Antioxidant Nutraceutical Strategies in the Prevention of Oxidative Stress Related Eye Diseases
Abstract
1. Introduction
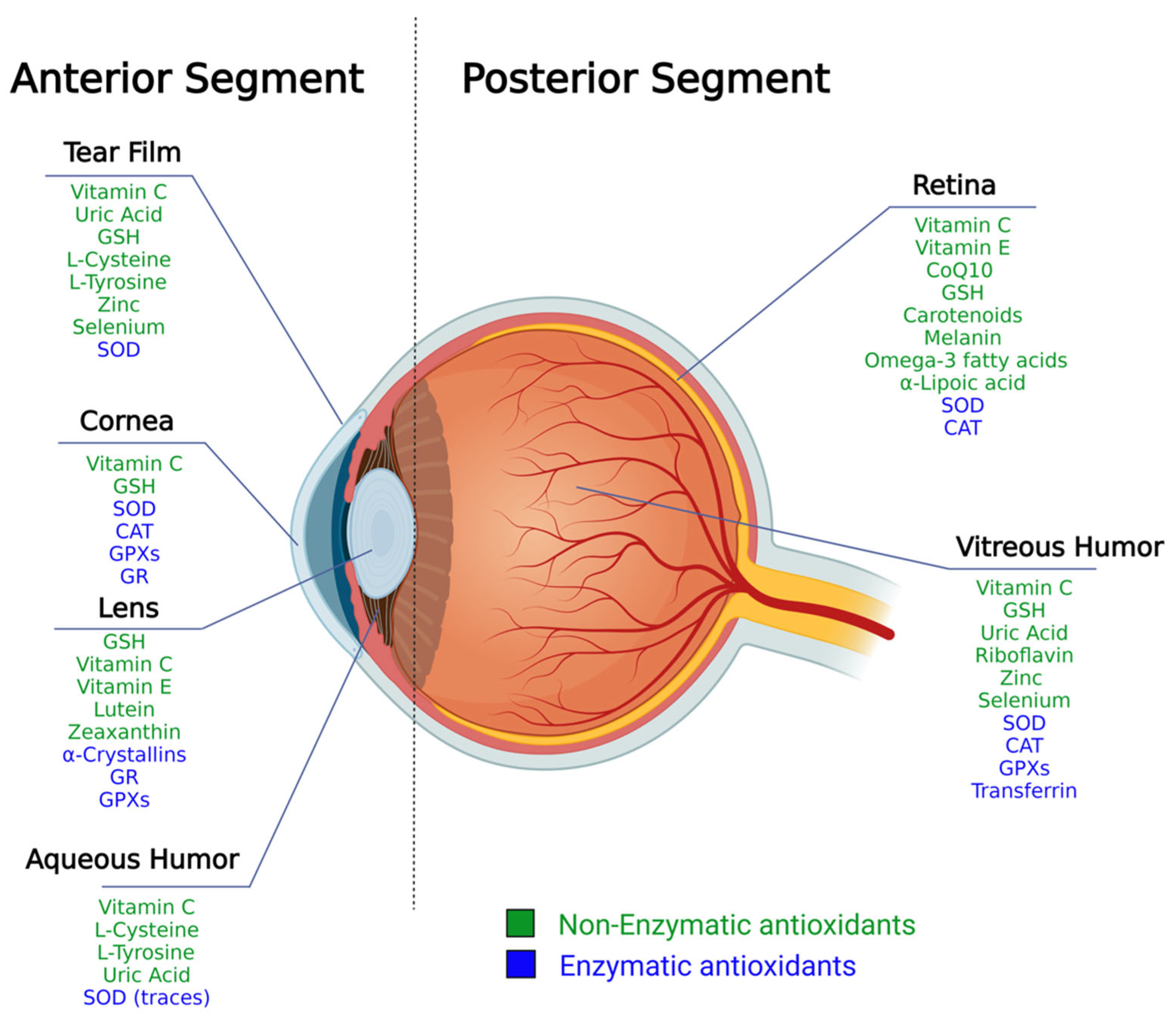
2. The Structure of the Eye and Ocular Antioxidant Defense System
2.1. Antioxidants Arsenal in the Anterior Segment
2.2. Antioxidants Arsenal in the Posterior Segment
3. Oxidative Stress and Eye Pathologies
4. Nutraceutical Antioxidants for the Ophthalmic Field
- (i)
- Exogenous plant-derived antioxidants: they are defined as “essential” nutrients, as they are not synthesized by the human body and, thus, can be obtained exclusively through exogenous introduction into the body (usually nutrition). We sub-divided them by their chemical class:
- Polyphenols.
- Carotenoids.
- (ii)
- Water-soluble promoters of the endogenous antioxidant system: molecules either produced or not by the human organism, showing direct antioxidant activity and/or able to boost the endogenous antioxidant system.
- (iii)
- Lipophilic antioxidants: lipophilic molecules promoting the defence of cellular membranes.
5. Exogenous Plant-Derived Antioxidants
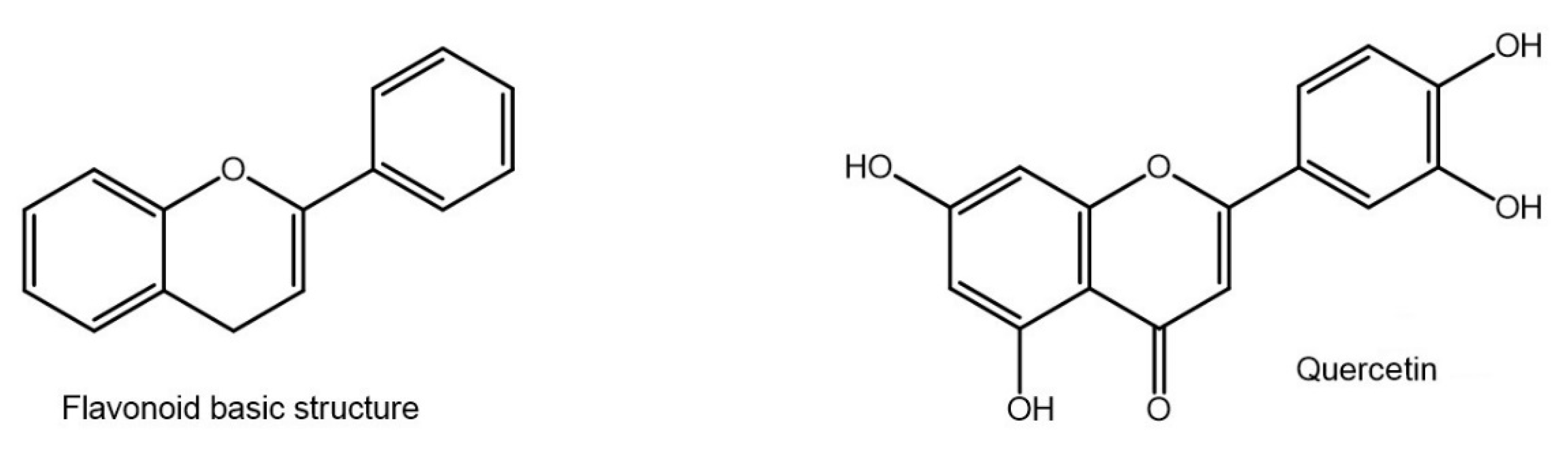
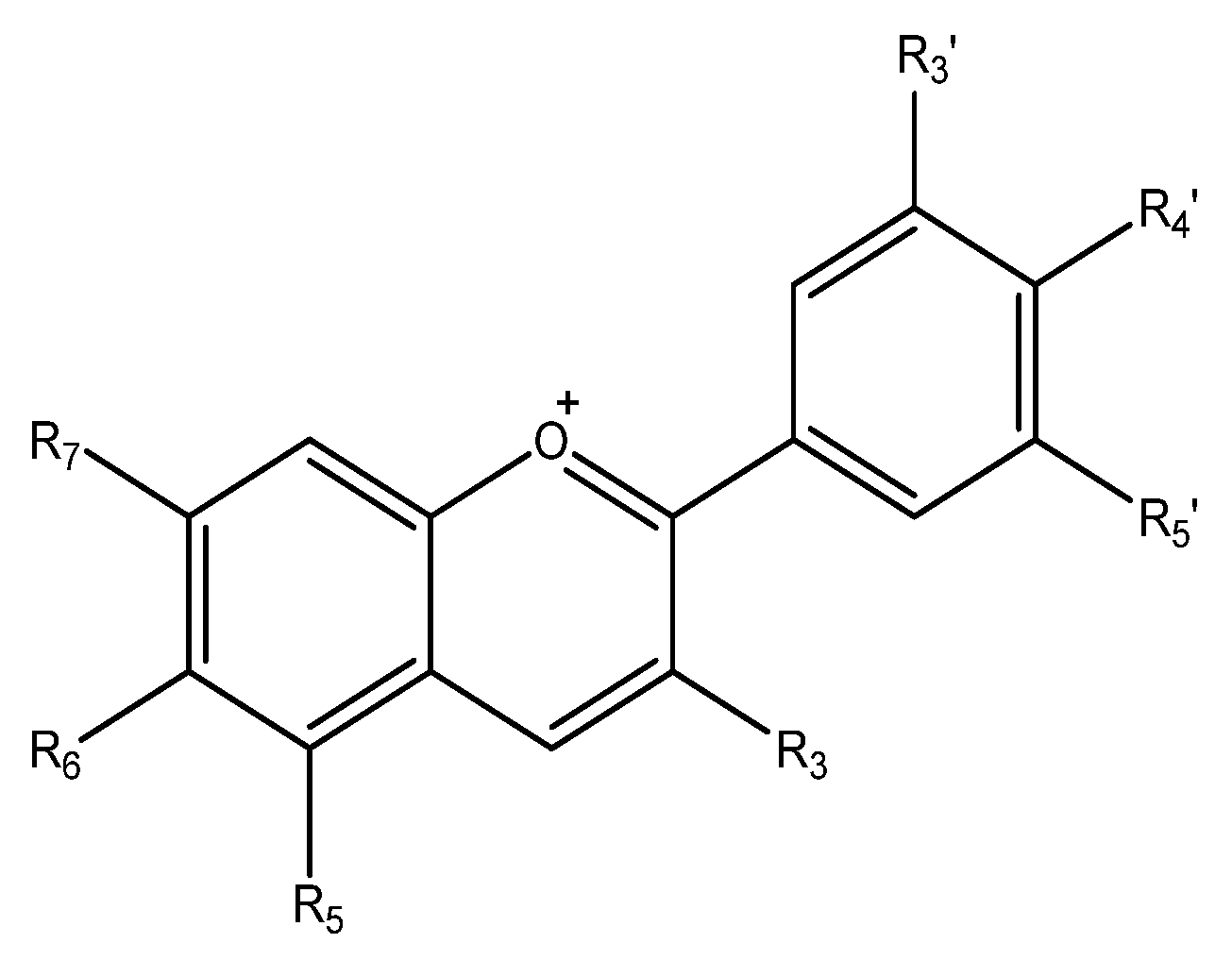
5.1. Flavonoids
5.2. Catechins
5.3. Anthocyanins
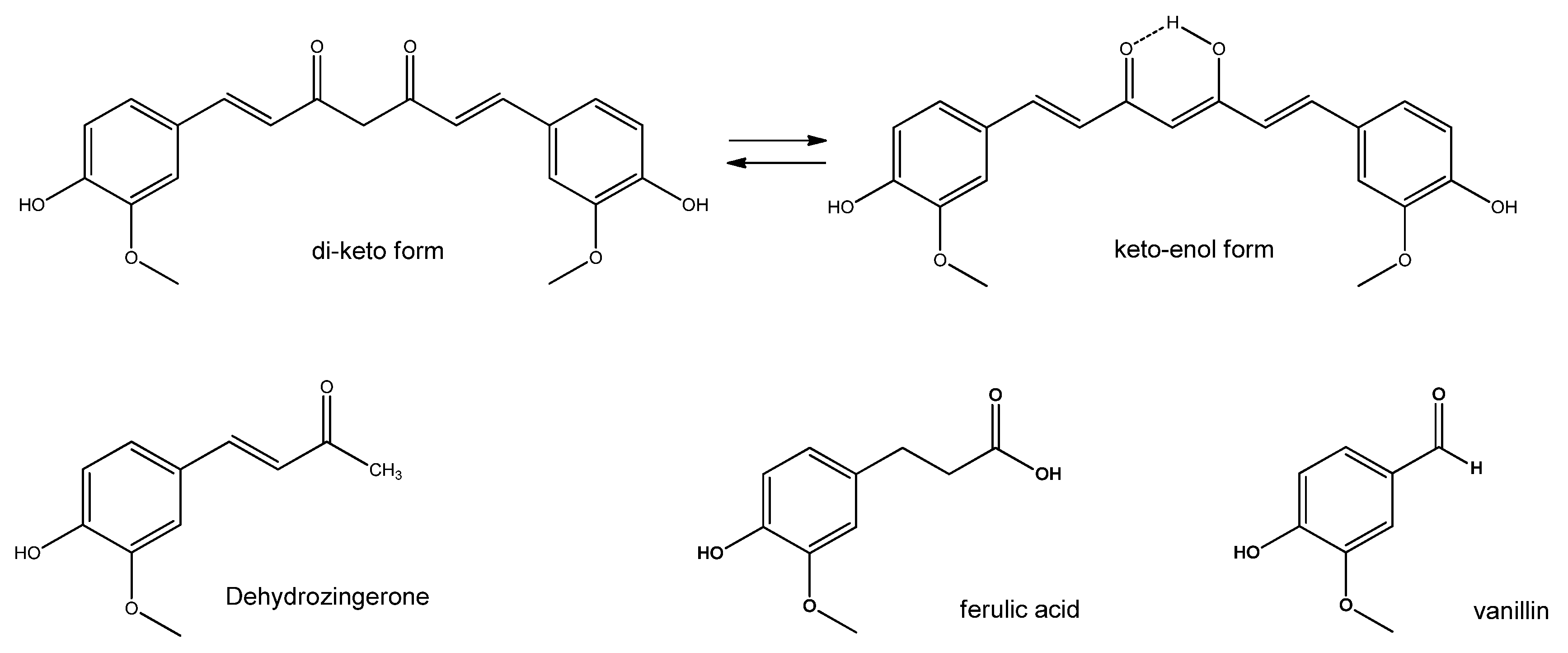
5.4. Curcumin
5.5. Resveratrol
5.6. Carotenoids
6. Water-Soluble Promoters of the Endogenous Antioxidant System
6.1. Zinc and Selenium
6.2. Ascorbic Acid
6.3. N-Acetyl Cysteine and Other Cysteine Derivatives
6.4. Riboflavin
7. Defense of Cellular Membranes with Lipophilic Antioxidants
7.1. Vitamin E
7.2. Omega-3 Fatty Acids
7.3. Coenzyme Q10
7.4. Alpha Lipoic Acid
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Ginter, E.; Simko, V.; Panakova, V. Antioxidants in health and disease. Bratisl. Lek. Listy 2014, 115, 603–606. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Willoughby, C.E.; Ponzin, D.; Ferrari, S.; Lobo, A.; Landau, K.; Omidi, Y. Anatomy and physiology of the human eye: Effects of mucopolysaccharidoses disease on structure and function—A review. Clin. Exp. Ophthalmol. 2010, 38, 2–11. [Google Scholar] [CrossRef]
- Aragona, P.; Rolando, M. Towards a dynamic customised therapy for ocular surface dysfunctions. Br. J. Ophthalmol. 2013, 97, 955–960. [Google Scholar] [CrossRef]
- Cancarini, A.; Fostinelli, J.; Napoli, L.; Gilberti, M.E.; Apostoli, P.; Semeraro, F. Trace elements and diabetes: Assessment of levels in tears and serum. Exp. Eye Res. 2017, 154, 47–52. [Google Scholar] [CrossRef]
- Barsouk, A.; Fraiture, B.; Peele, K.; Dalai, M.; Nebes, V.; Stolarski, C.; Kennerdell, J.S.; Jani, B.; Wall, J.R. Levels of manganese superoxide dismutase activity are increased in tears from patients with thyroid-associated ophthalmopathy. Orbit 1996, 15, 205–211. [Google Scholar] [CrossRef]
- Chen, Y.; Mehta, G.; Vasiliou, V. Antioxidant defenses in the ocular surface. Ocul. Surf. 2009, 7, 176–185. [Google Scholar] [CrossRef]
- Meister, A. Glutathione metabolism. Methods Enzymol. 1995, 251, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Bassnett, S.; Shi, Y.; Vrensen, G.F. Biological glass: Structural determinants of eye lens transparency. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2011, 366, 1250–1264. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Kim, J.-Y.; Martis, R.M.; Donaldson, P.J.; Lim, J.C. Characterisation of Glutathione Export from Human Donor Lenses. Transl. Vis. Sci. Technol. 2020, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Richer, S.P.; Rose, R.C. Water soluble antioxidants in mammalian aqueous humor: Interaction with UV B and hydrogen peroxide. Vis. Res. 1998, 38, 2881–2888. [Google Scholar] [CrossRef]
- Behndig, A.; Svensson, B.; Marklund, S.L.; Karlsson, K. Superoxide dismutase isoenzymes in the human eye. Investig. Ophthalmol. Vis. Sci. 1998, 39, 471–475. [Google Scholar]
- Sasaki, M.; Yuki, K.; Kurihara, T.; Miyake, S.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K.; Ozawa, Y. Biological role of lutein in the light-induced retinal degeneration. J. Nutr. Biochem. 2012, 23, 423–429. [Google Scholar] [CrossRef]
- Ozawa, Y. Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox Biol. 2020, 37, 101779. [Google Scholar] [CrossRef]
- Yeum, K.J.; Shang, F.M.; Schalch, W.M.; Russell, R.M.; Taylor, A. Fat-soluble nutrient concentrations in different layers of human cataractous lens. Curr. Eye Res. 1999, 19, 502–505. [Google Scholar] [CrossRef]
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Roberts, J.E.; Dennison, J. The Photobiology of Lutein and Zeaxanthin in the Eye. J. Ophthalmol. 2015, 2015, 687173. [Google Scholar] [CrossRef]
- Lim, J.C.; Caballero Arredondo, M.; Braakhuis, A.J.; Donaldson, P.J. Vitamin C and the Lens: New Insights into Delaying the Onset of Cataract. Nutrients 2020, 12, 3142. [Google Scholar] [CrossRef]
