Abstract
Nutrition during the prenatal period is crucial for the development of insulin resistance (IR) and its consequences in children. The relationship between intrauterine environment, fetal nutrition and the onset of IR, type 2 diabetes (T2D), obesity and metabolic syndrome later in life has been confirmed in many studies. The intake of carbohydrates, protein, fat and micronutrients during pregnancy seems to damage fetal metabolism programming; indeed, epigenetic mechanisms change glucose−insulin metabolism. Intrauterine growth restriction (IUGR) induced by unbalanced nutrient intake during prenatal life cause fetal adipose tissue and pancreatic beta-cell dysfunction. In this review we have summarized and discussed the role of maternal nutrition in preventing insulin resistance in youth.
1. Introduction
1.1. Definition
A Consensus Conference has defined insulin resistance (IR) as “the decreased tissue response to insulin-mediated cellular actions, so it is the inverse of insulin sensitivity” [1]. Therefore, IR represents a state in which normal plasma insulin concentrations are not able to maintain peripheral glucose removal and inhibit both hepatic glucose output and hepatic glucose-induced output of very low-density lipoproteins [1,2]. The impaired glucose metabolism in the liver and the declining beta-cell function play a crucial role in driving type 2 diabetes (T2D) development [3,4]. Thus, although IR represents the first step in the natural history of the spectrum of impaired glucose metabolism, so far there are no standard values accepted worldwide for IR in children; also, its frequency varies according to gender and race [5]. However, surrogate markers are widely adopted in the clinical setting, offering relevant tools that are useful for characterizing the risk of developing a complex spectrum of metabolic alteration.
1.2. Measurement
The evaluation of peripheral insulin sensitivity in vivo consists of the application of direct methods, such as the hyperinsulinemic-euglycemic clamp, the pancreatic suppression test and the minimal model approximation of the metabolism of glucose (MMAMG) [6]. The hyperinsulinemic-euglycemic clamp study is the “gold-standard” for whole body insulin sensitivity assessment; this method measures insulin-stimulated glucose disposal at a specific concentration of hyperinsulinemia [7]. Unfortunately, direct methods are elaborate, invasive, time-consuming and costly, so they are only used for research purposes [8].On the other hand, fasting plasma insulin (FPI), fasting insulin resistance index (FIRI), McAuley index (McA), homeostasis model assessment for insulin resistance (HOMA-IR), quantitative insulin-sensitivity check index (QUICKI), insulin sensitivity index (ISI) and whole body insulin sensitivity index (WBISI) are indirect methods that measure plasma insulin levels during fasting or after oral glucose load (Table 1) [9]. HOMA-IR is calculated as the result of the fasting insulin−glucose product divided by a different constant based on the measurement unit; it is the simplest method, and it depends on both the fasting glucose and insulin concentration [7]. All of these indexes have been validated according to the gold standard techniques, are well correlated with glucose-related metabolic abnormalities and might be used in clinical settings.

Table 1.
Indirect methods of evaluating peripheral insulin sensitivity and their corresponding formulae.
1.3. Pathophysiology
Insulin induces metabolic effects on target cells via PI3K (phosphoinositide-3-kinase)-Akt (protein kinase B) signaling, influencing glycogen synthesis, glycolysis, fatty acid and protein synthesis [10]. PI3K-Akt genes and many other genes might be involved in the pathophysiology of IRlike PPARG (peroxisome proliferator-activated receptor-γ), IRS1 (insulin receptor substrate 1) and GCKR (glucokinase regulator, [11] but at the present time data are still controversial [12]). The most important consequence of IR is the presence of a compensatory hyperinsulinemia state, characterized by an excessive and delayed rise in insulin secretion after meals, leading to triglyceride accumulation in hepatic and muscle tissues and a reduction of GLUT-4 translocation [13]. The link between an unfavorable prenatal environment and adverse health outcomes later in life has been confirmed in many studies. Indeed, poor nutritional status during the fetal period may cause structural and functional alterations in many organs, such as the liver, brain, muscle, pancreas and adipose tissue [14]. This hypothesis is known as “predictive adaptive response” (PAR) and describes the process by which the prenatal environment models fetal development and leads the organism to express a metabolic spearing phenotype based on the expected future environmental conditions [15]. Intrauterine growth restriction (IUGR) is the result of impaired fetal growth due to poor or unbalanced nutrient intake during intrauterine development [16]. This condition has many metabolic consequences regarding insulin activity; indeed, peripheral insulin sensitivity decreases, insulin-stimulated protein synthesis in muscles reduces and hepatic glucose production increases. If these conditions are persistent, the nutrient uptake and storage may lead to several complications later in life, such as obesity, T2D and IR [16,17]. These considerations are the core of the “thrifty phenotype” hypothesis, proposed by Hales and Barker in 1992 [18].
1.4. Maternal Nutrition and Intrauterine Fetal Growth
Maternal nutrition during pregnancy plays a fundamental role in both providing the fetus the essential nutrients for growth and establishing the phenotypic and metabolic characteristics of the fetus during postnatal life (Figure 1) [19]. The correlation between nutrition during different ages of life and the probability of developing metabolic alterations such as IR, obesity, hypertension and T2D, is now well known. Intrauterine nutrition represents the first phase and probably one of the most important and decisive ones for the metabolic programming of the fetus [20]. Fetal exposure to an inadequate or unbalanced intake of macro and micronutrients might cause changes in the fetal epigenome. Epigenetic changes determine modifications in the gene expression and, consequently, in the metabolic programming of the fetus, predisposing it to the onset of metabolic diseases later in life. It has been observed that the early stages of embryo development represent a time window in which cells are most susceptible to epigenetic changes [21]. However, despite the fact that the first trimester represents a crucial period in the metabolic programming of the fetus, it is also true that fetal exposure to unfavorable intra-uterine conditions continues to influence epigenetic modifications in the next stages of development. Indeed, it has been shown that inadequate intake of micronutrients like vitamin B12 and B9 during the second trimester of pregnancy are associated with an increased HOMA-IR in the offspring. According to the thrifty phenotype theory, intrauterine malnutrition could be correlated with an increased risk of obesity onset and metabolic syndrome in postnatal life [22,23]. The result is an increased incidence of IR and T2D among younger subjects, leading to a raised cardiovascular risk and increased likelihood of metabolic complications in adulthood [24,25]. We know that those born small for gestational age (SGA) exhibit catch-up growth 6 months after birth, mainly via fat storage in the abdominal area. Moreover, these children demonstrated hyperinsulinemia and insulin resistance that predispose them to several metabolic diseases like T2D later in life. This condition may be explained by the thrifty phenotype hypothesis. Probably, this process is derived from DNA methylation, leading to the repression of gene expression involved in the regulation of glucose metabolism [26]. Furthermore, postnatal exposure to overnutrition contributes to the impairment of beta-cell function and insulin sensitivity. Many studies have demonstrated that maternal obesity represents an independent risk factor for the high BMI of offspring. Moreover, in humans, it has been demonstrated that infants exposed to overnutrition in utero could manifest an increased risks of obesity, diabetes and other complications, including non-alcoholic fatty liver disease (NAFLD) [27,28]. To better understand the concept of fetal malnutrition, we will discuss in more detail about some models of maternal diet to understand how these are correlated with the onset of metabolic alterations during postnatal life.
