Is There a Link between Obesity Indices and Skin Autofluorescence? A Response from the ILERVAS Project
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Definition of Cardiovascular Risk Factors
2.3. Anthropometric Measures
2.4. Skin Autofluorescence
2.5. Statistical Methods
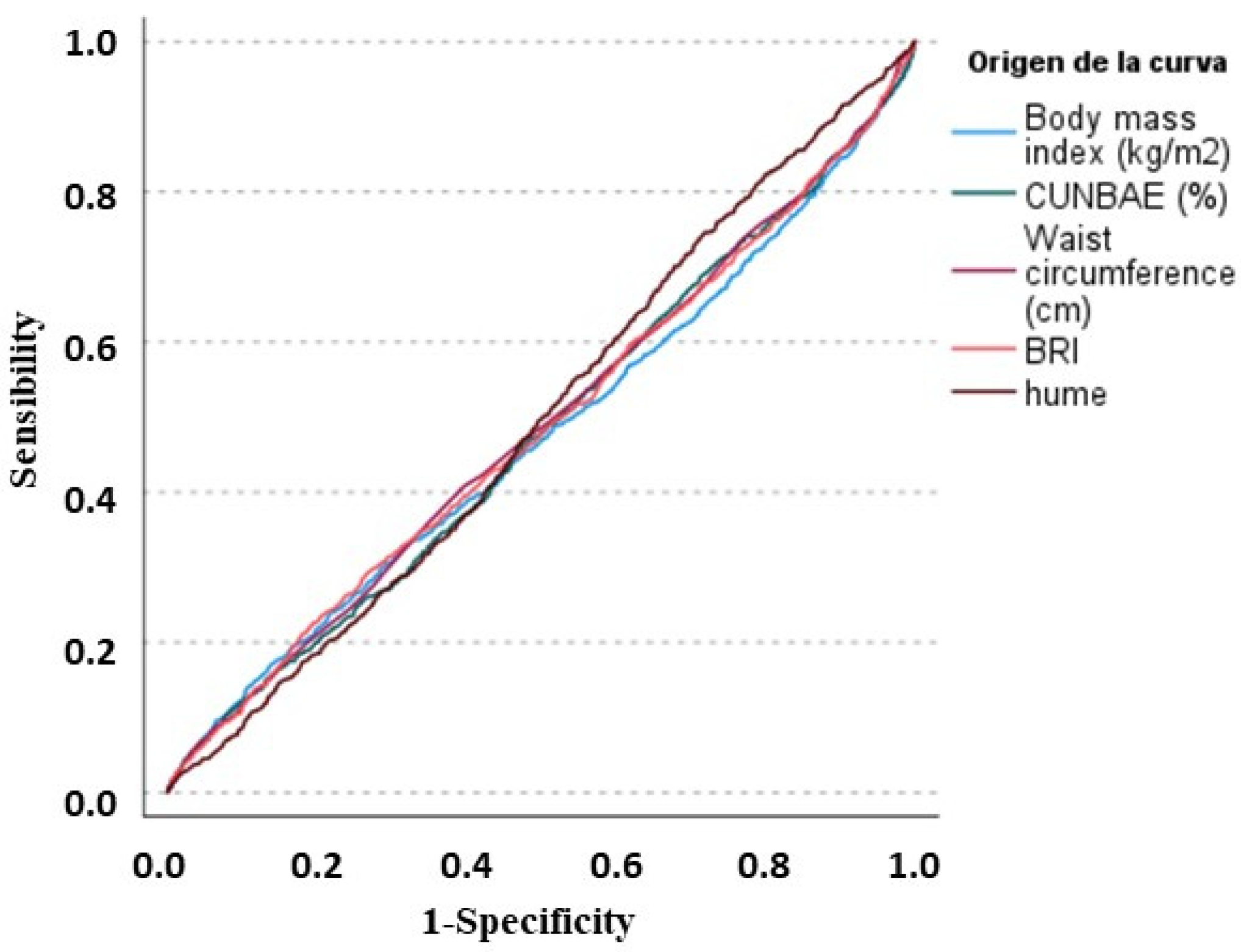
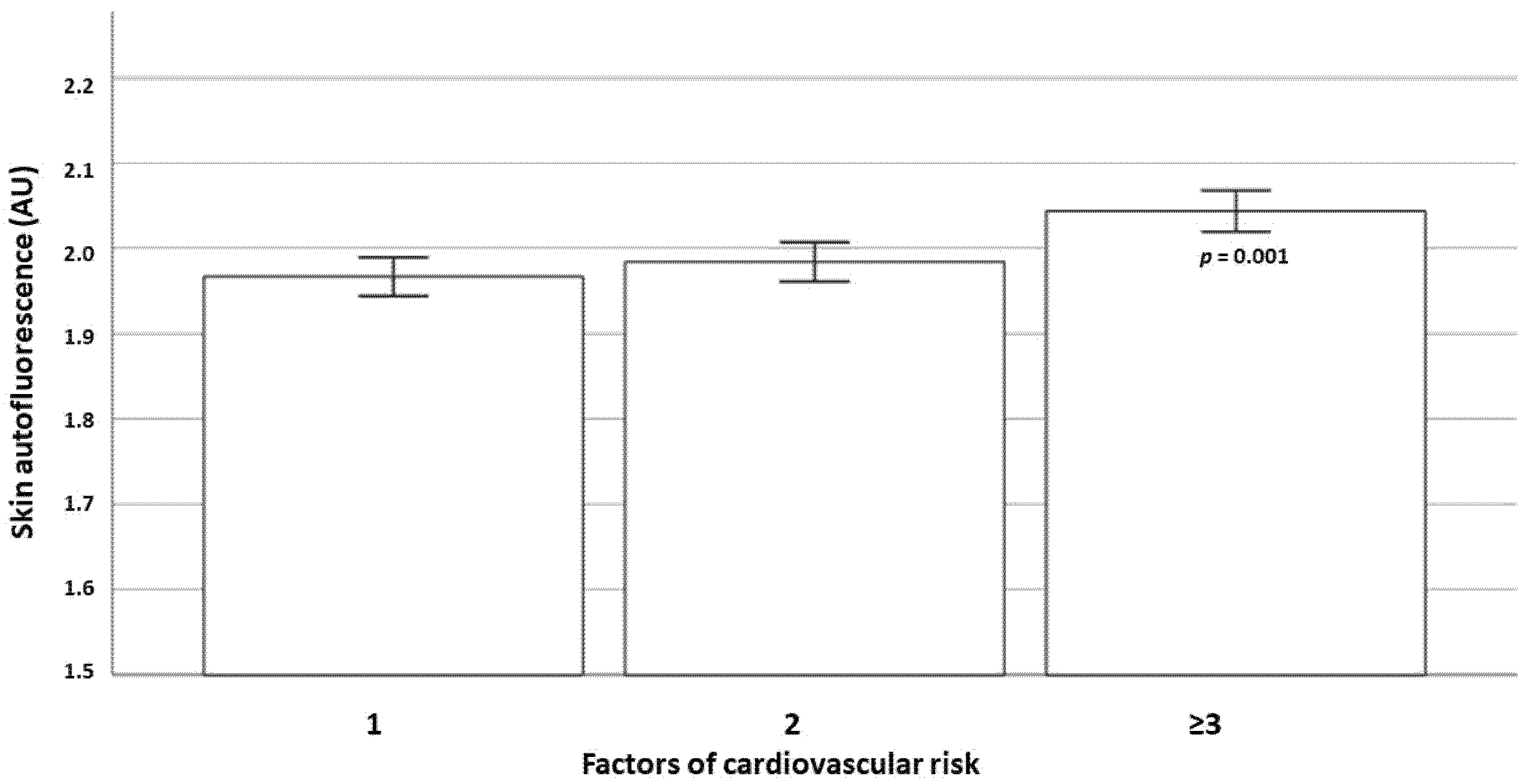
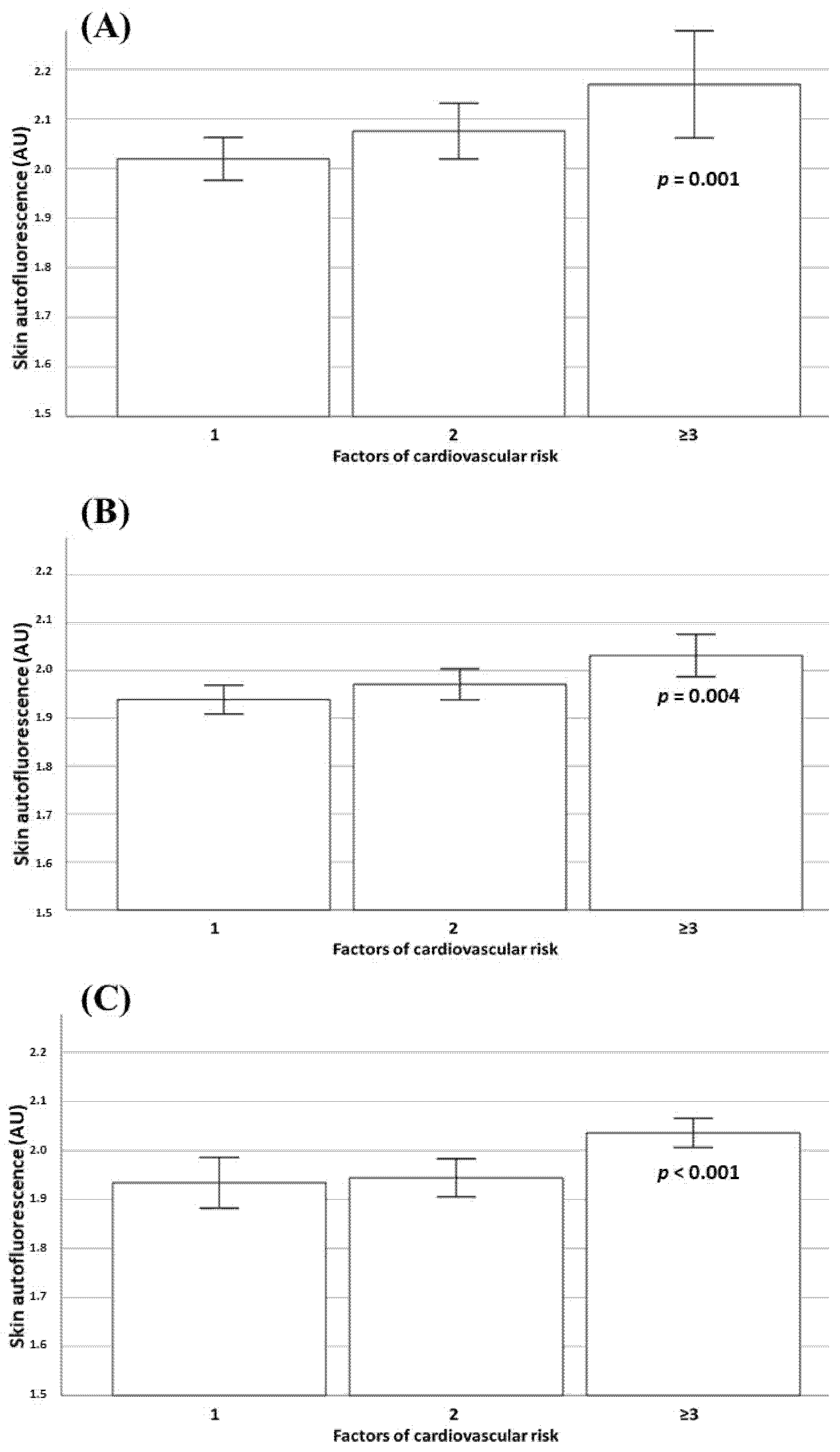
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manson, J.E.; Willett, W.C.; Stampfer, M.J.; Colditz, G.A.; Hunter, D.J.; Hankinson, S.E.; Hennekens, C.H.; Speizer, F.E. Body weight and mortality among women. N. Engl. J. Med. 1995, 333, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, L.; Zheng, R.; Zheng, Y. The prevalence, metabolic risk and effects of lifestyle intervention for metabolically healthy obesity: A systematic review and meta-analysis: A PRISMA-compliant article. Medicine 2017, 96, e8838. [Google Scholar] [CrossRef] [PubMed]
- Aung, K.; Lorenzo, C.; Hinojosa, M.A.; Haffner, S.M. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J. Clin. Endocrinol. Metab. 2014, 99, 462–468. [Google Scholar] [CrossRef]
