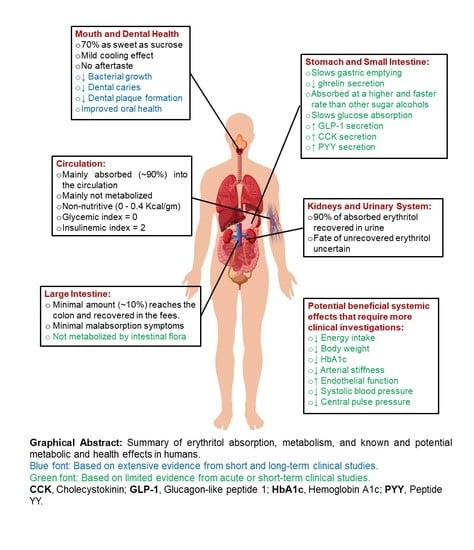
Erythritol: An In-Depth Discussion of Its Potential to Be a Beneficial Dietary Component
Abstract
1. Introduction
2. Erythritol-Naturally Occurring and Endogenously Produced
3. Commercial Production of Erythritol
4. Erythritol Safety
5. The Metabolism of Erythritol
6. Health Effect of Erythritol
6.1. Effects of Erythritol on Tooth Decay
6.2. Effects of Erythritol on Glycemia and Insulin Secretion
6.3. Effects of Erythritol on Energy Intake and Body Weight
6.4. Effects of Erythritol on Risk Factors for Cardiometabolic Diseases
6.5. Circulating Erythritol as a Candidate Predictor of Metabolic Risks
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sterk, R. Consumer Attitudes on Sweeteners Changing. Available online: https://www.foodbusinessnews.net/articles/6528-consumer-attitudes-on-sweeteners (accessed on 30 January 2022).
- Goodman, S.; Vanderlee, L.; Jones, A.; White, C.M.; Hammond, D. Perceived Healthiness of Sweeteners among Young Adults in Canada. Can. J. Diet. Pract. Res 2021, 82, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Campos, A. What exactly is a “natural” sweetener? It’s Not What You Might Think. Available online: https://www.whatsugar.com/post/whats-natural-sweetener-its-not-what-you-might-think (accessed on 30 January 2022).
- Heller, L. HFCS Is Not ‘Natural’, Says FDA. Available online: https://www.beveragedaily.com/Article/2008/04/02/HFCS-is-not-natural-says-FDA (accessed on 30 January 2022).
- Crowley, L. HFCS Is Natural, Says FDA in a Letter. Available online: https://www.foodnavigator.com/Article/2008/07/08/HFCS-is-natural-says-FDA-in-a-letter (accessed on 30 January 2022).
- Hug, J.J.; Krug, D.; Muller, R. Bacteria as genetically programmable producers of bioactive natural products. Nat. Rev. Chem. 2020, 4, 172–193. [Google Scholar] [CrossRef]
- Agriculture Research Services-US Department of Agriculture. Sugar, Tubinado. 2019. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170674/nutrients (accessed on 9 January 2022).
- Sigala, D.M.; Hieronimus, B.; Medici, V.; Lee, V.; Nunez, M.V.; Bremer, A.A.; Cox, C.L.; Price, C.A.; Benyam, Y.; Chaudhari, A.J.; et al. Consuming Sucrose- or HFCS-sweetened Beverages Increases Hepatic Lipid and Decreases Insulin Sensitivity in Adults. J. Clin. Endocrinol. Metab 2021, 106, 3248–3264. [Google Scholar] [CrossRef]
- Sipple, L.R.; Racette, C.M.; Schiano, A.N.; Drake, M.A. Consumer perception of ice cream and frozen desserts in the “better-for-you” category. J. Dairy Sci 2022, 105, 154–169. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. GRAS notice 76: Erythritol. 2001. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=76&sort=GRN_No&order=DESC&startrow=1&type=basic&search=erythritol (accessed on 15 January 2022).
- European Commission-Health & Consumer Protection Directorate. Opinion of the Scientific Committee on Food on Erythritol. 2003. Available online: https://ec.europa.eu/food/system/files/2020-12/sci-com_scf_out175_en.pdf (accessed on 15 January 2022).
- Daza-Serna, L.; Serna-Loaiza, S.; Masi, A.; Mach, R.L.; Mach-Aigner, A.R.; Friedl, A. From the culture broth to the erythritol crystals: An opportunity for circular economy. Appl. Microbiol. Biotechnol. 2021, 105, 4467–4486. [Google Scholar] [CrossRef] [PubMed]
- Economic Research Services-US Department of Agriculture. Sugar and Sweeteners Yearbook Tables. 2018. Available online: https://www.ers.usda.gov/data-products/sugar-and-sweeteners-yearbook-tables/ (accessed on 9 January 2022).
- Ellis, E. Natural Sweeteners: Erythritol. Available online: https://www.todaysdietitian.com/newarchives/0819p12.shtml (accessed on 20 January 2022).
- US Food and Drug Administration. GRAS Notice 789: Erythritol. 2019. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=789&sort=GRN_No&order=DESC&startrow=1&type=basic&search=erythritol (accessed on 15 January 2022).
- Grembecka, M. Sugar alcohols-their role in the modern world of sweeteners: A review. Eur. Food Res. Technol. 2015, 241, 15–16. [Google Scholar] [CrossRef]
- Shindou, T.; Sasaki, Y.; Eguchi, T.; Euguchi, T.; Hagiwara, K.; Ichikawa, T. Identification of erythritol by HPLC and GC-MS and quantitative measurement in pulps of various fruits. J. Agr. Food Chem. 1989, 37, 1474–1476. [Google Scholar] [CrossRef]
- Shindou, T.; Sasaki, Y.; Miki, H.; Eguchi, T.; Hagiwara, K.; Ichikawa, T. Determination of erythritol in fermented foods by high performance liquid chromatography. J. Food. Hyg. Soc. Jpn. 1988, 29, 419–422_411. [Google Scholar] [CrossRef]
- DeCock, P. 10 Erythritol. In Sweeteners and Sugar Alternatives in Food Technology; John Wiley & Sons: Hoboken, NJ, USA; West Sussex, UK, 2012. [Google Scholar]
- Ortiz, S.R.; Field, M.S. Chronic Dietary Erythritol Exposure Elevates Plasma Erythritol Concentration in Mice but Does Not Cause Weight Gain or Modify Glucose Homeostasis. J. Nutr. 2021, 151, 2114–2124. [Google Scholar] [CrossRef]
- Verhoeven, N.M.; Huck, J.H.; Roos, B.; Struys, E.A.; Salomons, G.S.; Douwes, A.C.; van der Knaap, M.S.; Jakobs, C. Transaldolase deficiency: Liver cirrhosis associated with a new inborn error in the pentose phosphate pathway. Am. J. Hum. Genet. 2001, 68, 1086–1092. [Google Scholar] [CrossRef]
- Hootman, K.C.; Trezzi, J.P.; Kraemer, L.; Burwell, L.S.; Dong, X.; Guertin, K.A.; Jaeger, C.; Stover, P.J.; Hiller, K.; Cassano, P.A. Erythritol is a pentose-phosphate pathway metabolite and associated with adiposity gain in young adults. Proc. Natl. Acad. Sci. USA 2017, 114, E4233–E4240. [Google Scholar] [CrossRef] [PubMed]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.M.; Krüger, A.; Tauqeer Alam, M. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Schlicker, L.; Szebenyi, D.M.; Ortiz, S.R.; Heinz, A.; Hiller, K.; Field, M.S. Unexpected roles for ADH1 and SORD in catalyzing the final step of erythritol biosynthesis. J. Biol. Chem. 2019, 294, 16095–16108. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.; Zannini, E.; Arendt, E.K.; Coffey, A. A review of polyols–biotechnological production, food applications, regulation, labeling and health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2034–2051. [Google Scholar] [CrossRef] [PubMed]
- Regnat, K.; Mach, R.L.; Mach-Aigner, A.R. Erythritol as sweetener-wherefrom and whereto? Appl. Microbiol. Biotechnol. 2018, 102, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Rzechonek, D.A.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A.M. Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 2018, 38, 620–633. [Google Scholar] [CrossRef]
- Ali, B. Consumers’ Attitude Towards Microbial Applications in Food Production. Available online: http://simbaproject.eu/consumers-attitudes-microbial-applications/#:~:text=Preliminary%20results%20show%20that%20about,for%20microbial%2Dbased%20food%20products. (accessed on 30 January 2022).
- Stephanopoulos, G. Synthetic biology and metabolic engineering. ACS Synth. Biol 2012, 1, 514–525. [Google Scholar] [CrossRef]
- World Health Organization-International Programme on Chemical Safety. Safety Evaluation of Certain Food Additives and Contaminants—WHO Food Additive Series: 44. 2000. Available online: https://inchem.org/documents/jecfa/jecmono/v44jec03.htm (accessed on 5 January 2022).
- US Food and Drug Administration. GRAS Notice 297: Erythritol Fatty Acid Esters. 2009. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=297&sort=GRN_No&order=DESC&startrow=1&type=basic&search=erythritol (accessed on 15 January 2022).
- US Food and Drug Administration. GRAS Notice 208: Erythritol. 2006. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=208&sort=GRN_No&order=DESC&startrow=1&type=basic&search=erythritol (accessed on 15 January 2022).
- US Food and Drug Administration. GRAS Notice 382: Erythritol. 2011. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=382&sort=GRN_No&order=DESC&startrow=1&type=basic&search=erythritol (accessed on 15 January 2022).
- The European Parliament and the Council of European Unioin. Directive 2006/52/EC of the European Parliament and of the Council of 5 July 2006 Amending Directive 95/2/EC on food Additives Other than Colours and Sweeteners and Directive 94/35/EC on Sweeteners for Use in Foodstuffs. 2006. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:204:0010:0022:EN:PDF (accessed on 15 January 2022).
- The European Food Safety Authority. Statement in relation to the safety of erythritol (E 968) in light of new data, including a new paediatric study on the gastrointestinal tolerability of erythritol. EFSA J. 2010, 8, 1650. [Google Scholar] [CrossRef]
- The European Food Safety Authority. Scientific Opinion on the safety of the proposed extension of use of erythritol (E 968) as a food additive. EFSA J. 2015, 13, 4033. [Google Scholar] [CrossRef]
- Munro, I.C.; Berndt, W.O.; Borzelleca, J.F.; Flamm, G.; Lynch, B.S.; Kennepohl, E.; Bar, E.A.; Modderman, J. Erythritol: An interpretive summary of biochemical, metabolic, toxicological and clinical data. Food Chem. Toxicol 1998, 36, 1139–1174. [Google Scholar] [CrossRef]
- Lina, B.; Bos-Kuijpers, M.; Til, H.; Bär, A. Chronic toxicity and carcinogenicity study of erythritol in rats. Regul. Toxicol. Pharmacol. 1996, 24, S264–S279. [Google Scholar] [CrossRef] [PubMed]
- Oku, T.; Okazaki, M. Laxative threshold of sugar alcohol erythritol in human subjects. Nutr. Res. 1996, 16, 577–589. [Google Scholar] [CrossRef]
- Tetzloff, W.; Dauchy, F.; Medimagh, S.; Carr, D.; Bar, A. Tolerance to subchronic, high-dose ingestion of erythritol in human volunteers. Regul. Toxicol. Pharm. 1996, 24, S286–S295. [Google Scholar] [CrossRef] [PubMed]
- Arrigoni, E.; Brouns, F.; Amado, R. Human gut microbiota does not ferment erythritol. Br. J. Nutr. 2005, 94, 643–646. [Google Scholar] [CrossRef]
- Hiele, M.; Ghoos, Y.; Rutgeerts, P.; Vantrappen, G. Metabolism of erythritol in humans: Comparison with glucose and lactitol. Br. J. Nutr. 1993, 69, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kawano, R.; Okamura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Asano, M.; Yamazaki, M.; Takakuwa, H.; Sasano, R. Erythritol Ameliorates Small Intestinal Inflammation Induced by High-Fat Diets and Improves Glucose Tolerance. Int. J. Mol. Sci. 2021, 22, 5558. [Google Scholar] [CrossRef]
- Wolnerhanssen, B.K.; Meyer-Gerspach, A.C.; Beglinger, C.; Islam, M.S. Metabolic effects of the natural sweeteners xylitol and erythritol: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 60, 1986–1998. [Google Scholar] [CrossRef]
- Noda, K.; Nakayama, K.; Oku, T. Serum glucose and insulin levels and erythritol balance after oral administration of erythritol in healthy subjects. Eur. J. Clin. Nutr. 1994, 48, 286–292. [Google Scholar]
- The European Association of Polyol Producers, E. ERYTHRITOL (E 968). Available online: https://polyols-eu.org/polyols/erythritol/ (accessed on 18 January 2022).
- Muhlemann, H.R.; Regolati, B.; Marthaler, T.M. The effect on rat fissure caries of xylitol and sorbitol. Helv. Odontol. Acta 1970, 14, 48–50. [Google Scholar]
- Fosdick, L.S.; Englander, H.R.; Hoerman, K.C.; Kesel, R.G. A comparison of pH values of in vivo dental plaque after sucrose and sorbitol mouth rinses. J. Am. Dent. Assoc. 1957, 55, 191–195. [Google Scholar] [CrossRef]
- de Cock, P.; Makinen, K.; Honkala, E.; Saag, M.; Kennepohl, E.; Eapen, A. Erythritol Is More Effective than Xylitol and Sorbitol in Managing Oral Health Endpoints. Int. J. Dent. 2016, 2016, 9868421. [Google Scholar] [CrossRef] [PubMed]
- Runnel, R.; Makinen, K.K.; Honkala, S.; Olak, J.; Makinen, P.L.; Nommela, R.; Vahlberg, T.; Honkala, E.; Saag, M. Effect of three-year consumption of erythritol, xylitol and sorbitol candies on various plaque and salivary caries-related variables. J. Dent. 2013, 41, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Makinen, K.K.; Saag, M.; Isotupa, K.P.; Olak, J.; Nommela, R.; Soderling, E.; Makinen, P.L. Similarity of the effects of erythritol and xylitol on some risk factors of dental caries. Caries Res. 2005, 39, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Makinen, K.K.; Isotupa, K.P.; Kivilompolo, T.; Makinen, P.L.; Toivanen, J.; Soderling, E. Comparison of erythritol and xylitol saliva stimulants in the control of dental plaque and mutans streptococci. Caries Res. 2001, 35, 129–135. [Google Scholar] [CrossRef]
- Makinen, K.K.; Isotupa, K.P.; Kivilompolo, T.; Makinen, P.L.; Murtomaa, S.; Petaja, J.; Toivanen, J.; Soderling, E. The effect of polyol-combinant saliva stimulants on S. mutans levels in plaque and saliva of patients with mental retardation. Spec. Care Dentist. 2002, 22, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Hashino, E.; Kuboniwa, M.; Alghamdi, S.A.; Yamaguchi, M.; Yamamoto, R.; Cho, H.; Amano, A. Erythritol alters microstructure and metabolomic profiles of biofilm composed of Streptococcus gordonii and Porphyromonas gingivalis. Mol. Oral Microbiol. 2013, 28, 435–451. [Google Scholar] [CrossRef]
- Honkala, S.; Runnel, R.; Saag, M.; Olak, J.; Nommela, R.; Russak, S.; Makinen, P.L.; Vahlberg, T.; Falony, G.; Makinen, K.; et al. Effect of erythritol and xylitol on dental caries prevention in children. Caries Res. 2014, 48, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Honkala, S.; Runnel, R.; Olak, J.; Nommela, R.; Russak, S.; Saag, M.; Makinen, P.L.; Makinen, K.; Vahlberg, T.; et al. Long-Term Effect of Erythritol on Dental Caries Development during Childhood: A Posttreatment Survival Analysis. Caries Res. 2016, 50, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Wolnerhanssen, B.K.; Cajacob, L.; Keller, N.; Doody, A.; Rehfeld, J.F.; Drewe, J.; Peterli, R.; Beglinger, C.; Meyer-Gerspach, A.C. Gut hormone secretion, gastric emptying, and glycemic responses to erythritol and xylitol in lean and obese subjects. Am. J. Physiol. Endocrinol. Metab 2016, 310, E1053–E1061. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Miyashita, M.; Kawashima, Y.; Nakamura, T.; Saitou, N.; Modderman, J. Effects of oral administration of erythritol on patients with diabetes. Regul. Toxicol. Pharmacol. 1996, 24, S303–S308. [Google Scholar] [CrossRef]
- Livesey, G. Health potential of polyols as sugar replacers, with emphasis on low glycaemic properties. Nutr. Res. Rev. 2003, 16, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Wölnerhanssen, B.K.; Drewe, J.; Verbeure, W.; le Roux, C.W.; Dellatorre-Teixeira, L.; Rehfeld, J.F.; Holst, J.J.; Hartmann, B.; Tack, J.; Peterli, R. Gastric emptying of solutions containing the natural sweetener erythritol and effects on gut hormone secretion in humans: A pilot dose-ranging study. Diabetes Obes. Metab. 2021, 23, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Tang, B.; Stewart, A.J.; Tao, Y.; Shao, Y.; Cui, Y.; Yue, H.; Pei, J.; Liu, Z.; Mei, L.; et al. Erythritol Attenuates Postprandial Blood Glucose by Inhibiting alpha-Glucosidase. J. Agric. Food Chem. 2018, 66, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Kim, H.Y.; Cho, E.J. Erythritol attenuates the diabetic oxidative stress through modulating glucose metabolism and lipid peroxidation in streptozotocin-induced diabetic rats. J. Agr. Food Chem. 2002, 50, 5485–5489. [Google Scholar] [CrossRef]
- Chukwuma, C.I.; Mopuri, R.; Nagiah, S.; Chuturgoon, A.A.; Islam, M.S. Erythritol reduces small intestinal glucose absorption, increases muscle glucose uptake, improves glucose metabolic enzymes activities and increases expression of Glut-4 and IRS-1 in type 2 diabetic rats. Eur. J. Nutr 2018, 57, 2431–2444. [Google Scholar] [CrossRef]
- Bordier, V.; Teysseire, F.; Schlotterbeck, G.; Senner, F.; Beglinger, C.; Meyer-Gerspach, A.C.; Wölnerhanssen, B.K. Effect of a Chronic Intake of the Natural Sweeteners Xylitol and Erythritol on Glucose Absorption in Humans with Obesity. Nutrients 2021, 13, 3950. [Google Scholar] [CrossRef]
- Overduin, J.; Collet, T.H.; Medic, N.; Henning, E.; Keogh, J.M.; Forsyth, F.; Stephenson, C.; Kanning, M.W.; Ruijschop, R.; Farooqi, I.S.; et al. Failure of sucrose replacement with the non-nutritive sweetener erythritol to alter GLP-1 or PYY release or test meal size in lean or obese people. Appetite 2016, 107, 596–603. [Google Scholar] [CrossRef]
- Sorrentino, Z.A.; Smith, G.; Palm, L.; Motwani, K.; Butterfield, J.; Archer, C.; Henderson, R.; Heldermon, C.; Gautam, S.; Brantly, M.L. An Erythritol-Sweetened Beverage Induces Satiety and Suppresses Ghrelin Compared to Aspartame in Healthy Non-Obese Subjects: A Pilot Study. Cureus 2020, 12, e11409. [Google Scholar] [CrossRef]
- Overduin, J.; Tylee, T.S.; Frayo, R.S.; Cummings, D.E. Hyperosmolarity in the small intestine contributes to postprandial ghrelin suppression. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 306, G1108–G1116. [Google Scholar] [CrossRef]
- Little, T.; Gopinath, A.; Patel, E.; McGlone, A.; Lassman, D.; D’amato, M.; McLaughlin, J.; Thompson, D. Gastric emptying of hexose sugars: Role of osmolality, molecular structure and the CCK1 receptor. Neurogastroent Motil 2010, 22, 1183-e314. [Google Scholar] [CrossRef]
- Han, Y.; Park, H.; Choi, B.R.; Ji, Y.; Kwon, E.Y.; Choi, M.S. Alteration of Microbiome Profile by D-Allulose in Amelioration of High-Fat-Diet-Induced Obesity in Mice. Nutrients 2020, 12, 352. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Han, Y.; Kwon, E.Y.; Choi, M.S. d-allulose Ameliorates Metabolic Dysfunction in C57BL/KsJ-db/db Mice. Molecules 2020, 25, 3656. [Google Scholar] [CrossRef]
- Mitsutomi, K.; Masaki, T.; Shimasaki, T.; Gotoh, K.; Chiba, S.; Kakuma, T.; Shibata, H. Effects of a nonnutritive sweetener on body adiposity and energy metabolism in mice with diet-induced obesity. Metabolism 2014, 63, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Flint, N.; Hamburg, N.M.; Holbrook, M.; Dorsey, P.G.; LeLeiko, R.M.; Berger, A.; de Cock, P.; Bosscher, D.; Vita, J.A. Effects of erythritol on endothelial function in patients with type 2 diabetes mellitus: A pilot study. Acta Diabetol. 2014, 51, 513–516. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Flammer, A.J.; Anderson, T.; Celermajer, D.S.; Creager, M.A.; Deanfield, J.; Ganz, P.; Hamburg, N.M.; Lüscher, T.F.; Shechter, M.; Taddei, S. The assessment of endothelial function: From research into clinical practice. Circulation 2012, 126, 753–767. [Google Scholar] [CrossRef] [PubMed]
- den Hartog, G.J.; Boots, A.W.; Adam-Perrot, A.; Brouns, F.; Verkooijen, I.W.; Weseler, A.R.; Haenen, G.R.; Bast, A. Erythritol is a sweet antioxidant. Nutrition 2010, 26, 449–458. [Google Scholar] [CrossRef]
- Boesten, D.M.; Berger, A.; de Cock, P.; Dong, H.; Hammock, B.D.; den Hartog, G.J.; Bast, A. Multi-targeted mechanisms underlying the endothelial protective effects of the diabetic-safe sweetener erythritol. PLoS ONE 2013, 8, e65741. [Google Scholar] [CrossRef]
- Menni, C.; Fauman, E.; Erte, I.; Perry, J.R.; Kastenmüller, G.; Shin, S.-Y.; Petersen, A.-K.; Hyde, C.; Psatha, M.; Ward, K.J. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 2013, 62, 4270–4276. [Google Scholar] [CrossRef]
- Shao, M.; Lu, H.; Yang, M.; Liu, Y.; Yin, P.; Li, G.; Wang, Y.; Chen, L.; Chen, Q.; Zhao, C. Serum and urine metabolomics reveal potential biomarkers of T2DM patients with nephropathy. Ann. Transl. Med. 2020, 8, 199. [Google Scholar] [CrossRef]
- Chen, L.; Cheng, C.-Y.; Choi, H.; Ikram, M.K.; Sabanayagam, C.; Tan, G.S.; Tian, D.; Zhang, L.; Venkatesan, G.; Tai, E.S. Plasma metabonomic profiling of diabetic retinopathy. Diabetes 2016, 65, 1099–1108. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Yu, B.; Zheng, Z.; Chang, P.; Tin, A.; Köttgen, A.; Wagenknecht, L.E.; Coresh, J.; Boerwinkle, E.; Selvin, E. Serum metabolomic profile of incident diabetes. Diabetologia 2018, 61, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, C.; Nambi, V.; Morrison, A.C.; Folsom, A.R.; Ballantyne, C.M.; Boerwinkle, E.; Yu, B. Metabolomic pattern predicts incident coronary heart disease: Findings from the Atherosclerosis Risk in Communities Study. Arter. Throm. Vas 2019, 39, 1475–1482. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, S.R.; Field, M.S. Mammalian metabolism of erythritol: A predictive biomarker of metabolic dysfunction. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Gupte, S.A. Targeting the pentose phosphate pathway in syndrome X-related cardiovascular complications. Drug Dev. Res. 2010, 71, 161–167. [Google Scholar] [CrossRef]
- Ge, T.; Yang, J.; Zhou, S.; Wang, Y.; Li, Y.; Tong, X. The role of the pentose phosphate pathway in diabetes and cancer. Front. Endocrinol. 2020, 11, 365. [Google Scholar] [CrossRef]
- Dore, M.P.; Parodi, G.; Portoghese, M.; Pes, G.M. The Controversial Role of Glucose-6-Phosphate Dehydrogenase Deficiency on Cardiovascular Disease: A Narrative Review. Oxidative Med. Cell. Longev. 2021, 2021, 5529256. [Google Scholar] [CrossRef]
- Bonner, R.; Albajrami, O.; Hudspeth, J.; Upadhyay, A. Diabetic Kidney Disease. Prim. Care 2020, 47, 645–659. [Google Scholar] [CrossRef]
- Hammes, H.P. Diabetic retinopathy: Hyperglycaemia, oxidative stress and beyond. Diabetologia 2018, 61, 29–38. [Google Scholar] [CrossRef]
- André, P.; Balkau, B.; Vol, S.; Charles, M.A.; Eschwege, E.; Group, D.S. γ-glutamyltransferase activity and development of the metabolic syndrome (International Diabetes Federation Definition) in middle-aged Men Women: Data Epidemiol. Study on the Insulin Resistance Syndrome (DESIR) cohort. Diabetes Care 2007, 30, 2355–2361. [Google Scholar] [CrossRef]
- Liangpunsakul, S.; Chalasani, N. Unexplained elevations in alanine aminotransferase in individuals with the metabolic syndrome: Results from the third National Health and Nutrition Survey (NHANES III). Am. J. Med. Sci. 2005, 329, 111–116. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Apekey, T.A.; Khan, H. Liver enzymes and risk of cardiovascular disease in the general population: A meta-analysis of prospective cohort studies. Atherosclerosis 2014, 236, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Medici, V.; Bremer, A.A.; Lee, V.; Lam, H.D.; Nunez, M.V.; Chen, G.X.; Keim, N.L.; Havel, P.J. A dose-response study of consuming high-fructose corn syrup–sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am. J. Clin. Nutr. 2015, 101, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Bruun, J.M.; Maersk, M.; Belza, A.; Astrup, A.; Richelsen, B. Consumption of sucrose-sweetened soft drinks increases plasma levels of uric acid in overweight and obese subjects: A 6-month randomised controlled trial. Eur. J. Clin. Nutr. 2015, 69, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Maersk, M.; Belza, A.; Stodkilde-Jorgensen, H.; Ringgaard, S.; Chabanova, E.; Thomsen, H.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am. J. Clin. Nutr. 2012, 95, 283–289. [Google Scholar] [CrossRef]
- Raatz, S.K.; Johnson, L.K.; Picklo, M.J. Consumption of Honey, Sucrose, and High-Fructose Corn Syrup Produces Similar Metabolic Effects in Glucose-Tolerant and -Intolerant Individuals. J. Nutr. 2015, 145, 2265–2272. [Google Scholar] [CrossRef]
- Raben, A.; Macdonald, I.; Astrup, A. Replacement of dietary fat by sucrose or starch: Effects on 14 d ad libitum energy intake, energy expenditure and body weight in formerly obese and never-obese subjects. Int. J. Obes. Relat. Metab Disord. 1997, 21, 846–859. [Google Scholar] [CrossRef]
- Raben, A.; Vasilaras, T.H.; Moller, A.C.; Astrup, A. Sucrose compared with artificial sweeteners: Different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am. J. Clin. Nutr. 2002, 76, 721–729. [Google Scholar] [CrossRef]
- Reiser, S.; Hallfrisch, J.; Michaelis, O.E.t.; Lazar, F.L.; Martin, R.E.; Prather, E.S. Isocaloric exchange of dietary starch and sucrose in humans. I. Effects on levels of fasting blood lipids. Am. J. Clin. Nutr. 1979, 32, 1659–1669. [Google Scholar] [CrossRef]
- Reiser, S.; Handler, H.B.; Gardner, L.B.; Hallfrisch, J.G.; Michaelis, O.E.; Prather, E.S. Isocaloric exchange of dietary starch and sucrose in humans. II. Effect on fasting blood insulin, glucose, and glucagon and on insulin and glucose response to a sucrose load. Am. J. Clin. Nutr. 1979, 32, 2206–2216. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazi, T.A.; Stanhope, K.L. Erythritol: An In-Depth Discussion of Its Potential to Be a Beneficial Dietary Component. Nutrients 2023, 15, 204. https://doi.org/10.3390/nu15010204
Mazi TA, Stanhope KL. Erythritol: An In-Depth Discussion of Its Potential to Be a Beneficial Dietary Component. Nutrients. 2023; 15(1):204. https://doi.org/10.3390/nu15010204
Chicago/Turabian StyleMazi, Tagreed A., and Kimber L. Stanhope. 2023. "Erythritol: An In-Depth Discussion of Its Potential to Be a Beneficial Dietary Component" Nutrients 15, no. 1: 204. https://doi.org/10.3390/nu15010204
APA StyleMazi, T. A., & Stanhope, K. L. (2023). Erythritol: An In-Depth Discussion of Its Potential to Be a Beneficial Dietary Component. Nutrients, 15(1), 204. https://doi.org/10.3390/nu15010204