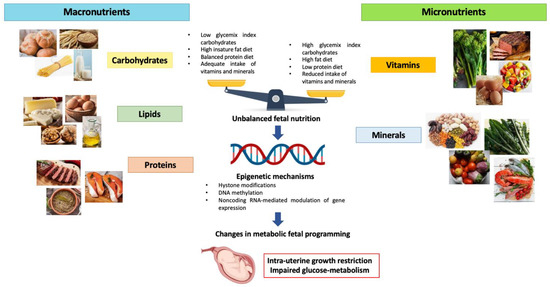
Figure 1.
Effects of unbalanced maternal nutrition on intrauterine fetal growth and fetal metabolic programming.
1.5. Maternal High Glycemix Index Diet
Numerous studies have demonstrated the correlation between maternal diet with a high glycemic content and the increased risk of developing IR in offspring [29]. Glycemic index (GI) represents the glycemic response after the ingestion of a carbohydrate-containing food [30,31]. Carbohydrates are distinguished in those that are absorbed quickly and cause a rapid rise in blood sugar (high GI) and others that release glucose more slowly (low GI). Sweets and processed grains are considered high-GI foods because they produce elevated blood glucose concentration, while foods rich in soluble fiber contribute to a lower GI. In adult populations, the intake of high GI and glycemic load (GL) foods has been associated with an increased risk of obesity and T2D [32]. Conversely, low-GI foods, such as fiber-containing foods, have been found to be protective [33,34]. It was seen that maternal blood glucose levels in pregnancy are more negatively influenced by the intake of high-GI carbohydrates. For this reason, primary interventions during pregnancy, such as dietary interventions, are important to improve perinatal outcomes. For example, using low-GI foods can reduce postprandial glycemia. Maslova et al. examined the association of maternal GI and GL during pregnancy with offspring body mass index (BMI) in the first 7 years of life in a large cohort study. It was observed that higher maternal GI and GL were likely related to an increase in IR and adiposity in children between 9 and 16 years exposed to maternal hyperglycemia in utero [35]. Moreover, it was supposed that a maternal diet with a high GI may influence the expression of leptin (LEP) and fat mass and the obesity-associated gene (FTO), as well as other appetite-related genes, like agouti-related peptide (Agrp), neuropeptide Y (Npy), pro-opiomelanocortin cocaine (Pomc), amphetamine-regulated transcript (Cart) and leptin receptor (Lepr) in specific tissues [36]. Leptin is a protein hormone synthesized in the white adipose tissue that acts on the hypothalamic receptors by regulating the sense of satiety [37,38,39]. Sideratou et al. observed using a mouse model that the glycemic qualities of carbohydrates consumed during pregnancy and lactation modifies the expression of appetite-related genes in the offspring. It was observed that an increased FTO expression is associated with a high GI diet during both pregnancy and postnatal ages; in particular, the expression is almost 4-fold higher in the placenta of female mice during pregnancy and >2-fold higher in the hypothalamus of pups fed with a high GI diet from weaning to about 2 years of age. By contrast, Lep expression in the visceral adipose tissue of offspring was >3-fold higher if mothers were fed with a low-GI diet throughout pregnancy and lactation [36]. These findings imply that consumption of rapidly digestible starchy foods may increase the risk of early-onset obesity and IR in humans. On the other hand, newer studies have proposed that a low-carbohydrate diet with an elevated content of fibers could be more effective for the prevention of T2D onset compared to a low-fat diet. Toh et al., in their study on mouse models, showed that a maternal high-fiber intake reduced the probability of diabetes onset in offspring that consumed a diabetogenic diet after weaning, especially in the male sex [40].
1.6. Maternal High-Fat Diet
Many animal studies have demonstrated the metabolic effects of a high-fat (HF) maternal diet on the fetus [41,42]. Saullo et al. have observed that offspring adiposity is significantly affected by a maternal HF diet and that the consumption of an HF diet during pregnancy influences the development of visceral white adipose tissue in offspring, especially when the pups also have an HF diet consumption during postnatal life [43,44]. Peng et al., in their study conducted on mice, demonstrated a correlation between fetal exposure to overnutrition and an increased risk for obesity and related metabolic diseases, such as hepatic steatosis and NAFLD [45]. In summary, a maternal HF diet leads to disrupted one-carbon (1C) metabolism in their children through epigenetic shifts, leading to metabolic gene expression changes. Methionine cycle dysfunction causes abnormal methyl group delivery to its substrate, modifying lipid metabolism through the turning off of the fatty acid oxidation process involving L-carnitine depletion and, thus, increasing the risk for offspring to develop NAFLD later in life. Moreover, a greater susceptibility of the male sex to disrupted 1C metabolism and methionine cycles has also been demonstrated [46]. Maternal overnutrition increases susceptibility to obesity and diabetes in their offspring [47]. In their study, Zhang et al. showed that maternal HF diet impairs placental fatty acid beta-oxidation (FAO) [48]. The placenta plays a central role in linking the mother and fetus, and it has been demonstrated that placental fatty acid metabolism influences the metabolic outcomes of the fetus [49]. It has been observed that maternal HF diet downregulates mRNA and protein expressions of carnitine palmitoyltransferase 2 (CPT2), an enzyme with an important role in FAO, by suppressing the AMPK/Sirt1/PGC1α signaling pathway in the placenta; therefore, decreased FAO in the placenta was related to an increased placental weight and a fetal growth restriction due to an intracellular lipid accumulation [50,51,52,53]. This would cause an increased risk of many metabolic changes in children at weaning age such as high body weight, glucose intolerance, hyperinsulinemia and hypercholesteremia.