- Smith, A.R.; Shenvi, S.V.; Widlansky, M.; Suh, J.H.; Hagen, T.M. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr. Med. Chem. 2004, 11, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.J.; Negi, D.S.; Short, S.M.; van Kuijk, F.J.; Dratz, E.A.; Thomas, D.W. Vitamin E distribution in ocular tissues following long-term dietary depletion and supplementation as determined by microdissection and gas chromatography-mass spectrometry. Exp. Eye Res. 1988, 47, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Saenz de Viteri, M.; Hernandez, M.; Bilbao-Malavé, V.; Fernandez-Robredo, P.; González-Zamora, J.; Garcia-Garcia, L.; Ispizua, N.; Recalde, S.; Garcia-Layana, A. A Higher Proportion of Eicosapentaenoic Acid (EPA) When Combined with Docosahexaenoic Acid (DHA) in Omega-3 Dietary Supplements Provides Higher Antioxidant Effects in Human Retinal Cells. Antioxidants 2020, 9, 828. [Google Scholar] [CrossRef] [PubMed]
- Pietras-Baczewska, A.; Nowomiejska, K.; Sztanke, M.; Toro, M.; Rejdak, R. Antioxidants in the retina and vitreous—current state of knowledge. Ophthalmol. J. 2020, 5, 81–86. [Google Scholar] [CrossRef]
- Gipson, I.K. The ocular surface: The challenge to enable and protect vision: The Friedenwald lecture. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4390–4391. [Google Scholar] [CrossRef] [PubMed]
- Barbazetto, I.A.; Liang, J.; Chang, S.; Zheng, L.; Spector, A.; Dillon, J.P. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp. Eye Res. 2004, 78, 917–924. [Google Scholar] [CrossRef]
- Slingsby, C.; Wistow, G.J.; Clark, A.R. Evolution of crystallins for a role in the vertebrate eye lens. Protein Sci. A Publ. Protein Soc. 2013, 22, 367–380. [Google Scholar] [CrossRef]
- Schafheimer, N.; Wang, Z.; Schey, K.; King, J. Tyrosine/cysteine cluster sensitizing human γD-crystallin to ultraviolet radiation-induced photoaggregation in vitro. Biochemistry 2014, 53, 979–990. [Google Scholar] [CrossRef]
- Schafheimer, N.; King, J. Tryptophan cluster protects human γD-crystallin from ultraviolet radiation-induced photoaggregation in vitro. Photochem. Photobiol. 2013, 89, 1106–1115. [Google Scholar] [CrossRef]
- Chen, J.; Callis, P.R.; King, J. Mechanism of the very efficient quenching of tryptophan fluorescence in human gamma D- and gamma S-crystallins: The gamma-crystallin fold may have evolved to protect tryptophan residues from ultraviolet photodamage. Biochemistry 2009, 48, 3708–3716. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Truscott, R.J.W. Photo-oxidation of proteins and its role in cataractogenesis. J. Photochem. Photobiol. B Biol. 2001, 63, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Picciani, R.G.; Lee, R.K.; Bhattacharya, S.K. Aqueous humor dynamics: A review. Open Ophthalmol. J. 2010, 4, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Y.; Cringle, S.J. Oxygen distribution and consumption within the retina in vascularised and avascular retinas and in animal models of retinal disease. Prog. Retin. Eye Res. 2001, 20, 175–208. [Google Scholar] [CrossRef]
- Hu, D.N.; Simon, J.D.; Sarna, T. Role of ocular melanin in ophthalmic physiology and pathology. Photochem. Photobiol. 2008, 84, 639–644. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Parish, C.A.; Hashimoto, M.; Nakanishi, K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2988–2995. [Google Scholar]
- Tram, N.K.; Swindle-Reilly, K.E. Rheological Properties and Age-Related Changes of the Human Vitreous Humor. Front. Bioeng. Biotechnol. 2018, 6, 199. [Google Scholar] [CrossRef]
- Ankamah, E.; Sebag, J.; Ng, E.; Nolan, J.M. Vitreous Antioxidants, Degeneration, and Vitreo-Retinopathy: Exploring the Links. Antioxidants 2019, 9, 7. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc is an Antioxidant and Anti-Inflammatory Agent: Its Role in Human Health. Front. Nutr. 2014, 1, 14. [Google Scholar] [CrossRef]
- Goodman, D.; Ness, S. The Role of Oxidative Stress in the Aging Eye. Life 2023, 13, 837. [Google Scholar] [CrossRef]
- Dogru, M.; Kojima, T.; Simsek, C.; Tsubota, K. Potential Role of Oxidative Stress in Ocular Surface Inflammation and Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2018, 59, Des163–Des168. [Google Scholar] [CrossRef] [PubMed]
- Abokyi, S.; To, C.H.; Lam, T.T.; Tse, D.Y. Central Role of Oxidative Stress in Age-Related Macular Degeneration: Evidence from a Review of the Molecular Mechanisms and Animal Models. Oxid. Med. Cell. Longev. 2020, 2020, 7901270. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, M.; Li, X.; Shao, Y. Aging and diabetic retinopathy: Inherently intertwined pathophysiological processes. Exp. Gerontol. 2023, 175, 112138. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Sperduto, R.D.; Sangiovanni, J.P.; Kurinij, N.; Davis, M.D. Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology 2013, 120, 1604–1611.e1604. [Google Scholar] [CrossRef] [PubMed]
- Clemons, T.E.; Kurinij, N.; Sperduto, R.D. Associations of mortality with ocular disorders and an intervention of high-dose antioxidants and zinc in the Age-Related Eye Disease Study: AREDS Report No. 13. Arch. Ophthalmol. 2004, 122, 716–726. [Google Scholar] [CrossRef]
