.; Yokoyama, T.; Kawa, S.; Kiyosawa, K. Pancreatic exocrine insufficiency: A rare cause of nonalcoholic steatohepatitis. Am. J. Gastroenterol. 2008, 103, 245–246. [Google Scholar] [CrossRef]
- Saheki, T.; Moriyama, M.; Funahashi, A.; Kuroda, E. AGC2 (Citrin) Deficiency-From Recognition of the Disease till Construction of Therapeutic Procedures. Biomolecules 2020, 10, 1100. [Google Scholar] [CrossRef]
- Saheki, T.; Iijima, M.; Li, M.X.; Kobayashi, K.; Horiuchi, M.; Ushikai, M.; Okumura, F.; Meng, X.J.; Inoue, I.; Tajima, A.; et al. Citrin/mitochondrial glycerol 3-phosphate double-knockout mice recapitulate features of human citrin deficiency. J. Biol. Chem. 2007, 282, 25041–25052. [Google Scholar] [CrossRef] [PubMed]
- Inui, Y.; Kuwajima, M.; Kawata, S.; Fukuda, K.; Maeda, Y.; Igura, T.; Kono, N.; Tarui, S.; Matsuzawa, Y. Impaired ketogenesis in patients with adult-type citrullinemia. Gastroenterology 1994, 107, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Peters, J.M.; Iritani, N.; Nakajima, T.; Furihata, K.; Hashimoto, T.; Gonzalez, F.J. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα). J. Biol. Chem. 1998, 273, 5678–5684. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Moriya, K.; Kiyosawa, K.; Koike, K.; Gonzalez, F.J.; Aoyama, T. PPARα activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. J. Clin. Investig. 2008, 118, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Badizadegan, K.; Perez-Atayde, A.R. Focal glycogenosis of the liver in disorders of ureagenesis: Its occurrence and diagnostic significance. Hepatology 1997, 26, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Aoyama, T.; Kimura, S.; Gonzalez, F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol. Ther. 2017, 179, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Kimura, T.; Fujimori, N.; Nagaya, T.; Komatsu, M.; Tanaka, E. Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World J. Gastroenterol. 2019, 25, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Kimura, T.; Yazaki, M.; Tanaka, N.; Yang, Y.; Nakajima, T.; Horiuchi, A.; Fang, Z.Z.; Joshita, S.; Matsumoto, A.; et al. Steatogenesis in adult-onset type II citrullinemia is associated with down-regulation of PPARα. Biochim. Biophys. Acta 2015, 1852, 473–481. [Google Scholar] [CrossRef]
- Koek, G.H.; Liedorp, P.R.; Bast, A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin. Chim. Acta 2011, 412, 1297–1305. [Google Scholar] [CrossRef]
- Cui, R.; Iso, H.; Pi, J.; Kumagai, Y.; Yamagishi, K.; Tanigawa, T.; Shimamoto, T. Metabolic syndrome and urinary cGMP excretion in general population. Atherosclerosis 2007, 190, 423–428. [Google Scholar] [CrossRef]
- Pignitter, M.; Gorren, A.C.F.; Nedeianu, S.; Schmidt, K.; Mayer, B. Inefficient spin trapping of superoxide in the presence of nitric-oxide: Implications for studies on nitric-oxide synthase uncoupling. Free Radic. Biol. Med. 2006, 41, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Erez, A.; Nagamani, S.C.; Lee, B. Argininosuccinate lyase deficiency-argininosuccinic aciduria and beyond. Am. J. Med. Genet. C Semin. Med. Genet. 2011, 157C, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Sumida, Y.; Niki, E.; Naito, Y.; Yoshikawa, T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radic. Res. 2013, 47, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Ishida, N.; Uchiyama, K.; Yamaguchi, K.; Itoh, Y.; Shichiri, M.; Yoshida, Y.; Hagihara, Y.; Naito, Y.; Yoshikawa, T.; et al. Fatty liver induced by free radicals and lipid peroxidation. Free Radic. Res. 2012, 46, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Saheki, T.; Kobayashi, K.; Terashi, M.; Ohura, T.; Yanagawa, Y.; Okano, Y.; Hattori, T.; Fujimoto, H.; Mutoh, K.; Kizaki, Z.; et al. Reduced carbohydrate intake in citrin-deficient subjects. J. Inherit. Metab. Dis. 2008, 31, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Ashmore, C.; Batzios, S.; Daly, A.; Dawson, C.; Dixon, M.; Evans, S.; Green, D.; Gribben, J.; Hunjan, I.; et al. Dietary Management, Clinical Status and Outcome of Patients with Citrin Deficiency in the UK. Nutrients 2020, 12, 3313. [Google Scholar] [CrossRef]