1.7. Maternal Low-Protein Diet
The association between low-protein maternal diet and increased susceptibility to developing T2D during postnatal life has been noted in numerous human and animal studies [54]. Indeed, it has been observed that intrauterine exposure to malnutrition or inadequate nutrition is more associated with the development of reduced glucose tolerance in adult life. These observations clearly show that T2D can develop even in the absence of obesity [55,56]. In Western countries, the rise in popularity of vegan and vegetarian diets, which are characterized by a low-protein content, contributes to an increasing prevalence of maternal low-protein diets. Vegetarian mothers consume a low-protein diet [23,57] and give birth to babies with lower birth weight, exposing them to an increased risk of impaired glucose metabolism and T2D [58]. The correlation between fetal exposure to a reduced availability of nutrients and the development of impaired glucose metabolism in adulthood could be explained by the theory of the “thrifty phenotype” [59]. The dominant hypothesis in the metabolic programming of postnatal life attributes a central role to the fetal epigenome. In animal studies, it has been observed that intra-uterine exposure to a low-protein maternal diet determines modifications in methylations in the promoter regions of genes involved in glucose metabolism, influencing their expression, [60]. In studies conducted in mice exposed to low-protein diets during pregnancy, a reduction in pancreatic beta-cells and a consequent reduction in insulin synthesis in the offspring was observed. Indeed, the adequate function of pancreatic beta-cells depends both on their structural and functional integrity and a balanced nutritional rapport [61]. Consequently, the lack of protein in the maternal diet significantly contributes to the reduced secretion of insulin [62]. The reduced islet area and number of beta-cells are mainly due to the diminished expression of the FoxO1 and Pdx1 genes, or to the altered expression of the Reg1 pathway genes [63,64]. Furthermore, a low-protein maternal diet has demonstrated higher rates of beta-cell apoptosis [65,66,67] caused by increased oxidative stress and mitochondrial dysfunction [68]. Zambrano et al. have evidenced that a maternal low-protein diet was correlated to IR mainly in male rats; instead, the female rats had a greater insulin sensitivity [69]. This study hypothesized that the mechanism underlying the occurrence of IR in male rats could be mitochondrial dysfunction of pancreatic beta-cells [70]. These findings have also been observed in numerous human studies. Indeed, a higher incidence of T2D was observed in males, demonstrating that females are characterized by a greater insulin sensitivity and lower susceptibility to the onset of IR. These differences might depend on the protective effect of estrogens and this data could be supported by the fact that after menopause, the risk of T2D onset is comparable to that of men. Furthermore, sex chromosomes control adiposity and glucose homeostasis. The mechanisms by which estrogens rule insulin sensitivity are well known and consist of modifications of the signal transduction and metabolic pathways in both the central nervous system and fat, muscle and liver tissue [71]. A low-protein maternal diet not only has consequences for glucose metabolism, but also seems to affect the adequate development of skeletal muscle tissue and normal differentiation of bone marrow cells [72]. Furthermore, thyroid hormone synthesis and the hypothalamic−pituitary−gonadal axis are also influenced by a low-protein maternal diet [73]. The correlation between maternal malnutrition and thyroid dysfunction in offspring in later life has been highlighted in numerous studies. Indeed, it has been observed that a low-protein maternal diet, especially during lactation, is associated with the onset of hyperthyroidism; this could be due to an increased activity of 5-iodothyronine deiodinase, resulting in higher levels of triiodothyronine, the bioactive hormone [74]. Alterations in the reproductive function of offspring may be correlated to the impaired expression of genes associated with steroidogenesis, folliculogesis and steroid hormone receptors in the gonads [75,76]. The data in the literature show that a low-protein maternal diet leads to a decrease in the availability of essential amino acid levels in the maternal circulation and, consequently, in the fetus. Under these conditions, the fetus modifies its growth and metabolic programming. Therefore, protein intake is able to affect metabolic risk during childhood and adulthood through different pathways. Further studies are needed to completely characterize these metabolic alterations.
