Abstract
Alpha-lipoic acid (ALA) is a natural compound with antioxidant and pro-oxidant properties which has effects on the regulation of insulin sensitivity and insulin secretion. ALA is widely prescribed in patients with diabetic polyneuropathy due to its positive effects on nerve conduction and alleviation of symptoms. It is, moreover, also prescribed in other insulin resistance conditions such as metabolic syndrome (SM), polycystic ovary syndrome (PCOS) and obesity. However, several cases of Insulin Autoimmune Syndrome (IAS) have been reported in subjects taking ALA. The aim of the present review is to describe the main chemical and biological functions of ALA in glucose metabolism, focusing on its antioxidant activity, its role in modulating insulin sensitivity and secretion and in symptomatic peripheral diabetic polyneuropathy. We also provide a potential explanation for increased risk for the development of IAS.
1. Introduction
Alpha-lipoic Acid (ALA) is a natural compound with diverse biochemical functions, especially in its reduced form dihydro-lipoic acid (DHLA). It acts as a metal chelator, regenerates endogenous antioxidants such as vitamins C and E and is a modulator of the signalling transduction of several pathways [1]. Many studies have indicated its potential role in the regulation of glucose metabolism, highlighting its effects on insulin sensitivity [2], insulin secretion [3], the reduction of circulating lipid levels [4] and the increase of nitric oxygen [5]. Further, ALA also seems to play a role in improving peripheral diabetic polyneuropathy [6]. It is, therefore, widely prescribed in both type 1 and type 2 diabetes (T1D; T2D) [7,8] diabetic neuropathy and in other insulin resistance conditions such as metabolic syndrome (MS), polycystic ovary syndrome (PCOS) and obesity [9,10,11].
However, numerous reports have been published in the last 15 years describing cases of Insulin Autoimmune Syndrome (IAS) in genetically predisposed subjects taking ALA. The first case was reported in Japan [12] and followed by reports from other countries, including Italy [13,14]. Our group has also described two new cases of IAS triggered by ALA and associated with HLA-DRB1*04:03, in two women [15].
Here we aim to review the main chemical and biological functions of ALA in glucose metabolism, focusing on its antioxidant activity, its role in modulating insulin sensitivity and secretion and in improving symptomatic peripheral diabetic polyneuropathy.
2. Chemistry and Metabolism of ALA
Alpha-lipoic acid, or 1,2 dithiolane-3-pentanoic acid or thioctic acid, is an endogenous cofactor of important enzyme complexes necessary to produce energy. Initially described as a vitamin used for bacterial growth, it was isolated for the first time in 1951 by the biochemist Lester Reed from a liver sample [16].
The term alpha-lipoic acid derives from the fact that it is soluble in fats. Its molecule consists of two atoms of sulphur and eight of carbon. It is amphiphilic and due to this property is readily taken up in tissues, including the nervous system, as it can cross the blood-brain central barrier [17,18]. It has an asymmetric carbon atom with two optical isomers, the dextrorotatory (R-ALA or +ALA) and the levo-rotatory (S-ALA or—ALA). R-ALA form is naturally available and can be found free or conjugated to lysine residues, making it an essential cofactor, while synthetic ALA is a racemic mixture of +ALA and—ALA (+/− ALA). The oxidized and reduced form (ALA/DHLA) works as a powerful antioxidant inactivating free radicals and reactive oxygen species (ROS) and enhancing the activity of other endogenous antioxidants (vitamin C, E, glutathione) [18,19].
Although its biosynthesis pathway is not yet entirely clear, there is evidence of ALA synthesis in the mitochondria of mammalian cells [20]. ALA synthesis starts from a fatty acid of eight carbon atoms and cysteine [21,22] probably in the liver, which also seems to be responsible for its catabolism [22].
ALA is found in high concentrations in both animal and plant sources, although the highest content is found in animal tissues with a high metabolic activity, such as the heart, while muscle tissue has lower concentrations [23]. Good animal sources of ALA are pork and calf meat, with the highest amounts found in heart, liver and kidneys. Good plant sources are spinach, broccoli, tomatoes, peas, brussels sprouts and rice bran (in descending order of content) [17]. However, in foods, the R enantiomer of alpha-lipoic acid is often covalently linked to the lysine residues of proteins, which reduces its systemic bioavailability [23].
3. ALA: Cellular Energy and Oxidative Stress
ALA is both an anti-oxidant and a pro-oxidant agent [24,25], inactivating free radicals and reactive oxygen species and modulating several signalling cascades involved in aging. ALA has diverse functions which can vary in relation to other compounds present in the cell, e.g., ALA can stimulate glucose uptake especially in muscles and adipocytes.
In describing the properties of this molecule, we will start by analysing the basic biochemical ones and then move on to those involved in glucose metabolism.
ALA is a cofactor of the pyruvate-dehydrogenase complex, which converts pyruvate obtained from glycolysis to acetyl-CoA. Lipoic acid in its oxidized form (ALA/lipoate) is first reduced (DHLA) and then oxidized again [26]. Through this biochemical reaction the pyruvate obtained from glycolysis can, by transforming into acetyl-CoA, start the Krebs cycle, which promotes the production of ATP and therefore the energy necessary for the cell to perform its functions. It has therefore been hypothesized that ALA could reduce the Warburg effect in cancer cells by stimulating the transformation of pyruvate into acetyl-CoA, rather than lactate, through the cofactor activity of the enzyme pyruvate-dehydrogenase (PDH) [27].
ALA acts as both a direct and indirect antioxidant: it is a scavenger of hydroxyl radicals, hypochlorous acid, singlet oxygen and peroxyl radicals [19,27]. It also increases the intracellular amount of glutathione and ascorbate [25,28], thus indirectly increasing the antioxidant ability of the cell and chelates iron and copper [24]. There is also some evidence that ALA can reduce the advanced glycation end products in neuronal cells with promising results for the treatment of Alzheimer’s disease [27].
ALA’s effects on cellular metabolism and oxidative stress probably have several systemic implications for both glucose and lipid metabolism. In the following sections we will discuss in depth how these properties can interact with glucose metabolism. We will first analyse ALA’s effects on insulin sensitivity and secretion, then discuss related diseases such as insulin resistance and diabetes. With regard to lipids, ALA seems to reduce both total cholesterol and triglycerides in animal models [4], suggesting a possible role also in the treatment of dyslipidaemias [29].
4. ALA and Glucose Metabolism
4.1. Effect of ALA on Glucose Uptake
Several studies over the past years have demonstrated that both ALA and DHLA can increase glucose uptake [2,30,31]. In particular, Eason et al. demonstrated that glucose uptake increased by 300% when muscles of ob/ob mice, a model of severe insulin resistance, were incubated with ALA alone, while no changes occurred when muscles were incubated only with insulin, thus demonstrating the effect of ALA on insulin resistance [30].
Further studies have confirmed this effect and several molecular pathways have been explored. One of these pathways includes the activation of the insulin receptor cascade. Treatment with R (+) alpha-lipoic acid stimulates the activity of PI3K and the phosphorylation of insulin receptor substrate- 1 (IRS-1) in 3T3-L1 adipocytes [31]. The phosphorylation of the substrate of the insulin receptor involves the activation of further intracellular mediators and subsequently the translocation of GLUT4 [31]. Diesel et al. revealed a direct binding site for alpha-lipoic acid at the tyrosine kinase domain of the insulin receptor in hepatocytes, suggesting a stabilizing function in loop A that is involved in ATP binding [32]. Thus, ALA can be considered an insulin-mimetic agent [1], since the bond of insulin receptor (IR) and IRS-1 phosphorylation could subsequently lead to GLUT4 translocation and increased glucose uptake. However, there are some controversies over ALA’s action on insulin signalling. In a preadipocyte cell culture exposed to ALA [33], Akt is phosphorylated within 30 min while the insulin receptor IR and the IRS-1 are not phosphorylated. Other research findings in adipocyte cell cultures demonstrate that IR is indeed phosphorylated but this is specific to ALA, the reduced form of the compound and not to DHLA [34].
Konrad et al. described two different mechanisms through which ALA induces glucose uptake: ALA induces PI3K and AKT phosphorylation, which determine GLUT4 translocation and it also increases p38 MAPK activity, which determines GLUT4 activation [2]. Therefore, GLUT4 activity seems to be a common element to several hypotheses since the activation of the insulin receptor cascade would also secondarily determine an increase in glucose uptake by GLUT4.
Furthermore, ALA has different effects on AMPK expression in peripheral tissues such as skeletal muscles and hypothalamus. A three-day ALA treatment increases glucose uptake, evaluated by euglycemic hyper-insulinemic clamp, reduces plasma lactate and increases α-2 AMPK activity in the skeletal muscle [35] while decreasing AMPK activity in the hypothalamus [36].
AMPK is a cellular fuel sensor whose activation is associated with ATP production through fatty acid oxidation. Moreover, it can induce the translocation of GLUT4 to the cell membrane and plays an important role in mitochondrial biogenesis [37,38]. ALA seems to share the same mechanisms as metformin, which can also activate AMPK in hepatocytes and skeletal muscles [39].
Moreover, within the cell ALA could carry out additional functions associated with glucose metabolism; in particular, it could influence mitochondrial biogenesis and endoplasmic reticulum (ER) activity. In fact, ALA stimulates the expression of mitochondrial markers such as TFAM, PPARɣ and PGC1α in C2C12 cells (a myoblast cell line) and the expression of gene encoding for representative antioxidant enzymes such as glutathione peroxidase (GPX1) and superoxide dismutase 1 (SOD1) [40]. In glucose-treated rats, the decrease in PPARγ protein levels caused by oxidative stress is prevented or attenuated if they are fed with ALA [41]. However, to date, the contribution of ALA-induced PPARγ expression to insulin sensitivity cannot be clearly quantified. In the endoplasmic reticulum, ALA increases the endogenous expression of DNAJB3 cochaperone, heat shock proteins (HSP25 and HSP27) in C2C12 cells [40,42]. DNAJB3 cochaperone has been associated with reduced metabolic stress, improved insulin signalling and glucose uptake in previous in vitro and in vivo studies (3T3-L1 adipocytes and human obese subjects) [43,44]. However, it is unclear whether DNAJB3 exerts a direct effect on ER stress or acts indirectly through other pathways. Similarly, low HSP expression could play a critical role in the induction of insulin resistance and diabetes [45]. ALA’s pro-oxidant properties also include the induction of HSP and the activation of PI3K.