- Okamoto, M.; Okano, Y.; Okano, M.; Yazaki, M.; Inui, A.; Ohura, T.; Murayama, K.; Watanabe, Y.; Tokuhara, D.; Takeshima, Y. Food Preferences of Patients with Citrin Deficiency. Nutrients 2021, 13, 3123. [Google Scholar] [CrossRef]
- Okano, Y.; Okamoto, M.; Yazaki, M.; Inui, A.; Ohura, T.; Murayama, K.; Watanabe, Y.; Tokuhara, D.; Takeshima, Y. Analysis of daily energy, protein, fat, and carbohydrate intake in citrin-deficient patients: Towards prevention of adult-onset type II citrullinemia. Mol. Genet. Metab. 2021, 133, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Z.; Deng, M.; Chen, F.P.; Wen, F.; Guo, L.; Cao, S.L.; Gong, J.; Xu, H.; Jiang, G.Y.; Zhong, L.; et al. Genotypic and phenotypic features of citrin deficiency: Five-year experience in a Chinese pediatric center. Int. J. Mol. Med. 2011, 28, 33–40. [Google Scholar] [CrossRef]
- Otsuka, H.; Sasai, H.; Abdelkreem, E.; Kawamoto, N.; Kawamoto, M.; Kamiya, T.; Tanimoto, Y.; Kikuchi, A.; Kure, S.; Numakura, C.; et al. Effectiveness of Medium-Chain Triglyceride Oil Therapy in Two Japanese Citrin-Deficient Siblings: Evaluation Using Oral Glucose Tolerance Tests. Tohoku J. Exp. Med. 2016, 240, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, M.; Takei, Y.; Kobayashi, K.; Saheki, T.; Ikeda, S. Risk of worsened encephalopathy after intravenous glycerol therapy in patients with adult-onset type II citrullinemia (CTLN2). Intern. Med. 2005, 44, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kagawa, T.; Kobayashi, K.; Hirabayashi, H.; Yui, M.; Begum, L.; Mine, T.; Takagi, S.; Saheki, T.; Shinohara, Y. A case of adult-onset type II citrullinemia: Deterioration of clinical course after infusion of hyperosmotic and high sugar solutions. Med. Sci. Monit. 2006, 12, CS13-5. [Google Scholar] [PubMed]
- Hayasaka, K.; Numakura, C.; Toyota, K.; Kimura, T. Treatment with lactose (galactose)-restricted and medium-chain triglyceride-supplemented formula for neonatal intrahepatic cholestasis caused by citrin deficiency. JIMD Rep. 2012, 2, 37–44. [Google Scholar]
- Hayasaka, K.; Numakura, C.; Toyota, K.; Kakizaki, S.; Watanabe, H.; Haga, H.; Takahashi, H.; Takahashi, Y.; Kaneko, M.; Yamakawa, M.; et al. Medium-chain triglyceride supplementation under a low-carbohydrate formula is a promising therapy for adult-onset type II citrullinemia. Mol. Genet. Metab. Rep. 2014, 1, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, K. Metabolic basis and treatment of citrin deficiency. J. Inherit Metab Dis 2021, 44, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Abuduxikuer, K.; Chen, R.; Wang, Z.L.; Wang, J.S. Risk factors associated with mortality in neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD) and clinical implications. BMC Pediatr. 2019, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, K.; Numakura, C. Adult-onset type II citrullinemia: Current insights and therapy. Appl. Clin. Genet. 2018, 11, 163–170. [Google Scholar] [CrossRef]
- Hayasaka, K.; Numakura, C.; Yamakawa, M.; Mitsui, T.; Watanabe, H.; Haga, H.; Yazaki, M.; Ohira, H.; Ochiai, Y.; Tahara, T.; et al. Medium-chain triglycerides supplement therapy with a low-carbohydrate formula can supply energy and enhance ammonia detoxification in the hepatocytes of patients with adult-onset type II citrullinemia. J. Inherit. Metab. Dis. 2018, 41, 777–784. [Google Scholar] [CrossRef]
- Mutoh, K.; Kurokawa, K.; Kobayashi, K.; Saheki, T. Treatment of a citrin-deficient patient at the early stage of adult-onset type II citrullinaemia with arginine and sodium pyruvate. I Inherit. Metab. Dis. 2008, 31 (Suppl. S2), S343–S347. [Google Scholar] [CrossRef]
- Yazaki, M.; Kinoshita, M.; Ogawa, S.; Fujimi, S.; Matsushima, A.; Hineno, A.; Tazawa, K.; Fukushima, K.; Kimura, R.; Yanagida, M.; et al. A 73-year-old patient with adult-onset type II citrullinemia successfully treated by sodium pyruvate and arginine. Clin. Neurol. Neurosurg. 2013, 115, 1542–1545. [Google Scholar] [CrossRef]
- Kogure, T.; Kondo, Y.; Kakazu, E.; Ninomiya, M.; Kimura, O.; Kobayashi, N.; Shimosegawa, T. Three cases of adult-onset type II citrullinemia treated with different therapies: Efficacy of sodium pyruvate and low-carbohydrate diet. Hepatol. Res. 2014, 44, 707–712. [Google Scholar] [PubMed]
- Yazaki, M.; Ikeda, S.; Kobayashi, K.; Saheki, T. Therapeutic approaches for patients with adult-onset type II citrullinemia (CTLN2): Effectiveness of treatment with low-carbohydrate diet and sodium pyruvate. Rinsho Shinkeigaku 2010, 50, 844–847. [Google Scholar] [PubMed]
- Dennery, P.A. Pharmacological interventions for the treatment of neonatal jaundice. Semin. Neonatal. 2002, 7, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Gharehbaghi, M.M.; Sani, A.M.; Refeey, M. Evaluating the effects of different doses of ursodeoxycholic acid on neonatal jaundice. Turk. J. Pediatr. 2020, 62, 424–430. [Google Scholar]
- Cuperus, F.J.; Hafkamp, A.M.; Havinga, R.; Vitek, L.; Zelenka, J.; Tiribelli, C.; Ostrow, J.D.; Verkade, H.J. Effective treatment of unconjugated hyperbilirubinemia with oral bile salts in Gunn rats. Gastroenterology 2009, 136, 673–682.e1. [Google Scholar]
- Zhang, M.; Gong, J.; Wang, J. Citrin deficiency presenting as acute liver failure in an eight-month-old infant. World J. Gastroenterol. 2015, 21, 7331–7334. [Google Scholar] [CrossRef] [PubMed]
- Kimura, N.; Kubo, N.; Narumi, S.; Toyoki, Y.; Ishido, K.; Kudo, D.; Umehara, M.; Yakoshi, Y.; Hakamada, K. Liver transplantation versus conservative treatment for adult-onset type II citrullinemia: Our experience and a review of the literature. Transpl. Proc. 2013, 45, 3432–3437. [Google Scholar]
- Maharaj, R.; Kota, V.; Singh, B.; Kapoor, D.; Nageswara Rao, P.B.; Moode, J.; Dekate, J.; Nathani, P. Living Donor Liver Transplantation in a Paediatric Patient with Citrullinaemia Type 2. J. Clin. Exp. Hepatol. 2020, 10, 525–528. [Google Scholar] [CrossRef]
- Yazaki, M.; Hineno, A.; Matsushima, A.; Ozawa, K.; Kishida, D.; Tazawa, K.; Fukushima, K.; Urata, K.; Ikegami, T.; Miyagawa, S.; et al. First two cases of adult-onset type II citrullinemia successfully treated by deceased-donor liver transplantation in Japan. Hepatol. Res. 2012, 42, 934–939. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, T.; Gonzalez, F.J.; Tanaka, N. PPARs as Metabolic Regulators in the Liver: Lessons from Liver-Specific PPAR-Null Mice. Int. J. Mol. Sci. 2020, 21, 2061. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komatsu, M.; Tanaka, N.; Kimura, T.; Yazaki, M. Citrin Deficiency: Clinical and Nutritional Features. Nutrients 2023, 15, 2284. https://doi.org/10.3390/nu15102284
Komatsu M, Tanaka N, Kimura T, Yazaki M. Citrin Deficiency: Clinical and Nutritional Features. Nutrients. 2023; 15(10):2284. https://doi.org/10.3390/nu15102284
Chicago/Turabian StyleKomatsu, Michiharu, Naoki Tanaka, Takefumi Kimura, and Masahide Yazaki. 2023. "Citrin Deficiency: Clinical and Nutritional Features" Nutrients 15, no. 10: 2284. https://doi.org/10.3390/nu15102284
APA StyleKomatsu, M., Tanaka, N., Kimura, T., & Yazaki, M. (2023). Citrin Deficiency: Clinical and Nutritional Features. Nutrients, 15(10), 2284. https://doi.org/10.3390/nu15102284