1.8. Role of Micronutrients
Micronutrients are nutritive substances that must be ingested in small quantities in the diet, and they are necessary for a series of physiological functions essential for the metabolism and biochemical processes of the cells [77]. An unbalanced diet may cause a reduced intake of micronutrients, resulting in negative effects on fetal growth and development (IUGR, low birth weight (LBW), congenital malformations, impaired cognitive development, immunodeficiencies) and maternal health [78,79]. Micronutrients are divided into vitamins and minerals. Impaired dietary intake or lack of any of them might lead to important consequences. Several clinical trials and perspective studies have examined the correlation of their unbalanced uptake with the risk of developing metabolic alterations during postnatal life. Particularly, a relevant inquiry has focused on the role of vitamin B12, folic acid and the levels of circulating homocysteine [80]. All of these elements play an essential role in the 1C metabolism [81]. Alterations in 1C metabolism seem to be implicated in epigenetic modifications and, consequently, in long-term metabolic programming [82]. One-carbon metabolism is characterized by interdependent metabolic pathways, such as the folate and methionine cycles, which regulate several cell cycle mechanisms, such as the synthesis of purine and pyrimidine bases and amino acids and the methylation of DNA, RNA and histones. The main mechanism underlying these metabolic pathways is represented by the transfer of a carbon unit (methyl group), and S-adenosyl methionine (SAM) represents the main coenzyme involved in methylation processes [83,84]. The one-carbon metabolism strictly depends on elements introduced with the diet, such as vitamins of group B (vitamin B9, B12, B6), methionine and betaine, each performing the function of cofactors or substrates. Vitamin B12 and vitamin B9 represent the major coenzymes for the methionine cycle, whereby homocysteine is converted to methionine by the enzyme methionine synthase following donation of the methyl group from 5-methyltetrahydrofolate, which is converted to tetrahydrofolate. In the case of vitamin B12 deficiency, 5-methyltetrahydrofolate cannot be converted to tetrahydrofolate and methionine synthesis cannot take place [85,86,87,88]. Vitamin B9 belongs to the group known as folates, a set of essential nutrients that participate in the synthesis of DNA and proteins. A deficiency of folic acid (and also of vitamin B6 and B12) induces an increase in homocysteine concentrations in the blood, a non-essential amino acid. Of note, several studies have clearly shown that high homocysteine concentrations result in neurotoxic, vasculotoxic and, therefore, teratogenic effects [89]. Vitamin B12 (also named cobalamin) is a water-soluble vitamin essential for red blood formation, homocysteine metabolism and the normal development and function of the central nervous system. Vitamin B12 and folate act as methyl donors in 1C metabolism, which influence cell growth and differentiation by DNA synthesis and epigenetic regulation [90]. Vitamin B12 and vitamin B9 deficiency is related to an increase in homocysteine levels. Another indicator of vitamin B12 deficiency is methylmalonic acid (MMA). Low vitamin B12 levels and high homocysteine levels during pregnancy are associated with an increased risk for LBW, SGA and IUGR and cardiovascular and metabolic disorders in adulthood (T2D, hypertension, coronary artery disease, etc.). Interestingly, the associations were time-dependent, showing a stronger correlation during the second trimester [91,92]. Moreover, vitamin B12 deficiency has been associated with congenital malformations such as neural tube defects, poor neurocognitive development and even maternal obesity and dyslipidemia. In addition, low maternal vitamin B12 level has been associated with lower offspring B12 concentrations in cord blood and during childhood as well as an increased risk of diabetes (IR in childhood) [93]. Indeed, an altered ratio of folate to vitamin B12 (high folate and low vitamin B12) has been associated with a high risk of gestational diabetes (GDM) with a consequent increased risk for the offspring of obesity and IR [92]. Finally, hyperhomocysteinemia could have an adverse effect on endothelial function mediated by oxidative stress. This can cause alterations in placental flow and, consequently, in fetal growth. Maher et al. have highlighted the relationship between folate and B12 status and GDM, which represents an alteration in glucose metabolism characterized by a state of IR and impaired glucose tolerance, diagnosed for the first time during the pregnancy [94,95]. The precise mechanisms linking the altered ratio folate/vitamin B12 and increased GDM risk is still not well known [96]. Vitamin B12 deficiency and blockage of methionine synthesis resulting in increased homocysteine levels may be contributing factors. A study into non-diabetic obese male and female adults found that B12 concentrations negatively correlated with fasting plasma glucose levels and the risk of IR [95,96]. Moreover, it was observed that high maternal folate concentrations at 28 weeks of gestation and low maternal B12 concentrations at 18 weeks of gestation were associated with high HOMA-IR in offspring. Consequently, the offspring of women with a combination of high folate and low B12 concentrations were found to be characterized by a significantly impaired insulin sensitivity status. These data support the idea that an inadequate or insufficient intake of micronutrients during intrauterine life leads to epigenetic changes in the fetus that may promote the development of chronic metabolic disease later in life [97]. Regarding minerals, the importance of an adequate supply of substances such as calcium, iodine, magnesium and zinc is well known. Calcium is a micronutrient that plays an important role in the bone mineralization of both the fetus and the mother, and whose action is closely related to vitamin D. Currently the recommended dose during pregnancy is 1 g/day and foods containing the highest amount of calcium are dairy products; consequently, in the case of vegetarian or vegan diets, a supplementation is recommended. Vitamin D deficiency is defined by values below 50 nmol/L according to the WHO and this is associated with several negative pregnancy and offspring outcomes, such as preeclampsia, GDM, preterm birth, LBW and SGA. Currently, the recommended daily dose is 600 IU/day. Iodine is an element present in very small amounts in our body which performs important functions for fetal growth and development. It represents the essential component of thyroid hormones, regulating carbohydrate, fat and protein metabolism, basal metabolism and development of the central nervous system. During the first weeks of gestation, the fetal thyroid hormone requirement is ensured by maternal synthesis until fetal synthesis is sufficient (about 17–19 weeks). It is very important to promptly recognize any iodine and thyroid hormone deficiency because even subclinical maternal hypothyroidism might have very serious consequences on the fetus, such as impaired neurocognitive development. The main food sources in which iodine is present are fish, meat, dairy products and iodized salt and the recommended daily dose during pregnancy is 200 μg. Magnesium is another important microelement that plays a role in glucose homeostasis. Indeed, it would seem to improve insulin sensitivity and reduce insulin resistance. An alteration in the magnesium-dependent channels may contribute to the onset of GDM. It is therefore important to ensure an adequate intake of magnesium, with the main food sources being legumes, nuts, seeds and green, leafy vegetables. In addition to magnesium, zinc would also appear to be involved in glucose metabolism and contribute to the control of GDM [97]. Thus, it is evident that micronutrients play a central role in metabolic control. Therefore, it is important to ensure an adequate intake of these substances in the diet, especially during pregnancy, in order to prevent the onset of metabolic diseases later in life and improve maternal and child health outcomes.