Thus, the effect of ALA on GLUT4 is certainly the major determinant of the increase in glucose uptake, making ALA an insulin mimetic agent. However, it is still unclear how ALA interacts with the insulin receptor and insulin receptor cascade. It is possible that ancillary effects of ALA on mitochondria and the endoplasmic reticulum further improve insulin sensitivity. The effects of ALA on glucose metabolism seem not to be limited to a peripheral insulin mimetic effect, as some evidence suggests that it also acts on beta-cells.
4.2. Effect of ALA on Pancreatic Beta-Cells
Several studies over the last two decades have explored changes in insulin secretion during ALA treatment. Most of these studies investigated the effect of ALA in vitro in the presence of substances toxic for beta-cell function, such as oleic acid (OA), which can interfere with mitochondria and insulin secretion [46] and 2-deoxy-D-ribose (dRib), a strongly reducing sugar which induces apoptosis and oxidative stress in beta-cell lines [47]. Pre-treatment with ALA (10 mM) prevented OA-induced decrease in insulin secretion in MIN6 cells [48] and reversed low mRNA insulin levels in a dose-dependent manner in dRib [49] stimulated HIT-T15 cells. These studies suggest that in beta-cells ALA acts as an antidote to toxic substances which reduce insulin secretion.
This hypothesis has also been confirmed in studies in vivo. In T2D rats fed with fructose, ALA did not affect insulin sensitivity but induced greater insulin excursions after glucose administration [50]. In a more recent study, the co-administration of lypo-polisaccharide S (LPS) (an inducer of chronic subacute hepatic inflammation, which can also impair pancreatic insulin secretion) [51] and ALA produced no changes in insulin sensitivity [52], assessed by euglycemic hyper-insulinemic clamp [53], but determined a restoration of first-phase and second-phase insulin secretion [54] (assessed by hyperglycaemic clamp), compared to rats treated with LPS alone. In aged rat islets, ALA significantly increased insulin secretion and decreased reactive oxygen species [55]. We can consider the latter as a model of oxidative stress induced cell damage, even though it differs from the other studies due to the absence of exogenous toxic substances.
In relation to the structure of pancreatic beta-cells, further studies suggest that ALA could exert a protective effect against pancreatic injury induced by Ciclosporin A [56] and Valproic acid [57]. Ciclosporin A is a potent immunosuppressive drug, listed among the possible etiologic causes of post-transplant diabetes mellitus (PTDM). In this specific context, ALA seems to protect islets from atrophy and increase insulin-immunoreactive granules.
IL-1β is produced by activated macrophages and could be the responsible for early beta-cell injury in type 1 diabetes. When ALA was administered to isolated islets from NOD mice treated with IL-1β, insulin secretion returned to normal. However, this effect was dose-dependent since although a 10−9 M lipoic acid concentration was effective, 10−6 M and 10−12 M concentrations produced no effects. Interestingly, when the 10−9 mM lipoic acid concentration was tested alone without the addition of IL-1β,insulin secretion was not restored, but, in fact, decreased significantly (68%) compared to the control condition [58]. The latter observation suggests that the effect of ALA on insulin secretion can change in relation to more complex intracellular dynamics [59].
The administration of ALA alone leads to similar results in vitro, as shown in two different clonal beta-cell types (HIT-T15 cells [60] and in MIN6 cells [3]), as ALA inhibited glucose-induced insulin secretion in both. While in HIT-T15 cells this finding was also accompanied by a reduction in beta-cell apoptosis, in MIN6 cells this effect was associated with AMPK α-subunit phosphorylation and activation and was compared to other well-known AMPK activators, such as AICAR and metformin. In high glucose conditions all these compounds, administered chronically, decreased insulin secretion and, moreover, ALA treatment produced an acute decrease in glucose-stimulated insulin secretion (GSIS) while AICAR and metformin did not. Thus, ALA’s effects on beta-cell AMPK parallels its effects in skeletal muscle, even though with opposite effects, as it increases insulin sensitivity while decreasing insulin secretion. The administration of ALA alone, therefore, seems to worsen insulin secretion, at least in beta-cell lines. However, we could consider the overall effect as protective, because improved insulin sensitivity accompanied by a smaller demand for insulin production may protect beta-cells from exhaustion [7,61,62].
In addition, a recent study by Azzam et al. suggests that lipoic acid and ascorbic acid—among other antioxidant compounds—have a significant inhibitory activity on the fibril aggregation of amylin [63], which is physiologically co-localized in granules with insulin in beta-cells. Thus, both ALA and ascorbic acid could play a protective role in β-cell overload, which has been correlated with plasma amylin levels in T2D [64]. Furthermore, ALA successfully counteracts other mechanisms of amyloidosis such as the formation of β-amyloid fibrils [65].
The effects of ALA on insulin secretion, therefore, vary according to the oxidative balance of the cell or the presence of other substances. In vitro and in vivo models suggest a role in the prevention or attenuation of the pathogenetic mechanisms that lead to type 1 and type 2 diabetes, diabetes associated with liver diseases and post-transplant diabetes.
4.3. ALA for the Treatment of Diabetes
Diabetes is a chronic disease characterized by chronic hyperglycaemia. While Type 1 diabetes is the result of a reduced or absent insulin secretion, Type 2 diabetes is characterized by both insulin resistance and reduced insulin secretion in different proportions [66].
Given the positive effects of ALA on insulin sensitivity and the protective effects on damaged beta-cells, we would expect some therapeutic efficacy in the management of diabetes.
A metanalysis [67] of six studies including uncomplicated T2D patients, showed no differences in terms of HbA1c between the control group and the group treated with ALA. Most of the patients enrolled were taking other antidiabetic drugs like metformin, sulphonyl-ureas and insulin in different combinations. None of these studies specifically included drug-naïve patients and only one study showed an improvement in HbA1c and other metabolic parameters in patients with complicated T2D treated with ALA [68]. The latter study evaluated 90 elderly type 2 diabetic patients with acute cerebral infarction randomized to receive ALA or vitamin C for 3 weeks plus insulin therapy. With the same insulin doses, FPG, 2HPG and HbA1c decreased after treatment in both groups, but the changes in the ALA group were greater compared with the control group.
Another metanalysis [69] reported a significant decrease in FPG, HbA1c, insulin concentrations, HOMA-IR, triglycerides and LDL cholesterol in individuals with metabolic diseases treated with ALA. In this case, the overall population analysed included subjects who were only overweight or had diabetes, metabolic syndrome, or polycystic ovarian syndrome. Half of the total number of eligible studies (i.e., 12 papers) examined the effects of ALA on glucose control and/or lipid profiles in T2D patients, only a few evaluated changes in HbA1c and the rest evaluated only FPG or HOMA-IR.
Therefore, despite the promising results in animal and in vitro models, treatment with ALA has had controversial and mostly disappointing results in studies on the treatment of diabetes in humans.
A recent study [70], not included in the previous metanalysis, confirms these data in glibenclamide/metformin-treated patients as no statistically significant differences were found in terms of HbA1c and glutathione peroxidase (Gpx) between ALA group and placebo group. The authors, however, argue that the negative results are due to low doses of ALA.A dose dependent effect could also be assumed in the study by Porasuphatana [71], which included metformin and sulphonyl-ureas-treated patients randomly divided into five groups to receive either placebo or four different doses of ALA. The changes between pre- and post-treatment in each group failed to reach a statistical significance due to the small sample size. Only when all patients in the ALA groups were pooled and compared to the placebo group was there a significant difference in terms of HbA1c and a significant correlation between ALA doses and HbA1c. Studies evaluating the effect of ALA on HbA1c in humans are listed in Table 1.
Thus, further studies with high doses of ALA are needed and the drug should be tested as add-on therapy to newer and safer antidiabetic drugs such as SGLT2 inhibitors and GLP-1 receptor agonists.
Although the effects on HbA1c are limited, the studies carried out with hyper-insulinemic euglycemic clamp in T2D showed increased insulin sensitivity both after acute parenteral administration [72] and after one-month oral treatment [73,74]. In particular, in the study by Kamenova [74], glucose disposal rate after treatment did not differ statistically from that of a control group of normal glucose-tolerant subjects. This discrepancy between ALA’s effect on insulin sensitivity and glycaemic control is not surprising. Insulin resistance and glucose control are different parameters and insulin resistance counts only partially towards the pathogenesis of diabetes and its manifestations. Thus, ALA could be effective in those phases in which the insulin sensitivity defect is predominant but not when beta-cell failure has already occurred. This would also explain the difference in results between the two previously mentioned meta-analyses, as the latter probably included a higher number of insulin resistant patients with normal insulin secretion. This ambivalent effect has also been observed in prediabetes, which is also characterized by a beta-cell defect [75]. In this setting, ALA had no effect on body weight, percentage body fat, blood pressure and glucose level compared to placebo, but decreased plasma insulin and HOMA-IR [76]. In women with gestational diabetes, however, the administration of ALA 100–300 mg per day led to a reduction in fasting glucose levels, although no insulin data were available [77,78]. Studies evaluating the effect of ALA on insulin sensitivity and secretion in human are listed respectively in Table 2 and Table 3.


Table 1.
Studies on ALA’s effects on HbA1c *.
Table 1.
