Real-World Intake of Dietary Sugars Is Associated with Reduced Cortisol Reactivity Following an Acute Physiological Stressor
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Pre-Screening Questionnaires
2.2.2. Australian Eating Survey Food Frequency Questionnaire (AES FFQ)
2.2.3. Cold Pressor Test (CPT)
2.2.4. Saliva Sampling and Analysis
2.3. Procedure
2.4. Statistical Analyses
3. Results
- Step 1: Cortisol ~ 1 + (1 | Participant)
- Step 2: Cortisol ~ 1 + Time + I(Time2) + (1 + Time + I(Time2) | Participant)
- Step 3: Cortisol ~ 1 + Time + Gender + Saturated Fats + Sugars + BMI + I(Time2) + (1 + Time + I(Time2) | Participant)
- Step 4: Cortisol~1 + Time + Gender + Saturated Fats + Sugars + BMI + I(Time2) + Time:Saturated Fats + Time:Sugars + I(Time2):Saturated Fats + I(Time2):Sugars + (1 + Time + I(Time2) | Participant)
4. Discussion
4.1. General Discussion
4.2. Limitations
4.3. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sparling, T.M.; Cheng, B.; Deeney, M.; Santoso, M.V.; Pfeiffer, E.; Emerson, J.A.; Amadi, F.M.; Mitu, K.; Corvalan, C.; Verdeli, H.; et al. Global Mental Health and Nutrition: Moving Toward a Convergent Research Agenda. Front. Public Health 2021, 9, 722290. [Google Scholar] [CrossRef] [PubMed]
- Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; Abegaz, K.H.; Abolhassani, H.; Aboyans, V.; et al. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities to Reduce the Global Crises of Obesity and Diabetes. Nat. Food 2020, 1, 38–50. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and Evolution of the Western Diet: Health Implications for the 21st Century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Adan, R.A.H.; van der Beek, E.M.; Buitelaar, J.K.; Cryan, J.F.; Hebebrand, J.; Higgs, S.; Schellekens, H.; Dickson, S.L. Nutritional Psychiatry: Towards Improving Mental Health by What You Eat. Eur. Neuropsychopharmacol. 2019, 29, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. A Review of Lifestyle Factors That Contribute to Important Pathways Associated with Major Depression: Diet, Sleep and Exercise. J. Affect. Disord. 2013, 148, 12–27. [Google Scholar] [CrossRef]
- Firth, J.; Siddiqi, N.; Koyanagi, A.; Siskind, D.; Rosenbaum, S.; Galletly, C.; Allan, S.; Caneo, C.; Carney, R.; Carvalho, A.F.; et al. The Lancet Psychiatry Commission: A Blueprint for Protecting Physical Health in People with Mental Illness. Lancet Psychiatry 2019, 6, 675–712. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Teasdale, S.B.; Ward, P.B.; Veronese, N.; Shivappa, N.; Hebert, J.R.; Berk, M.; Yung, A.R.; Sarris, J. Diet as a Hot Topic in Psychiatry: A Population-Scale Study of Nutritional Intake and Inflammatory Potential in Severe Mental Illness. World Psychiatry 2018, 17, 365–367. [Google Scholar] [CrossRef]
- Li, Y.; Lv, M.R.; Wei, Y.J.; Sun, L.; Zhang, J.X.; Zhang, H.G.; Li, B. Dietary Patterns and Depression Risk: A Meta-Analysis. Psychiatry Res. 2017, 253, 373–382. [Google Scholar] [CrossRef]
- Rahe, C.; Unrath, M.; Berger, K. Dietary Patterns and the Risk of Depression in Adults: A Systematic Review of Observational Studies. Eur. J. Nutr. 2014, 53, 997–1013. [Google Scholar] [CrossRef]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional Psychiatry: The Present State of the Evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N. Nutritional Psychiatry: Where to Next? EBioMedicine 2017, 17, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.; Molero, P.; Ortuño Sánchez-Pedreño, F.; Van der Does, W.; Angel Martínez-González, M. Diet Quality and Depression Risk: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef]
- Malhi, G.S.; Bassett, D.; Boyce, P.; Bryant, R.; Fitzgerald, P.B.; Fritz, K.; Hopwood, M.; Lyndon, B.; Mulder, R.; Murray, G.; et al. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines for Mood Disorders. Aust. N. Z. J. Psychiatry 2015, 49, 1087–1206. [Google Scholar] [CrossRef]
- Andrews, G.; Bell, C.; Boyce, P.; Gale, C.; Lampe, L.; Marwat, O.; Rapee, R.; Wilkins, G. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines for the Treatment of Panic Disorder, Social Anxiety Disorder and Generalised Anxiety Disorder. Aust. N. Z. J. Psychiatry 2018, 52, 1109–1172. [Google Scholar] [CrossRef]
- Bremner, J.; Moazzami, K.; Wittbrodt, M.; Nye, J.; Lima, B.; Gillespie, C.; Rapaport, M.; Pearce, B.; Shah, A.; Vaccarino, V. Diet, Stress and Mental Health. Nutrients 2020, 12, 2428. [Google Scholar] [CrossRef]
- Arab, A.; Mehrabani, S.; Moradi, S.; Amani, R. The Association between Diet and Mood: A Systematic Review of Current Literature. Psychiatry Res. 2019, 271, 428–437. [Google Scholar] [CrossRef]
- Dionysopoulou, S.; Charmandari, E.; Bargiota, A.; Vlachos, N.; Mastorakos, G.; Valsamakis, G. The Role of Hypothalamic Inflammation in Diet-Induced Obesity and Its Association with Cognitive and Mood Disorders. Nutrients 2021, 13, 498. [Google Scholar] [CrossRef]
- Morera, L.P.; Marchiori, G.N.; Medrano, L.A.; Defagó, M.D. Stress, Dietary Patterns and Cardiovascular Disease: A Mini-Review. Front. Neurosci. 2019, 13, 1226. [Google Scholar] [CrossRef]
- Schweren, L.J.S.; Larsson, H.; Vinke, P.C.; Li, L.; Kvalvik, L.G.; Arias-Vasquez, A.; Haavik, J.; Hartman, C.A. Diet Quality, Stress and Common Mental Health Problems: A Cohort Study of 121,008 Adults. Clin. Nutr. 2021, 40, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Herbison, C.E.; Allen, K.; Robinson, M.; Newnham, J.; Pennell, C. The Impact of Life Stress on Adult Depression and Anxiety Is Dependent on Gender and Timing of Exposure. Dev. Psychopathol. 2017, 29, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Kendler, K.S.; Karkowski, L.M.; Prescott, C.A. Causal Relationship between Stressful Life Events and the Onset of Major Depression. Am. J. Psychiatry 1999, 156, 837–841. [Google Scholar] [CrossRef]
- Plieger, T.; Melchers, M.; Montag, C.; Meermann, R.; Reuter, M. Life Stress as Potential Risk Factor for Depression and Burnout. Burn. Res. 2015, 2, 19–24. [Google Scholar] [CrossRef]
- Epel, E.; McEwen, B.; Seeman, T.; Matthews, K.; Castellazzo, G.; Brownell, K.D.; Bell, J.; Ickovics, J.R. Stress and Body Shape: Stress-Induced Cortisol Secretion Is Consistently Greater among Women with Central Fat. Psychosom. Med. 2000, 62, 623–632. [Google Scholar] [CrossRef]
- Epel, E.; Lapidus, R.; McEwen, B.; Brownell, K. Stress May Add Bite to Appetite in Women: A Laboratory Study of Stress-Induced Cortisol and Eating Behavior. Psychoneuroendocrinology 2001, 26, 37–49. [Google Scholar] [CrossRef]
- Oliver, G.; Wardle, J.; Gibson, E.L. Stress and Food Choice: A Laboratory Study. Psychosom. Med. 2000, 62, 853–865. [Google Scholar] [CrossRef]
- Zellner, D.A.; Loaiza, S.; Gonzalez, Z.; Pita, J.; Morales, J.; Pecora, D.; Wolf, A. Food Selection Changes under Stress. Physiol. Behav. 2006, 87, 789–793. [Google Scholar] [CrossRef]
- Zellner, D.A.; Saito, S.; Gonzalez, J. The Effect of Stress on Men’s Food Selection. Appetite 2007, 49, 696–699. [Google Scholar] [CrossRef]
- Adam, T.C.; Epel, E.S. Stress, Eating and the Reward System. Physiol. Behav. 2007, 91, 449–458. [Google Scholar] [CrossRef]
- Dallman, M.F.; Pecoraro, N.; Akana, S.F.; La Fleur, S.E.; Gomez, F.; Houshyar, H.; Bell, M.E.; Bhatnagar, S.; Laugero, K.D.; Manalo, S. Chronic Stress and Obesity: A New View of “Comfort Food”. Proc. Natl. Acad. Sci. USA 2003, 100, 11696–11701. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, N.; Reyes, F.; Gomez, F.; Bhargava, A.; Dallman, M.F. Chronic Stress Promotes Palatable Feeding, Which Reduces Signs of Stress: Feedforward and Feedback Effects of Chronic Stress. Endocrinology 2004, 145, 3754–3762. [Google Scholar] [CrossRef]
- Wilson, M.E.; Fisher, J.; Fischer, A.; Lee, V.; Harris, R.B.; Bartness, T.J. Quantifying Food Intake in Socially Housed Monkeys: Social Status Effects on Caloric Consumption. Physiol. Behav. 2008, 94, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Arce, M.; Michopoulos, V.; Shepard, K.N.; Ha, Q.C.; Wilson, M.E. Diet Choice, Cortisol Reactivity, and Emotional Feeding in Socially Housed Rhesus Monkeys. Physiol. Behav. 2010, 101, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, J.A.; Finch, L.E.; Cummings, J.R. Did That Brownie Do Its Job? Stress, Eating, and the Biobehavioral Effects of Comfort Food. In Emerging Trends in the Social and Behavioral Sciences; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 1–15. [Google Scholar] [CrossRef]
- Finch, L.E.; Tomiyama, A.J. Stress-Induced Eating Dampens Physiological and Behavioral Stress Responses. In Nutrition in the Prevention and Treatment of Abdominal Obesity; Academic Press: Cambridge, MA, USA, 2014; pp. 189–195. [Google Scholar] [CrossRef]
- Gonzalez, M.J.; Miranda-Massari, J.R. Diet and Stress. Psychiatr. Clin. North Am. 2014, 37, 579–589. [Google Scholar] [CrossRef]
- Singh, K. Nutrient and Stress Management. J. Nutr. Food Sci. 2016, 6, 4. [Google Scholar] [CrossRef]
- Appiakannan, H.S.; Rasimowicz, M.L.; Harrison, C.B.; Weber, E.T. Differential Effects of High-Fat Diet on Glucose Tolerance, Food Intake, and Glucocorticoid Regulation in Male C57BL/6J and BALB/CJ Mice. Physiol. Behav. 2020, 215, 112773. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, B.M.; Brindley, D.N.; Tannenbaum, G.S.; Dallman, M.F.; McArthur, M.D.; Meaney, M.J. High-Fat Feeding Alters Both Basal and Stress-Induced Hypothalamic- Pituitary-Adrenal Activity in the Rat. Am. J. Physiol.-Endocrinol. Metab. 1997, 273, 1168–1177. [Google Scholar] [CrossRef]
- Kamara, K.; Eskay, R.; Castonguay, T. High-Fat Diets and Stress Responsivity. Physiol. Behav. 1998, 64, 1–6. [Google Scholar] [CrossRef]
- Legendre, A.; Harris, R.B.S. Exaggerated Response to Mild Stress in Rats Fed High-Fat Diet. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 291, 1288–1294. [Google Scholar] [CrossRef]
- von Dawans, B.; Zimmer, P.; Domes, G. Effects of Glucose Intake on Stress Reactivity in Young, Healthy Men. Psychoneuroendocrinology 2021, 126, 105062. [Google Scholar] [CrossRef] [PubMed]
- Zänkert, S.; Kudielka, B.M.; Wüst, S. Effect of Sugar Administration on Cortisol Responses to Acute Psychosocial Stress. Psychoneuroendocrinology 2020, 115, 104607. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bono, E.; Rohleder, N.; Hellhammer, D.H.; Salvador, A.; Kirschbaum, C. Glucose but Not Protein or Fat Load Amplifies the Cortisol Response to Psychosocial Stress. Horm. Behav. 2002, 41, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Straznicky, N.E.; Louis, W.J.; McGrade, P.; Howes, L.G. The Effects of Dietary Lipid Modification on Blood Pressure, Cardiovascular Reactivity and Sympathetic Activity in Man. J. Hypertens. 1993, 11, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Jakulj, F.; Zernicke, K.; Bacon, S.L.; Van Wielingen, L.E.; Key, B.L.; West, S.G.; Campbell, T.S. A High-Fat Meal Increases Cardiovascular Reactivity to Psychological Stress in Healthy Young Adults. J. Nutr. 2007, 137, 935–939. [Google Scholar] [CrossRef]
- La Fleur, S.E.; Houshyar, H.; Roy, M.; Dallman, M.F. Choice of Lard, but Not Total Lard Calories, Damps Adrenocorticotropin Responses to Restraint. Endocrinology 2005, 146, 2193–2199. [Google Scholar] [CrossRef]
- Foster, M.T.; Warne, J.P.; Ginsberg, A.B.; Horneman, H.F.; Pecoraro, N.C.; Akana, S.F.; Dallman, M.F. Palatable Foods, Stress, and Energy Stores Sculpt Corticotropin-Releasing Factor, Adrenocorticotropic and Corticosterone Concentrations after Restraint. Endocrinology 2009, 150, 2325–2333. [Google Scholar] [CrossRef]
- Tryon, M.S.; Stanhope, K.L.; Epel, E.S.; Mason, A.E.; Brown, R.; Medici, V.; Havel, P.J.; Laugero, K.D. Excessive Sugar Consumption May Be a Difficult Habit to Break: A View from the Brain and Body. J. Clin. Endocrinol. Metab. 2015, 100, 2239–2247. [Google Scholar] [CrossRef]
- Schwabe, L.; Haddad, L.; Schachinger, H. HPA Axis Activation by a Socially Evaluated Cold-Pressor Test. Psychoneuroendocrinology 2008, 33, 890–895. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Gold, P.W. The Concepts of Stress and Stress System Disorders: Overview of Physical and Behavioral Homeostasis. JAMA J. Am. Med. Assoc. 1992, 267, 1244–1252. [Google Scholar] [CrossRef]
- Zänkert, S.; Bellingrath, S.; Wüst, S.; Kudielka, B.M. HPA Axis Responses to Psychological Challenge Linking Stress and Disease: What Do We Know on Sources of Intra- and Interindividual Variability? Psychoneuroendocrinology 2019, 105, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Flaa, A.; Ekeberg, Ø.; Kjeldsen, S.E.; Rostrup, M. Personality May Influence Reactivity to Stress. Biopsychosoc. Med. 2007, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Snieder, H.; de Geus, E. Genetic Influences on Cardiovascular Stress Reactivity. Neurosci. Biobehav. Rev. 2010, 35, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Stylianakis, A.A. The Effect of Chronic Stress on the Adolescent Brain, Learning, and Memory. PhD Thesis, University of New South Wales, Sydney, NSW, Australia, 2021. [Google Scholar] [CrossRef]
- Lovibond, S.H.; Lovibond, P.F. Manual for the Depression Anxiety Stress Scales, 2nd ed.; Psychology Foundation: Sydney, Australia, 1995; ISBN 7334-1423-0. [Google Scholar]
- Aleknaviciute, J.; Tulen, J.H.M.; De Rijke, Y.B.; Bouwkamp, C.G.; van der Kroeg, M.; Timmermans, M.; Wester, V.L.; Bergink, V.; Hoogendijk, W.J.G.; Tiemeier, H.; et al. The Levonorgestrel-Releasing Intrauterine Device Potentiates Stress Reactivity. Psychoneuroendocrinology 2017, 80, 39–45. [Google Scholar] [CrossRef]
- Hill, E.E.; Zack, E.; Battaglini, C.; Viru, M.; Viru, A.; Hackney, A.C. Exercise and Circulating Cortisol Levels: The Intensity Threshold Effect. J. Endocrinol. Investig. 2008, 31, 587–591. [Google Scholar] [CrossRef]
- Lovallo, W.R.; Whitsett, T.L.; Al’Absi, M.; Sung, B.H.; Vincent, A.S.; Wilson, M.F. Caffeine Stimulation of Cortisol Secretion across the Waking Hours in Relation to Caffeine Intake Levels. Psychosom. Med. 2005, 67, 734–739. [Google Scholar] [CrossRef]
- Stachowicz, M.; Lebiedzińska, A. The Effect of Diet Components on the Level of Cortisol. Eur. Food Res. Technol. 2016, 242, 2001–2009. [Google Scholar] [CrossRef]
- Watson, J.F.; Collins, C.E.; Sibbritt, D.W.; Dibley, M.J.; Garg, M.L. Reproducibility and Comparative Validity of a Food Frequency Questionnaire for Australian Children and Adolescents. Int. J. Behav. Nutr. Phys. Act. 2009, 6, 62. [Google Scholar] [CrossRef]
- Collins, C.E.; Burrows, T.L.; Truby, H.; Morgan, P.J.; Wright, I.M.R.; Davies, P.S.W.; Callister, R. Comparison of Energy Intake in Toddlers Assessed by Food Frequency Questionnaire and Total Energy Expenditure Measured by the Doubly Labeled Water Method. J. Acad. Nutr. Diet. 2013, 113, 459–463. [Google Scholar] [CrossRef]
- Collins, C.E.; Burrows, T.L.; Rollo, M.E.; Boggess, M.M.; Watson, J.F.; Guest, M.; Duncanson, K.; Pezdirc, K.; Hutchesson, M.J. The Comparative Validity and Reproducibility of a Diet Quality Index for Adults: The Australian Recommended Food Score. Nutrients 2015, 7, 785–798. [Google Scholar] [CrossRef]
- Collins, C.E.; Boggess, M.M.; Watson, J.F.; Guest, M.; Duncanson, K.; Pezdirc, K.; Rollo, M.; Hutchesson, M.J.; Burrows, T.L. Reproducibility and Comparative Validity of a Food Frequency Questionnaire for Australian Adults. Clin. Nutr. 2014, 33, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.; Watson, J.; Burrows, T.; Guest, M.; Collins, C.E. The Development and Evaluation of the Australian Child and Adolescent Recommended Food Score: A Cross-Sectional Study. Nutr. J. 2012, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Goldfein, K.R.; Slavin, J.L. Why Sugar Is Added to Food: Food Science 101. Compr. Rev. Food Sci. Food Saf. 2015, 14, 644–656. [Google Scholar] [CrossRef]
- Hess, J.; Latulippe, M.E.; Ayoob, K.; Slavin, J. The Confusing World of Dietary Sugars: Definitions, Intakes, Food Sources and International Dietary Recommendations. Food Funct. 2012, 3, 477–486. [Google Scholar] [CrossRef]
- Sjörs, A.; Ljung, T.; Jonsdottir, I.H. Diurnal Salivary Cortisol in Relation to Perceived Stress at Home and at Work in Healthy Men and Women. Biol. Psychol. 2014, 99, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, M. GAMLj: General Analyses for Linear Models. Jamovi Modul. 2019. Available online: https://gamlj.github.io/ (accessed on 2 March 2021).
- The Jamovi Project. Jamovi (Version 1.8.1). 2021. [Computer Software]. Available online: https://www.jamovi.org/ (accessed on 2 March 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 2 March 2022).
- Satterthwaite, F.E. An Approximate Distribution of Estimates of Variance Components. Biom. Bull. 1946, 2, 110–114. [Google Scholar] [CrossRef]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models.; Cambrige University Press: Cambridge, UK, 2006; ISBN 9780511790942. [Google Scholar]
- Goldstein, H. Multilevel Mixed Linear Model Analysis Using Iterative Generalized Least Squares. Biometrika 1986, 73, 43–56. [Google Scholar] [CrossRef]
- Bryk, A.S.; Raudenbush, S.W. Application of Hierarchical Linear Models to Assessing Change. Psychol. Bull. 1987, 101, 147–158. [Google Scholar] [CrossRef]
- Singer, J.D.; Willet, J.B. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence; Oxford University Press: Oxford, UK, 2003; ISBN 9780195152968. [Google Scholar]
- Snijders, T.A.B.; Bosker, R. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling; Sage Publications: London, UK, 1999; ISBN 0-7619-5889-4. [Google Scholar]
- Mathieu, J.E.; Aguinis, H.; Culpepper, S.A.; Chen, G. Understanding and Estimating the Power to Detect Cross-Level Interaction Effects in Multilevel Modeling. J. Appl. Psychol. 2012, 97, 951–966. [Google Scholar] [CrossRef]
- Nezlek, J.B.; Mroziński, B. Applications of Multilevel Modeling in Psychological Science: Intensive Repeated Measures Designs. L’Année Psychol. 2020, 120, 39–72. [Google Scholar] [CrossRef]
- Verma, R.; Balhara, Y.S.; Gupta, C. Gender Differences in Stress Response: Role of Developmental and Biological Determinants. Ind. Psychiatry J. 2012, 20, 4. [Google Scholar] [CrossRef]
- Bryant, R.A.; McGrath, C.; Felmingham, K.L. The Roles of Noradrenergic and Glucocorticoid Activation in the Development of Intrusive Memories. PLoS ONE 2013, 8, e62675. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Pliego, L.E.; Del Socorro Camarillo-Romero, E.; Montenegro-Morales, L.P.; De Jesus Garduño-García, J. Dietary Patterns Associated with Body Mass Index (BMI) and Lifestyle in Mexican Adolescents. BMC Public Health 2016, 16, 850. [Google Scholar] [CrossRef]
- Newby, P.K.; Muller, D.; Hallfrisch, J.; Qiao, N.; Andres, R.; Tucker, K.L. Dietary Patterns and Changes in Body Mass Index and Waist Circumference in Adults. Am. J. Clin. Nutr. 2003, 77, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Majeed, F. Association of BMI with Diet and Physical Activity of Female Medical Students at the University of Dammam, Kingdom of Saudi Arabia. J. Taibah Univ. Med. Sci. 2015, 10, 188–196. [Google Scholar] [CrossRef]
- Browne, W.J.; Draper, D. A Comparison of Bayesian and Likelihood-Based Methods for Fitting Multilevel Models. Bayesian Anal. 2006, 1, 473–514. [Google Scholar] [CrossRef]
- Maas, C.J.M.; Hox, J.J. Sufficient Sample Sizes for Multilevel Modeling. Methodology 2005, 1, 85–91. [Google Scholar] [CrossRef]
- McNeish, D.; Stapleton, L.M. The Effect of Small Sample Size on Two-Level Model Estimates: A Review and Illustration. Educ. Psychol. Rev. 2016, 28, 295–314. [Google Scholar] [CrossRef]
- McNeish, D. Small Sample Methods for Multilevel Modeling: A Colloquial Elucidation of REML and the Kenward-Roger Correction. Multivar. Behav. Res. 2017, 52, 661–670. [Google Scholar] [CrossRef]
- Biesanz, J.C.; Deeb-Sossa, N.; Papadakis, A.A.; Bollen, K.A.; Curran, P.J. The Role of Coding Time in Estimating and Interpreting Growth Curve Models. Psychol. Methods 2004, 9, 30–52. [Google Scholar] [CrossRef]
- King, K.M.; Littlefield, A.K.; McCabe, C.J.; Mills, K.L.; Flournoy, J.; Chassin, L. Longitudinal Modeling in Developmental Neuroimaging Research: Common Challenges, and Solutions from Developmental Psychology. Dev. Cogn. Neurosci. 2018, 33, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Byrk, A.S.; Raudenbush, S.W.; Congdon, R. HLM 7 for Windows; Scientific Software International: Chicago, IL, USA, 2008. [Google Scholar]
- Cohen, J. Partialed Products Are Interactions; Partialed Powers Are Curve Components. Psychol. Bull. 1978, 85, 858–866. [Google Scholar] [CrossRef]
- Aiken, L.; West, S. Multiple Regression: Testing and Interpreting Interactions; Sage Publications: Newbury Park, CA, USA, 1991; ISBN 0-7619-0712-2. [Google Scholar]
- West, S.G.; Finch, J.F.; Curran, P.J. Structural Equation Models with Nonnormal Variables: Problems and Remedies. In Structural Equation Modeling: Concepts, Issues, and Applications; Hoyle, R.H., Ed.; Sage Publications, Inc.: Thousand Oaks, CA, USA, 1995; pp. 56–75. [Google Scholar]
- Kim, H.-Y. Statistical Notes for Clinical Researchers: Assessing Normal Distribution (2) Using Skewness and Kurtosis. Restor. Dent. Endod. 2013, 38, 52. [Google Scholar] [CrossRef] [PubMed]
- Kline, R.B. Principles and Practice of Structural Equation Modeling, 2nd ed.; Guilford: New York, NY, USA, 2004. [Google Scholar]
- World Health Organization. Guideline: Sugars Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2015. Available online: https://www.ncbi.nlm.nih.gov/books/NBK285537/ (accessed on 24 December 2022).
- Drewnowski, A.; Rehm, C.D. Consumption of Added Sugars among Us Children and Adults by Food Purchase Location and Food Source. Am. J. Clin. Nutr. 2014, 100, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Christiansen, A.M.; Ostrander, M.M.; Jones, A.A.; Jones, K.R.; Choi, D.C.; Krause, E.G.; Evanson, N.K.; Furay, A.R.; Davis, J.F.; et al. Pleasurable Behaviors Reduce Stress via Brain Reward Pathways. Proc. Natl. Acad. Sci. USA 2010, 107, 20529–20534. [Google Scholar] [CrossRef] [PubMed]
- Laugero, K.; Bell, M.E.; Bhatnagar, S.; Soriano, L.; Dallman, M.F. Sucrose Ingestion Normalizes Central Expression of Corticotropin-Releasing-Factor Messenger Ribonucleic Acid and Energy Balance in Adrenalectomized Rats: A Glucocorticoid-Metabolic-Brain Axis? Endocrinology 2001, 142, 2796–2804. [Google Scholar] [CrossRef] [PubMed]
- Laugero, K.; Gomez, F.; Manalo, S.; Dallman, M.F. Corticosterone Infused Intracerebroventricularly Inhibits Energy Storage and Stimulates the Hypothalamo-Pituitary Axis in Adrenalectomized Rats Drinking Sucrose. Endocrinology 2002, 143, 4552–4562. [Google Scholar] [CrossRef][Green Version]
- Markus, R.; Panhuysen, G.; Tuiten, A.; Koppeschaar, H. Effects of Food on Cortisol and Mood in Vulnerable Subjects under Controllable and Uncontrollable Stress. Physiol. Behav. 2000, 70, 333–342. [Google Scholar] [CrossRef]
- Warne, J.P. Shaping the Stress Response: Interplay of Palatable Food Choices, Glucocorticoids, Insulin and Abdominal Obesity. Mol. Cell. Endocrinol. 2009, 300, 137–146. [Google Scholar] [CrossRef]
- Oliver, G.; Wardle, J. Perceived Effects of Stress on Food Choice. Physiol. Behav. 1999, 66, 511–515. [Google Scholar] [CrossRef]
- Rutters, F.; Nieuwenhuizen, A.G.; Lemmens, S.G.T.; Born, J.M.; Westerterp-Plantenga, M.S. Acute Stress-Related Changes in Eating in the Absence of Hunger. Obesity 2009, 17, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yang, H.Y.; Kim, A.J.; Lim, Y. Academic Stress Levels Were Positively Associated with Sweet Food Consumption among Korean High-School Students. Nutrition 2013, 29, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Polivy, J.; Herman, C.P.; McFarlane, T. Effects of Anxiety on Eating: Does Palatability Moderate Distress-Induced Overeating in Dieters? J. Abnorm. Psychol. 1994, 103, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Leigh Gibson, E. Emotional Influences on Food Choice: Sensory, Physiological and Psychological Pathways. Physiol. Behav. 2006, 89, 53–61. [Google Scholar] [CrossRef]
- Macht, M.; Mueller, J. Immediate Effects of Chocolate on Experimentally Induced Mood States. Appetite 2007, 49, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M. Self-Medication with Sucrose. Curr. Opin. Behav. Sci. 2016, 9, 78–83. [Google Scholar] [CrossRef]
- Bell, M.E.; Bhatnagar, S.; Liang, J.; Soriano, L.; Nagy, T.R.; Dallman, M.F. Voluntary Sucrose Ingestion, like Corticosterone Replacement, Prevents the Metabolic Deficits of Adrenalectomy. J. Neuroendocrinol. 2000, 12, 461–470. [Google Scholar] [CrossRef]
- Dallman, M.F.; Pecoraro, N.C.; La Fleur, S.E.; Warne, J.P.; Ginsberg, A.B.; Akana, S.F.; Laugero, K.C.; Houshyar, H.; Strack, A.M.; Bhatnagar, S.; et al. Glucocorticoids, Chronic Stress, and Obesity. Prog. Brain Res. 2006, 153, 75–105. [Google Scholar] [CrossRef]
- Dallman, M.F.; Akana, S.F.; Strack, A.M.; Scribner, K.S.; Pecoraro, N.; La Fleur, S.E.; Houshyar, H.; Gomez, F. Chronic Stress-Induced Effects of Corticosterone on Brain: Direct and Indirect. Ann. N. Y. Acad. Sci. 2004, 1018, 141–150. [Google Scholar] [CrossRef]
- Peters, A.; Schweiger, U.; Pellerin, L.; Hubold, C.; Oltmanns, K.M.; Conrad, M.; Schultes, B.; Born, J.; Fehm, H.L. The Selfish Brain: Competition for Energy Resources. Neurosci. Biobehav. Rev. 2004, 28, 143–180. [Google Scholar] [CrossRef]
- Ulrich-Lai, Y.M.; Ostrander, M.M.; Thomas, I.M.; Packard, B.A.; Furay, A.R.; Dolgas, C.M.; Van Hooren, D.C.; Figueiredo, H.F.; Mueller, N.K.; Choi, D.C.; et al. Daily Limited Access to Sweetened Drink Attenuates Hypothalamic-Pituitary- Adrenocortical Axis Stress Responses. Endocrinology 2007, 148, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Drolet, G.; Dumont, E.C.; Gosselin, I.; Kinkead, R.; Laforest, S.; Trottier, J.F. Role of Endogenous Opioid System in the Regulation of the Stress Response. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2001, 25, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Daubenmier, J.; Lustig, R.H.; Hecht, F.M.; Kristeller, J.; Woolley, J.; Adam, T.; Dallman, M.; Epel, E. A New Biomarker of Hedonic Eating? A Preliminary Investigation of Cortisol and Nausea Responses to Acute Opioid Blockade. Appetite 2014, 74, 92–100. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Erratum: Brain on Stress: How the Social Environment Gets under the Skin. Proc. Natl. Acad. Sci. USA 2013, 110, 1561. [Google Scholar] [CrossRef]
- Pruessner, J.C.; Dedovic, K.; Khalili-Mahani, N.; Engert, V.; Pruessner, M.; Buss, C.; Renwick, R.; Dagher, A.; Meaney, M.J.; Lupien, S. Deactivation of the Limbic System During Acute Psychosocial Stress: Evidence from Positron Emission Tomography and Functional Magnetic Resonance Imaging Studies. Biol. Psychiatry 2008, 63, 234–240. [Google Scholar] [CrossRef]
- Daubenmier, J.; Kristeller, J.; Hecht, F.M.; Maninger, N.; Kuwata, M.; Jhaveri, K.; Lustig, R.H.; Kemeny, M.; Karan, L.; Epel, E. Mindfulness Intervention for Stress Eating to Reduce Cortisol and Abdominal Fat among Overweight and Obese Women: An Exploratory Randomized Controlled Study. J. Obes. 2011, 2011, 651936. [Google Scholar] [CrossRef]
- Dallman, M.F.; Pecoraro, N.C.; La Fleur, S.E. Chronic Stress and Comfort Foods: Self-Medication and Abdominal Obesity. Brain. Behav. Immun. 2005, 19, 275–280. [Google Scholar] [CrossRef]
- Friedman, J.M. A War on Obesity, Not the Obese. Science 2003, 299, 856–858. [Google Scholar] [CrossRef]
- Stunkard, A.J.; Faith, M.S.; Allison, K.C. Depression and Obesity. Biol. Psychiatry 2003, 54, 330–337. [Google Scholar] [CrossRef]
- Bjorntorp, P. “Portal” Adipose Tissue as a Generator of Risk Factors for Cardiovascular Disease and Diabetes. Arteriosclerosis 1990, 10, 493–496. [Google Scholar] [CrossRef]
- Wajchenberg, B.L.; Lé, B.; Wajchenberg, O. Subcutaneous and Visceral Adipose Tissue. Endocr. Rev. 2000, 21, 697–738. [Google Scholar]
- Maniam, J.; Morris, M.J. Palatable Cafeteria Diet Ameliorates Anxiety and Depression-like Symptoms Following an Adverse Early Environment. Psychoneuroendocrinology 2010, 35, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Konttinen, H.; Männistö, S.; Sarlio-Lähteenkorva, S.; Silventoinen, K.; Haukkala, A. Emotional Eating, Depressive Symptoms and Self-Reported Food Consumption. A Population-Based Study. Appetite 2010, 54, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Schuman, M.; Gitlin, M.J.; Fairbanks, L. Sweets, Chocolate, and Atypical Depressive Traits. J. Nerv. Ment. Dis. 1987, 175, 491–495. [Google Scholar] [CrossRef]
- Parker, G.; Roy, K.; Mitchell, P.; Wilhelm, K.; Malhi, G.; Hadzi-Pavlovic, D. Atypical Depression: A Reappraisal. Am. J. Psychiatry 2002, 159, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Dash, S.; Allender, S.; Jacka, F.; Hoare, E. Diet and Mental Health During Emerging Adulthood: A Systematic Review. Emerg. Adulthood 2022, 10, 645–659. [Google Scholar] [CrossRef]
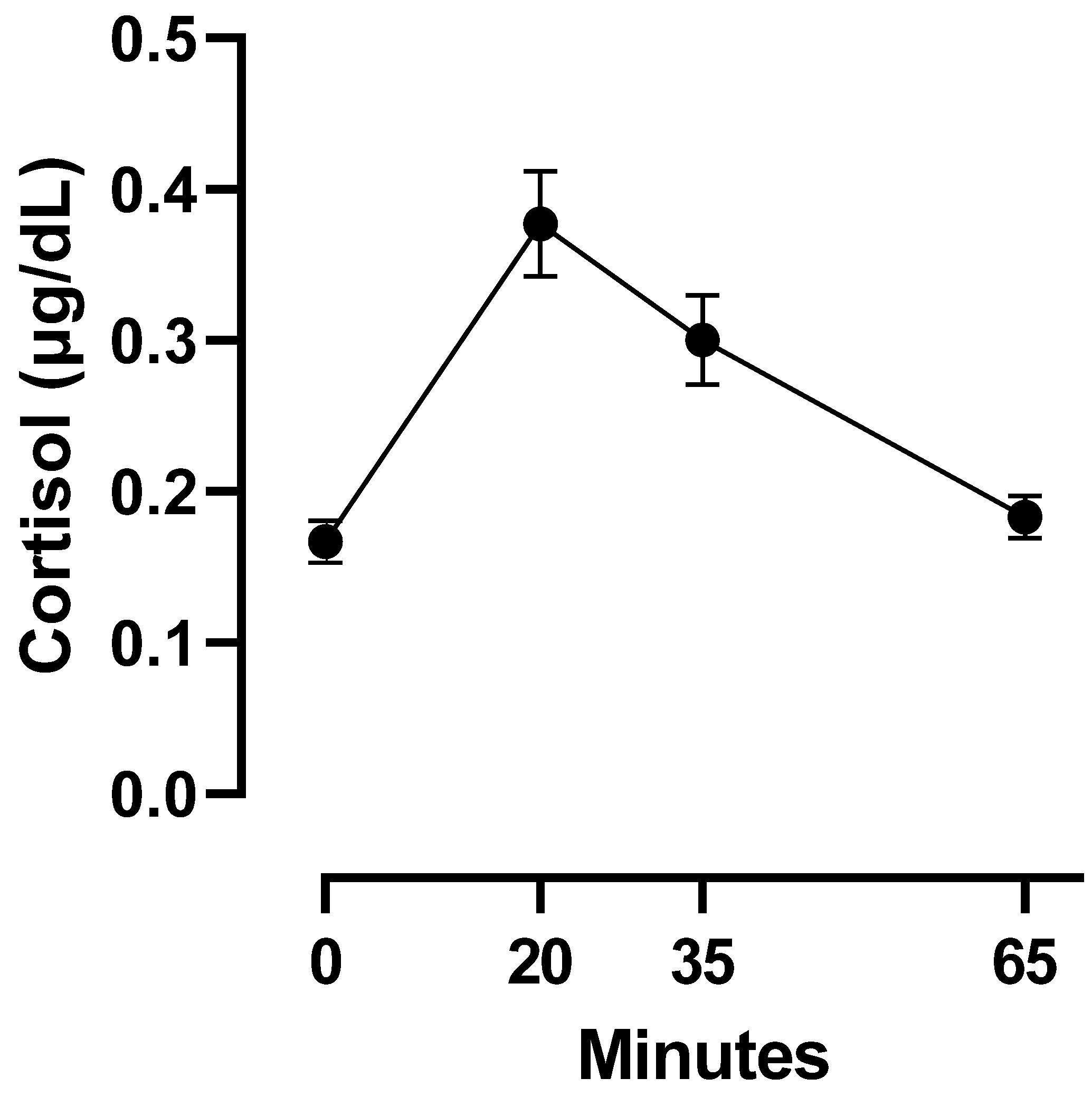
| BMI | Saturated Fats | Sugars | Cortisol_0 | Cortisol_20 | Cortisol_35 | Cortisol_65 | |
|---|---|---|---|---|---|---|---|
| (kg/m2) | (% Daily Energy Intake) | (% Daily Energy Intake) | (µg/dL) | (µg/dL) | (µg/dL) | (µg/dL) | |
| N | 54 | 54 | 54 | 54 | 54 | 49 | 54 |
| Missing | 0 | 0 | 0 | 0 | 0 | 5 | 0 |
| Mean | 23.05 | 13.78 | 18.3 | 0.167 | 0.377 | 0.3 | 0.183 |
| Standard Deviation | 4.33 | 2.42 | 5.92 | 0.102 | 0.259 | 0.213 | 0.102 |
| Minimum | 16.76 | 8 | 8.93 | 0.012 | 0.097 | 0.074 | 0.052 |
| Maximum | 36.16 | 19 | 39.49 | 0.635 | 1.06 | 0.879 | 0.546 |
| BMI | Saturated Fats | Sugars | Cortisol_0 | Cortisol_20 | Cortisol_35 | Cortisol_65 | |
|---|---|---|---|---|---|---|---|
| (kg/m2) | (% Daily Energy Intake) | (% Daily Energy Intake) | (µg/dL) | (µg/dL) | (µg/dL) | (µg/dL) | |
| BMI | — | ||||||
| Saturated Fats | −0.185 | — | |||||
| Sugars | 0.334 * | −0.259 | — | ||||
| Cortisol_0 | 0.085 | 0.101 | −0.014 | — | |||
| Cortisol_20 | −0.28 * | −0.068 | −0.366 ** | 0.12 | — | ||
| Cortisol_35 | −0.211 | −0.12 | −0.307 * | 0.01 | 0.823 *** | — | |
| Cortisol_65 | −0.065 | −0.023 | −0.307 * | 0.037 | 0.718 *** | 0.865 *** | — |
| Fixed Effects Parameter Estimates | ||||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | ||||||||
| Names | Effect | Estimate | SE | Lower | Upper | df | t | p |
| (Intercept) | (Intercept) | 0.329 | 0.026 | 0.278 | 0.381 | 53.187 | 12.511 | <0.001 |
| Time | Time | 0.082 | 0.016 | 0.051 | 0.113 | 56.381 | 5.13 | <0.001 |
| Gender | Female—Male | 0.004 | 0.024 | −0.044 | 0.051 | 90.293 | 0.156 | 0.876 |
| Saturated Fats | Saturated Fats | −0.013 | 0.011 | −0.035 | 0.009 | 51.64 | −1.183 | 0.242 |
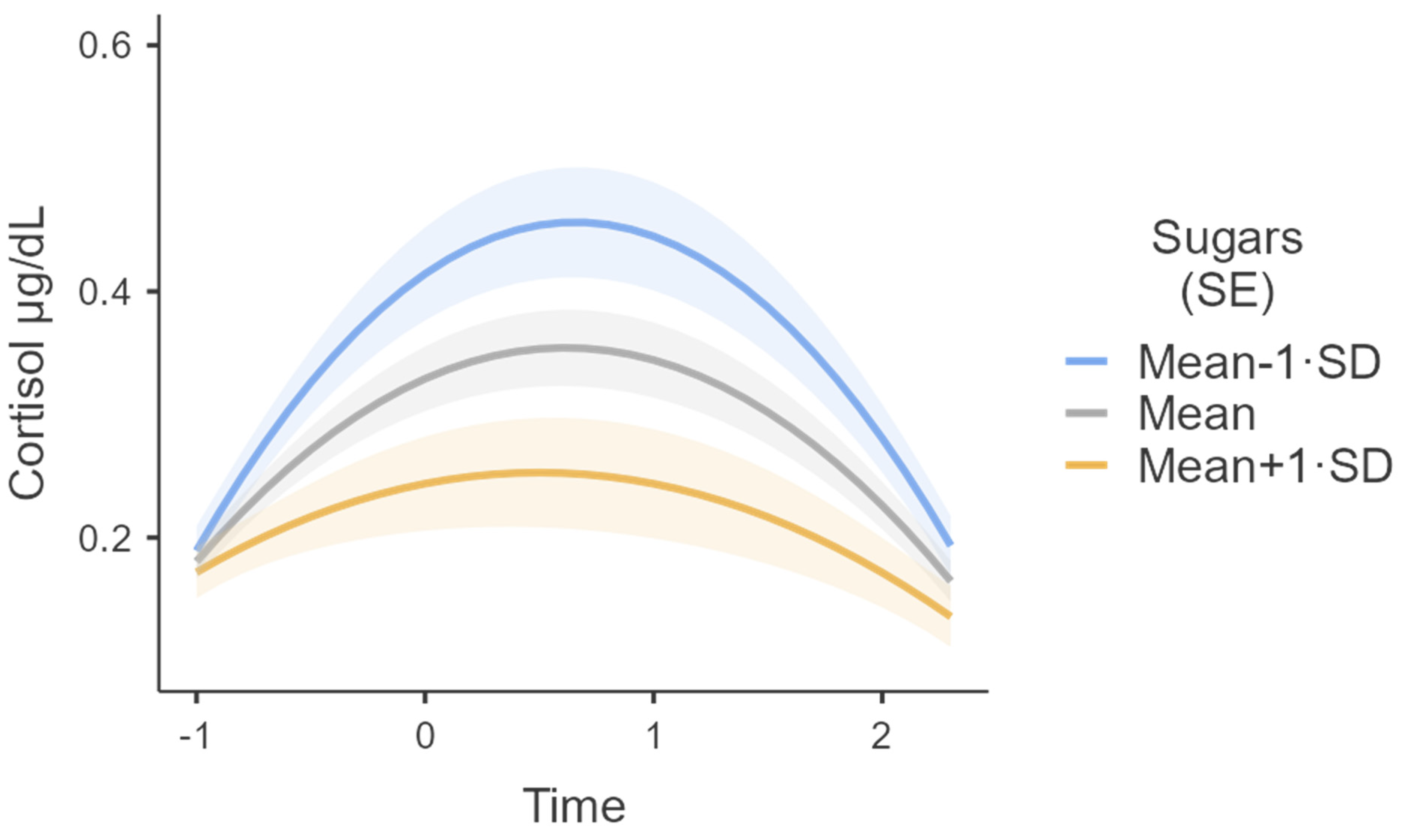
| Sugars | Sugars | −0.014 | 0.005 | −0.024 | −0.005 | 54.298 | −3.102 | 0.003 |
| BMI | BMI | 0.002 | 0.003 | −0.003 | 0.008 | 90.846 | 0.724 | 0.471 |
| Time2 | Time2 | −0.067 | 0.01 | −0.087 | −0.046 | 55.533 | −6.45 | <0.001 |
| Time ∗ Saturated Fats | Time ∗ Saturated Fats | −0.01 | 0.007 | −0.024 | 0.003 | 55.446 | −1.494 | 0.141 |
| Time ∗ Sugars | Time ∗ Sugars | −0.008 | 0.003 | −0.013 | −0.002 | 56.193 | −2.776 | 0.007 |
| Saturated Fats ∗ Time2 | Saturated Fats ∗ Time2 | 0.006 | 0.004 | −0.002 | 0.015 | 54.555 | 1.463 | 0.149 |
| Sugars ∗ Time2 | Sugars ∗ Time2 | 0.005 | 0.002 | 0.002 | 0.009 | 55.336 | 2.858 | 0.006 |
| Random Components | ||||
|---|---|---|---|---|
| Groups | Name | SD | Variance | ICC |
| Participant | (Intercept) | 0.178 | 0.032 | 0.756 |
| Time | 0.093 | 0.009 | ||
| Time2 | 0.063 | 0.004 | ||
| Residual | 0.101 | 0.01 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Polito, N.; Stylianakis, A.A.; Richardson, R.; Baker, K.D. Real-World Intake of Dietary Sugars Is Associated with Reduced Cortisol Reactivity Following an Acute Physiological Stressor. Nutrients 2023, 15, 209. https://doi.org/10.3390/nu15010209
Di Polito N, Stylianakis AA, Richardson R, Baker KD. Real-World Intake of Dietary Sugars Is Associated with Reduced Cortisol Reactivity Following an Acute Physiological Stressor. Nutrients. 2023; 15(1):209. https://doi.org/10.3390/nu15010209
Chicago/Turabian StyleDi Polito, Nicola, Anthea A. Stylianakis, Rick Richardson, and Kathryn D. Baker. 2023. "Real-World Intake of Dietary Sugars Is Associated with Reduced Cortisol Reactivity Following an Acute Physiological Stressor" Nutrients 15, no. 1: 209. https://doi.org/10.3390/nu15010209
APA StyleDi Polito, N., Stylianakis, A. A., Richardson, R., & Baker, K. D. (2023). Real-World Intake of Dietary Sugars Is Associated with Reduced Cortisol Reactivity Following an Acute Physiological Stressor. Nutrients, 15(1), 209. https://doi.org/10.3390/nu15010209