2. Conclusions
During the last few years, many observational studies and clinical trials on animals have been conducted which demonstrate the correlation between nutrition during various ages and the onset of glucose metabolism alterations. Therefore, the crucial importance of maternal nutrition during pregnancy and its effects on the metabolic programming of the fetus is now widely accepted. Particularly, an unbalanced maternal diet is known to correlate with both inadequate intrauterine growth and a higher risk of IR onset during postnatal life and, consequently, an increased risk of developing T2D and metabolic syndrome. In order to obtain a healthy and balanced maternal diet, an adequate supply of both macronutrients and micronutrients must be guaranteed. Particularly, it has been shown that a diet rich in foods containing low glycemic index carbohydrates, low saturated fat and an adequate protein content is correlated with better metabolic outcomes in the fetus. On the other hand, micronutrients in the maternal diet are also relevant components. Indeed, many studies have demonstrated that micronutrient deficiency, particularly vitamins B6, B9 and B12, affects the mechanisms of epigenetic regulation, promoting the development of metabolic alterations later in life. It is evident the great importance of adopting preventive strategies aimed at achieving a balanced diet in order to reduce the risk of metabolic diseases and, therefore, cardiovascular risk in adulthood. Furthermore, importance must be placed on an adequate intake of micronutrients, which has been shown to be decisive during intrauterine development.
Author Contributions
Conceptualization, A.B.; Writing—original draft, A.Q. and M.G.; Writing—review & editing, I.C. and D.I.; Methodology, C.G.; Supervision, C.G. and F.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levy-Marchal, C.; Arslanian, S.; Cutfield, W.; Sinaiko, A.; Druet, C.; Marcovecchio, M.L.; Chiarelli, F. Insulin Resistance in Children: Consensus, Perspective, and Future Directions. J. Clin. Endocrinol. Metab. 2010, 95, 5189–5198. [Google Scholar] [CrossRef]
- Tagi, V.M.; Chiarelli, F. Obesity and insulin resistance in children. Curr. Opin. Pediatr. 2020, 32, 582–588. [Google Scholar] [CrossRef]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.-H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Kurtoğlu, S.; Hatipoğlu, N.; Mazicioglu, M.M.; Kendirici, M.; Keskin, M.; Kondolot, M. Insulin Resistance in Obese Children and Adolescents: HOMA-IR Cut-Off Levels in the Prepubertal and Pubertal Periods—Original Article. J. Clin. Res. Pediatr. Endocrinol. 2010, 2, 100–106. [Google Scholar] [CrossRef]
- Ascaso, J.F.; Pardo, S.; Real, J.T.; Lorente, R.I.; Priego, A.; Carmena, R. Diagnosing Insulin Resistance by Simple Quantitative Methods in Subjects With Normal Glucose Metabolism. Diabetes Care 2003, 26, 3320–3325. [Google Scholar] [CrossRef]
- Brown, R.; Yanovski, J.A. Estimation of insulin sensitivity in children: Methods, measures and controversies. Pediatr. Diabetes 2014, 15, 151–161. [Google Scholar] [CrossRef][Green Version]
- Blasetti, A.; Franchini, S.; Comegna, L.; Prezioso, G.; Chiarelli, F. Role of nutrition in preventing insulin resistance in children. J. Pediatr. Endocrinol. Metab. 2016, 29, 247–257. [Google Scholar] [CrossRef]
- Tagi, V.M.; Giannini, C.; Chiarelli, F. Insulin Resistance in Children. Front Endocrinol (Lausanne). Front. Endocrinol. 2019, 10, 342. [Google Scholar] [CrossRef]
- Monetti, M.; Nagaraj, N.; Sharma, K.; Mann, M. Large-scale phosphosite quantification in tissues by a spike-in SILAC method. Nat. Methods 2011, 8, 655–658. [Google Scholar] [CrossRef]
- Watanabe, R.M. The Genetics of Insulin Resistance: Where’s Waldo? Curr. Diabetes Rep. 2010, 10, 476–484. [Google Scholar] [CrossRef]
- Komurcu-Bayrak, E. Impact of Genetic Polymorphisms on Insulin Resistance. In Insulin Resistance; InTech: Singapore, 2012. [Google Scholar]
- Eyzaguirre, F.; Mericq, V. Insulin Resistance Markers in Children. Horm. Res. Paediatr. 2009, 71, 65–74. [Google Scholar] [CrossRef]
- Vaiserman, A.M. Early-life nutritional programming of longevity. J. Dev. Orig. Health Dis. 2014, 5, 325–338. [Google Scholar] [CrossRef]
- Bateson, P.; Gluckman, P.; Hanson, M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J. Physiol. 2014, 592, 2357–2368. [Google Scholar] [CrossRef]
- Darendeliler, F. IUGR: Genetic influences, metabolic problems, environmental associations/triggers, current and future management. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101260. [Google Scholar] [CrossRef]
- Vaiserman, A.; Lushchak, O. Prenatal Malnutrition-Induced Epigenetic Dysregulation as a Risk Factor for Type 2 Diabetes. Int. J. Genom. 2019, 2019, 3821409. [Google Scholar] [CrossRef]
- Hales, C.; Barker, D. Type 2 (non-insulin-dependent) diabetes mellitus: The thrifty phenotype hypothesis. Int. J. Epidemiol. 2013, 42, 1215–1222. [Google Scholar] [CrossRef]
- Huang, L.T. Maternal and Early-Life Nutrition and Health. Int. J. Environ. Res. Public Health 2020, 17, 7982. [Google Scholar] [CrossRef]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal programming of the metabolic syndrome. Taiwan J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef]
- Ryznar, R.J.; Phibbs, L.; Van Winkle, L.J. Epigenetic Modifications at the Center of the Barker Hypothesis and Their Transgenerational Implications. Int. J. Environ. Res. Public Health 2021, 18, 12728. [Google Scholar] [CrossRef]