- Stefan, N.; Fritsche, A.; Schick, F.; Häring, H.U. Phenotypes of prediabetes and stratification of cardiometabolic risk. Lancet Diabetes Endocrinol. 2016, 4, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Lee, M.J.; Kang, Y.M.; Jang, J.E.; Leem, J.; Hwang, J.Y.; Kim, E.H.; Park, J.Y.; Kim, H.K.; Lee, W.J. The risk of incident type 2 diabetes in a Korean metabolically healthy obese population: The role of systemic inflammation. J. Clin. Endocrinol. Metab. 2015, 100, 934–941. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Metabolic Inflammation and Insulin Resistance in Obesity. Circ. Res. 2020, 126, 1549–1564. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef]
- Scherer, P.E. The many secret lives of adipocytes: Implications for diabetes. Diabetologia 2019, 62, 223–232. [Google Scholar] [CrossRef]
- Sun, K.; Kusminski, C.M.; Scherer, P.E. Adipose tissue remodeling and obesity. J. Clin. Investig. 2011, 121, 2094–2101. [Google Scholar] [CrossRef]
- Hwang, I.; Kim, J.B. Two Faces of White Adipose Tissue with Heterogeneous Adipogenic Progenitors. Diabetes Metab J. 2019, 43, 752–762. [Google Scholar] [CrossRef]