Studies on ALA’s effects on HbA1c *.
| Country/Population | Intervention | Duration | Other Antidiabetic Drugs | Results | |
|---|---|---|---|---|---|
| [6] Ziegler et al., 1997 | Germany/T2D | ALA 800 mg or placebo (oral administration) per day | 4 months | 14 patients on oral antidiabetics while 47 patients on insulin therapy | No HbA1c differences between ALA and placebo |
| [79] Heinisch et al., 2010 | Austria/T2D | ALA 600 mg or placebo (oral administration) per day | 3 weeks | 23 patients on oral antidiabetics (metformin, glitazones and sulphonylureas), 5 patients on insulin and one patient no therapy | No HbA1c differences between ALA and placebo |
| [80] Hegazy et al., 2013 | Egypt/T1D | ALA 600 mg or placebo (oral administration) per day | 4 months | Insulin therapy | No HbA1c differences between ALA and placebo |
| [71] Porasuphatana et al., 2011 | Thailand/T2D | ALA 300 mg, 600 mg, 900 mg, 1200 mg or placebo (oral administration) per day | 6 months | Metformin and/or sulphonylureas | No differences between placebo and other groups, HbA1c significantly decreased in all treatment groups compared to placebo |
| [81] Udupa et al., 2012 | India/T2D | ALA 300 mg or Vitamin E or Omega-3 or placebo (oral administration) per day | 3 months | Metformin plus Glimepiride | No HbA1c differences between ALA and placebo |
| [82] Huang et al., 2013 | China/T2D | ALA 600 mg or placebo (intravenous infusion) per day | 3 months | Short term continuous subcutaneous insulin infusion | No HbA1c differences between ALA and placebo |
| [68] Zhao et al., 2014 | China/T2D complicated by acute cerebral infarction | ALA 600 mg or placebo (intravenous infusion) per day | 1 month | Insulin therapy | ALA significantly decreased HbA1c compared to placebo |
| [70] Mendoza-Núñez et al., 2019 | Mexico/T2D | ALA 600 mg or placebo (oral administration) per day | 6 months | Metformin/glibenclamide | No HbA1c differences between ALA and placebo |
* Glycated Haemoglobin (HbA1c) estimates blood glucose levels of an individual over the last 3 months.

Table 2.
Studies on ALA’s effect on insulin sensitivity (M and insulin sensitivity indexes) **.

Table 3.
Studies on ALA’s effect on insulin secretion (ISR) ***.
Therefore, alpha-lipoic acid could be considered in the prevention of glucose metabolism alterations rather than in their treatment. In the next section, we will analyse the effects of ALA on syndromes characterized by alteration of insulin levels and signalling without impaired glucose levels.
4.4. ALA for Treatment of PCOS and Conditions of Insulin Resistance
Polycystic ovary syndrome is characterized by hyperandrogenism and ovarian dysfunction [85]. It is also well known that these pathological findings often overlap with obesity (32% of PCO patients in USA) [86] and insulin resistance (up to 75% according to some authors) [87].Moreover, insulin resistance is an important contributor to the development of T2D [88] and its prevalence is also increased in PCOS [10,89]. A number of studies have performed a detailed analysis of the metabolic profile of obese PCO patients, showing that these subjects have reduced insulin sensitivity, but increased insulin secretion compared to obese adolescents without PCOS, as both first-phase and second-phase insulin secretion were higher compared to controls [90]. Therefore, the prevalence of a beta-cell defect appears to be low in these patients.
Of all the studies exploring the effect of ALA treatment in PCOS, only one performed a euglycemic hyper-insulinemic clamp, which is the gold standard for the evaluation of insulin sensitivity [91], showing a significant improvement in insulin resistance, without changes in body weight, after ALA treatment [83].
Other studies report an improvement in insulin sensitivity expressed by HOMA-I or maximal insulin response during OGTT. In a group of obese PCOS patients, ALA administration significantly decreased insulin, glucose, BMI and HOMA index after 12 weeks compared to baseline values. The degree of improvement was higher especially in those with a family history for diabetes [9]. This could suggest a protection against diabetes onset in subjects at risk of diabetes. In another study by the same group [92], overweight patients were treated withmyo-inositol1 g/die, or alpha-lipoic-acid 400 mg/die, or myo-inositol 1 gr/die + alpha-lipoic acid 400 mg/die. All treatments determined a reduction in HOMA-IR and maximal insulin response. No significant differences were found in the ALA groups in subgroups with and without T2D familiar history. ALA treatment, however, does not affect sexual hormone parameters while myo-inositol does.
The combination of alpha-lipoic acid and inositol has been evaluated in several studies and beneficial effects have been reported despite different dosages and length of observation [93,94,95]. HOMA-IR decreased especially in the studies that documented a condition of greater insulin resistance at baseline. For example, in the study by Fruzzetti et al. [93], HOMA-I significantly decreased only in insulin resistant subjects, while no changes were observed in the group of subjects without insulin resistance.
In addition, the combination of ALA and inositol also determined a decrease in menstrual abnormalities [94,95]. Cycle length was progressively reduced in oligomenorrheic women after 24 months of treatment [94] and there was also a significant decrease in BMI [93,94]. Hormonal parameters also improved in the study by De Cicco et al. [96] in which treatment was administered at a double dose (ALA 800 mg and myo-inositol 2000 mg). They found that androstenedione and DHEA-S significantly decreased and mean SHBG increased and AMH serum levels decreased significantly compared to baseline. Promising results were also obtained in patients undergoing in vitro fertilization [97,98]. Moreover, the association of alpha-lipoic acid, myo-inositol and metformin (1.7 g) determined a greater improvement in hyperandrogenism, BMI and HOMA index than metformin alone (3 g) [99].
Few studies have been conducted on the effects of this molecule in other states of insulin resistance. In a study by Xiao et al. [84], there were no beneficial effects on lipid-induced impairment in insulin sensitivity and secretion, evaluated with clamp techniques (hyperglycaemic clamp followed by euglycemic hyper-insulinemic clamp the same day) in overweight and obese patients without T2D.
By contrast, other studies [100,101,102] show better metabolic profiles following ALA treatment. In a cross-over, double-blind placebo-controlled study, ALA 1200 mg positively affected blood pressure, BMI, waist circumference and triglyceride levels compared to placebo [100]. Moreover, two metanalyses [101,102] have confirmed a significant—even though minimal- reduction of BMI and weight with ALA treatment. In the metanalysis by Kucukgoncu et al., overall weight loss was 1.27 kg greater with ALA treatment than in the placebo group and BMI difference was 0.40 kg/m2 between the two groups. In the metanalysis by Namazi et al., there was a slight effect on body weight and BMI: respectively 0.69 kg and 0.39 kg/m2 compared to placebo. However, the reduction in waist circumference was not significant and there was no correlation between weight loss and ALA dose. Interestingly, both metanalyses indicate that shorter treatment duration achieves greater BMI reduction than longer intervention. Therefore, we could conclude that ALA is effective for weight loss but with no certain durability of the results.
5. ALA and Diabetic Neuropathy
Diabetic neuropathy is a known microvascular complication of diabetes. Data on the benefits of alpha-lipoic acid for both diabetic polyneuropathy (DPN) and diabetic autonomic neuropathy (DAN) started emerging in the 1990s [103,104]. DPN is one of the main clinical presentations [105] and is characterized by “negative” symptoms such as hypoesthesia and “positive” symptoms such as paraesthesia, dysesthesia, allodynia, or pain. The latter has been identified as the major determinant of depressive symptoms in people with diabetes, as it interferes with the ability to enjoy life, with daily activities and with sleep [106,107]. Electroneurography is the main diagnostic tool, confirming the presence of DPN in symptomatic patients and highlighting alterations in peripheral nerve conduction [105]. Systematic reviews and metanalyses [108,109] have evidenced the beneficial effects of ALA in the treatment of diabetic polyneuropathy (DPN). ALA, administered at a dosage of 600 mg or more either intravenously or orally, improves the Total Symptoms Score (TSS) and Neuropathy Impairment Score (NIS) in people with DPN [108,109]. ALA was shown to improve night pain, paraesthesia, muscle atrophy and difficulty in walking in a cohort of 20 patients. Better results were obtained in patients with lower HbA1c <7% than in those with higher HbA1c >7% [110].
The effects of ALA appear not to be limited solely to the symptoms of diabetic neuropathy. Indeed, ALA can be considered as the only treatment that acts on the pathogenesis of the disease while the others—α2δ ligands, tricyclic antidepressants and opioids— are only symptomatic treatments for pain. The mechanism may be related to an improvement in nerve blood flow and distal nerve conduction mediated by the antioxidant action [111,112].A systematic review including 15 articles, examined the effect of ALA 300–600 mg i.v. per day for two to four weeks, on motor nerve conduction velocity (MNCV) and sensory nerve conduction velocity (SNCV). Both these parameters increased after ALA administration even though most of the studies included in this review were of poor methodological quality [113]. Despite these positive premises, to date, no pharmacological therapies (ALA included) have been able to modify the history of the disease or reverse it [114] and, according to the American Academy of Neurology, there are no conclusive data for the use of ALA or B vitamins in the treatment of pain [115].
The manifestations of autonomic diabetic neuropathy are very disparate since different systems can be involved. Cardiovascular autonomic neuropathy (CAN), for example, can manifest with tachycardia, reduced heart rate variability, QT lengthening, orthostatic hypotension, reverse dipping and reduced sympathetic-adrenergic response to hypoglycaemia. CAN is a risk factor for cardiovascular morbidity and all-cause mortality [116]. In the Deutsche Kardiale Autonome Neuropathie Studie [117], diabetic patients with CAN—on oral anti-hyperglycaemic therapy—treated with ALA, experienced an improvement in heart rate variability, while QTc decreased but not significantly.
As regards the use of ALA in the management of other microvascular complications of diabetes, the results are rather disappointing. In the REPITON trial [118], a daily dose of 600 mg ALA did not prevent the occurrence of clinically significant macular oedema in diabetic patients. Similarly, a recent metanalysis concluded that ALA supplementation does not improve biological indices that reflect diabetic nephropathy in humans, even though with limited evidence [119].
The possibilities for use of this molecule for glyco-metabolic pathologies are therefore ample, but attention must be paid to possible side effects.
6. ALA and Insulin Autoimmune Syndrome
Insulin Autoimmune Syndrome is a rare cause of endogenous hyper-insulinemic hypoglycaemia [120] It was first described in 1970 in Japan by Hirata and colleagues. Since then, several cases have been identified and nowadays IAS is reported to be the third most common cause of spontaneous hypoglycaemia in Japan [121].
IAS is characterized by the presence of a high titre of insulin autoantibodies (IAA) which bind to insulin and proinsulin. They thus interfere with insulin action, determining a transient phase of hyperglycaemia and a prolonged pancreatic secretion of insulin and C-peptide. When IAA dissociate from insulin, hypoglycaemia occurs [122]. It can be lasting and even more dangerous than that seen in patients with insulinoma.
In 2007, Uchigata et al. published a report on the first case of ALA-induced Insulin Autoimmune Syndrome [123]. To date, 49 cases can be retrieved by a comprehensive literature search [124]. The majority of cases have been reported in Japan, while in Europe most cases have been described in Italy, two by our group in 2018 [15].The pathogenesis of ALA-induced IAS has not been fully elucidated. It seems that certain individuals could be more susceptible to the development of the disease after the introduction of substances containing sulfhydryl groups, such as ALA. The presence of the Human Leukocyte Antigen HLA-DR4 [125] and, in particular, the DRB1*04:06 and DRB1*04:03 alleles (most of the European cases) are associated with an increased risk of IAS. In Japan, 97% of affected patients are HLA-DR4-positive and 43% are also DRB1*0406-positive [12].In Europe, the predominant allele is *0403. This difference is also linked to the different prevalence of these two alleles: in Japan the frequency of the allele*04:06 is reported to range between 5.3% and 13.2% while in Europe it is between 0.1 and 1%. Conversely the frequency of allele*0403 is 1.6–12.3% in Japan while its prevalence is 0.4% to 3.9% in Europe [126]. Before ALA-induced IAS was discovered, other sulfhydryl compounds, such as methimazole, had been associated with IAS [127]. These compounds, due to the reducing properties conferred by sulfhydryl groups, could linearize insulin α chain by cleaving its disulphide bond. In this way hidden fragments of human insulin would be exposed and recognised by T lymphocytes, triggering an immune response and eventually antibody production. Matsushita et al. [128] detected the insulin fragment (TSICSLYQLE) with the highest affinity to DRB1*0406. Conversely the allele DRB1*0405, which differs from the latter for some amino acid residues, exhibits an IC50 value 44 times higher [128]. HLA- DRB1*0403 is closely related to HLA-DRB1*04:06 and it has been suggested that HLA-DRB1*04:03 is an evolutionary predecessor of HLA-DRB1*04:06 [129]. Interestingly, allele DRB1*0403 is protective against type 1 diabetes [130] and primary adrenal insufficiency [131] while both DRB1*0403 and DRB1*0406 could predispose to pemphigus [132]. To date, the exact interactions of ALA and methimazole with insulin in IAS are unclear, but it can be asserted that both a reducing ambient and a genetic predisposition seem to be necessary for the development of the disease.
In case reports, the time to onset of IAS after taking ALA ranges from 1 week to 4 months (7–120 days) with no obvious association between dose and time to onset. Hypoglycaemic episodes are generally postprandial, but they have also been reported in the fasting state. Insulin levels are very high and C-peptide levels are normal or high. After PEG precipitation, recovery of insulin is low (5–10%) compared to control samples (70%) [13]. The reason for high C-peptide levels is still controversial. C-peptide may cross-react with the same IAA and may also be “incorrectly” reported as “free” C-peptide by some immunoassays [122]. Furthermore, the initial transient hyperglycaemia—due to insulin binding to antibodies—in a usually euglycemic subject could stimulate beta-cells and determine C-peptide release. The severity of hypoglycaemia is linked to antibody titres and their affinity: low affinity antibodies are more likely to cause hypoglycaemia [122,133]. However, in some clinical situations IAA are not pathogenetic. In a study on T2D patients, insulin antibodies were detected in 48 of 118 patients (40.7%) on insulin therapy and were unexpectedly found in seven of 263 insulin naïve patients (2.7%) [134].
IAS usually resolves once the trigger is removed. There is no agreement as to whether to introduce a therapy or even which therapy to use. In case reports, diazoxide, prednisone and other immunosuppressants were administered after ALA suspension. In all cases, hypoglycaemic events disappeared after a comparable period of time (one week to three months) [124]. Thus, the introduction of drugs is not mandatory, but based on the severity of symptoms and the patient’s general clinical condition. However, the positioning of a flash glucose monitoring (FGM) device could be a reasonable option to monitor the possible persistence of hypoglycaemic episodes over time [135]. In only one case report ALA was wrongly re-administered after disease remission in a South Korean 67-year-old woman with type 2 diabetes and neuropathy. She underwent two ALA re-challenges after the first administration and in both cases developed Insulin Autoimmune Syndrome [136].
Recently the European Commission has asked the European Food Safety Authority (EFSA) to review existing data on the possible link between ALA administration and IAS and provide advice on the dietary intake of foods supplemented with alpha-lipoic acid. A panel published by EFSA in April 2021 [124] concluded that the addition of ALA to foods is likely to lead to an increased risk of development of IAS in individuals with certain genetic polymorphisms. On the other hand, no link has been found between the intake of foods naturally containing ALA with IAS. Furthermore, an ALA dose below which IAS is not expected to occur cannot be derived based on the available data. This is probably due to the autoimmune pathogenesis of the disease.
Therefore, we can conclude that the safe daily administration of ALA would require the analysis of HLA, although this is rarely feasible in clinical practice. An accurate evaluation of the medical history of the patient could help identify any pathologies sharing the same HLA predisposing alleles of ALA-induced IAS, such as pemphigus, thus avoiding the introduction of the drug.
This Figure 1 summarizes the main effects of ALA on glucose metabolism: it can activate the insulin receptor by extracellular binding, but it can also cross the cell membrane and activate AMPK. The latter interaction is responsible for GLUT4 expression on cell surface and increased glucose uptake. This promotes glucose entry into the cell and glycolysis. ALA then contributes to the beginning of Krebs cycle through its interaction with pyruvate dehydrogenase. On the other hand, ALA could modify insulin structure through the cleavage of disulphide bond and the subsequent exposition of hidden fragments to immune system. In this way lymphatic organs produce IAA which bind insulin and proinsulin and determine IAS.
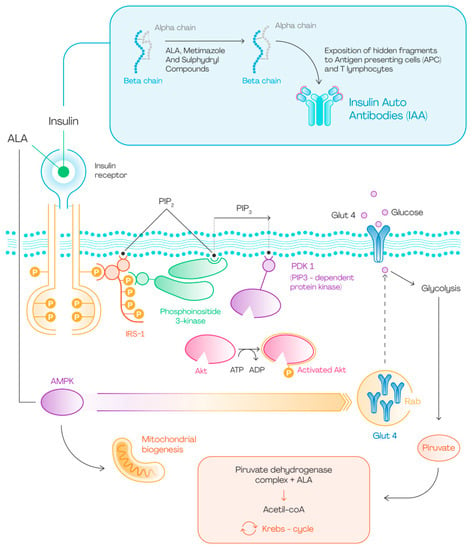
Figure 1.
ALA and glucose metabolism.
7. Conclusions
Alpha-lipoic acid has pleiotropic effects on glucose metabolism (Figure 1), many of which are still under investigation. This compound is widely prescribed for the treatment of insulin resistant states, such as polycystic ovary syndrome and for diabetic neuropathy, due to the amount of supporting evidence.
Blood glucose is the result of many sophisticated intra- and extra-cellular processes on which ALA seems to have only a partial impact. We have seen how the effect on insulin secretion primarily depends on the degree of cellular oxidative balance [55], but that this molecule can have only a limited effect on beta-cell metabolism tout court [67]. To date, the evidence on ALA’s validity as an antihyperglycemic drug is rather disappointing, but there are few human studies on the addition of alpha-lipoic acid to more recent therapies such as SGLT2i and GLP1-RA. Further studies are needed to determine its possible role in the treatment of diabetes, identifying any sub-categories of diabetic patients who could benefit from this type of therapy [137].
However, it cannot be excluded that alpha-lipoic acid could be used to prevent rather than treat the alteration in insulin sensitivity that may precede the development of diabetes by years. The studies on ALA in PCOS seem to confirm this hypothesis. For example, overweight and obese PCO patients are characterized by increased insulin secretion and reduced insulin sensitivity [90] but have an increased risk of developing diabetes [88]. Family history is known to be a risk factor for its development and ALA has been shown to improve insulin sensitivity and reduce blood glycaemia particularly in those patients with diabetic relatives [9]. Another area where ALA treatment has become consolidated is diabetic neuropathy, due to its beneficial effects both on the pathogenesis of the disease and on pain control [113]. However, curative treatments for this pathology are still lacking and the response rates to this and other treatments are often incomplete [115].
Considering the effects of this molecule on glucose metabolism, we cannot disregard its possible interference with the immune system leading to severe hypoglycaemia. In recent years there have been many reports of ALA-induced Insulin Autoimmune Syndrome, which, however, seems to occur only in genetically predisposed individuals [124]. The exact interactions of ALA with insulin in this disease are still unclear. It probably reduces disulphide bonds of insulin α chain displaying usually hidden amino acid residues to the immune system, leading to the production of autoantibodies [128]. Great attention must be paid to the possible onset of insulin autoimmune syndrome. The analysis of HLA would make the introduction of the drug safe, but this is rarely feasible in clinical practice. When this test is not available, we believe that this molecule should be prescribed with great care particularly in pregnancy and in fragile subjects in which a hypoglycaemic crisis could have particularly serious consequences. We consider it reasonable to discourage its use in patients with a history of pemphigus. It is also important that physicians become familiar with the early symptoms and signs of this pathology in order to avoid the onset of the most severe forms. Any hypoglycaemic event in a person taking alpha-lipoic acid must lead to suspicion of IAS and to immediate discontinuation of therapy.
Author Contributions
T.M., S.M. and I.I.; writing—original draft preparation: U.C., S.M., I.I., G.D.G. and E.C.N.; writing—review and editing: F.C., A.G. (Andrea Giaccari), A.P., C.M.A.C., A.G. (Antonio Gasbarrini) and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the Università Cattolica del Sacro Cuore (Fondi Ateneo Linea D.1 2018 R4124500690; Fondi Ateneo Linea D.1, 2019 R4124500845 and Fondi Ateneo Linea D.1, 2021 R4124501111); the Italian Ministry of Education, University and Research (GR-2018-12365577 to T.M. and RF-2019-12369293 to A. Giaccari); and the Italian Ministry of University and Research (PRIN 2020SH2ZZA to A. Giaccari).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to thank Serena Rotunno for editorial assistance in the writing of this article and Adele Manca for the design of Figure 1.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ALA | alpha-lipoic acid |
| DHLA | dihydro-lipoic acid |
| T1D | type 1 diabetes |
| T2D | type 2 diabetes |
| MS | metabolic syndrome |
| PCOS | polycystic ovary syndrome |
| IAS | insulin autoimmune syndrome |
| IAA | insulin autoantibodies |
| IRS-1 | insulin receptor substrate—1 |
| IR | insulin receptor |
| HSP | heat shock proteins |
| PTDM | post-transplant diabetes mellitus |
| DPN | diabetic polyneuropathy |
| DAN | diabetic autonomic neuropathy |
| NIS | neuropathy impairment score |
| TSS | total symptoms score |
| CAN | cardiovascular autonomic neuropathy |
| FGM | flash glucose monitoring |
References
- Rochette, L.; Ghibu, S.; Richard, C.; Zeller, M.; Cottin, Y.; Vergely, C. Direct and indirect antioxidant properties of alpha-lipoic acid and therapeutic potential. Mol. Nutr. Food Res. 2013, 57, 114–125. [Google Scholar] [CrossRef]
- Konrad, D.; Somwar, R.; Sweeney, G.; Yaworsky, K.; Hayashi, M.; Ramlal, T.; Klip, A. The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: Potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes 2001, 50, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Targonsky, E.D.; Dai, F.; Koshkin, V.; Karaman, G.T.; Gyulkhandanyan, A.V.; Zhang, Y.; Chan, C.B.; Wheeler, M.B. α-lipoic acid regulates AMP-activated protein kinase and inhibits insulin secretion from beta cells. Diabetologia 2006, 49, 1587–1598. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghelani, H.; Razmovski-Naumovski, V.; Nammi, S. Chronic treatment of (R)-α-lipoic acid reduces blood glucose and lipid levels in high-fat diet and low-dose streptozotocin-induced metabolic syndrome and type 2 diabetes in Sprague-Dawley rats. Pharmacol. Res. Perspect. 2017, 5, e00306. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.; Li, X.; Qu, Z.C.; Perriott, L.; Whitesell, R.R.; May, J.M. Uptake, recycling, and antioxidant actions of α-lipoic acid in endothelial cells. Free Radic. Biol. Med. 2002, 33, 83–93. [Google Scholar] [CrossRef]
- Ziegler, D.; Hanefeld, M.; Ruhnau, K.J.; Hasche, H.; Lobisch, M.; Schütte, K.; Kerum, G.; Malessa, R. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: A 7-month multicenter randomized controlled trial (ALADIN III Study). ALADIN III Study Group. Alpha-Lipoic Acid in Diabetic Neuropathy. Diabetes Care 1999, 22, 1296–1301. [Google Scholar] [CrossRef]
- Mezza, T.; Cinti, F.; Cefalo, C.M.A.; Pontecorvi, A.; Kulkarni, R.N.; Giaccari, A. β-Cell Fate in Human Insulin Resistance and Type 2 Diabetes: A Perspective on Islet Plasticity. Diabetes 2019, 68, 1121–1129. [Google Scholar] [CrossRef]
- Mezza, T.; Cefalo, C.M.A.; Cinti, F.; Quero, G.; Pontecorvi, A.; Alfieri, S.; Holst, J.J.; Giaccari, A. Endocrine and Metabolic Insights from Pancreatic Surgery. Trends Endocrinol. Metab. 2020, 31, 760–772. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Shefer, K.; Della Casa, D.; Prati, A.; Napolitano, A.; Manzo, A.; Despini, G.; Simoncini, T. Modulatory effects of alpha-lipoic acid (ALA) administration on insulin sensitivity in obese PCOS patients. J. Endocrinol. Investig. 2018, 41, 583–590. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Policola, C.; Prioletta, A.; Sorice, G.; Mezza, T.; Lassandro, A.; Della Casa, S.; Pontecorvi, A.; Giaccari, A. Low levels of 25(OH)D and insulin-resistance: 2 unrelated features or a cause-effect in PCOS? Clin. Nutr. 2012, 31, 476–480. [Google Scholar] [CrossRef]
- Moffa, S.; Mezza, T.; Cefalo, C.M.A.; Cinti, F.; Impronta, F.; Sorice, G.P.; Santoro, A.; Di Giuseppe, G.; Pontecorvi, A.; Giaccari, A. The Interplay between Immune System and Microbiota in Diabetes. Mediat. Inflamm. 2019, 2019, 9367404. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Miyamoto, T.; Kakizawa, T.; Shigematsu, S.; Hashizume, K. Insulin Autoimmune Syndrome possibly caused by alpha lipoic acid. Intern. Med. 2007, 46, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Gullo, D.; Evans, J.L.; Sortino, G.; Goldfine, I.D.; Vigneri, R. Insulin autoimmune syndrome (Hirata Disease) in European Caucasians taking α-lipoic acid. Clin. Endocrinol. 2014, 81, 204–209. [Google Scholar] [CrossRef]
- Bresciani, E.; Bussi, A.; Bazzigaluppi, E.; Balestrieri, G. Insulin autoimmune syndrome induced by α-lipoic acid in a Caucasian woman: Case report. Diabetes Care 2011, 34, e146. [Google Scholar] [CrossRef] [PubMed]
- Moffa, S.; Improta, I.; Rocchetti, S.; Mezza, T.; Giaccari, A. Potential cause-effect relationship between insulin autoimmune syndrome and alpha lipoic acid: Two case reports. Nutrition 2019, 57, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Berkson, B.M. Thioctic acid in treatment of hepatotoxic mushroom (Phalloides) poisoning. N. Engl. J. Med. 1979, 300, 371. [Google Scholar] [CrossRef] [PubMed]
- Amenta, F.; Traini, E.; Tomassoni, D.; Mignini, F. Pharmacokinetics of different formulations of tioctic (alpha-lipoic) acid in healthy volunteers. Clin. Exp. Hypertens. 2008, 30, 767–775. [Google Scholar] [CrossRef]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta 2009, 1790, 1149–1160. [Google Scholar] [CrossRef]
- Kagan, V.E.; Shvedova, A.; Serbinova, E.; Khan, S.; Swanson, C.; Powell, R.; Packer, L. Dihydrolipoic acid—A universal antioxidant both in the membrane and in the aqueous phase. Reduction of peroxyl, ascorbyl and chromanoxyl radicals. Biochem. Pharmacol. 1992, 44, 1637–1649. [Google Scholar] [CrossRef]
- Morikawa, T.; Yasuno, R.; Wada, H. Do mammalian cells synthesize lipoic acid? Identification of a mouse cDNA encoding a lipoic acid synthase located in mitochondria. FEBS Lett. 2001, 498, 16–21. [Google Scholar] [CrossRef]
- Bilska, A.; Wlodek, L. Lipoic acid—The drug of the future? Pharmacol. Rep. 2005, 57, 570–577. [Google Scholar] [PubMed]
- Biewenga, G.P.; Haenen, G.R.; Bast, A. The pharmacology of the antioxidant lipoic acid. Gen. Pharmacol. 1997, 29, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Mattulat, A.; Baltes, W. Determination of lipoic acid in meat of commercial quality. Z. Lebensm. Unters. Forsch. 1992, 194, 326–329. [Google Scholar] [CrossRef]
- Bast, A.; Haenen, G.R. Lipoic acid: A multifunctional antioxidant. Biofactors 2003, 17, 207–213. [Google Scholar] [CrossRef]
- Palaniappan, A.R.; Dai, A. Mitochondrial ageing and the beneficial role of α-lipoic acid. Neurochem. Res. 2007, 32, 1552–1558. [Google Scholar] [CrossRef]
- Behal, R.H.; Buxton, D.B.; Robertson, J.G.; Olson, M.S. Regulation of the pyruvate dehydrogenase multienzyme complex. Annu. Rev. Nutr. 1993, 13, 497–520. [Google Scholar] [CrossRef]
- Tibullo, D.; Li Volti, G.; Giallongo, C.; Grasso, S.; Tomassoni, D.; Anfuso, C.D.; Lupo, G.; Amenta, F.; Avola, R.; Bramanti, V. Biochemical and clinical relevance of alpha lipoic acid: Antioxidant and anti-inflammatory activity, molecular pathways and therapeutic potential. Inflamm. Res. 2017, 66, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.P.; Wells, W.W. α-Lipoic acid dependent regeneration of ascorbic acid from dehydroascorbic acid in rat liver mitochondria. J. Bioenerg. Biomembr. 1996, 28, 77–85. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Shab-Bidar, S.; Kord-Varkaneh, H.; Khorshidi, M.; Djafarian, K. Effect of alpha-lipoic acid supplementation on lipid profile: A systematic review and meta-analysis of controlled clinical trials. Nutrition 2019, 59, 121–130. [Google Scholar] [CrossRef]
- Eason, R.C.; Archer, H.E.; Akhtar, S.; Bailey, C.J. Lipoic acid increases glucose uptake by skeletal muscles of obese-diabetic ob/ob mice. Diabetes Obes. Metab. 2002, 4, 29–35. [Google Scholar] [CrossRef]
- Yaworsky, K.; Somwar, R.; Ramlal, T.; Tritschler, H.J.; Klip, A. Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by alpha-lipoic acid in 3T3-L1 adipocytes. Diabetologia 2000, 43, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Diesel, B.; Kulhanek-Heinze, S.; Holtje, M.; Brandt, B.; Höltje, H.-D.; Vollmar, A.M.; Kiemer, A.K. α-lipoic acid as a directly binding activator of the insulin receptor: Protection from hepatocyte apoptosis. Biochemistry 2007, 46, 2146–2155. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.J.; Moon, H.E.; Moini, H.; Packer, L.; Yoon, D.Y.; Chung, A.S. α-lipoic acid inhibits adipocyte differentiation by regulating pro-adipogenic transcription factors via mitogen-activated protein kinase pathways. J. Biol. Chem. 2003, 278, 34823–34833. [Google Scholar] [CrossRef]
- Moini, H.; Tirosh, O.; Park, Y.C.; Cho, K.J.; Packer, L. R-α-lipoic acid action on cell redox status, the insulin receptor, and glucose uptake in 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2002, 397, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Song, K.-H.; Koh, E.H.; Won, J.C.; Kim, H.S.; Park, H.-S.; Kim, M.-S.; Kim, S.-W.; Lee, K.-U.; Park, J.-Y. α-lipoic acid increases insulin sensitivity by activating AMPK in skeletal muscle. Biochem. Biophys. Res. Commun. 2005, 332, 885–891. [Google Scholar] [CrossRef]
- Kim, M.-S.; Park, J.-Y.; Namkoong, C.; Jang, P.-G.; Ryu, J.-W.; Song, H.-S.; Yun, J.-Y.; Nam-Goong, I.S.; Ha, J.; Park, I.-S.; et al. Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat. Med. 2004, 10, 727–733. [Google Scholar] [CrossRef]
- Bergeron, R.; Ren, J.M.; Cadman, K.S.; Moore, I.K.; Perret, P.; Pypaert, M.; Young, L.H.; Semenkovich, C.F.; Shulman, G.I. Chronic activation of AMP kinase results in NRF-1 activation and mitochondrial biogenesis. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1340–E1346. [Google Scholar] [CrossRef]
- Zong, H.; Ren, J.M.; Young, L.H.; Pypaert, M.; Mu, J.; Birnbaum, M.J.; Shulman, G.I. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA 2002, 99, 15983–15987. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Diane, A.; Mahmoud, N.; Bensmail, I.; Khattab, N.; Abunada, H.A.; Dehbi, M. Alpha lipoic acid attenuates ER stress and improves glucose uptake through DNAJB3 cochaperone. Sci. Rep. 2020, 10, 20482. [Google Scholar] [CrossRef]
- El Midaoui, A.; Wu, L.; Wang, R.; de Champlain, J. Modulation of cardiac and aortic peroxisome proliferator-activated receptor-gamma expression by oxidative stress in chronically glucose-fed rats. Am. J. Hypertens. 2006, 19, 407–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupte, A.A.; Bomhoff, G.L.; Morris, J.K.; Gorres, B.K.; Geiger, P.C. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J. Appl. Physiol. 2009, 106, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Cherian, P.; Al-Khairi, I.; Tiss, A.; Khadir, A.; Kavalakatt, S.; Warsame, S.; Dehbi, M.; Behbehani, K.; Abubaker, J. DNAJB3/HSP-40 cochaperone improves insulin signaling and enhances glucose uptake in vitro through JNK repression. Sci. Rep. 2015, 5, 14448. [Google Scholar] [CrossRef] [PubMed]
- Arredouani, A.; Diane, A.; Khattab, N.; Bensmail, I.; Aoude, I.; Chikri, M.; Mohammad, R.; Abou-Samra, A.B.; Dehbi, M. DNAJB3 attenuates metabolic stress and promotes glucose uptake by eliciting Glut4 translocation. Sci. Rep. 2019, 9, 4772. [Google Scholar] [CrossRef] [PubMed]
- Hooper, P.L.; Hooper, P.L. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones 2009, 14, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Koshkin, V.; Dai, F.F.; Robson-Doucette, C.A.; Chan, C.B.; Wheeler, M.B. Limited mitochondrial permeabilization is an early manifestation of palmitate-induced lipotoxicity in pancreatic β-cells. J. Biol. Chem. 2008, 283, 7936–7948. [Google Scholar] [CrossRef]
- Koh, G.; Lee, D.H.; Woo, J.T. 2-Deoxy-D-ribose induces cellular damage by increasing oxidative stress and protein glycation in a pancreatic β-cell line. Metabolism 2010, 59, 325–332. [Google Scholar] [CrossRef]
- Shen, W.; Liu, K.; Tian, C.; Yang, L.; Li, X.; Ren, J.; Packer, L.; Head, E.; Sharman, E.; Liu, J. Protective effects of R-alpha-lipoic acid and acetyl-L-carnitine in MIN6 and isolated rat islet cells chronically exposed to oleic acid. J. Cell. Biochem. 2008, 104, 1232–1243. [Google Scholar] [CrossRef]
- Koh, G.; Yang, E.J.; Kim, M.K.; Lee, S.A.; Lee, D.H. Alpha-lipoic acid treatment reverses 2-deoxy-D-ribose-induced oxidative damage and suppression of insulin expression in pancreatic beta-cells. Biol. Pharm. Bull. 2013, 36, 1570–1576. [Google Scholar] [CrossRef]
- Cummings, B.P.; Stanhope, K.L.; Graham, J.L.; Evans, J.L.; Baskin, D.G.; Griffen, S.C.; Havel, P.J. Dietary fructose accelerates the development of diabetes in UCD-T2DM rats: Amelioration by the antioxidant, α-lipoic acid. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1343–R1350. [Google Scholar] [CrossRef]
- Hsieh, P.S.; Chan, J.Y.; Shyu, J.F.; Chen, Y.T.; Loh, C.H. Mild portal endotoxaemia induces subacute hepatic inflammation and pancreatic β-cell dysfunction in rats. Eur. J. Clin. Investig. 2008, 38, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.F.; Hsieh, C.H.; Hsieh, Y.J.; Chen, Y.T.; Peng, Y.J.; Hsieh, P.S. α-Lipoic acid prevents mild portal endotoxaemia-induced hepatic inflammation and beta cell dysfunction. Eur. J. Clin. Investig. 2012, 42, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Mezza, T.; Muscogiuri, G.; Sorice, G.P.; Clemente, G.; Hu, J.; Pontecorvi, A.; Holst, J.J.; Giaccari, A.; Kulkarni, R.N. Insulin resistance alters islet morphology in nondiabetic humans. Diabetes 2014, 63, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Mezza, T.; Ferraro, P.M.; Di Giuseppe, G.; Moffa, S.; Cefalo, C.M.; Cinti, F.; Impronta, F.; Capece, U.; Quero, G.; Pontecorvi, A.; et al. Pancreaticoduodenectomy model demonstrates a fundamental role of dysfunctional β cells in predicting diabetes. J. Clin. Investig. 2021, 131, e146788. [Google Scholar] [CrossRef] [PubMed]
- Nobakht-Haghighi, N.; Rahimifard, M.; Baeeri, M.; Rezvanfar, M.A.; Nodeh, S.M.; Haghi-Aminjan, H.; Hamurtekin, E.; Abdollahi, M. Regulation of aging and oxidative stress pathways in aged pancreatic islets using alpha-lipoic acid. Mol. Cell. Biochem. 2018, 449, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Abd-Ellah, H.F.; Abou-Zeid, N.R. Role of alpha-lipoic acid in ameliorating Cyclosporine A-induced pancreatic injury in albino rats: A structural, ultrastructural, and morphometric study. Ultrastruct. Pathol. 2017, 41, 196–208. [Google Scholar] [CrossRef]
- Ghoneim, F.M.; Alrefai, H.; Elsamanoudy, A.Z.; Abo El-Khair, S.M.; Khalaf, H.A. The Protective Role of Prenatal Alpha Lipoic Acid Supplementation against Pancreatic Oxidative Damage in Offspring of Valproic Acid-Treated Rats: Histological and Molecular Study. Biology 2020, 9, 239. [Google Scholar] [CrossRef]
- Schroeder, M.M.; Belloto, R.J., Jr.; Hudson, R.A.; McInerney, M.F. Effects of antioxidants coenzyme Q10 and lipoic acid on interleukin-1β-mediated inhibition of glucose-stimulated insulin release from cultured mouse pancreatic islets. Immunopharmacol. Immunotoxicol. 2005, 27, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Brusco, N.; Sebastiani, G.; Di Giuseppe, G.; Licata, G.; Grieco, G.E.; Fignani, D.; Nigi, L.; Formichi, C.; Aiello, E.; Auddino, S.; et al. Intra-islet insulin synthesis defects are associated with endoplasmic reticulum stress and loss of beta cell identity in human diabetes. Diabetologia 2022. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Liu, Y.; Guo, T.; Chen, P.; Ma, K.; Zhou, C. α-lipoic acid inhibits high glucose-induced apoptosis in HIT-T15 cells. Dev. Growth Differ. 2012, 54, 557–565. [Google Scholar] [CrossRef]
- Cinti, F.; Mezza, T.; Severi, I.; Suleiman, M.; Cefalo, C.; Sorice, G.; Moffa, S.; Impronta, F.; Quero, G.; Alfieri, S.; et al. Noradrenergic fibers are associated with beta-cell dedifferentiation and impaired beta-cell function in humans. Metabolism 2021, 114, 154414. [Google Scholar] [CrossRef] [PubMed]
- Mezza, T.; Sorice, G.P.; Conte, C.; Sun, V.A.; Cefalo, C.M.A.; Moffa, S.; Pontecorvi, A.; Mari, A.; Kulkarni, R.N.; Giaccari, A. β-Cell Glucose Sensitivity Is Linked to Insulin/Glucagon Bihormonal Cells in Nondiabetic Humans. J. Clin. Endocrinol. Metab. 2016, 101, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Azzam, S.K.; Jang, H.; Choi, M.C.; Alsafar, H.; Lukman, S.; Lee, S. Inhibition of Human Amylin Aggregation and Cellular Toxicity by Lipoic Acid and Ascorbic Acid. Mol. Pharm. 2018, 15, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Marzban, L.; Park, K.; Verchere, C.B. Islet amyloid polypeptide and type 2 diabetes. Exp. Gerontol. 2003, 38, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hirohata, M.; Yamada, M. α-lipoic acid exhibits anti-amyloidogenicity for beta-amyloid fibrils in vitro. Biochem. Biophys. Res. Commun. 2006, 341, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Prim. 2015, 1, 15019. [Google Scholar] [CrossRef]
- Ebada, M.A.; Fayed, N.; Fayed, L.; Alkanj, S.; Abdelkarim, A.; Farwati, H.; Hanafy, A.; Negida, A.; Ebada, M.; Noser, Y. Efficacy of Alpha-lipoic Acid in The Management of Diabetes Mellitus: A Systematic Review and Meta-analysis. Iran. J. Pharm. Res. 2019, 18, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hu, F.X. alpha-Lipoic acid treatment of aged type 2 diabetes mellitus complicated with acute cerebral infarction. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3715–3719. [Google Scholar] [PubMed]
- Akbari, M.; Ostadmohammadi, V.; Lankarani, K.B.; Tabrizi, R.; Kolahdooz, F.; Khatibi, S.R.; Asemi, Z. The effects of alpha-lipoic acid supplementation on glucose control and lipid profiles among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. Metabolism 2018, 87, 56–69. [Google Scholar] [CrossRef]
- Mendoza-Nunez, V.M.; Garcia-Martinez, B.I.; Rosado-Perez, J.; Santiago-Osorio, E.; Pedraza-Chaverri, J.; Hernandez-Abad, V.J. The Effect of 600 mg Alpha-lipoic Acid Supplementation on Oxidative Stress, Inflammation, and RAGE in Older Adults with Type 2 Diabetes Mellitus. Oxidative Med. Cell. Longev. 2019, 2019, 3276958. [Google Scholar] [CrossRef]
- Porasuphatana, S.; Suddee, S.; Nartnampong, A.; Konsil, J.; Harnwong, B.; Santaweesuk, A. Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alpha-lipoic acid: A randomized double-blinded placebo-controlled study. Asia Pac. J. Clin. Nutr. 2012, 21, 12–21. [Google Scholar] [PubMed]
- Jacob, S.; Henriksen, E.J.; Tritschler, H.J.; Augustin, H.J.; Dietze, G.J. Improvement of insulin-stimulated glucose-disposal in type 2 diabetes after repeated parenteral administration of thioctic acid. Exp. Clin. Endocrinol. Diabetes 1996, 104, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Ruus, P.; Hermann, R.; Tritschler, H.; Maerker, E.; Renn, W.; Augustin, H.; Dietze, G.; Rett, K. Oral administration of RAC-α-lipoic acid modulates insulin sensitivity in patients with type-2 diabetes mellitus: A placebo-controlled pilot trial. Free Radic. Biol. Med. 1999, 27, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kamenova, P. Improvement of insulin sensitivity in patients with type 2 diabetes mellitus after oral administration of alpha-lipoic acid. Hormones 2006, 5, 251–258. [Google Scholar] [CrossRef]
- Bacha, F.; Lee, S.; Gungor, N.; Arslanian, S.A. From pre-diabetes to type 2 diabetes in obese youth: Pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care 2010, 33, 2225–2231. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, L.E.; Chrapowitzky, L.; Rideout, T.C. Metabolic effects of α-lipoic acid supplementation in pre-diabetics: A randomized, placebo-controlled pilot study. Food Funct. 2019, 10, 5732–5738. [Google Scholar] [CrossRef]
- Mandani, M.; Badehnoosh, B.; Jalali-Mashayekhi, F.; Tavakoli-Far, B.; Khosrowbeygi, A. Alpha-lipoic acid supplementation effects on serum values of some oxidative stress biomarkers in women with gestational diabetes. Gynecol. Endocrinol. 2021, 37, 1111–1115. [Google Scholar] [CrossRef]
- Aslfalah, H.; Jamilian, M.; Rafiei, F.; Khosrowbeygi, A. Reduction in maternal serum values of glucose and gamma-glutamyltransferase after supplementation with alpha-lipoic acid in women with gestational diabetes mellitus. J. Obstet. Gynaecol. Res. 2019, 45, 313–317. [Google Scholar] [CrossRef]
- Heinisch, B.B.; Francesconi, M.; Mittermayer, F.; Schaller, G.; Gouya, G.; Wolzt, M.; Pleiner, J. Alpha-lipoic acid improves vascular endothelial function in patients with type 2 diabetes: A placebo-controlled randomized trial. Eur. J. Clin. Investig. 2010, 40, 148–154. [Google Scholar] [CrossRef]
- Hegazy, S.K.; Tolba, O.A.; Mostafa, T.M.; Eid, M.A.; El-Afify, D.R. Alpha-lipoic acid improves subclinical left ventricular dysfunction in asymptomatic patients with type 1 diabetes. Rev. Diabet. Stud. 2013, 10, 58–67. [Google Scholar] [CrossRef]
- Udupa, A.S.; Nahar, P.S.; Shah, S.H.; Kshirsagar, M.J.; Ghongane, B.B. Study of comparative effects of antioxidants on insulin sensitivity in type 2 diabetes mellitus. J. Clin. Diagn. Res. 2012, 6, 1469–1473. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wan, X.; Liu, J.; Deng, W.; Chen, A.; Liu, L.; Liu, J.; Wei, G.; Li, H.; Fang, D.; et al. Short-term continuous subcutaneous insulin infusion combined with insulin sensitizers rosiglitazone, metformin, or antioxidant α-lipoic acid in patients with newly diagnosed type 2 diabetes mellitus. Diabetes Technol. Ther. 2013, 15, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Masharani, U.; Gjerde, C.; Evans, J.L.; Youngren, J.F.; Goldfine, I.D. Effects of controlled-release alpha lipoic acid in lean, nondiabetic patients with polycystic ovary syndrome. J. Diabetes Sci. Technol. 2010, 4, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Giacca, A.; Lewis, G.F. Short-term oral α-lipoic acid does not prevent lipid-induced dysregulation of glucose homeostasis in obese and overweight nondiabetic men. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E736–E741. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef]
- Azziz, R.; Woods, K.S.; Reyna, R.; Key, T.J.; Knochenhauer, E.S.; Yildiz, B.O. The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinl. Metab. 2004, 89, 2745–2749. [Google Scholar] [CrossRef]
- Moghetti, P.; Tosi, F. Insulin resistance and PCOS: Chicken or egg? J. Endocrinol. Investig. 2021, 44, 233–244. [Google Scholar] [CrossRef]
- Solis-Herrera, C.; Triplitt, C.; Cersosimo, E.; DeFronzo, R.A.; Feingold, K.R.; Anawalt, B.; Boyce, A.; Chrousos, G.; de Herder, W.W.; Dhatariya, K.; et al. Pathogenesis of Type 2 Diabetes Mellitus. In Endotext; MDText.com, Inc.: Portland, OR, USA, 2000. [Google Scholar]
- Kakoly, N.S.; Khomami, M.B.; Joham, A.E.; Cooray, S.; Misso, M.L.; Norman, R.; Harrison, C.L.; Ranasinha, S.; Teede, H.; Moran, L.J. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: A systematic review and meta-regression. Hum. Reprod. Update 2018, 24, 455–467. [Google Scholar] [CrossRef]
- Lewy, V.D.; Danadian, K.; Witchel, S.F.; Arslanian, S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J. Pediatr. 2001, 138, 38–44. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Genazzani, A.D.; Prati, A.; Santagni, S.; Ricchieri, F.; Chierchia, E.; Rattighieri, E.; Campedelli, A.; Simoncini, T.; Artini, P.G. Differential insulin response to myo-inositol administration in obese polycystic ovary syndrome patients. Gynecol. Endocrinol. 2012, 28, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Fruzzetti, F.; Capozzi, A.; Canu, A.; Lello, S. Treatment with d-chiro-inositol and alpha lipoic acid in the management of polycystic ovary syndrome. Gynecol. Endocrinol. 2019, 35, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Fruzzetti, F.; Fidecicchi, T.; Palla, G.; Gambacciani, M. Long-term treatment with α-lipoic acid and myo-inositol positively affects clinical and metabolic features of polycystic ovary syndrome. Gynecol. Endocrinol. 2020, 36, 152–155. [Google Scholar] [CrossRef]
- Cianci, A.; Panella, M.; Fichera, M.; Falduzzi, C.; Bartolo, M.; Caruso, S. d-chiro-Inositol and alpha lipoic acid treatment of metabolic and menses disorders in women with PCOS. Gynecol. Endocrinol. 2015, 31, 483–486. [Google Scholar] [CrossRef]
- De Cicco, S.; Immediata, V.; Romualdi, D.; Policola, C.; Tropea, A.; Di Florio, C.; Tagliaferri, V.; Scarinci, E.; Della Casa, S.; Lanzone, A.; et al. Myoinositol combined with alpha-lipoic acid may improve the clinical and endocrine features of polycystic ovary syndrome through an insulin-independent action. Gynecol. Endocrinol. 2017, 33, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Rago, R.; Marcucci, I.; Leto, G.; Caponecchia, L.; Salacone, P.; Bonanni, P.; Fiori, C.; Sorrenti, G.; Sebastianelli, A. Effect of myo-inositol and alpha-lipoic acid on oocyte quality in polycystic ovary syndrome non-obese women undergoing in vitro fertilization: A pilot study. J. Biol. Regul. Homeost. Agents 2015, 29, 913–923. [Google Scholar] [PubMed]
- Artini, P.G.; Obino, M.E.R.; Micelli, E.; Malacarne, E.; Vacca, C.; Papini, F.; Cela, V. Effect of d-chiro-inositol and alpha-lipoic acid combination on COH outcomes in overweight/obese PCOS women. Gynecol. Endocrinol. 2020, 36, 755–759. [Google Scholar] [CrossRef]
- Cappelli, V.; Di Sabatino, A.; Musacchio, M.C.; De Leo, V. Valutazione di una nuova associazione tra insulino-sensibilizzanti e acido alpha-lipoico in donne obese affette da PCOS [Evaluation of a new association between insulin-sensitizers and alpha-lipoic acid in obese women affected by PCOS]. Minerva Ginecol. 2013, 65, 425–433. [Google Scholar]
- Li, N.; Yan, W.; Hu, X.; Huang, Y.; Wang, F.; Zhang, W.; Wang, Q.; Wang, X.; Sun, K. Effects of oral α-lipoic acid administration on body weight in overweight or obese subjects: A crossover randomized, double-blind, placebo-controlled trial. Clin. Endocrinol. 2017, 86, 680–687. [Google Scholar] [CrossRef]
- Kucukgoncu, S.; Zhou, E.; Lucas, K.B.; Tek, C. Alpha-lipoic acid (ALA) as a supplementation for weight loss: Results from a meta-analysis of randomized controlled trials. Obes. Rev. 2017, 18, 594–601. [Google Scholar] [CrossRef]
- Namazi, N.; Larijani, B.; Azadbakht, L. Alpha-lipoic acid supplement in obesity treatment: A systematic review and meta-analysis of clinical trials. Clin. Nutr. 2018, 37, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Gries, F.A. α-lipoic acid in the treatment of diabetic peripheral and cardiac autonomic neuropathy. Diabetes 1997, 46 (Suppl. 2), S62–S66. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.; Nowak, H.; Kempler, P.; Vargha, P.; Low, P.A. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: A meta-analysis. Diabet. Med. 2004, 21, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef]
- D’Amato, C.; Morganti, R.; Greco, C.; Di Gennaro, F.; Cacciotti, L.; Longo, S.; Mataluni, G.; Lauro, D.; Marfia, G.A.; Spallone, V. Diabetic peripheral neuropathic pain is a stronger predictor of depression than other diabetic complications and comorbidities. Diabetes Vasc. Dis. Res. 2016, 13, 418–428. [Google Scholar] [CrossRef]
- Zelman, D.C.; Brandenburg, N.A.; Gore, M. Sleep impairment in patients with painful diabetic peripheral neuropathy. Clin. J. Pain 2006, 22, 681–685. [Google Scholar] [CrossRef]
- Mijnhout, G.S.; Alkhalaf, A.; Kleefstra, N.; Bilo, H.J. Alpha lipoic acid: A new treatment for neuropathic pain in patients with diabetes? Neth. J. Med. 2010, 68, 158–162. [Google Scholar]
- Nguyen, N.; Takemoto, J.K. A Case for Alpha-Lipoic Acid as an Alternative Treatment for Diabetic Polyneuropathy. J. Pharm. Pharm. Sci. 2018, 21, 177s–191s. [Google Scholar] [CrossRef]
- Ibrahimpasic, K. Alpha lipoic acid and glycaemic control in diabetic neuropathies at type 2 diabetes treatment. Med. Arch. 2013, 67, 7–9. [Google Scholar] [CrossRef]
- Nagamatsu, M.; Nickander, K.K.; Schmelzer, J.D.; Raya, A.; Wittrock, D.A.; Tritschler, H.; Low, P.A. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care 1995, 18, 1160–1167. [Google Scholar] [CrossRef]
- Mitsui, Y.; Schmelzer, J.D.; Zollman, P.J.; Mitsui, M.; Tritschler, H.J.; Low, P.A. Alpha-lipoic acid provides neuroprotection from ischemia-reperfusion injury of peripheral nerve. J. Neurol. Sci. 1999, 163, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Bai, J.; Liu, W.; Hu, Y. A systematic review and meta-analysis of alpha-lipoic acid in the treatment of diabetic peripheral neuropathy. Eur. J. Endocrinol. 2012, 167, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef]
- Bril, V.; England, J.; Franklin, G.M.; Backonja, M.; Cohen, J.; Del Toro, D.; Feldman, E.; Iverson, D.J.; Perkins, B.; Russell, J.W.; et al. Evidence-based guideline: Treatment of painful diabetic neuropathy: Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R 2011, 3, 345–352.e21. [Google Scholar] [CrossRef] [PubMed]
- Spallone, V.; Ziegler, D.; Freeman, R.; Bernardi, L.; Frontoni, S.; Pop-Busui, R.; Stevens, M.; Kempler, P.; Hilsted, J.; Tesfaye, S.; et al. Cardiovascular autonomic neuropathy in diabetes: Clinical impact, assessment, diagnosis, and management. Diabetes Metab. Res. Rev. 2011, 27, 639–653. [Google Scholar] [CrossRef]
- Ziegler, D.; Schatz, H.; Conrad, F.; Gries, F.A.; Ulrich, H.; Reichel, G. Effects of treatment with the antioxidant α-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN Study). Deutsche KardialeAutonomeNeuropathie. Diabetes Care 1997, 20, 369–373. [Google Scholar] [CrossRef]
- Haritoglou, C.; Gerss, J.; Hammes, H.P.; Kampik, A.; Ulbig, M.W.; Group, R.S. Alpha-lipoic acid for the prevention of diabetic macular edema. Ophthalmologica 2011, 226, 127–137. [Google Scholar] [CrossRef]
- Vakali, E.; Rigopoulos, D.; Carrillo, A.E.; Flouris, A.D.; Dinas, P.C. Effects of Alpha-lipoic Acid Supplementation on Human Diabetic Nephropathy: A Systematic Review and Meta-analysis. Curr. Diabetes Rev. 2022, 18, e140921196457. [Google Scholar] [CrossRef]
- Uchigata, Y.; Hirata, Y. Insulin autoimmune syndrome (IAS, Hirata disease). Ann. Med. Interne 1999, 150, 245–253. [Google Scholar]
- Yamada, Y.; Kitayama, K.; Oyachi, M.; Higuchi, S.; Kawakita, R.; Kanamori, Y.; Yorifuji, T. Nationwide survey of endogenous hyperinsulinemic hypoglycemia in Japan (2017–2018): Congenital hyperinsulinism, insulinoma, non-insulinoma pancreatogenous hypoglycemia syndrome and insulin autoimmune syndrome (Hirata’s disease). J. Diabetes Investig. 2020, 11, 554–563. [Google Scholar] [CrossRef]
- Ismail, A.A. The insulin autoimmune syndrome (IAS) as a cause of hypoglycaemia: An update on the pathophysiology, biochemical investigations and diagnosis. Clin. Chem. Lab. Med. 2016, 54, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Uchigata, Y. The novel agent, alpha lipoic acid, can cause the development of insulin autoimmune syndrome. Intern. Med. 2007, 46, 1321–1322. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Scientific opinion on the relationship between intake of alpha-lipoic acid (thioctic acid) and the risk of insulin autoimmune syndrome. EFSA J. 2021, 19, e06577. [Google Scholar] [CrossRef] [PubMed]
- Uchigata, Y.; Kuwata, S.; Tokunaga, K.; Omon, Y.; Hirata, Y.; Kuwata, S.; Tokunaga, K.; Miyamoto, M.; Juji, T. Strong association of insulin autoimmune syndrome with HLA-DR4. Lancet 1992, 339, 393–394. [Google Scholar] [CrossRef]
- Gonzalez-Galarza, F.F.; McCabe, A.; Santos, E.; Jones, J.; Takeshita, L.; Ortega-Rivera, N.D.; Del Cid-Pavon, G.M.; Ramsbottom, K.; Ghattaoraya, G.; Alfirevic, A.; et al. Allele frequency net database (AFND) 2020 update: Gold-standard data classification, open access genotype data and new query tools. Nucleic Acids Res. 2020, 48, D783–D788. [Google Scholar] [CrossRef]
- Burden, A.C.; Rosenthal, F.D. Methimazole and insulin autoimmune syndrome. Lancet 1983, 2, 1311. [Google Scholar] [CrossRef]
- Matsushita, S.; Takahashi, K.; Motoki, M.; Komoriya, K.; Ikagawa, S.; Nishimura, Y. Allele specificity of structural requirement for peptides bound to HLA-DRB1*0405 and -DRB1*0406 complexes: Implication for the HLA-associated susceptibility to methimazole-induced insulin autoimmune syndrome. J. Exp. Med. 1994, 180, 873–883. [Google Scholar] [CrossRef]
- Uchigata, Y.; Hirata, Y.; Omori, Y.; Iwamoto, Y.; Tokunaga, K. Worldwide differences in the incidence of insulin autoimmune syndrome (Hirata disease) with respect to the evolution of HLA-DR4 alleles. Hum. Immunol. 2000, 61, 154–157. [Google Scholar] [CrossRef]
- Roep, B.O.; Schipper, R.; Verduyn, W.; Bruining, G.J.; Schreuder, G.M.; de Vries, R.R. HLA-DRB1*0403 is associated with dominant protection against IDDM in the general Dutch population and subjects with high-risk DQA1*0301-DQB1*0302/DQA1*0501-DQB1*0201 genotype. Tissue Antigens 1999, 54, 88–90. [Google Scholar] [CrossRef][Green Version]
- Falorni, A.; Brozzetti, A.; Perniola, R. From Genetic Predisposition to Molecular Mechanisms of Autoimmune Primary Adrenal Insufficiency. Cortisol Excess Insufficiency 2016, 46, 115–132. [Google Scholar] [CrossRef]
- Miyagawa, S.; Higashimine, I.; Iida, T.; Yamashina, Y.; Fukumoto, T.; Shirai, T. HLA-DRB1*04 and DRB1*14 alleles are associated with susceptibility to pemphigus among Japanese. J. Investig. Dermatol. 1997, 109, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Redmon, J.B.; Nuttall, F.Q. Autoimmune hypoglycemia. Endocrinol. Metab. Clin. N. Am. 1999, 28, 603–618. [Google Scholar] [CrossRef]
- Hattori, N.; Duhita, M.R.; Mukai, A.; Matsueda, M.; Shimatsu, A. Development of insulin antibodies and changes in titers over a long-term period in patients with type 2 diabetes. Clin. Chim. Acta 2014, 433, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Cappellani, D.; Sardella, C.; Campopiano, M.C.; Falorni, A.; Marchetti, P.; Macchia, E. Spontaneously remitting insulin autoimmune syndrome ina patient taking alpha-lipoic acid. Endocrinol. Diabetes Metab. Case Rep. 2018, 2018, 1. [Google Scholar] [CrossRef]
- Bae, S.M.; Bae, M.N.; Kim, E.Y.; Kim, I.K.; Seo, M.W.; Shin, J.K.; Cho, S.R.; Jeong, G.H. Recurrent Insulin Autoimmune Syndrome Caused by alpha-Lipoic Acid in Type 2 Diabetes. Endocrinol. Metab. 2013, 28, 326–330. [Google Scholar] [CrossRef]
- Di Giuseppe, G.; Ciccarelli, G.; Cefalo, C.M.; Cinti, F.; Moffa, S.; Impronta, F.; Capece, U.; Pontecorvi, A.; Giaccari, A.; Mezza, T. Prediabetes: How pathophysiology drives potential intervention on a subclinical disease with feared clinical consequences. Minerva Endocrinol. 2021, 46, 272–292. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).