- Wells, J.C.K. The thrifty phenotype as an adaptive maternal effect. Biol. Rev. 2007, 82, 143–172. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J.P. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Kislal, S.; Shook, L.L.; Edlow, A.G. Perinatal exposure to maternal obesity: Lasting cardiometabolic impact on offspring. Prenat. Diagn. 2020, 40, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Garde, E.; Lopez-Bermejo, A.; de Zegher, F.; Ibañez, L. Differential DNA methylation profile in infants born small-for-gestational-age: Association with markers of adiposity and insulin resistance from birth to age 24 months. BMJ Open Diabetes Res. Care 2020, 8, e001402. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Hagström, H.; Simon, T.G.; Roelstraete, B.; Stephansson, O.; Söderling, J.; Ludvigsson, J.F. Maternal obesity increases the risk and severity of NAFLD in offspring. J. Hepatol. 2021, 75, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhang, Y.; Wang, L.; Yang, W.; Li, C.; Gu, P.; Xia, Y.; Yan, J.; Shen, Y.; Zhao, Q.; et al. Maternal dietary glycaemic change during gestation influences insulin-related gene methylation in the placental tissue: A genome-wide methylation analysis. Genes Nutr. 2019, 14, 17. [Google Scholar] [CrossRef]
- Jenkins avid, J.A.; Mente, A.; Bangdiwala, I.S.; Rangarajan, S.; Srichaikul, K.; Mohan, V.; Avezum, A.; Díaz, R.; Rosengren, A.; Lanas, F.; et al. Glycemic Index, Glycemic Load, and Cardiovascular Disease and Mortality. N. Engl. J. Med. 2021, 385, 378–380. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- Choudhury, R.P.; Akbar, N. Beyond diabetes: A relationship between cardiovascular outcomes and glycaemic index. Cardiovasc. Res. 2021, 117, e97–e98. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; A Khan, T.; Blanco, S.; Mejia; Mirrahimi, A.; A Jenkins, D.J.; Livesey, G.; et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: Systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374, n1651. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.I.; E Mills, K.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.-L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Maslova, E.; Hansen, S.; Grunnet, L.G.; Strøm, M.; Bjerregaard, A.A.; Hjort, L.; Kampmann, F.B.; Madsen, C.M.; Baun Thuesen, A.C.; Bech, B.H.; et al. Maternal glycemic index and glycemic load in pregnancy and offspring metabolic health in childhood and adolescence—A cohort study of 68,471 mother–offspring dyads from the Danish National Birth Cohort. Eur. J. Clin. Nutr. 2019, 73, 1049–1062. [Google Scholar] [CrossRef] [PubMed]
- Sideratou, T.; Atkinson, F.; Campbell, G.J.; Petocz, P.; Bell-Anderson, K.S.; Brand-Miller, J. Glycaemic Index of Maternal Dietary Carbohydrate Differentially Alters Fto and Lep Expression in Offspring in C57BL/6 Mice. Nutrients 2018, 10, 1342. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S. Leptin Function and Regulation. In Comprehensive Physiology; Wiley: New York, NY, USA, 2017; pp. 351–369. [Google Scholar]
- Hoggard, N.; Haggarty, P.; Thomas, L.; Lea, R. Leptin expression in placental and fetal tissues: Does leptin have a functional role? Biochem. Soc. Trans. 2001, 29, 57. [Google Scholar] [CrossRef]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and obesity: Role and clinical implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Toh, H.; Thomson, J.A.; Jiang, P. Maternal High-Fiber Diet Protects Offspring against Type 2 Diabetes. Nutrients 2020, 13, 94. [Google Scholar] [CrossRef]
- Gawlińska, K.; Gawliński, D.; Filip, M.; Przegaliński, E. Przegaliński, E. Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr. Rev. 2021, 79, 709–725. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Hsu, C.-N. Maternal High-Fat Diet and Offspring Hypertension. Int. J. Mol. Sci. 2022, 23, 8179. [Google Scholar] [CrossRef]
- Saullo, C.; da Cruz, L.L.; Damasceno, D.C.; Volpato, G.T.; Sinzato, Y.K.; Karki, B.; Gallego, F.Q.; Vesentini, G. Effects of a maternal high-fat diet on adipose tissue in murine offspring: A systematic review and meta-analysis. Biochimie 2022, 201, 18–32. [Google Scholar] [CrossRef]
- Barbosa, C.M.; Figueiredo, V.P.; Barbosa, M.A.; Cardoso, L.M.; Alzamora, A.C. Maternal high-fat diet triggers metabolic syndrome disorders that are transferred to first and second offspring generations. Br. J. Nutr. 2020, 123, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Xu, H.; Wu, J.; Li, J.; Zhou, Y.; Ding, Z.; Siwko, S.K.; Yuan, X.; Schalinske, K.L.; Alpini, G.; et al. Maternal high-fat diet disrupted one-carbon metabolism in offspring, contributing to nonalcoholic fatty liver disease. Liver Int. 2021, 41, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, J.; Xu, H.; Wang, X.; He, L.; McCauley, N.; Zhang, K.K.; Xie, L. Offspring NAFLD Liver Phospholipid Profiles are Differentially Programmed by Maternal High-fat Diet and Maternal One Carbon Supplement. J. Nutr. Biochem. 2022, 111, 109187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Wu, H.; Gao, Y.; Zheng, J.; Zhang, J. Maternal High-Fat Diet Impairs Placental Fatty Acid β-Oxidation and Metabolic Homeostasis in the Offspring. Front Nutr. 2022, 9, 849684. [Google Scholar] [CrossRef]
- Duttaroy, A.K.; Basak, S. Maternal dietary fatty acids and their roles in human placental development. Prostaglandins Leukot. Essent. Fat. Acids 2020, 155, 102080. [Google Scholar] [CrossRef]