- Dragoljevic, D.; Westerterp, M.; Veiga, C.B.; Nagareddy, P.; Murphy, A.J. Disordered haematopoiesis and cardiovascular disease: A focus on myelopoiesis. Clin. Sci. 2018, 132, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Engström, G.; Hedblad, B.; Stavenow, L.; Jonsson, S.; Lind, P.; Janzon, L.; Lindgärde, F. Incidence of obesity-associated cardiovascular disease is related to inflammation-sensitive plasma proteins: A population-based cohort study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Betriu, À.; Yeramian, A.; Fernández, E.; Purroy, F.; Sánchez-de-la-Torre, M.; Pamplona, R.; Miquel, E.; Kerkeni, M.; Hernández, C.; et al. Skin Autofluorescence Measurement in Subclinical Atheromatous Disease: Results from the ILERVAS Project. J. Atheroscler. Thromb. 2019, 26, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Fishman, S.L.; Sonmez, H.; Basman, C.; Singh, V.; Poretsky, L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- van Waateringe, R.P.; Slagter, S.N.; van der Klauw, M.M.; van Vliet-Ostaptchouk, J.V.; Graaff, R.; Paterson, A.D.; Lutgers, H.L.; Wolffenbuttel, B.H.R. Lifestyle and clinical determinants of skin autofluorescence in a population-based cohort study. Eur. J. Clin. Investig. 2016, 46, 481–490. [Google Scholar] [CrossRef]