- The Age-Related Eye Disease Study 2 (AREDS2) Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L. The Age-Related Eye Disease Study 2 (AREDS2): Study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012, 119, 2282–2289. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Oh, H.N.; Kim, C.E.; Lee, J.H.; Yang, J.W. Effects of Quercetin in a Mouse Model of Experimental Dry Eye. Cornea 2015, 34, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Abengózar-Vela, A.; Schaumburg, C.S.; Stern, M.E.; Calonge, M.; Enríquez-de-Salamanca, A.; González-García, M.J. Topical Quercetin and Resveratrol Protect the Ocular Surface in Experimental Dry Eye Disease. Ocul. Immunol. Inflamm. 2019, 27, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Beyer-Mears, A.; Farnsworth, P.N. Diminished sugar cataractogenesis by quercetin. Exp. Eye Res. 1979, 28, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.; McLauchlan, W.R.; Williamson, G. Quercetin inhibits hydrogen peroxide-induced oxidation of the rat lens. Free Radic. Biol. Med. 1999, 26, 639–645. [Google Scholar] [CrossRef]
- Cornish, K.M.; Williamson, G.; Sanderson, J. Quercetin metabolism in the lens: Role in inhibition of hydrogen peroxide induced cataract. Free Radic. Biol. Med. 2002, 33, 63–70. [Google Scholar] [CrossRef]
- Lija, Y.; Biju, P.G.; Reeni, A.; Cibin, T.R.; Sahasranamam, V.; Abraham, A. Modulation of selenite cataract by the flavonoid fraction of Emilia sonchifolia in experimental animal models. Phytother. Res. PTR 2006, 20, 1091–1095. [Google Scholar] [CrossRef]
- Isai, M.; Sakthivel, M.; Ramesh, E.; Thomas, P.A.; Geraldine, P. Prevention of selenite-induced cataractogenesis by rutin in Wistar rats. Mol. Vis. 2009, 15, 2570–2577. [Google Scholar]
- Stefek, M. Natural flavonoids as potential multifunctional agents in prevention of diabetic cataract. Interdiscip. Toxicol. 2011, 4, 69–77. [Google Scholar] [CrossRef]
- Tewari, D.; Samoilă, O.; Gocan, D.; Mocan, A.; Moldovan, C.; Devkota, H.P.; Atanasov, A.G.; Zengin, G.; Echeverría, J.; Vodnar, D.; et al. Medicinal Plants and Natural Products Used in Cataract Management. Front. Pharmacol. 2019, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Saccà, S.C.; Izzotti, A.; Vernazza, S.; Tirendi, S.; Scarfì, S.; Gandolfi, S.; Bassi, A.M. Can Polyphenols in Eye Drops Be Useful for Trabecular Protection from Oxidative Damage? J. Clin. Med. 2020, 9, 3584. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Cavet, M.E.; Harrington, K.L.; Vollmer, T.R.; Ward, K.W.; Zhang, J.Z. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol. Vis. 2011, 17, 533–542. [Google Scholar] [PubMed]
- Lee, H.S.; Chauhan, S.K.; Okanobo, A.; Nallasamy, N.; Dana, R. Therapeutic efficacy of topical epigallocatechin gallate in murine dry eye. Cornea 2011, 30, 1465–1472. [Google Scholar] [CrossRef]
- Chaudhury, S.; Ghosh, I.; Saha, G.; Dasgupta, S. EGCG prevents tryptophan oxidation of cataractous ocular lens human γ-crystallin in presence of H2O2. Int. J. Biol. Macromol. 2015, 77, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Masmali, A.M.; Alanazi, S.A.; Alotaibi, A.G.; Fagehi, R.; Abusharaha, A.; El-Hiti, G.A. The acute effect of a single dose of green tea on the quality and quantity of tears in normal eye subjects. Clin. Ophthalmol. 2019, 13, 605–610. [Google Scholar] [CrossRef]
- Nagao, T.; Komine, Y.; Soga, S.; Meguro, S.; Hase, T.; Tanaka, Y.; Tokimitsu, I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005, 81, 122–129. [Google Scholar] [CrossRef]
- Nejabat, M.; Reza, S.A.; Zadmehr, M.; Yasemi, M.; Sobhani, Z. Efficacy of Green Tea Extract for Treatment of Dry Eye and Meibomian Gland Dysfunction; A Double-blind Randomized Controlled Clinical Trial Study. J. Clin. Diagn. Res. 2017, 11, Nc05–Nc08. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Togni, S.; Franceschi, F.; Kawada, S.; Inaba, Y.; Eggenhoffner, R.; Giacomelli, L. The effect of a natural, standardized bilberry extract (Mirtoselect®) in dry eye: A randomized, double blinded, placebo-controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2518–2525. [Google Scholar]
- Hitoe, S.; Tanaka, J.; Shimoda, H. MaquiBright™ standardized maqui berry extract significantly increases tear fluid production and ameliorates dry eye-related symptoms in a clinical pilot trial. Panminerva Med. 2014, 56, 1–6. [Google Scholar] [PubMed]
- Yamashita, S.I.; Suzuki, N.; Yamamoto, K.; Iio, S.I.; Yamada, T. Effects of MaquiBright® on improving eye dryness and fatigue in humans: A randomized, double-blind, placebo-controlled trial. J. Tradit. Complement. Med. 2019, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal-Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef]
- Rodriguez, E.L.; Zhang, C.; Woolfork, A.G.; Li, Z.; Bi, C.; Kaur, H.; Juritsch, A.F.; Moreau, R.; Hage, D.S. Analysis of curcumin and piperine in biological samples by reversed-phase liquid chromatography with multi-wavelength detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1162, 122487. [Google Scholar] [CrossRef]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and curcumin-like molecules: From spice to drugs. Curr. Med. Chem. 2014, 21, 204–222. [Google Scholar] [CrossRef]
- Liu, X.F.; Hao, J.L.; Xie, T.; Mukhtar, N.J.; Zhang, W.; Malik, T.H.; Lu, C.W.; Zhou, D.D. Curcumin, A Potential Therapeutic Candidate for Anterior Segment Eye Diseases: A Review. Front. Pharmacol. 2017, 8, 66. [Google Scholar] [CrossRef]