- Lewis, R.; Desoye, G. Placental Lipid and Fatty Acid Transfer in Maternal Overnutrition. Ann. Nutr. Metab. 2017, 70, 228–231. [Google Scholar] [CrossRef]
- Perazzolo, S.; Hirschmugl, B.; Wadsack, C.; Desoye, G.; Lewis, R.M.; Sengers, B.G. The influence of placental metabolism on fatty acid transfer to the fetus. J. Lipid Res. 2017, 58, 443–454. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Cook, T.; Knipp, G.T. Effect of Placental Fatty Acid Metabolism and Regulation by Peroxisome Proliferator Activated Receptor on Pregnancy and Fetal Outcomes. J. Pharm. Sci. 2007, 96, 2582–2606. [Google Scholar] [CrossRef]
- Lewis, R.M.; Wadsack, C.; Desoye, G. Placental fatty acid transfer. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 78–82. [Google Scholar] [CrossRef]
- Vipin, V.A.; Blesson, C.S.; Yallampalli, C. Maternal low protein diet and fetal programming of lean type 2 diabetes. World J. Diabetes 2022, 13, 185–202. [Google Scholar] [CrossRef]
- George, A.M.; Jacob, A.G.; Fogelfeld, L. Lean diabetes mellitus: An emerging entity in the era of obesity. World J. Diabetes 2015, 6, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Ranabir, S.; Barma, P.; Prasad, L.; Singh, T. Clinical and biochemical profile of lean type 2 diabetes mellitus. Indian J. Endocrinol. Metab. 2011, 15, S40. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, G.B.; Clari, R.; Vigotti, F.N.; Leone, F.; Attini, R.; Cabiddu, G.; Mauro, G.; Castelluccia, N.; Colombi, N.; Capizzi, I.; et al. Vegan-vegetarian diets in pregnancy: Danger or panacea? A systematic narrative review. BJOG Int. J. Obstet. Gynaecol. 2015, 122, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Sanders, T.A.; Obeid, O. The influence of maternal vegetarian diet on essential fatty acid status of the newborn. Eur. J. Clin. Nutr. 1994, 48, 358–368. [Google Scholar] [PubMed]
- Sebastiani, G.; Barbero, A.H.; Borràs-Novell, C.; Alsina, M.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Tutusaus, M.P.; Martínez, S.F.; Gómez-Roig, M.D.; García-Algar, Ó. The Effects of Vegetarian and Vegan Diet during Pregnancy on the Health of Mothers and Offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, K.; Lillycrop, K.A.; Silver, M.J. Fetal programming and epigenetics. Curr. Opin. Endocr. Metab. Res. 2020, 13, 1–6. [Google Scholar] [CrossRef]
- Cox, A.R.; Beamish, C.A.; Carter, D.E.; Arany, E.J.; Hill, D.J. Cellular mechanisms underlying failed beta cell regeneration in offspring of protein-restricted pregnant mice. Exp. Biol. Med. 2013, 238, 1147–1159. [Google Scholar] [CrossRef]
- Dumortier, O.; Blondeau, B.; Duvillié, B.; Reusens, B.; Bréant, B.; Remacle, C. Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet. Diabetologia 2007, 50, 2495–2503. [Google Scholar] [CrossRef]
- Rees, W.D.; Hay, S.M.; Cruickshank, M.; Reusens, B.; Remacle, C.; Antipatis, C.; Grant, G. Maternal protein intake in the pregnant rat programs the insulin axis and body composition in the offspring. Metabolism 2006, 55, 642–649. [Google Scholar] [CrossRef]
- Sparre, T.; Reusens, B.; Cherif, H.; Larsen, M.R.; Roepstorff, P.; Fey, S.J.; Larsen, P.M.; Remacle, C.; Nerup, J. Intrauterine programming of fetal islet gene expression in rats?effects of maternal protein restriction during gestation revealed by proteome analysis. Diabetologia 2003, 46, 1497–1511. [Google Scholar] [CrossRef]
- Mateus Gonçalves, L.; Vettorazzi, J.F.; Vanzela, E.C.; Figueiredo, M.S.; Batista, T.M.; Zoppi, C.C.; Boschero, A.C.; Carneiro, E.M. Amino acid restriction increases β-cell death under challenging conditions. J. Cell Physiol. 2019, 234, 16679–16684. [Google Scholar] [CrossRef] [PubMed]
- Merezak, S.; A Hardikar, A.; Yajnik, C.S.; Remacle, C.; Reusens, B. Intrauterine low protein diet increases fetal beta-cell sensitivity to NO and IL-1 beta: The protective role of taurine. J. Endocrinol. 2001, 171, 299–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alejandro, E.U.; Gregg, B.; Wallen, T.; Kumusoglu, D.; Meister, D.; Chen, A.; Merrins, M.J.; Satin, L.S.; Liu, M.; Arvan, P.; et al. Maternal diet–induced microRNAs and mTOR underlie β cell dysfunction in offspring. J. Clin. Investig. 2014, 124, 4395–4410. [Google Scholar] [CrossRef] [PubMed]
- Theys, N.; Bouckenooghe, T.; Ahn, M.T.; Remacle, C.; Reusens, B. Maternal low-protein diet alters pancreatic islet mitochondrial function in a sex-specific manner in the adult rat. Am. J. Physiol. Integr. Comp. Physiol. 2009, 297, R1516–R1525. [Google Scholar] [CrossRef]
- Zambrano, E.; Bautista, C.J.; Deás, M.; Martínez-Samayoa, P.M.; González-Zamorano, M.; Ledesma, H.; Morales, J.; Larrea, F.; Nathanielsz, P.W. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J. Physiol. 2006, 571, 221–230. [Google Scholar] [CrossRef]
- Vistisen, B.; I Hellgren, L.; Vadset, T.; Scheede-Bergdahl, C.; Helge, J.W.; Dela, F.; Stallknecht, B. Effect of gender on lipid-induced insulin resistance in obese subjects. Eur. J. Endocrinol. 2008, 158, 61–68. [Google Scholar] [CrossRef]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T., Jr. Sex-Specific Differences in Lipid and Glucose Metabolism. Front. Endocrinol. 2015, 5, 241. [Google Scholar] [CrossRef]
- Oreffo, R.O.; Lashbrooke, B.; I Roach, H.; Clarke, N.M.; Cooper, C. Maternal protein deficiency affects mesenchymal stem cell activity in the developing offspring. Bone 2003, 33, 100–107. [Google Scholar] [CrossRef]