- den Engelsen, C.; van den Donk, M.; Gorter, K.J.; Salomé, P.L.; Rutten, G.E. Advanced glycation end products measured by skin autofluorescence in a population with central obesity. Derm. Endocrinol. 2012, 4, 33–38. [Google Scholar] [CrossRef]
- Sánchez, E.; Baena-Fustegueras, J.A.; de la Fuente, M.C.; Gutiérrez, L.; Bueno, M.; Ros, S.; Lecube, A. Advanced glycation end-products in morbid obesity and after bariatric surgery: When glycemic memory starts to fail. Endocrinol. Diabetes Nutr. 2017, 64, 4–10. [Google Scholar] [CrossRef]
- Betriu, À.; Farràs, C.; Abajo, M.; Martinez-Alonso, M.; Arroyo, D.; Barbé, F.; Buti, M.; Lecube, A.; Portero, M.; Purroy, F.; et al. Randomised intervention study to assess the prevalence of subclinical vascular disease and hidden kidney disease and its impact on morbidity and mortality: The ILERVAS project. Nefrologia 2016, 36, 389–396. [Google Scholar] [CrossRef]
- Sánchez, E.; Sánchez, M.; Betriu, À.; Rius, F.; Torres, G.; Purroy, F.; Pamplona, R.; Ortega, M.; López-Cano, C.; Hernández, M.; et al. Are Obesity Indices Useful for Detecting Subclinical Atheromatosis in a Middle-Aged Population? Obes. Facts 2020, 13, 29–39. [Google Scholar] [CrossRef]
- WHO Consultation on Obesity. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000; pp. 1–253.
- Ma, W.Y.; Yang, C.Y.; Shih, S.R.; Hsieh, H.J.; Hung, C.S.; Chiu, F.C.; Lin, M.S.; Liu, P.H.; Hua, C.H.; Hsein, Y.C.; et al. Measurement of Waist Circumference: Midabdominal or iliac crest? Diabetes Care 2013, 36, 1660–1666. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Silva, C.; Catalán, V.; Rodríguez, A.; Galofré, J.C.; Escalada, J.; Valentí, V.; Rotellar, F.; Romero, S.; Ramírez, B.; et al. Clinical usefulness of a new equation for estimating body fat. Diabetes Care 2012, 35, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.M.; Bredlau, C.; Bosy-Westphal, A.; Mueller, M.; Shen, W.; Gallagher, D.; Maeda, Y.; McDougall, A.; Peterson, C.M.; Ravussin, E.; et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obesity 2013, 21, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Hume, R. Prediction of lean body mass from height and weight. J. Clin. Pathol. 1966, 19, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple non-invasive assessment of advanced glycation endproduct accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Lentferink, Y.E.; van Teeseling, L.; Knibbe, C.A.J.; van der Vorst, M.M.J. Skin autofluorescence in children with and without obesity. J. Pediatr. Endocrinol. Metab. 2019, 32, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Gogas Yavuz, D.; Apaydin, T.; Imre, E.; Uygur, M.M.; Yazici, D. Skin Autofluorescence and Carotid Intima-Media Thickness Evaluation Following Bariatric Surgery in Patients with Severe Obesity. Obes. Surg. 2021, 31, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Son, M.; Ahn, H.; Oh, S.; Yum, Y.; Choi, C.H.; Park, K.Y.; Byun, K. Age-related accumulation of advanced glycation end-products-albumin, S100β, and the expressions of advanced glycation end product receptor differ in visceral and subcutaneous fat. Biochem. Biophys. Res. Commun. 2016, 477, 271–276. [Google Scholar] [CrossRef]
- Semba, R.D.; Ferrucci, L.; Sun, K.; Beck, J.; Dalal, M.; Varadhan, R.; Walston, J.; Guralnik, J.M.; Fried, L.P. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging. Clin. Exp. Res. 2009, 21, 182–190. [Google Scholar] [CrossRef]
- Koyama, H.; Shoji, T.; Yokoyama, H.; Motoyama, K.; Mori, K.; Fukumoto, S.; Emoto, M.; Shoji, T.; Tamei, H.; Matsuki, H.; et al. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2587–2593. [Google Scholar] [CrossRef]
- Kiuchi, K.; Nejima, J.; Takano, T.; Ohta, M.; Hashimoto, H. Increased serum concentrations of advanced glycation end products: A marker of coronary artery disease activity in type 2 diabetic patients. Heart 2001, 85, 87–91. [Google Scholar] [CrossRef]
- Sánchez, E.; Betriu, À.; Salas-Salvadó, J.; Pamplona, R.; Barbé, F.; Purroy, F.; Farràs, C.; Fernández, E.; López-Cano, C.; Mizab, C.; et al. Mediterranean diet, physical activity and subcutaneous advanced glycation end-products’ accumulation: A cross-sectional analysis in the ILERVAS project. Eur. J. Nutr. 2020, 59, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Baye, E.; Kiriakova, V.; Uribarri, J.; Moran, L.J.; de Courten, B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: Meta-analysis of randomised controlled trials. Sci. Rep. 2017, 7, 2266. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Coughlan, M.T. Dietary Advanced Glycation End Products: Digestion, Metabolism and Modulation of Gut Microbial Ecology. Nutrients 2019, 11, 215. [Google Scholar] [CrossRef] [PubMed]
| Normal SAF (n = 3139) | High SAF (n = 1115) | p-Value | |
|---|---|---|---|
| Women, n (%) | 1576 (50.2) | 548 (49.1) | 0.543 |
| Age (years) | 57 (52–63) | 57 (54–61) | 0.003 |
| Smoking habits (current and former), n (%) | 1840 (58.6) | 856 (76.8) | <0.001 |
| Obesity diagnosis, n (%) | 941 (30.0) | 311 (27.9) | 0.189 |
| Blood hypertension diagnosis, n (%) | 1296 (41.3) | 390 (35.0) | <0.001 |
| Antihypertensive drugs, n (%) | 1036 (33.0) | 313 (28.1) | 0.002 |
| Dyslipidemia diagnosis, n (%) | 1635 (52.1) | 495 (44.4) | <0.001 |
| Lipid-lowering agents, n (%) | 555 (17.7) | 157 (14.1) | 0.006 |
| Prediabetes diagnosis, n (%) | 1081 (34.4) | 363 (32.6) | 0.311 |
| Normal SAF | High SAF | p-Value | |
|---|---|---|---|
| Total adiposity | |||
| BMI (kg/m2) | 28.4 (25.7–31.6) | 28.1 (24.7–31.8) | 0.104 |
| CUN-BAE (%) | 35.9 (30.1–42.3) | 35.3 (29.1–42.1) | 0.132 |
| Visceral adipose tissue | |||
| Waist circumference (cm) | 101 (94–108) | 100 (94–108) | 0.19 |
| Body roundness index | 5.68 (4.73–6.83) | 5.58 (4.50–6.94) | 0.127 |
| Lean body mass | |||
| Hume index (kg) | 49.5 (43.0–56.2) | 49.5 (43.4–55.5) | 0.693 |
| r | p | |
|---|---|---|
| BMI (kg/m2) | −0.019 | 0.221 |
| CUN-BAE (%) | 0.080 | <0.001 |
| Waist circumference (cm) | 0.005 | 0.746 |
| Body roundness index | 0.065 | <0.001 |
| Lean body mass (kg) | −0.122 | <0.001 |
| Odds Ratio (95% CI) | p | |
|---|---|---|
| Sex Women | Ref. | |
| Men | 0.94 (0.79 to 1.11) | 0.435 |
| Age (years) | 1.00 (0.99 to 1.01) | 0.992 |
| Body mass index (kg/m2) | 1.03 (1.01 to 1.05) | 0.006 |
| Smoking status Never | Ref. | |
| Current or former | 1.09 (0.092 to 1.28) | 0.314 |
| Cardiovascular risk factors 1 | Ref. | |
| 2 | 1.15 (0.98 to 1.36) | 0.087 |
| ≥3 | 1.28 (1.04 to 1.58) | 0.019 |
| Hosmer–Lemeshow test of fit | 0.492 | |
| Area under the ROC curve | 0.74 (0.72 to 0.77) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, E.; Sánchez, M.; López-Cano, C.; Bermúdez-López, M.; Valdivielso, J.M.; Farràs-Sallés, C.; Pamplona, R.; Torres, G.; Mauricio, D.; Castro, E.; et al. Is There a Link between Obesity Indices and Skin Autofluorescence? A Response from the ILERVAS Project. Nutrients 2023, 15, 203. https://doi.org/10.3390/nu15010203
Sánchez E, Sánchez M, López-Cano C, Bermúdez-López M, Valdivielso JM, Farràs-Sallés C, Pamplona R, Torres G, Mauricio D, Castro E, et al. Is There a Link between Obesity Indices and Skin Autofluorescence? A Response from the ILERVAS Project. Nutrients. 2023; 15(1):203. https://doi.org/10.3390/nu15010203
Chicago/Turabian StyleSánchez, Enric, Marta Sánchez, Carolina López-Cano, Marcelino Bermúdez-López, José Manuel Valdivielso, Cristina Farràs-Sallés, Reinald Pamplona, Gerard Torres, Dídac Mauricio, Eva Castro, and et al. 2023. "Is There a Link between Obesity Indices and Skin Autofluorescence? A Response from the ILERVAS Project" Nutrients 15, no. 1: 203. https://doi.org/10.3390/nu15010203
APA StyleSánchez, E., Sánchez, M., López-Cano, C., Bermúdez-López, M., Valdivielso, J. M., Farràs-Sallés, C., Pamplona, R., Torres, G., Mauricio, D., Castro, E., Fernández, E., & Lecube, A. (2023). Is There a Link between Obesity Indices and Skin Autofluorescence? A Response from the ILERVAS Project. Nutrients, 15(1), 203. https://doi.org/10.3390/nu15010203