- Guo, C.; Li, M.; Qi, X.; Lin, G.; Cui, F.; Li, F.; Wu, X. Intranasal delivery of nanomicelle curcumin promotes corneal epithelial wound healing in streptozotocin-induced diabetic mice. Sci. Rep. 2016, 6, 29753. [Google Scholar] [CrossRef]
- Chung, S.H.; Choi, S.H.; Choi, J.A.; Chuck, R.S.; Joo, C.K. Curcumin suppresses ovalbumin-induced allergic conjunctivitis. Mol. Vis. 2012, 18, 1966–1972. [Google Scholar]
- Chen, M.; Hu, D.N.; Pan, Z.; Lu, C.W.; Xue, C.Y.; Aass, I. Curcumin protects against hyperosmoticity-induced IL-1beta elevation in human corneal epithelial cell via MAPK pathways. Exp. Eye Res. 2010, 90, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Palsamy, P.; Subramanian, S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim. Biophys. Acta 2011, 1812, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Chanvitayapongs, S.; Draczynska-Lusiak, B.; Sun, A.Y. Amelioration of oxidative stress by antioxidants and resveratrol in PC12 cells. Neuroreport 1997, 8, 1499–1502. [Google Scholar] [CrossRef] [PubMed]
- Murcia, M.A.; Martínez-Tomé, M. Antioxidant activity of resveratrol compared with common food additives. J. Food Prot. 2001, 64, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, S.; Sprinz, H.; Brede, O. Efficiency and mechanism of the antioxidant action of trans-resveratrol and its analogues in the radical liposome oxidation. Arch. Biochem. Biophys. 2001, 391, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Wang, N.; Wu, T.; Ge, Q.; Chen, L. Antioxidative Stress Mechanisms behind Resveratrol: A Multidimensional Analysis. J. Food Qual. 2021, 2021, 5571733. [Google Scholar] [CrossRef]
- De la Lastra, C.A.; Villegas, I. Resveratrol as an antioxidant and pro-oxidant agent: Mechanisms and clinical implications. Biochem. Soc. Trans. 2007, 35, 1156–1160. [Google Scholar] [CrossRef]
- Goutham, G.; Manikandan, R.; Beulaja, M.; Thiagarajan, R.; Arulvasu, C.; Arumugam, M.; Setzer, W.N.; Daglia, M.; Nabavi, S.F.; Nabavi, S.M. A focus on resveratrol and ocular problems, especially cataract: From chemistry to medical uses and clinical relevance. Biomed. Pharmacother. 2017, 86, 232–241. [Google Scholar] [CrossRef]
- Cordova, A.C.; Jackson, L.S.; Berke-Schlessel, D.W.; Sumpio, B.E. The cardiovascular protective effect of red wine. J. Am. Coll. Surg. 2005, 200, 428–439. [Google Scholar] [CrossRef]
- Luna, C.; Li, G.; Liton, P.B.; Qiu, J.; Epstein, D.L.; Challa, P.; Gonzalez, P. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2009, 47, 198–204. [Google Scholar] [CrossRef]
- Doganay, S.; Borazan, M.; Iraz, M.; Cigremis, Y. The effect of resveratrol in experimental cataract model formed by sodium selenite. Curr. Eye Res. 2006, 31, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bodakhe, S.H. Resveratrol delay the cataract formation against naphthalene-induced experimental cataract in the albino rats. J. Biochem. Mol. Toxicol. 2020, 34, e22420. [Google Scholar] [CrossRef] [PubMed]
- Yar, A.S.; Menevse, S.; Dogan, I.; Alp, E.; Ergin, V.; Cumaoglu, A.; Aricioglu, A.; Ekmekci, A.; Menevse, A. Investigation of ocular neovascularization-related genes and oxidative stress in diabetic rat eye tissues after resveratrol treatment. J. Med. Food 2012, 15, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, Y.S.; Roh, G.S.; Choi, W.S.; Cho, G.J. Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol. 2012, 90, e31–e37. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Valko, M. Health protective effects of carotenoids and their interactions with other biological antioxidants. Eur. J. Med. Chem. 2013, 70, 102–110. [Google Scholar] [CrossRef]
- Ahmed, S.S.; Lott, M.N.; Marcus, D.M. The Macular Xanthophylls. Surv. Ophthalmol. 2005, 50, 183–193. [Google Scholar] [CrossRef]
- Bone, R.A.; Landrum, J.T.; Tarsis, S.L. Preliminary identification of the human macular pigment. Vis. Res. 1985, 25, 1531–1535. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Asp. Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Galasso, C.; Orefice, I.; Pellone, P.; Cirino, P.; Miele, R.; Ianora, A.; Brunet, C.; Sansone, C. On the Neuroprotective Role of Astaxanthin: New Perspectives? Mar. Drugs 2018, 16, 247. [Google Scholar] [CrossRef]
- Johnson, E.J. A possible role for lutein and zeaxanthin in cognitive function in the elderly. Am. J. Clin. Nutr. 2012, 96, 1161s–1165s. [Google Scholar] [CrossRef]
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, Zeaxanthin and Meso-zeaxanthin Supplementation Associated with Macular Pigment Optical Density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.M.; Tharmarajah, S.; Jia, Y.; Semba, R.D.; Schaumberg, D.A.; Robinson, K.A. The Effect of Lutein/Zeaxanthin Intake on Human Macular Pigment Optical Density: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 2244–2254. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Qin, T.; Liu, Z.; Caceres, M.A.; Ronchi, C.F.; Chen, C.Y.O.; Yeum, K.-J.; Taylor, A.; Blumberg, J.B.; Liu, Y.; et al. Lutein and zeaxanthin supplementation reduces H2O2-induced oxidative damage in human lens epithelial cells. Mol. Vis. 2011, 17, 3180–3190. [Google Scholar]
- Xiong, R.; Yuan, Y.; Zhu, Z.; Wu, Y.; Ha, J.; Han, X.; Wang, W.; He, M. Micronutrients and Diabetic Retinopathy: Evidence From The National Health and Nutrition Examination Survey and a Meta-analysis. Am. J. Ophthalmol. 2022, 238, 141–156. [Google Scholar] [CrossRef] [PubMed]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta 2007, 1768, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, E. Extensive Bioactivity of Astaxanthin from Haematococcus pluvialis in Human. Adv. Exp. Med. Biol. 2021, 1261, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef]
- Kikuchi, K.; Dong, Z.; Shinmei, Y.; Murata, M.; Kanda, A.; Noda, K.; Harada, T.; Ishida, S. Cytoprotective Effect of Astaxanthin in a Model of Normal Intraocular Pressure Glaucoma. J. Ophthalmol. 2020, 2020, 9539681. [Google Scholar] [CrossRef]
- Tanaka-Gonome, T.; Xie, Y.; Yamauchi, K.; Maeda-Monai, N.; Tanabu, R.; Kudo, T.; Nakazawa, M. The protective effect of astaxanthin on the ganglion cell complex in glutamate/aspartate transporter deficient mice, a model of normal tension glaucoma, analyzed by spectral domain-optical coherence tomography. Biochem. Biophys. Rep. 2020, 23, 100777. [Google Scholar] [CrossRef]
- Lennikov, A.; Kitaichi, N.; Fukase, R.; Murata, M.; Noda, K.; Ando, R.; Ohguchi, T.; Kawakita, T.; Ohno, S.; Ishida, S. Amelioration of ultraviolet-induced photokeratitis in mice treated with astaxanthin eye drops. Mol. Vis. 2012, 18, 455–464. [Google Scholar] [PubMed]
- Shimokawa, T.; Fukuta, T.; Inagi, T.; Kogure, K. Protective effect of high-affinity liposomes encapsulating astaxanthin against corneal disorder in the in vivo rat dry eye disease model. J. Clin. Biochem. Nutr. 2020, 66, 224–232. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Higuchi, A.; Inoue, H.; Kawakita, T.; Ogishima, T.; Tsubota, K. Selenium compound protects corneal epithelium against oxidative stress. PLoS ONE 2012, 7, e45612. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, A.; Takahashi, K.; Hirashima, M.; Kawakita, T.; Tsubota, K. Selenoprotein P controls oxidative stress in cornea. PLoS ONE 2010, 5, e9911. [Google Scholar] [CrossRef] [PubMed]
- Marreiro, D.D.; Cruz, K.J.; Morais, J.B.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Newsome, D.A.; Miceli, M.V.; Tate, D.J., Jr.; Alcock, N.W.; Oliver, P.D. Zinc content of human retinal pigment epithelium decreases with age and macular degeneration, but superoxide dismutase activity increases. J. Trace Elem. Exp. Med. 1996, 8, 193–199. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Zhao, J.; Han, X.Y.; Zhou, X.H.; Wu, J.; Ji, L.N. Relationship Between Serum Zinc Level and Microvascular Complications in Patients with Type 2 Diabetes. Chin. Med. J. 2015, 128, 3276–3282. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kanwar, M.; Chan, P.S.; Zhang, J.P. Inhibition of retinopathy and retinal metabolic abnormalities in diabetic rats with AREDS-based micronutrients. Arch. Ophthalmol. 2008, 126, 1266–1272. [Google Scholar] [CrossRef]
- Engin, K.N. Alpha-tocopherol: Looking beyond an antioxidant. Mol. Vis. 2009, 15, 855–860. [Google Scholar]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Winkler, B.S.; Orselli, S.M.; Rex, T.S. The redox couple between glutathione and ascorbic acid: A chemical and physiological perspective. Free Radic. Biol. Med. 1994, 17, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Drouin, G.; Godin, J.R.; Pagé, B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.C.; Ervin, A.M.; Tao, J.; Davis, R.M. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst. Rev. 2012, 6, Cd004567. [Google Scholar] [CrossRef]
- Meyer, C.H.; Sekundo, W. Nutritional supplementation to prevent cataract formation. Dev. Ophthalmol. 2005, 38, 103–119. [Google Scholar] [CrossRef]
- Sperduto, R.D.; Hu, T.S.; Milton, R.C.; Zhao, J.L.; Everett, D.F.; Cheng, Q.F.; Blot, W.J.; Bing, L.; Taylor, P.R.; Li, J.Y.; et al. The Linxian cataract studies. Two nutrition intervention trials. Arch. Ophthalmol. 1993, 111, 1246–1253. [Google Scholar] [CrossRef]
- Williams, D.L. Oxidation, antioxidants and cataract formation: A literature review. Vet. Ophthalmol. 2006, 9, 292–298. [Google Scholar] [CrossRef]
- Taylor, A.; Jacques, P.F.; Nowell, T.; Perrone, G.; Blumberg, J.; Handelman, G.; Jozwiak, B.; Nadler, D. Vitamin C in human and guinea pig aqueous, lens and plasma in relation to intake. Curr. Eye Res. 1997, 16, 857–864. [Google Scholar] [CrossRef]
- Tsao, Y.T.; Wu, W.C.; Chen, K.J.; Liu, C.F.; Hsueh, Y.J.; Cheng, C.M.; Chen, H.C. An Assessment of Cataract Severity Based on Antioxidant Status and Ascorbic Acid Levels in Aqueous Humor. Antioxidants 2022, 11, 397. [Google Scholar] [CrossRef]
- Nagata, M.; Tanioka, H.; Mibu, H.; Hikida, M.; Akiba, M.; Yamamoto, I. Effect of ascorbic acid 2-O-alpha-glucoside on hydrocortisone-induced cataract formation in developing chick embryos: I. Comparison of the preventive effect of ascorbic acid derivatives. J. Ocul. Pharmacol. 1993, 9, 59–68. [Google Scholar] [CrossRef]
- Nagata, M.; Hikida, M.; Mibu, H.; Muto, N.; Yamamoto, I. Effect of ascorbic acid 2-O-alpha-glucoside on hydrocortisone-induced cataract formation in developing chick embryos: II. Influence on glutathione and lipid peroxide contents in the lens. J. Ocul. Pharmacol. 1994, 10, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Jaber, B.; Hamed, S.; AlKhatib, H.S. Preparation and evaluation of ascorbyl glucoside and ascorbic acid solid in oil nanodispersions for corneal epithelial wound healing. Int. J. Pharm. 2022, 627, 122227. [Google Scholar] [CrossRef] [PubMed]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta 2013, 1830, 4117–4129. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, L.; Gazzaniga, A. Toxicological, pharmacokinetic and metabolic studies on acetylcysteine. Eur. J. Respir. Dis. Suppl. 1980, 111, 45–51. [Google Scholar] [PubMed]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The mechanism of action of N-acetylcysteine (NAC): The emerging role of H(2)S and sulfane sulfur species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef]
- Eghtedari, Y.; Oh, L.J.; Girolamo, N.D.; Watson, S.L. The role of topical N-acetylcysteine in ocular therapeutics. Surv. Ophthalmol. 2022, 67, 608–622. [Google Scholar] [CrossRef]
- Singh, P.; Tyagi, M.; Kumar, Y.; Gupta, K.K.; Sharma, P.D. Ocular chemical injuries and their management. Oman J. Ophthalmol. 2013, 6, 83–86. [Google Scholar] [CrossRef]
- Messina, M.; Dua, H.S. Early results on the use of chitosan-N-acetylcysteine (Lacrimera(®)) in the management of dry eye disease of varied etiology. Int. Ophthalmol. 2019, 39, 693–696. [Google Scholar] [CrossRef]
- Wang, P.; Liu, X.C.; Yan, H.; Li, M.Y. Hyperoxia-induced lens damage in rabbit: Protective effects of N-acetylcysteine. Mol. Vis. 2009, 15, 2945–2952. [Google Scholar]
- Maddirala, Y.; Tobwala, S.; Karacal, H.; Ercal, N. Prevention and reversal of selenite-induced cataracts by N-acetylcysteine amide in Wistar rats. BMC Ophthalmol. 2017, 17, 54. [Google Scholar] [CrossRef]
- Terluk, M.R.; Ebeling, M.C.; Fisher, C.R.; Kapphahn, R.J.; Yuan, C.; Kartha, R.V.; Montezuma, S.R.; Ferrington, D.A. N-Acetyl-L-cysteine Protects Human Retinal Pigment Epithelial Cells from Oxidative Damage: Implications for Age-Related Macular Degeneration. Oxid. Med. Cell. Longev. 2019, 2019, 5174957. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Iftikhar, M.; Hafiz, G.; Akhlaq, A.; Tsai, G.; Wehling, D.; Lu, L.; Wall, G.M.; Singh, M.S.; Kong, X. Oral N-acetylcysteine improves cone function in retinitis pigmentosa patients in phase I trial. J. Clin. Investig. 2020, 130, 1527–1541. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Pan, W.H.; Liu, J.H.; Chen, M.M.; Liu, C.M.; Yeh, M.Y.; Tsai, S.K.; Young, M.S.; Zhang, X.M.; Chao, H.M. The effects and underlying mechanisms of S-allyl l-cysteine treatment of the retina after ischemia/reperfusion. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2012, 28, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.M.; Chen, I.L.; Liu, J.H. S-allyl L-cysteine protects the retina against kainate excitotoxicity in the rat. Am. J. Chin. Med. 2014, 42, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Savion, N.; Dahamshi, S.; Morein, M.; Kotev-Emeth, S. S-Allylmercapro-N-Acetylcysteine Attenuates the Oxidation-Induced Lens Opacification and Retinal Pigment Epithelial Cell Death In Vitro. Antioxidants 2019, 8, 25. [Google Scholar] [CrossRef]
- Olfat, N.; Ashoori, M.; Saedisomeolia, A. Riboflavin is an antioxidant: A review update. Br. J. Nutr. 2022, 128, 1887–1895. [Google Scholar] [CrossRef]
- Shi, C.; Wang, P.; Airen, S.; Brown, C.; Liu, Z.; Townsend, J.H.; Wang, J.; Jiang, H. Nutritional and medical food therapies for diabetic retinopathy. Eye Vis. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Di Nezza, F.; Caruso, C.; Costagliola, C.; Ambrosone, L. Reaction-diffusion model as framework for understanding the role of riboflavin in “eye defence” formulations. RSC Adv. 2020, 10, 14965–14971. [Google Scholar] [CrossRef]
- Bartollino, S.; Palazzo, M.; Semeraro, F.; Parolini, B.; Caruso, C.; Merolla, F.; Guerra, G.; Costagliola, C. Effects of an antioxidant protective topical formulation on retinal tissue of UV-exposed rabbits. Int. Ophthalmol. 2020, 40, 925–933. [Google Scholar] [CrossRef]
- Atkinson, J.; Epand, R.F.; Epand, R.M. Tocopherols and tocotrienols in membranes: A critical review. Free Radic. Biol. Med. 2008, 44, 739–764. [Google Scholar] [CrossRef]
- Wefers, H.; Sies, H. The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur. J. Biochem. 1988, 174, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Hayton, S.M.; Kriss, A.; Wade, A.; Muller, D.P.R. The effects of different levels of all-rac- and RRR-α-tocopheryl acetate (vitamin E) on visual function in rats. Clin. Neurophysiol. 2003, 114, 2124–2131. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.; Olson, C.G.; Euritt, C.P.; Koulen, P. Molecular Mechanisms Underlying the Therapeutic Role of Vitamin E in Age-Related Macular Degeneration. Front. Neurosci. 2022, 16, 890021. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2017, 7, Cd000254. [Google Scholar] [CrossRef]
- Robertson, J.M.; Donner, A.P.; Trevithick, J.R. Vitamin E intake and risk of cataracts in humans. Ann. N. Y. Acad. Sci. 1989, 570, 372–382. [Google Scholar] [CrossRef]
- Dziedziak, J.; Kasarełło, K.; Cudnoch-Jędrzejewska, A. Dietary Antioxidants in Age-Related Macular Degeneration and Glaucoma. Antioxidants 2021, 10, 1743. [Google Scholar] [CrossRef]
- Leung, H.H.; Galano, J.M.; Crauste, C.; Durand, T.; Lee, J.C. Combination of Lutein and Zeaxanthin, and DHA Regulated Polyunsaturated Fatty Acid Oxidation in H(2)O(2)-Stressed Retinal Cells. Neurochem. Res. 2020, 45, 1007–1019. [Google Scholar] [CrossRef]
- Clementi, M.E.; Lazzarino, G.; Sampaolese, B.; Brancato, A.; Tringali, G. DHA protects PC12 cells against oxidative stress and apoptotic signals through the activation of the NFE2L2/HO-1 axis. Int. J. Mol. Med. 2019, 43, 2523–2531. [Google Scholar] [CrossRef]
- Powell, N.; Chaudhary, S.; Zaidi, A. It Is Time for an Oil Change: Polyunsaturated Fatty Acids and Human Health. Mo. Med. 2021, 118, 426–430. [Google Scholar]
- Sala-Vila, A.; Díaz-López, A.; Valls-Pedret, C.; Cofán, M.; García-Layana, A.; Lamuela-Raventós, R.M.; Castañer, O.; Zanon-Moreno, V.; Martinez-Gonzalez, M.A.; Toledo, E.; et al. Dietary Marine ω-3 Fatty Acids and Incident Sight-Threatening Retinopathy in Middle-Aged and Older Individuals With Type 2 Diabetes: Prospective Investigation From the PREDIMED Trial. JAMA Ophthalmol. 2016, 134, 1142–1149. [Google Scholar] [CrossRef]
- Garcia-Medina, J.J.; Garcia-Medina, M.; Garrido-Fernandez, P.; Galvan-Espinosa, J.; Garcia-Maturana, C.; Zanon-Moreno, V.; Pinazo-Duran, M.D. A two-year follow-up of oral antioxidant supplementation in primary open-angle glaucoma: An open-label, randomized, controlled trial. Acta Ophthalmol. 2015, 93, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Romeo Villadóniga, S.; Rodríguez García, E.; Sagastagoia Epelde, O.; Álvarez Díaz, M.D.; Domingo Pedrol, J.C. Effects of Oral Supplementation with Docosahexaenoic Acid (DHA) plus Antioxidants in Pseudoexfoliative Glaucoma: A 6-Month Open-Label Randomized Trial. J. Ophthalmol. 2018, 2018, 8259371. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, C.; O’Keeffe, M. Omega-3 fatty acids in the management of dry eye disease-An updated systematic review and meta-analysis. Acta Ophthalmol. 2022, 101, e118–e134. [Google Scholar] [CrossRef]
- Christen, W.G.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Lee, I.-M.; Bubes, V.; Friedenberg, G.; Dushkes, R.; Smith, D.; Schaumberg, D.A.; et al. Efficacy of Marine ω-3 Fatty Acid Supplementation vs Placebo in Reducing Incidence of Dry Eye Disease in Healthy US Adults: A Randomized Clinical Trial. JAMA Ophthalmol. 2022, 140, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Peris-Martínez, C.; Piá-Ludeña, J.V.; Rog-Revert, M.J.; Fernández-López, E.; Domingo, J.C. Antioxidant and Anti-Inflammatory Effects of Oral Supplementation with a Highly-Concentrated Docosahexaenoic Acid (DHA) Triglyceride in Patients with Keratoconus: A Randomized Controlled Preliminary Study. Nutrients 2023, 15, 1300. [Google Scholar] [CrossRef]
- Chevalier, L.; Vachon, A.; Plourde, M. Pharmacokinetics of Supplemental Omega-3 Fatty Acids Esterified in Monoglycerides, Ethyl Esters, or Triglycerides in Adults in a Randomized Crossover Trial. J. Nutr. 2021, 151, 1111–1118. [Google Scholar] [CrossRef]
- Dyerberg, J.; Madsen, P.; Møller, J.M.; Aardestrup, I.; Schmidt, E.B. Bioavailability of marine n-3 fatty acid formulations. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 137–141. [Google Scholar] [CrossRef]
- Offman, E.; Davidson, M.; Abu-Rashid, M.; Chai, P.; Nilsson, C. Systemic Bioavailability and Dose Proportionality of Omega-3 Administered in Free Fatty Acid Form Compared With Ethyl Ester Form: Results of a Phase 1 Study in Healthy Volunteers. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 815–825. [Google Scholar] [CrossRef]
- Littarru, G.P.; Tiano, L. Bioenergetic and antioxidant properties of coenzyme Q10: Recent developments. Mol. Biotechnol. 2007, 37, 31–37. [Google Scholar] [CrossRef]
- Manzar, H.; Abdulhussein, D.; Yap, T.E.; Cordeiro, M.F. Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain. Int. J. Mol. Sci. 2020, 21, 9299. [Google Scholar] [CrossRef]
- Martucci, A.; Nucci, C. Evidence on neuroprotective properties of coenzyme Q10 in the treatment of glaucoma. Neural Regen. Res. 2019, 14, 197–200. [Google Scholar] [CrossRef]
- Zhang, X.; Tohari, A.M.; Marcheggiani, F.; Zhou, X.; Reilly, J.; Tiano, L.; Shu, X. Therapeutic Potential of Co-enzyme Q10 in Retinal Diseases. Curr. Med. Chem. 2017, 24, 4329–4339. [Google Scholar] [CrossRef] [PubMed]
- Cialdai, F.; Bolognini, D.; Vignali, L.; Iannotti, N.; Cacchione, S.; Magi, A.; Balsamo, M.; Vukich, M.; Neri, G.; Donati, A.; et al. Effect of space flight on the behavior of human retinal pigment epithelial ARPE-19 cells and evaluation of coenzyme Q10 treatment. Cell. Mol. Life Sci. 2021, 78, 7795–7812. [Google Scholar] [CrossRef] [PubMed]
- Biewenga, G.P.; Haenen, G.R.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997, 29, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Inman, D.M.; Lambert, W.S.; Calkins, D.J.; Horner, P.J. α-Lipoic acid antioxidant treatment limits glaucoma-related retinal ganglion cell death and dysfunction. PLoS ONE 2013, 8, e65389. [Google Scholar] [CrossRef]
- Santos, J.M.; Kowluru, R.A. Role of mitochondria biogenesis in the metabolic memory associated with the continued progression of diabetic retinopathy and its regulation by lipoic acid. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8791–8798. [Google Scholar] [CrossRef]
- Gomes, M.B.; Negrato, C.A. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol. Metab. Syndr. 2014, 6, 80. [Google Scholar] [CrossRef]
- Cagini, C.; Leontiadis, A.; Ricci, M.A.; Bartolini, A.; Dragoni, A.; Pellegrino, R.M. Study of alpha-lipoic acid penetration in the human aqueous after topical administration. Clin. Exp. Ophthalmol. 2010, 38, 572–576. [Google Scholar] [CrossRef]
- Ekinci, M.; Cagatay, H.H.; Ceylan, E.; Keles, S.; Koban, Y.; Gokce, G.; Huseyinoğlu, U.; Ozcan, E.; Oba, M.E. Reduction of conjunctival fibrosis after trabeculectomy using topical α-lipoic acid in rabbit eyes. J. Glaucoma 2014, 23, 372–379. [Google Scholar] [CrossRef]
- Alvarez-Rivera, F.; Fernández-Villanueva, D.; Concheiro, A.; Alvarez-Lorenzo, C. α-Lipoic Acid in Soluplus® Polymeric Nanomicelles for Ocular Treatment of Diabetes-Associated Corneal Diseases. J. Pharm. Sci. 2016, 105, 2855–2863. [Google Scholar] [CrossRef]






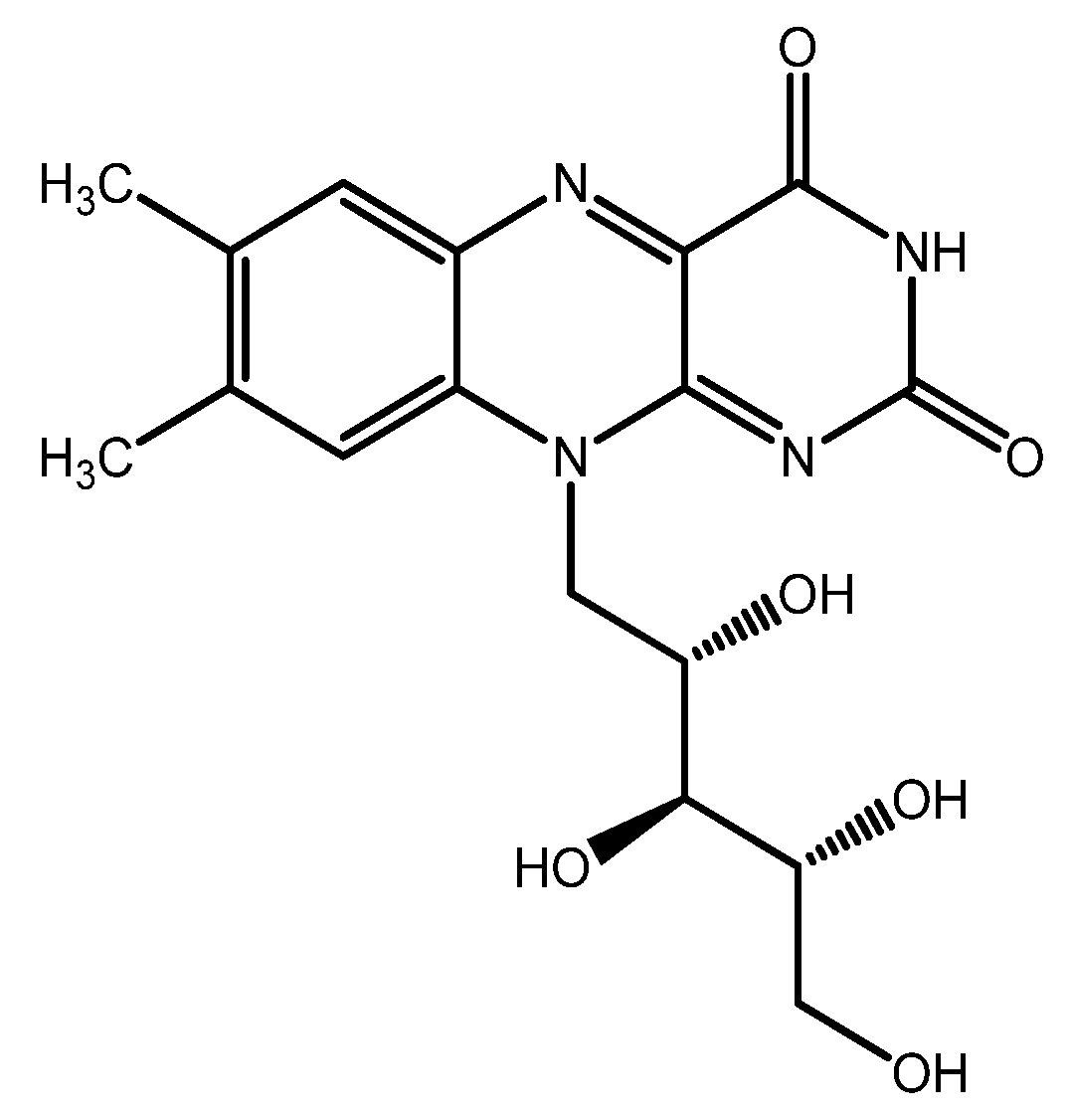
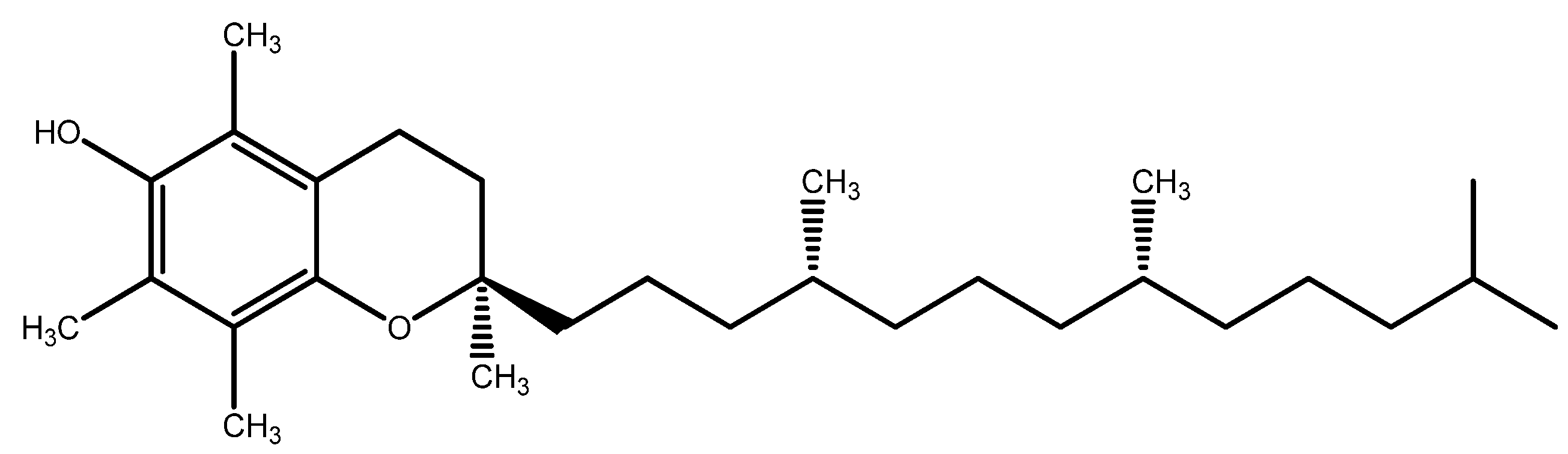
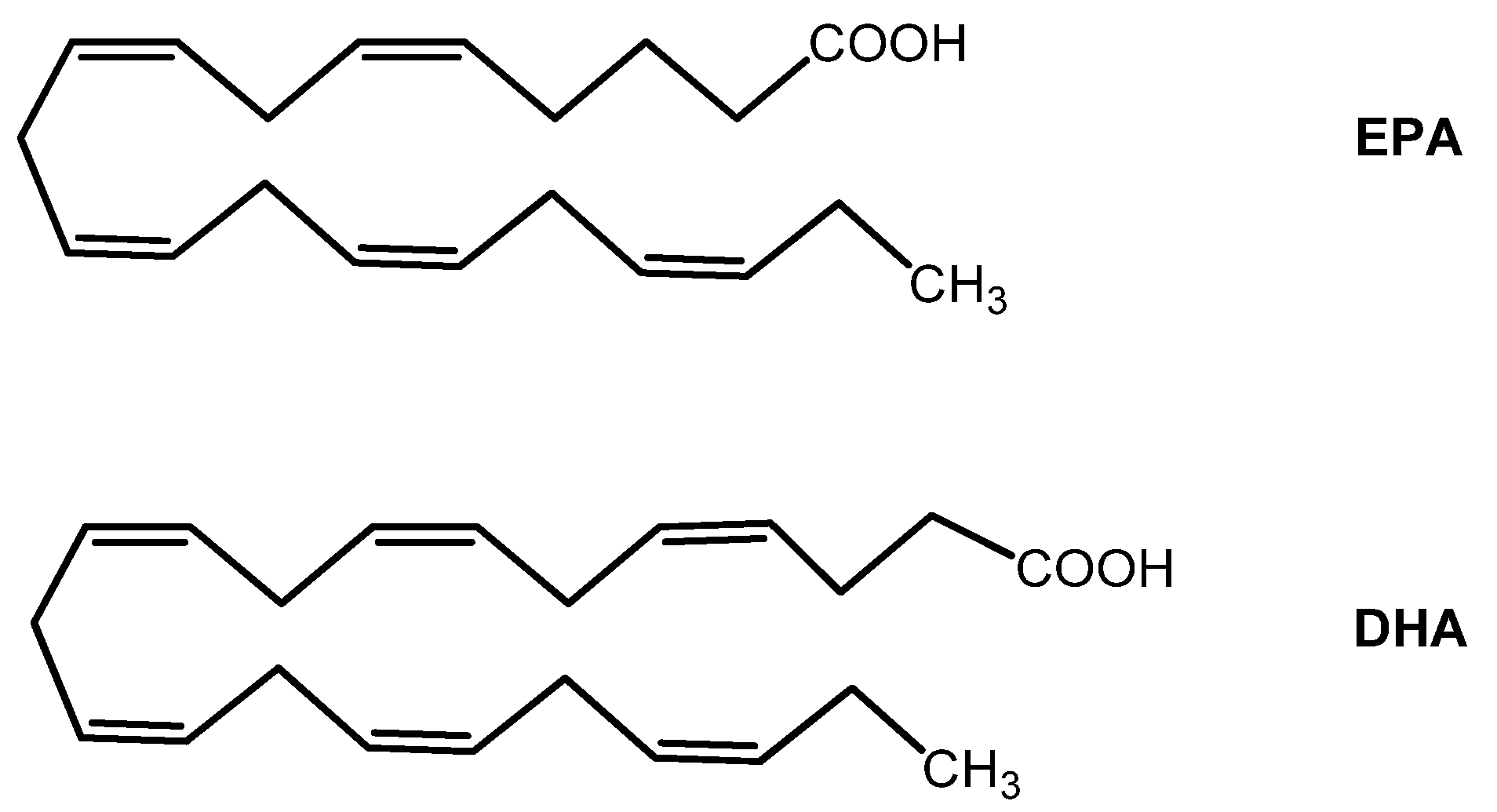
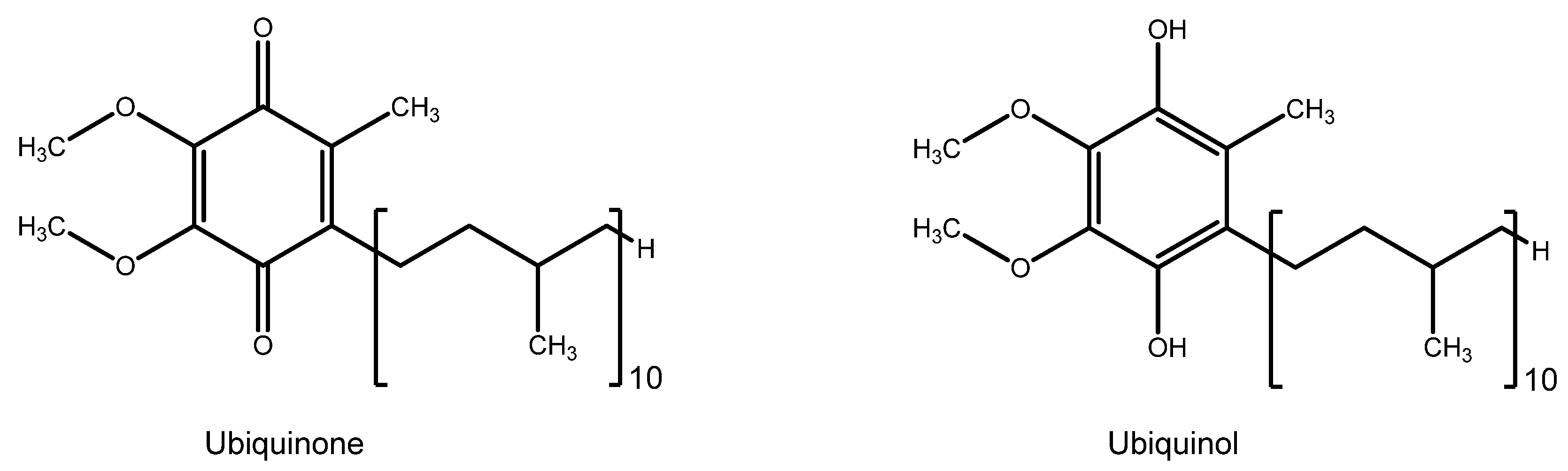

| Group | Antioxidant | Mechanism(s) of Antioxidant Action | Notes |
|---|---|---|---|
| Polyphenols | Flavonoids Catechins Anthocyanins | - Radical scavengers - Metal chelators - Inhibition of ROS-generation enzymes - Expression of antioxidant enzymes - Anti-inflammatory | |
| Curcumin | - Radical scavenger - Metal Chelator - Anti-inflammatory - Anti-angiogenetic | ||
| Resveratrol | - Radical scavenger - Pro-oxidant effects, beneficial in cancer - Expression of antioxidant enzymes - Anti-inflammatory | ||
| Carotenoids | Beta-carotene | - Cell membrane antioxidant - Regeneration of other antioxidants - Vitamin A precursor | - Included in the AREDS study |
| Lutein | - Cell membrane antioxidant - Regeneration of other antioxidants | - Xanthophylls subgroup - included in the AREDS2 study | |
| Zeaxanthin | - Cell membrane antioxidant - Regeneration of other antioxidants | - Xanthophylls subgroup - included in the AREDS2 study | |
| Astaxanthin | - Cell membrane antioxidant - Direct antioxidant - Anti-inflammatory - Neuroprotective | - Xanthophylls subgroup | |
| Water-Soluble Promoters of Endogenous Antioxidant System | Zinc | - A building block for redox system enzymes | - Included in the AREDS study |
| Selenium | - A building block for redox system enzymes | ||
| Vitamin C | - Direct antioxidant - Synergy with other antioxidants (Vitamin E, GSH, flavonoids) | - Included in the AREDS study - The most abundant antioxidant in the eye | |
| N-acetyl cysteine | - Protection from sulphydryl oxidation - Scavenger of superoxide and peroxide radicals - Precursor of GSH | ||
| Riboflavin | - Indirect antioxidant (supports the endogenous antioxidant system) - UV light absorption | ||
| Lipophilic Antioxidants in cellular membranes | Vitamin E | - Cell membrane antioxidant - Synergy with other antioxidants (Vitamin C, GSH) - UV light absorption | - Included in the AREDS study |
| Omega-3 fatty acids EPA and DHA | - Cell membrane antioxidant - Anti-inflammatory | - Included in the AREDS2 study - Nervous system and retina development | |
| Coenzyme Q10 | - Mitochondrial redox equilibrium regulator and antioxidant - Neuroprotective | - Energy metabolism | |
| Alpha Lipoic Acid | - Direct antioxidant - Regeneration of other antioxidants - Anti-inflammatory | - Energy metabolism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodella, U.; Honisch, C.; Gatto, C.; Ruzza, P.; D’Amato Tóthová, J. Antioxidant Nutraceutical Strategies in the Prevention of Oxidative Stress Related Eye Diseases. Nutrients 2023, 15, 2283. https://doi.org/10.3390/nu15102283
Rodella U, Honisch C, Gatto C, Ruzza P, D’Amato Tóthová J. Antioxidant Nutraceutical Strategies in the Prevention of Oxidative Stress Related Eye Diseases. Nutrients. 2023; 15(10):2283. https://doi.org/10.3390/nu15102283
Chicago/Turabian StyleRodella, Umberto, Claudia Honisch, Claudio Gatto, Paolo Ruzza, and Jana D’Amato Tóthová. 2023. "Antioxidant Nutraceutical Strategies in the Prevention of Oxidative Stress Related Eye Diseases" Nutrients 15, no. 10: 2283. https://doi.org/10.3390/nu15102283
APA StyleRodella, U., Honisch, C., Gatto, C., Ruzza, P., & D’Amato Tóthová, J. (2023). Antioxidant Nutraceutical Strategies in the Prevention of Oxidative Stress Related Eye Diseases. Nutrients, 15(10), 2283. https://doi.org/10.3390/nu15102283







