Diet Management of Patients with Chronic Kidney Disease in Bariatric Surgery
Abstract
1. Introduction
2. Obesity and Chronic Kidney Disease
3. Bariatric Surgery
4. Bariatric Surgery in Patients with Chronic Kidney Disease
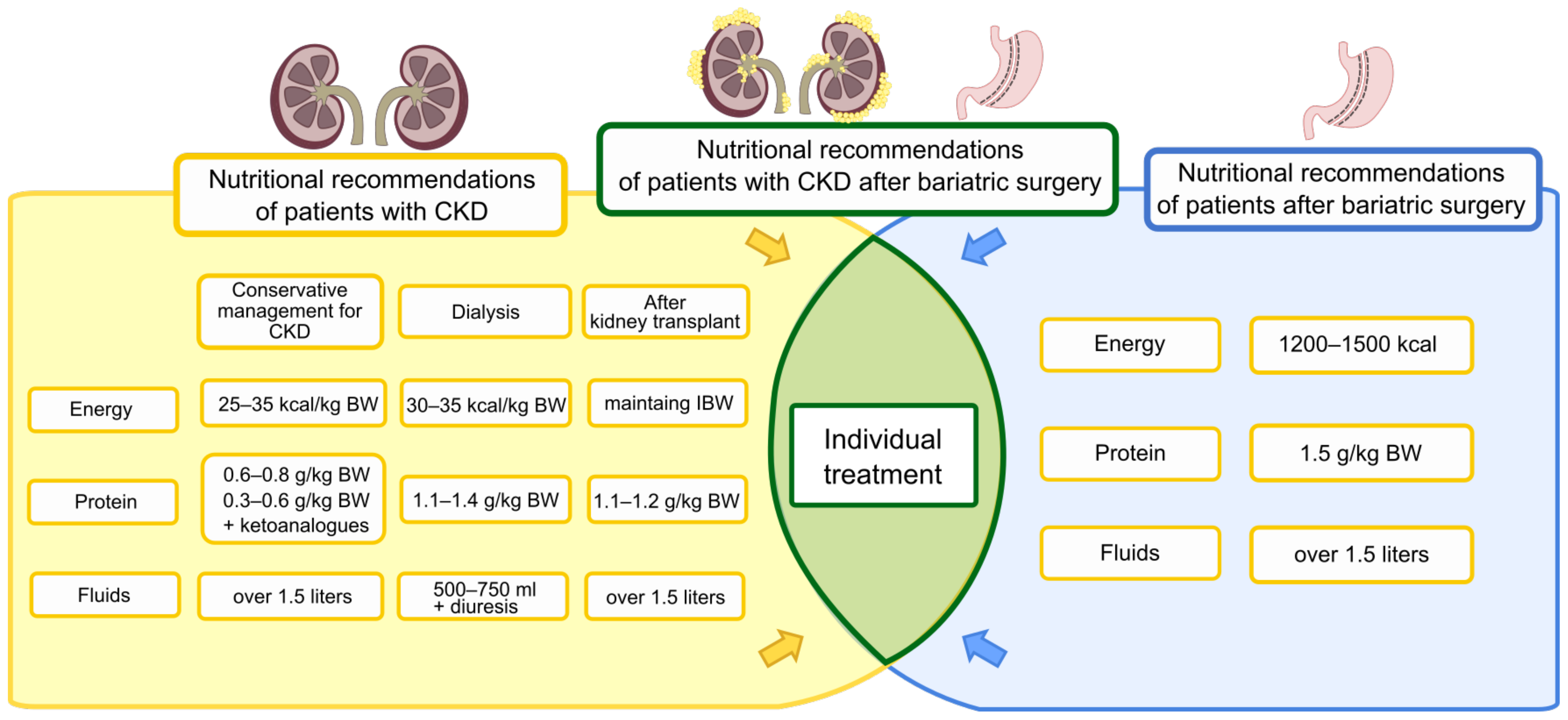
5. Diet before and after Bariatric Surgery
5.1. Patients without Chronic Kidney Disease
- (1)
- Carbohydrates—45–50% of energy,
- (2)
- Proteins—20–25% of energy, and
- (3)
- Fat—20–25% of energy (saturated fatty acids <7%, cholesterol <200 mg/day).
5.2. Patients with Chronic Kidney Disease
5.2.1. Energy Requirements
5.2.2. Protein
5.2.3. Phosphate
5.2.4. Calcium
5.2.5. Potassium
5.2.6. Sodium
5.2.7. Magnesium
5.2.8. Iron
5.2.9. Folic Acid
5.2.10. Vitamin B12
5.2.11. Vitamin D
5.2.12. Other Fat-Soluble Vitamins
5.2.13. Fluids
5.2.14. Dumping Syndrome
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 8 November 2022).
- Hammond, R.A.; Levine, R. The economic impact of obesity in the United States. Diabetes Metab. Syndr. Obes. 2010, 3, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Bhoyrul, S.; Lashock, J. The physical and fi scal impact of the obesity epidemic: The impact of comorbid conditions on patients and payers. J. Manag. Care Med. 2008, 11, 10–17. [Google Scholar]
- Conte, C.; Fabbrini, E.; Kars, M.; Mittendorfer, B.; Patterson, B.W.; Klein, S. Multiorgan insulin sensitivity in lean and obese subjects. Diabetes Care 2012, 35, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, D.E.; Telem, D.A.; Kushner, R.F.; Courcoulas, A.P. Benefits and Risks of Bariatric Surgery in Adults: A Review. JAMA 2020, 324, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Martin-Taboada, M.; Vila-Bedmar, R.; Medina-Gómez, G. From Obesity to Chronic Kidney Disease: How Can Adipose Tissue Affect Renal Function? Nephron 2021, 145, 609–613. [Google Scholar] [CrossRef]
- Czaja-Stolc, S.; Potrykus, M.; Stankiewicz, M.; Kaska, Ł.; Małgorzewicz, S. Pro-Inflammatory Profile of Adipokines in Obesity Contributes to Pathogenesis, Nutritional Disorders, and Cardiovascular Risk in Chronic Kidney Disease. Nutrients 2022, 14, 1457. [Google Scholar] [CrossRef]
- Oniscu, G.C.; Abramowicz, D.; Bolignano, D.; Gandolfini, I.; Hellemans, R.; Maggiore, U.; Nistor, I.; O’Neill, S.; Sever, M.S.; Koobasi, M.; et al. Management of obesity in kidney transplant candidates and recipients: A clinical practice guideline by the DESCARTES Working Group of ERA. Nephrol. Dial. Transpl. 2021, 37 (Suppl. 1), i1–i15. [Google Scholar] [CrossRef]
- Di Cocco, P.; Okoye, O.; Almario, J.; Benedetti, E.; Tzvetanov, I.G.; Spaggiari, M. Obesity in kidney transplantation. Transpl. Int. 2020, 33, 581–589. [Google Scholar] [CrossRef]
- Yamada, Y.; Ikenoue, T.; Saito, Y.; Fukuma, S. Undiagnosed and untreated chronic kidney disease and its impact on renal outcomes in the Japanese middle-aged general population. J. Epidemiol. Commun. Health 2019, 73, 1122–1127. [Google Scholar] [CrossRef]
- National Kidney Foundation. KDOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification and Stratification. Am. J. Kidney Dis. 2002, 39, 1–266. [Google Scholar]
- Rhee, C.M.; Ahmadi, S.F.; Kalantar-Zadeh, K. The dual roles of obesity in chronic kidney disease: A review of the current literature. Curr. Opin. Nephrol. Hypertens. 2016, 25, 208–216. [Google Scholar] [CrossRef]
- D’Agati, V.D.; Chagnac, A.; de Vries, A.P.; Levi, M.; Porrini, E.; Herman-Edelstein, M.; Praga, M. Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat. Rev. Nephrol. 2016, 12, 453–471. [Google Scholar] [CrossRef]
- Kittiskulnam, P.; Johansen, K.L. The obesity paradox: A further consideration in dialysis patients. Semin. Dial. 2019, 32, 485–489. [Google Scholar] [CrossRef]
- Potluri, K.; Hou, S. Obesity in kidney transplant recipients and candidates. Am. J. Kidney Dis. 2010, 56, 143–156. [Google Scholar] [CrossRef]
- Friedman, A.N.; Miskulin, D.C.; Rosenberg, I.H.; Levey, A.S. Demographics and trends in overweight and obesity in patients at time of kidney transplantation. Am. J. Kidney Dis. 2003, 41, 480–487. [Google Scholar] [CrossRef]
- Jarrar, F.; Tennankore, K.K.; Vinson, A.J. Combined Donor-Recipient Obesity and the Risk of Graft Loss After Kidney Transplantation. Transpl. Int. 2022, 35, 10656. [Google Scholar] [CrossRef]
- Bellini, M.I.; Paoletti, F.; Herbert, P.E. Obesity and bariatric intervention in patients with chronic renal disease. J. Int. Med. Res. 2019, 47, 2326–2341. [Google Scholar] [CrossRef]
- Taler, S.J.; Messersmith, E.E.; Leichtman, A.B.; Gillespie, B.W.; Kew, C.E.; Stegall, M.D.; Merion, R.M.; Matas, A.J.; Ibrahim, H.N.; RELIVE Study Group. Demographic, metabolic, and blood pressure characteristics of living kidney donors spanning five decades. Am. J. Transpl. 2013, 13, 390–398. [Google Scholar] [CrossRef]
- Locke, J.E.; Reed, R.D.; Massie, A.; MacLennan, P.A.; Sawinski, D.; Kumar, V.; Mehta, S.; Mannon, R.B.; Gaston, R.; Lewis, C.E.; et al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney Int. 2017, 91, 699–703. [Google Scholar] [CrossRef]
- Capehorn, M.S.; Haslam, D.W.; Welbourn, R. Obesity Treatment in the UK Health System. Curr. Obes. Rep. 2016, 5, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Metabolic surgery for type 2 diabetes with BMI < 35 kg/m2: An endocrinologist’s perspective. Obes. Surg. 2013, 23, 800–808. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric surgery: A systematic review and meta-analysis. JAMA 2004, 292, 1724–1737. [Google Scholar] [CrossRef] [PubMed]
- Pories, W.J. Bariatric surgery: Risks and rewards. J. Clin. Endocrinol. Metab. 2008, 93 (Suppl. 1), S89–S96. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Vitiello, A.; Zundel, N.; Buchwald, H.; Scopinaro, N. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes. Surg. 2017, 27, 2279–2289. [Google Scholar] [CrossRef] [PubMed]
- Angrisani, L.; Santonicola, A.; Iovino, P.; Vitiello, A.; Higa, K.; Himpens, J.; Buchwald, H.; Scopinaro, N. IFSO Worldwide Survey 2016: Primary, Endoluminal, and Revisional Procedures. Obes. Surg. 2018, 28, 3783–3794. [Google Scholar] [CrossRef]
- Scopinaro, N.; Gianetta, E.; Civalleri, D.; Bonalumi, U.; Bachi, V. Bilio-pancreatic bypass for obesity: II. Initial experience in man. Br. J. Surg. 1979, 66, 618–620. [Google Scholar] [CrossRef]
- Kremen, A.J.; Linner, J.H.; Nelson, C.H. An experimental evaluation of the nutritional importance of proximal and distal small intestine. Ann. Surg. 1954, 140, 439–448. [Google Scholar] [CrossRef]
- Cohen, R.V.; Schiavon, C.A.; Pinheiro, J.S.; Correa, J.L.; Rubino, F. Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22–34 kg/m2: A report of 2 cases. Surg. Obes. Relat. Dis. 2007, 3, 195–197. [Google Scholar] [CrossRef]
- Gagner, M. Hypoabsorption Not Malabsorption, Hypoabsorptive Surgery and Not Malabsorptive Surgery. Obes. Surg. 2016, 26, 2783–2784. [Google Scholar] [CrossRef]
- Fobi, M.A.; Lee, H.; Holness, R.; Cabinda, D. Gastric bypass operation for obesity. World J. Surg. 1998, 22, 925–935. [Google Scholar] [CrossRef]
- Rutledge, R.; Kular, K.; Manchanda, N. The Mini-Gastric Bypass original technique. Int. J. Surg. 2019, 61, 38–41. [Google Scholar] [CrossRef]
- Picot, J.; Jones, J.; Colquitt, J.L.; Gospodarevskaya, E.; Loveman, E.; Baxter, L.; Clegg, A.J. The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: A systematic review and economic evaluation. Health Technol. Assess 2009, 13, 215–357. [Google Scholar] [CrossRef]
- Ashrafian, H.; le Roux, C.W.; Darzi, A.; Athanasiou, T. Effects of bariatric surgery on cardiovascular function. Circulation 2008, 118, 2091–2102. [Google Scholar] [CrossRef]
- Cummings, D.E.; Cohen, R.V. Beyond BMI: The need for new guidelines governing the use of bariatric and metabolic surgery. Lancet Diabetes Endocrinol. 2014, 2, 175–181. [Google Scholar] [CrossRef]
- Pories, W.J.; MacDonald, K.G.; Flickinger, E.G.; Dohm, G.L.; Sinha, M.K.; Barakat, H.A.; May, H.J.; Khazanie, P.; Swanson, M.S.; Morgan, E.; et al. Is type II diabetes mellitus (NIDDM) a surgical disease? Ann. Surg. 1992, 215, 633–642. [Google Scholar] [CrossRef]
- Buchwald, H.; Estok, R.; Fahrbach, K.; Banel, D.; Jensen, M.D.; Pories, W.J.; Bantle, J.P.; Sledge, I. Weight and type 2 diabetes after bariatric surgery: Systematic review and meta-analysis. Am. J. Med. 2009, 122, 248–256.e5. [Google Scholar] [CrossRef]
- Van Huisstede, A.; Rudolphus, A.; Castro Cabezas, M.; Biter, L.U.; van de Geijn, G.J.; Taube, C.; Hiemstra, P.S.; Braunstahl, G.J. Effect of bariatric surgery on asthma control, lung function and bronchial and systemic inflammation in morbidly obese subjects with asthma. Thorax 2015, 70, 659–667. [Google Scholar] [CrossRef]
- Van Huisstede, A.; Rudolphus, A.; van Schadewijk, A.; Cabezas, M.C.; Mannaerts, G.H.; Taube, C.; Hiemstra, P.S.; Braunstahl, G.J. Bronchial and systemic inflammation in morbidly obese subjects with asthma: A biopsy study. Am. J. Respir. Crit. Care Med. 2014, 190, 951–954. [Google Scholar] [CrossRef]
- Jeng, E.I.; Aranda, J.M.; Ahmed, M.; Klodell, C.T. Left Ventricular Assist Device and Bariatric Surgery: A Bridge to Heart Transplant by Weight and Waiting Time Reduction. J. Card Surg. 2016, 31, 120–122. [Google Scholar] [CrossRef]
- Lim, C.P.; Fisher, O.M.; Falkenback, D. Bariatric surgery provides a “bridge to transplant” for morbidly obese patients with advanced heart failure and may obviate the need for transplantation. Obes. Surg. 2016, 26, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Singh, N.; Wharton, S.; Sharma, A.M. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity 2008, 16, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Pakiet, A.; Haliński, Ł.P.; Rostkowska, O.; Kaska, Ł.; Proczko-Stepaniak, M.; Śledziński, T.; Mika, A. The Effects of One-Anastomosis Gastric Bypass on Fatty Acids in the Serum of Patients with Morbid Obesity. Obes. Surg. 2021, 31, 4264–4271. [Google Scholar] [CrossRef] [PubMed]
- Celik, A.; Pouwels, S.; Karaca, F.C.; Çağıltay, E.; Ugale, S.; Etikan, İ.; Büyükbozkırlı, D.; Kılıç, Y.E. Time to Glycemic Control—an Observational Study of 3 Different Operations. Obes. Surg. 2017, 27, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Celik, A.; Ugale, S.; Ofluoglu, H.; Asci, M.; Celik, B.O.; Vural, E.; Aydin, M. Technical feasibility and safety profile of laparoscopic diverted sleeve gastrectomy with ileal transposition (DSIT). Obes. Surg. 2015, 25, 1184–1190. [Google Scholar] [CrossRef][Green Version]
- Celik, A.; Ugale, S.; Ofluoglu, H.; Vural, E.; Cagiltay, E.; Cat, H.; Asci, M.; Celik, B.O. Metabolic Outcomes of Laparoscopic Diverted Sleeve Gastrectomy with Ileal Transposition (DSIT) in Obese Type 2 Diabetic Patients. Obes. Surg. 2015, 25, 2018–2022. [Google Scholar] [CrossRef]
- Koulas, S.G.; Stefanou, C.K.; Stefanou, S.K.; Tepelenis, K.; Zikos, N.; Tepetes, K.; Kapsoritakis, A. Gut Microbiota in Patients with Morbid Obesity Before and After Bariatric Surgery: A Ten-Year Review Study (2009–2019). Obes. Surg. 2021, 31, 317–326. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Davids, M.; Winkelmeijer, M.; Verdoes, X.; Aydin, Ö.; de Brauw, M.; van de Laar, A.; Meijnikman, A.S.; Gerdes, V.E.A.; van Raalte, D.; et al. Compensatory intestinal antibody response against pro-inflammatory microbiota after bariatric surgery. Gut Microbes. 2022, 14, 2031696. [Google Scholar] [CrossRef]
- Kassam, A.F.; Mirza, A.; Kim, Y.; Hanseman, D.; Woodle, E.S.; Quillin, R.C.; Johnson, B.L.; Govil, A.; Cardi, M.; Schauer, D.P.; et al. Long-term outcomes in patients with obesity and renal disease after sleeve gastrectomy. Am. J. Transpl. 2020, 20, 422–429. [Google Scholar] [CrossRef]
- Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020, 104 (Suppl. 1), S11–S103. [Google Scholar] [CrossRef]
- Martin, W.P.; White, J.; López-Hernández, F.J.; Docherty, N.G.; le Roux, C.W. Metabolic Surgery to Treat Obesity in Diabetic Kidney Disease, Chronic Kidney Disease, and End-Stage Kidney Disease; What Are the Unanswered Questions? Front. Endocrinol. 2020, 11, 289. [Google Scholar] [CrossRef]
- Madsen, L.R.; Baggesen, L.M.; Richelsen, B.; Thomsen, R.W. Effect of Roux-en-Y gastric bypass surgery on diabetes remission and complications in individuals with type 2 diabetes: A Danish population-based matched cohort study. Diabetologia 2019, 62, 611–620. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Cetin, D.; Navaneethan, S.D.; Orzech, N.; Brethauer, S.A.; Schauer, P.R. Effects of bariatric surgery on diabetic nephropathy after 5 years of follow-up. Surg. Obes. Relat. Dis. 2013, 9, 7–14. [Google Scholar] [CrossRef]
- Canney, A.L.; Cohen, R.V.; Elliott, J.A.; Aboud, C.; Martin, W.P.; Docherty, N.G.; le Roux, C.W. Improvements in diabetic albuminuria and podocyte differentiation following Roux-en-Y gastric bypass surgery. Diab. Vasc. Dis. Res. 2020, 17, 1479164119879039. [Google Scholar] [CrossRef]
- Chang, A.R.; Chen, Y.; Still, C.; Wood, G.C.; Kirchner, H.L.; Lewis, M.; Kramer, H.; Hartle, J.E.; Carey, D.; Appel, L.J.; et al. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int. 2016, 90, 164–171. [Google Scholar] [CrossRef]
- Shulman, A.; Peltonen, M.; Sjöström, C.D.; Andersson-Assarsson, J.C.; Taube, M.; Sjöholm, K.; le Roux, C.W.; Carlsson, L.M.S.; Svensson, P.A. Incidence of end-stage renal disease following bariatric surgery in the Swedish Obese Subjects Study. Int. J. Obes. 2018, 42, 964–973. [Google Scholar] [CrossRef]
- Navaneethan, S.D.; Yehnert, H. Bariatric surgery and progression of chronic kidney disease. Surg. Obes. Relat. Dis. 2009, 5, 662–665. [Google Scholar] [CrossRef]
- Imam, T.H.; Fischer, H.; Jing, B.; Burchette, R.; Henry, S.; DeRose, S.F.; Coleman, K.J. Estimated GFR before and after Bariatric Surgery in CKD. Am. J. Kidney Dis. 2017, 69, 380–388. [Google Scholar] [CrossRef]
- Prasad, P.; Khullar, D.; Grover, R.; Chhabra, G.; Gupta, N.; Sinha, A.; Sharma, A.; Ahluwalia, V.; Chowbey, P. The Effect of Bariatric Surgery on Patients with Chronic Kidney Disease. Obes. Surg. 2020, 30, 4665–4668. [Google Scholar] [CrossRef]
- Sheetz, K.H.; Gerhardinger, L.; Dimick, J.B.; Waits, S.A. Bariatric Surgery and Long-term Survival in Patients with Obesity and End-stage Kidney Disease. JAMA Surg. 2020, 155, 581–588. [Google Scholar] [CrossRef]
- Cohen, J.B.; Lim, M.A.; Tewksbury, C.M.; Torres-Landa, S.; Trofe-Clark, J.; Abt, P.L.; Williams, N.N.; Dumon, K.R.; Goral, S. Bariatric surgery before and after kidney transplantation: Long-term weight loss and allograft outcomes. Surg. Obes. Relat. Dis. 2019, 15, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Schindel, H.; Winkler, J.; Yemini, R.; Carmeli, I.; Nesher, E.; Keidar, A. Survival benefit in bariatric surgery kidney recipients may be mediated through effects on kidney graft function and improvement of co-morbidities: A case-control study. Surg. Obes. Relat. Dis. 2019, 15, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Palamuthusingam, D.; Singh, A.; Palamuthusingam, P.; Hawley, C.M.; Pascoe, E.M.; Johnson, D.W.; Fahim, M. Postoperative outcomes after bariatric surgery in patients on chronic dialysis: A systematic review and meta-analysis. Obes. Res. Clin. Pract. 2021, 15, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Deledda, A.; Pintus, S.; Loviselli, A.; Fosci, M.; Fantola, G.; Velluzzi, F. Nutritional Management in Bariatric Surgery Patients. Int. J. Environ. Res. Public Health 2021, 18, 12049. [Google Scholar] [CrossRef] [PubMed]
- Bettini, S.; Belligoli, A.; Fabris, R.; Busetto, L. Diet approach before and after bariatric surgery. Rev. Endocr. Metab. Disord. 2020, 21, 297–306. [Google Scholar] [CrossRef]
- Watanabe, M.; Tuccinardi, D.; Ernesti, I.; Basciani, S.; Mariani, S.; Genco, A.; Manfrini, S.; Lubrano, C.; Gnessi, L. Scientific evidence underlying contraindications to the ketogenic diet: An update. Obes. Rev. 2020, 21, e13053. [Google Scholar] [CrossRef]
- Bruci, A.; Tuccinardi, D.; Tozzi, R.; Balena, A.; Santucci, S.; Frontani, R.; Mariani, S.; Basciani, S.; Spera, G.; Gnessi, L.; et al. Very Low-Calorie Ketogenic Diet: A Safe and Effective Tool for Weight Loss in Patients with Obesity and Mild Kidney Failure. Nutrients 2020, 12, 333. [Google Scholar] [CrossRef]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H.; Obesity Management Task Force of the European Association for the Study of Obesity. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Budzyński, A.; Major, P.; Głuszek, S.; Kaseja, K.; Koszutski, T.; Leśniak, S.; Lewandowski, T.; Lipka, M.; Lisik, W.; Makarewicz, M.; et al. Polish recommendations on bariatric and metabolic surgery. Med. Prakt. Chir. 2016, 6, 13–26. [Google Scholar]
- Busetto, L.; Dicker, D.; Azran, C.; Batterham, R.L.; Farpour-Lambert, N.; Fried, M.; Hjelmesæth, J.; Kinzl, J.; Leitner, D.R.; Makaronidis, J.M.; et al. Practical Recommendations of the Obesity Management Task Force of the European Association for the Study of Obesity for the Post-Bariatric Surgery Medical Management. Obes. Facts 2017, 10, 597–632. [Google Scholar] [CrossRef]
- Jastrzebska, W.; Boniecka, I.; Szostak-Wegierek, S. Validity and efficacy of diets used for preoperative weight reduction among patients qualified for bariatric surgery. Pol. Przegl. Chir. 2021, 93, 52–57. [Google Scholar] [CrossRef]
- Ladhani, M.; Craig, J.C.; Irving, M.; Clayton, P.A.; Wong, G. Obesity and the risk of cardiovascular and all-cause mortality in chronic kidney disease: A systematic review and meta-analysis. Nephrol. Dial. Transpl. 2017, 32, 439–449. [Google Scholar] [CrossRef]
- Rutkowski, B.; Durlik, M. Obesity and Organ Transplantation; Via Medica: Gdańsk, Poland, 2015. [Google Scholar]
- Dagan, S.S.; Goldenshluger, A.; Globus, I.; Schweiger, C.; Kessler, Y.; Kowen Sandbank, G.; Ben-Porat, T.; Sinai, T. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice. Adv. Nutr. 2017, 8, 382–394. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Garvey, W.T.; Hurley, D.L.; McMahon, M.M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S.; et al. American Association of Clinical Endocrinologists; Obesity Society; American Society for Metabolic & Bariatric Surgery. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patie—2013 update: Cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity 2013, 21 (Suppl. 1), S1–S27. [Google Scholar] [CrossRef]
- Chang, A.R.; Grams, M.E.; Navaneethan, S.D. Bariatric Surgery and Kidney-Related Outcomes. