Low-Dose Administration of Cannabigerol Attenuates Inflammation and Fibrosis Associated with Methionine/Choline Deficient Diet-Induced NASH Model via Modulation of Cannabinoid Receptor
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet, Reagents, and Primers
2.2. Animals, Diets and Cannabigerol Treatments
2.3. Liver Harvest
2.4. Histological Staining
2.5. Gene Expression
2.6. Immunofluorescence Staining
2.7. Triglyceride Assay
2.8. Statistical Analysis
3. Results:
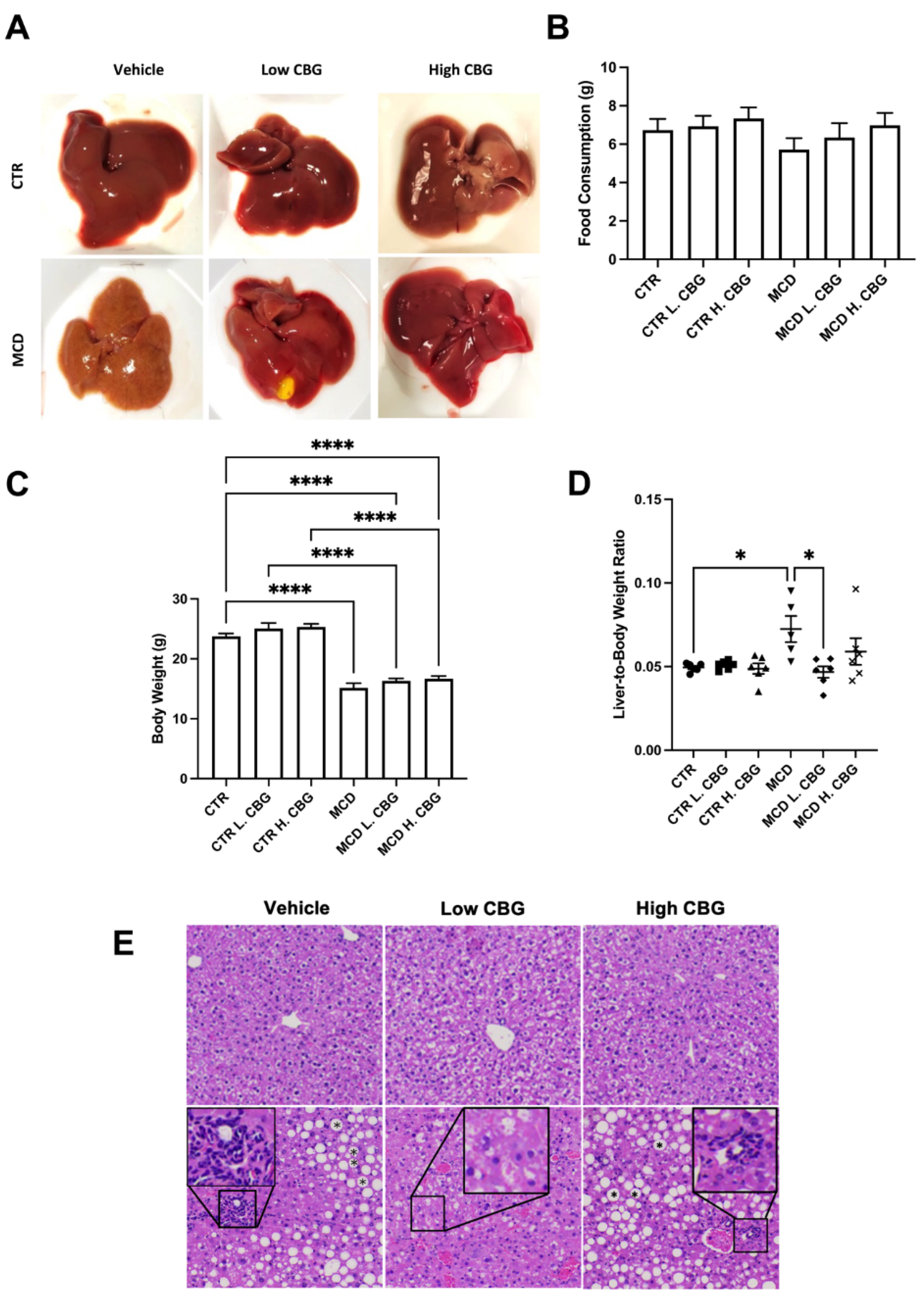
3.1. Low Dose of CBG Treatment Alleviated MCD Diet-Induced NASH Symptoms in C57BL/6 Mice
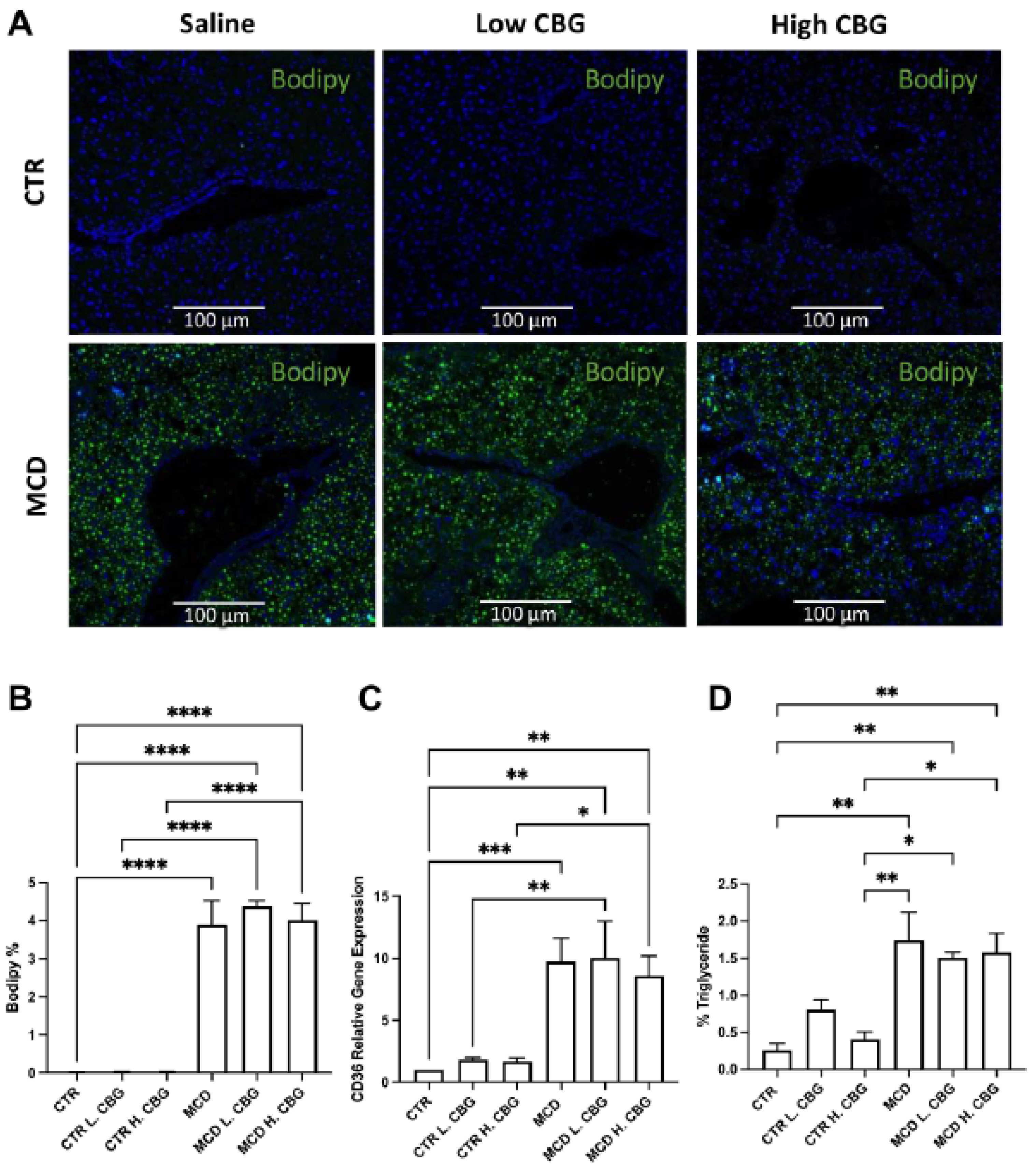
3.2. CBG Treatment Did Not Change the Steatosis Caused by MCD Diet
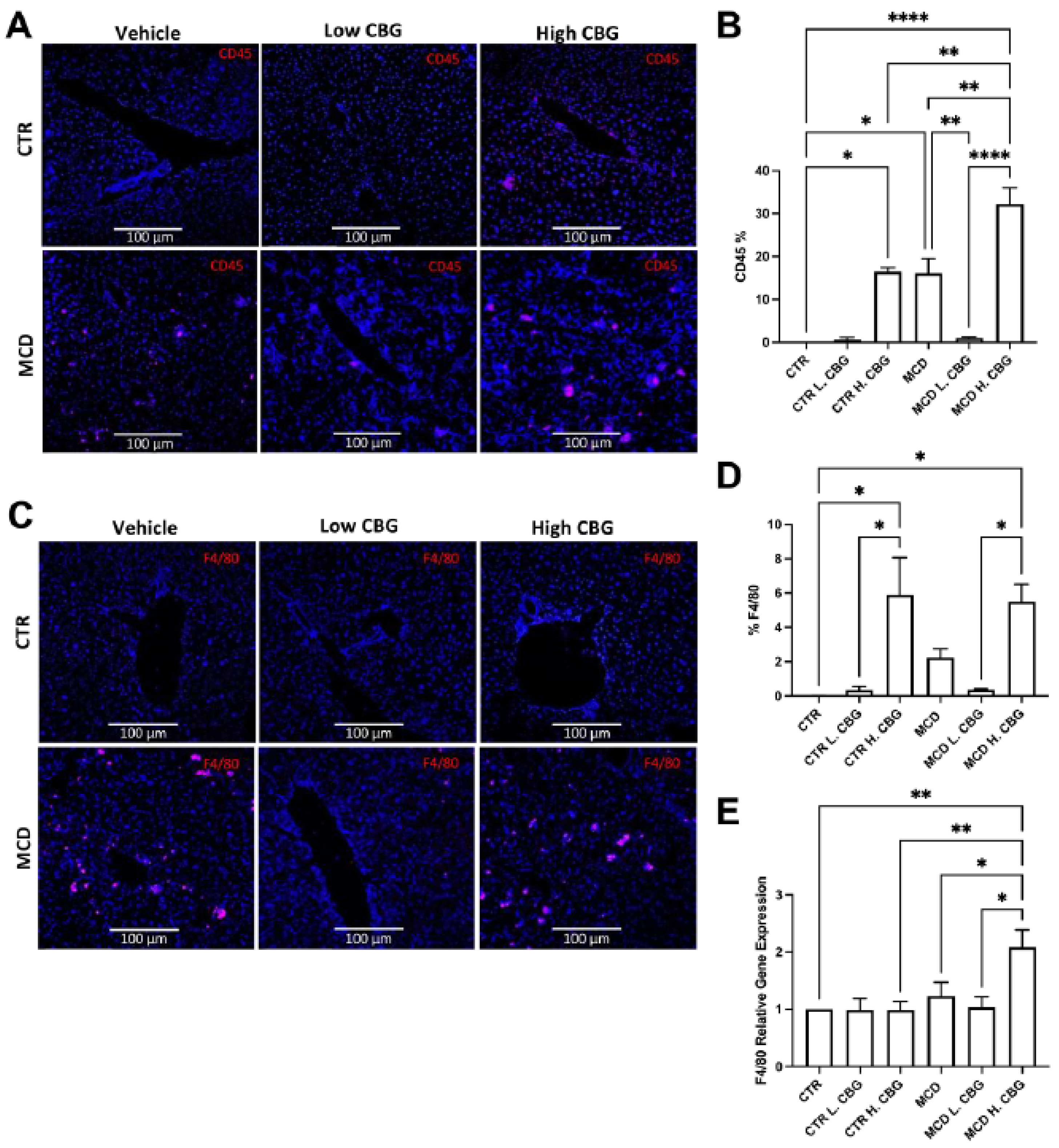
3.3. Hepatic Inflammation Was Reduced with the Low Dose of CBG, While the High Dose of CBG Enhanced Inflammation in Liver Tissues
3.4. A Low Dose of CBG Ameliorated Hepatic Fibrosis; While High Dose of CBG Increased Hepatic Fibrosis
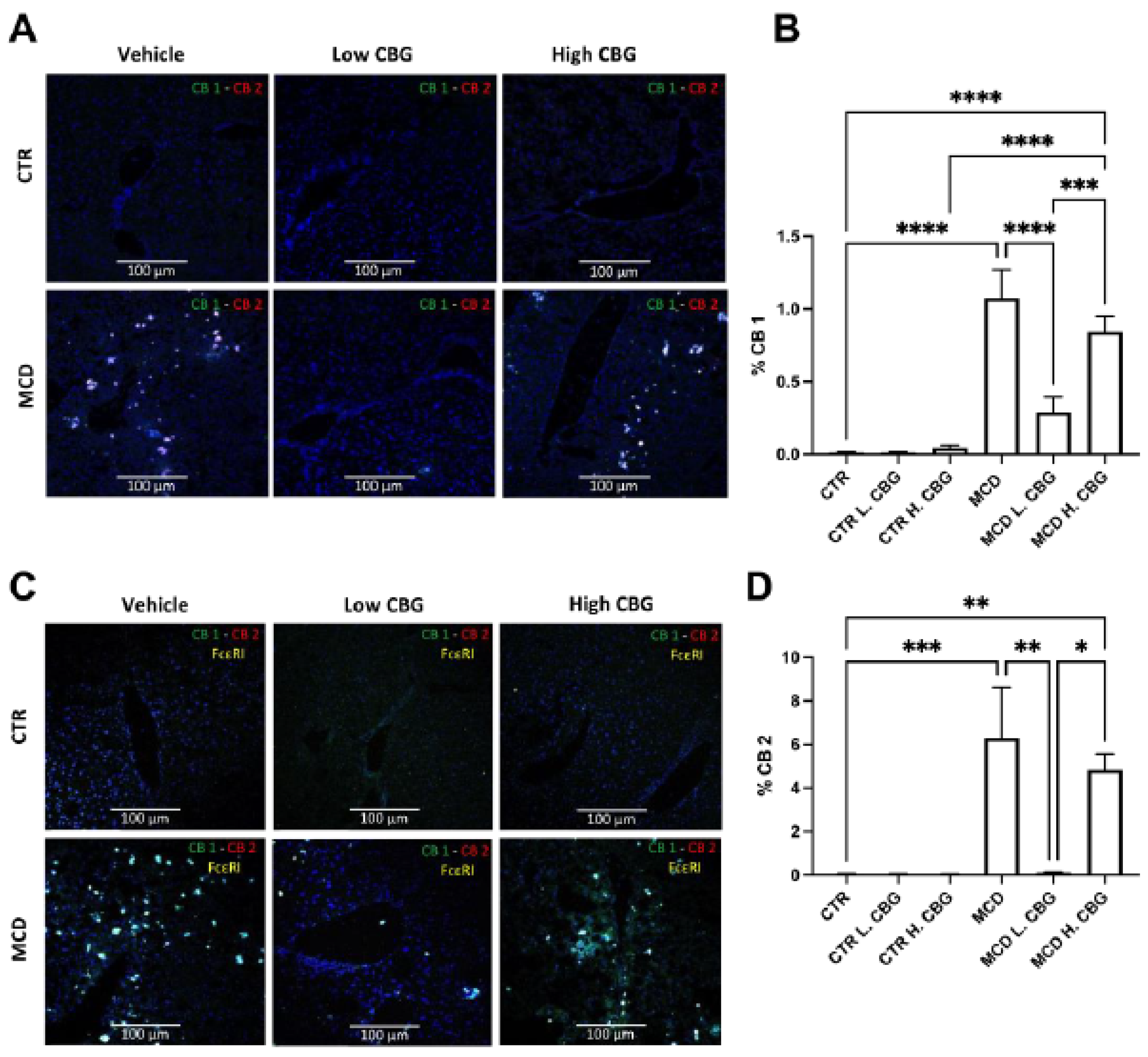
3.5. CB Receptors Showed Similar Trends Asthe Inflammatory Response in MCD-Fed Mice, and the Low Dose of CBG Alleviated the Expression of CB Receptors
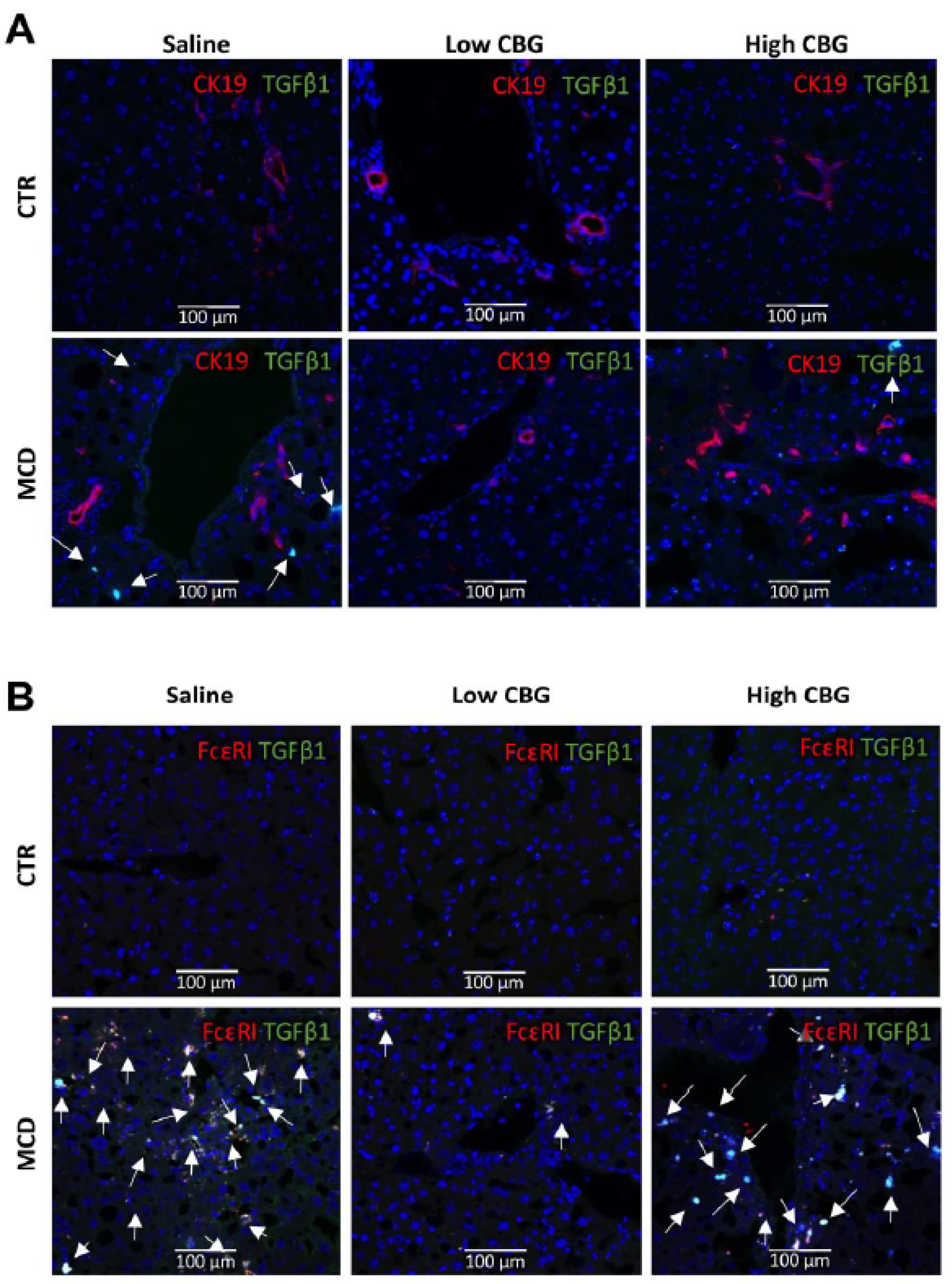
3.6. The Low Dose of CBG, Not the High Dose of CBG, Reduced Mast Cell Activation in MCD Diet via TGF-β1 Signaling
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Oseini, A.M.; Sanyal, A.J. Therapies in non-alcoholic steatohepatitis (NASH). Liver Int. 2017, 37, 97–103. [Google Scholar] [CrossRef]
- Kanwal, F.; Shubrook, J.H.; Younossi, Z.; Natarajan, Y.; Bugianesi, E.; Rinella, M.E.; Harrison, S.A.; Mantzoros, C.; Pfotenhauer, K.; Klein, S.; et al. Preparing for the NASH epidemic: A call to action. Metabolism 2021, 122, 154822. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcome. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, M.; Liu, Z.; Yuan, H.; Wu, X.; Shi, T.; Chen, X.; Zhang, T. Increasing prevalence of NAFLD/NASH among children, adolescents and young adults from 1990 to 2017: A population-based observational study. BMJ Open 2021, 11, e042843. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.J. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J. Gastroenterol. 2014, 20, 2515. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.P.; Ogawa, T.; Kawada, N. Reversibility of fibrosis, inflammation, and endoplasmic reticulum stress in the liver of rats fed a methionine–choline-deficient diet. Lab Investig. 2010, 90, 245–256. [Google Scholar] [CrossRef]
- Wang, S.; Friedman, S.L. Hepatic fibrosis: A convergent response to liver injury that is reversible. J. Hepatol. 2020, 73, 210–211. [Google Scholar] [CrossRef]
- Sharma, M.; Premkumar, M.; Kulkarni, A.V.; Kumar, P.; Reddy, D.N.; Rao, N.P. Drugs for Non-alcoholic Steatohepatitis (NASH): Quest for the Holy Grail. J. Clin. Transl. Hepatol. 2020, 9, 40. [Google Scholar] [CrossRef]
- Sumida, Y.; Yoneda, M. Current and future pharmacological therapies for NAFLD/NASH. J. Gastroenterol. 2018, 53, 362–376. [Google Scholar] [CrossRef]
- Germani, G.; Laryea, M.; Rubbia-Brandt, L.; Egawa, H.; Burra, P.; O’Grady, J.; Watt, K.D. Management of Recurrent and De Novo NAFLD/NASH After Liver Transplantation. Transplantation 2019, 103, 57–67. [Google Scholar] [CrossRef] [PubMed]
- American Liver Foundation. NASH Clinical Trial Overview and Trial Finder. American Liver Foundation. Published 2021. Available online: https://liverfoundation.org/for-patients/resources/nonalcoholic-steatohepatitis-nash-clinical-trials/ (accessed on 21 November 2021).
- Mayo Foundation for Medical Education and Research. Nonalcoholic Steatohepatitis (NASH) Clinical Trials—Mayo Clinic Research. Available online: https://www.mayo.edu/research/clinical-trials/diseases-conditions/nonalcoholic-steatohepatitis-(nash) (accessed on 21 November 2021).
- National Cancer Institute (NCI). Role of Lisinopril in Preventing the Progression of Non-Alcoholic Fatty Liver Disease (NAFLD): Relief-NAFLD; clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04550481 (accessed on 18 November 2021).
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Treatment of Nonalcoholic Steatohepatitis With Metformin; clinicaltrials.gov. 2011. Available online: https://clinicaltrials.gov/ct2/show/NCT00063232 (accessed on 18 November 2021).
- National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Vitamin E Pharmacokinetics and Biomarkers in Normal and Obese Women; clinicaltrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT00862433 (accessed on 18 November 2021).