- Lisboa, P.C.; Oliveira, E.; Fagundes, A.S.; Santos-Silva, A.P.; Conceição, E.S.; Passos, M.F.; Moura, E.G. Postnatal Low Protein Diet Programs Leptin Signaling in the Hypothalamic-Pituitary-Thyroid Axis and Pituitary TSH Response to Leptin in Adult Male Rats. Horm. Metab. Res. 2012, 44, 114–122. [Google Scholar] [CrossRef]
- Lisboa, P.C.; Fagundes, A.T.S.; Denolato, A.T.A.; Oliveira, E.; Bonomo, I.T.; Alves, S.B.; Curty, F.H.; Passos, M.C.F.; Moura, E. Neonatal Low-Protein Diet Changes Deiodinase Activities and Pituitary TSH Response to TRH in Adult Rats. Exp. Biol. Med. 2008, 233, 57–63. [Google Scholar] [CrossRef]
- Guzmán, C.; García-Becerra, R.; Aguilar-Medina, M.A.; Méndez, I.; Merchant-Larios, H.; Zambrano, E. Maternal Protein Restriction During Pregnancy and/or Lactation Negatively Affects Follicular Ovarian Development and Steroidogenesis in the Prepubertal Rat Offspring. Arch. Med Res. 2014, 45, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Jia, Y.; He, B.; Li, R.; Li, X.; Cai, D.; Song, H.; Zhang, R.; Zhao, R. Maternal Low-protein Diet Alters Ovarian Expression of Folliculogenic and Steroidogenic Genes and Their Regulatory MicroRNAs in Neonatal Piglets. Asian-Australas. J. Anim. Sci. 2014, 27, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Hardy, G.; Wong, T.; Morrissey, H.; Anderson, C.; Moltu, S.J.; Poindexter, B.; Lapillonne, A.; Ball, P.A. Parenteral Provision of Micronutrients to Pediatric Patients: An International Expert Consensus Paper. J. Parenter. Enter. Nutr. 2020, 44, S5–S23. [Google Scholar] [CrossRef] [PubMed]
- Fall, C.H.; Yajnik, C.S.; Rao, S.; Davies, A.A.; Brown, N.; Farrant, H.J. Micronutrients and Fetal Growth. J. Nutr. 2003, 133, 1747S–1756S. [Google Scholar] [CrossRef]
- McArdle, H.J.; Ashworth, C.J. Micronutrients in fetal growth and development. Br. Med. Bull. 1999, 55, 499–510. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Rush, E.C.; Katre, P.; Yajnik, C.S. Vitamin B12: One carbon metabolism, fetal growth and programming for chronic disease. Eur. J. Clin. Nutr. 2014, 68, 2–7. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Interventions with Vitamins B6, B12 and C in Pregnancy. Paediatr. Périnat. Epidemiol. 2012, 26, 55–74. [Google Scholar] [CrossRef]
- Molloy, A.M.; Kirke, P.N.; Brody, L.C.; Scott, J.M.; Mills, J.L. Effects of Folate and Vitamin B 12 Deficiencies During Pregnancy on Fetal, Infant, and Child Development. Food Nutr. Bull. 2008, 29 (Suppl. S1), S101–S111. [Google Scholar] [CrossRef]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle—Biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Jankovic-Karasoulos, T.; Furness, D.L.; Leemaqz, S.Y.; Dekker, G.A.; Grzeskowiak, L.E.; Grieger, J.A.; Andraweera, P.H.; McCullough, D.; McAninch, D.; McCowan, L.M.; et al. Maternal folate, one-carbon metabolism and pregnancy outcomes. Matern. Child Nutr. 2021, 17, e13064. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, U.; Katre, P.; Yajnik, C.S. Influence of Maternal Vitamin B12 and Folate on Growth and Insulin Resistance in the Offspring. In Maternal and Child Nutrition: The First 1000 Days; Karger Publishers: Basel, Switzerland, 2013; pp. 145–156. [Google Scholar]
- Sijilmassi, O. Folic acid deficiency and vision: A review. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Furness, D.; Fenech, M.; Dekker, G.; Khong, T.Y.; Roberts, C.; Hague, W. Folate, Vitamin B12, Vitamin B6 and homocysteine: Impact on pregnancy outcome. Matern. Child Nutr. 2013, 9, 155–166. [Google Scholar] [CrossRef]
- Dai, C.; Fei, Y.; Li, J.; Shi, Y.; Yang, X. A Novel Review of Homocysteine and Pregnancy Complications. BioMed Res. Int. 2021, 2021, 6652231. [Google Scholar] [CrossRef]
- Lai, J.S.; Pang, W.W.; Cai, S.; Lee, Y.S.; Chan, J.K.; Shek, L.P.; Yap, F.K.; Tan, K.H.; Godfrey, K.M.; van Dam, R.M.; et al. High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin. Nutr. 2018, 37, 940–947. [Google Scholar] [CrossRef]
- Wang, Z.P.; Shang, X.X.; Zhao, Z.T. Low maternal vitamin B12 is a risk factor for neural tube defects: A meta-analysis. J. Matern. Fetal Neonatal Med. 2012, 25, 389–394. [Google Scholar] [CrossRef]
- Shub, A. Diabetes and pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 2020, 60, 829–830. [Google Scholar] [CrossRef]
- Lebovitz, H. Insulin resistance: Definition and consequences. Exp. Clin. Endocrinol. Diabetes 2001, 109 (Suppl. S2), S135–S148. [Google Scholar] [CrossRef]
- Paul, L.; Selhub, J. Interaction between excess folate and low vitamin B12 status. Mol. Asp. Med. 2017, 53, 43–47. [Google Scholar] [CrossRef]
- Adaikalakoteswari, A.; Vatish, M.; Alam, M.T.; Ott, S.; Kumar, S.; Saravanan, P. Low Vitamin B12 in Pregnancy Is Associated With Adipose-Derived Circulating miRs Targeting PPARγ and Insulin Resistance. J. Clin. Endocrinol. Metab. 2017, 102, 4200–4209. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.P.; Christian, P.; Schulze, K.J.; Arguello, M.; LeClerq, S.C.; Khatry, S.K.; West, J.K.P. Low maternal vitamin B-12 status is associated with offspring insulin resistance regardless of antenatal micronutrient supplementation in rural Nepal. J. Nutr. 2011, 141, 1912–1917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Zhang, J.; Guan, Y.; Xing, Y. Early supplementation of folate and vitamin B12 improves insulin resistance in intrauterine growth retardation rats. Transl. Pediatr. 2022, 11, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.; Wright, C. Safety and efficacy of supplements in pregnancy. Nutr. Rev. 2020, 78, 813–826. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).