- Sebastiani, G.; Patel, K.; Ratziu, V.; Feld, J.J.; Neuschwander-Tetri, B.A.; Pinzani, M.; Petta, S.; Berzigotti, A.; Metrakos, P.; Shoukry, N.; et al. Current considerations for clinical management and care of non-alcoholic fatty liver disease: Insights from the 1st International Workshop of the Canadian NASH Network (CanNASH). Can Liver J. 2021, 5, e20210030. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V.; et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V. A brief history of cannabinoid and endocannabinoid pharmacology as inspired by the work of British scientists. Trends Pharmacol. Sci. 2006, 27, 134–140. [Google Scholar] [CrossRef]
- Wang, Y.; Mukhopadhyay, P.; Cao, Z.; Wang, H.; Feng, D.; Haskó, G.; Mechoulam, R.; Gao, B.; Pacher, P. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci. Rep. 2017, 7, 12064. [Google Scholar] [CrossRef]
- Abulseoud, O.A.; Zuccoli, M.L.; Zhang, L.; Barnes, A.; Huestis, M.A.; Lin, D.T. The acute effect of cannabis on plasma, liver and brain ammonia dynamics, a translational study. Eur. Neuropsychopharmacol. 2017, 27, 679–690. [Google Scholar] [CrossRef]
- Romero-Zerbo, S.Y.; García-Fernández, M.; Espinosa-Jiménez, V.; Pozo-Morales, M.; Escamilla-Sánchez, A.; Sánchez-Salido, L.; Lara, E.; Cobo-Vuilleumier, N.; Rafacho, A.; Olveira, G.; et al. The Atypical Cannabinoid Abn-CBD Reduces Inflammation and Protects Liver, Pancreas, and Adipose Tissue in a Mouse Model of Prediabetes and Non-alcoholic Fatty Liver Disease. Front. Endocrinol. 2020, 11, 103. [Google Scholar] [CrossRef]
- Huang, Y.; Wan, T.; Pang, N.; Zhou, Y.; Jiang, X.; Li, B.; Gu, Y.; Huang, Y.; Ye, X.; Lian, H.; et al. Cannabidiol protects livers against nonalcoholic steatohepatitis induced by high-fat high cholesterol diet via regulating NF-κB and NLRP3 inflammasome pathway. J. Cell Physiol. 2019, 234, 21224–21234. [Google Scholar] [CrossRef]
- Wu, J. Cannabis, cannabinoid receptors, and endocannabinoid system: Yesterday, today, and tomorrow. Acta Pharmacol. Sin. 2019, 40, 297–299. [Google Scholar] [CrossRef] [PubMed]
- Osei-Hyiaman, D.; Liu, J.; Zhou, L.; Godlewski, G.; Harvey-White, J.; Jeong, W.I.; Bátkai, S.; Marsicano, G.; Lutz, B.; Buettner, C.; et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. J. Clin. Investig. 2008, 118, 3160–3169. [Google Scholar] [CrossRef]
- Tam, J.; Liu, J.; Mukhopadhyay, B.; Cinar, R.; Godlewski, G.; Kunos, G. Endocannabinoids in liver disease. Hepatology 2011, 53, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sánchez, N.; Arrese, M.; Zamora-Valdés, D.; Uribe, M. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007, 27, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Patsenker, E.; Stickel, F. Cannabinoids in liver diseases: Endocannabinoids and Liver. Clin. Liver Dis. 2016, 7, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Michelotti, G.A.; Xie, G.; de Almeida, T.P.; Boursier, J.; Bohnic, B.; Guy, C.D.; Diehl, A.M. Mouse Models of Diet-Induced Nonalcoholic Steatohepatitis Reproduce the Heterogeneity of the Human Disease. PLoS ONE 2015, 10, e0127991. [Google Scholar] [CrossRef]
- Lau, J.K.C.; Zhang, X.; Yu, J. Animal models of non-alcoholic fatty liver disease: Current perspectives and recent advances: NAFLD animal models. J. Pathol. 2017, 241, 36–44. [Google Scholar] [CrossRef]
- Takahashi, Y. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2012, 18, 2300. [Google Scholar] [CrossRef]
- Wojcikowski, K.; Gobe, G. Animal studies on medicinal herbs: Predictability, dose conversion and potential value. Phytother. Res. 2014, 28, 22–27. [Google Scholar] [CrossRef]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.; Frank, C.; Black, T.; Jadhav, P.; Holbrook, L.; Laprairie, R. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci. Rep. 2020, 10, 20405. [Google Scholar] [CrossRef]
- Miquilena-Colina, M.E.; Lima-Cabello, E.; Sanchez-Campos, S.; García-Mediavilla, M.V.; Fernández-Bermejo, M.; Lozano-Rodríguez, T.; Vargas-Castrillón, J.; Buqué, X.; Ochoa, B.; Aspichueta, P.; et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 2011, 60, 1394–1402. [Google Scholar] [CrossRef]
- Rada, P.; González-Rodríguez, Á.; García-Monzón, C.; Valverde, Á.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef]
- Simental-Mendía, L.E.; Simental-Mendía, E.; Rodríguez-Hernández, H.; Rodríguez-Morán, M.; Guerrero-Romero, F. The product of triglycerides and glucose as biomarker for screening simple steatosis and NASH in asymptomatic women. Ann. Hepatol. 2017, 7, 715–720. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef]
- Baeck, C.; Wei, X.; Bartneck, M.; Fech, V.; Heymann, F.; Gassler, N.; Hittatiya, K.; Eulberg, D.; Luedde, T.; Trautwein, C.; et al. Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C+ macrophage infiltration in mice. Hepatology 2014, 59, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Varani, K.; Reyes-Resina, I.; Sanchez de Medina, V.; Rivas-Santisteban, R.; Sanchez-Carnerero Callado, C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol Action at Cannabinoid CB1 and CB2 Receptors and at CB1–CB2 Heteroreceptor Complexes. Front. Pharmacol. 2018, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Cascio, M.; Gauson, L.; Stevenson, L.; Ross, R.; Pertwee, R. Evidence that the plant cannabinoid cannabigerol is a highly potent α2-adrenoceptor agonist and moderately potent 5HT1A receptor antagonist: Novel pharmacological actions of cannabigerol. Br. J. Pharmacol. 2010, 159, 129–141. [Google Scholar] [CrossRef]
- Small-Howard, A.L.; Shimoda, L.M.N.; Adra, C.N.; Turner, H. Anti-inflammatory potential of CB1-mediated cAMP elevation in mast cells. Biochem. J. 2005, 388, 465–473. [Google Scholar] [CrossRef]
- Halova, I.; Draberova, L.; Draber, P. Mast Cell Chemotaxis—Chemoattractants and Signaling Pathways. Front. Immunol. 2012, 3, 119. [Google Scholar] [CrossRef]
- Cardoso, A.C.; Figueiredo-Mendes, C.; Villela-Nogueira, C.A. Current management of NAFLD/NASH. Liver Int. 2021, 41 (Suppl. 1), 89–94. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, R.; Xiong, Y.; Du, F.; Zhu, S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017, 16, 203. [Google Scholar] [CrossRef]
- Ganbold, M.; Owada, Y.; Ozawa, Y.; Shimamoto, Y.; Ferdousi, F.; Tominaga, K.; Zheng, Y.W.; Ohkohchi, N.; Isoda, H. Isorhamnetin Alleviates Steatosis and Fibrosis in Mice with Nonalcoholic Steatohepatitis. Sci. Rep. 2019, 9, 16210. [Google Scholar] [CrossRef]
- Rinella, M.E.; Green, R.M. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J. Hepatol. 2004, 40, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, K.; Kennedy, L.; Hargrove, L.; Demieville, J.; Thomson, J.; Alpini, G.; Francis, H. Updates on Dietary Models of Nonalcoholic Fatty Liver Disease: Current Studies and Insights. Gene Expr. 2018, 18, 5–17. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Aqawi, M.; Gallily, R.; Sionov, R.V.; Zaks, B.; Friedman, M.; Steinberg, D. Cannabigerol Prevents Quorum Sensing and Biofilm Formation of Vibrio harveyi. Front. Microbiol. 2020, 11, 858. [Google Scholar] [CrossRef]
- Jazz Pharmaceuticals. A Randomised, Partially-Blind, Placebo-Controlled, Pilot, Dose-Ranging Study To Assess The Effect Of Cannabidiol (CBD) On Liver Fat Levels In Subjects With Fatty Liver Disease; clinicaltrials.gov. 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT01284634 (accessed on 26 September 2022).
- Zhou, X.; Yang, L.; Fan, X.; Zhao, X.; Chang, N.; Yang, L.; Li, L. Neutrophil Chemotaxis and NETosis in Murine Chronic Liver Injury via Cannabinoid Receptor 1/Gαi/o/ROS/p38 MAPK Signaling Pathway. Cells 2020, 9, 373. [Google Scholar] [CrossRef]
- Osei-Hyiaman, D.; DePetrillo, M.; Pacher, P.; Liu, J.; Radaeva, S.; Bátkai, S.; Harvey-White, J.; Mackie, K.; Offertáler, L.; Wang, L.; et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Investig. 2005, 115, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Ravinet Trillou, C.; Arnone, M.; Delgorge, C.; Gonalons, N.; Keane, P.; Maffrand, J.P.; Soubrie, P. Anti-obesity effect of SR141716, a CB1 receptor antagonist, in diet-induced obese mice. Am. J. Physiol-Regul. Integr. Comp. Physiol. 2003, 284, R345–R353. [Google Scholar] [CrossRef]
- Kyritsi, K.; Kennedy, L.; Meadows, V.; Hargrove, L.; Demieville, J.; Pham, L.; Sybenga, A.; Kundu, D.; Cerritos, K.; Meng, F.; et al. Mast Cells Induce Ductular Reaction Mimicking Liver Injury in Mice Through Mast Cell–Derived Transforming Growth Factor Beta 1 Signaling. Hepatology 2021, 73, 2397–2410. [Google Scholar] [CrossRef] [PubMed]

| Formula | CTR | MCD |
|---|---|---|
| L-Amino acids (g/kg) | 156.4 | 156.4 |
| L-Methionine (g/kg) | 8.2 | 0.0 |
| Choline chloride (g/kg) | 350 g/kg * | 0.0 |
| Sucrose (g/kg) | 443.597 | 455.294 |
| Corn starch (g/kg) | 198.783 | 200.0 |
| Cellulose (g/kg) | 30.0 | 30.0 |
| Corn oil (g/kg) | 100.0 | 100.0 |
| Salt mix (g/kg) | 35.5 | 35.0 |
| Vitamin mix (g/kg) | 10.0 | 5.0 |
| Gene | Forward | Reverse |
|---|---|---|
| CD36 | AATTAGTAGAACCGGGCCAC | CCAACTCCCAGGTACAATCA |
| α-SMA | ACTGGGACGACATGGAAAAG | AGAGGCATAGAGGGACAGCA |
| TGF-β1 | GAGCCCGAAGCGGACTACTA | CACTGCTTCCCGAATGTCTGA |
| F4/80 | TGACAACCAGACGGCTTGTG | GCAGGCGAGGAAAAGATAGTGT |
| GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
| Antibody/ Fluorescent Dye | Company | Dilution | Clone | Catalog Number |
|---|---|---|---|---|
| α-SMA | Invitrogen | 1:100 | 1A4 | 14-9760-83 |
| CNR1 (CB1) | Invitrogen | 1:1000 | PA1-743 | PA585080 |
| CNR2 (CB2) | Invitrogen | 1:200 | PA5-18428 | PA518428 |
| F4/80 | Biolegend | 1:100 | BM8 | 123122 |
| Alexa Fluor 594 | Biolegend | 1:100 | Poly4053 | 405326 |
| FcεR1 | Biolegend | 1:200 | MAR-1 | 134316 |
| CD45 | Biolegend | 1:100 | OX-1 | 202201 |
| TGF-β1 | Invitrogen | 1:100 | N/A | 21898-1-AP |
| CK19 | DSHB | 1;100 | N/A | TROMA-III |
| Bodipy | ThermoFisher | 1:10,000 | N/A | D3922 |
| Alexa Fluor® 647 | ABCAM | 1:200 | N/A | ab150155 |
| Alexa Fluor® 555 | ABCAM | 1:200 | N/A | ab150130 |
| Alexa Fluor® 488 | ABCAM | 1:200 | N/A | ab150073 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljobaily, N.; Krutsinger, K.; Viereckl, M.J.; Joly, R.; Menlove, B.; Cone, B.; Suppes, A.; Han, Y. Low-Dose Administration of Cannabigerol Attenuates Inflammation and Fibrosis Associated with Methionine/Choline Deficient Diet-Induced NASH Model via Modulation of Cannabinoid Receptor. Nutrients 2023, 15, 178. https://doi.org/10.3390/nu15010178
Aljobaily N, Krutsinger K, Viereckl MJ, Joly R, Menlove B, Cone B, Suppes A, Han Y. Low-Dose Administration of Cannabigerol Attenuates Inflammation and Fibrosis Associated with Methionine/Choline Deficient Diet-Induced NASH Model via Modulation of Cannabinoid Receptor. Nutrients. 2023; 15(1):178. https://doi.org/10.3390/nu15010178
Chicago/Turabian StyleAljobaily, Nouf, Kelsey Krutsinger, Michael J. Viereckl, Raznin Joly, Bridger Menlove, Brexton Cone, Ailaina Suppes, and Yuyan Han. 2023. "Low-Dose Administration of Cannabigerol Attenuates Inflammation and Fibrosis Associated with Methionine/Choline Deficient Diet-Induced NASH Model via Modulation of Cannabinoid Receptor" Nutrients 15, no. 1: 178. https://doi.org/10.3390/nu15010178
APA StyleAljobaily, N., Krutsinger, K., Viereckl, M. J., Joly, R., Menlove, B., Cone, B., Suppes, A., & Han, Y. (2023). Low-Dose Administration of Cannabigerol Attenuates Inflammation and Fibrosis Associated with Methionine/Choline Deficient Diet-Induced NASH Model via Modulation of Cannabinoid Receptor. Nutrients, 15(1), 178. https://doi.org/10.3390/nu15010178






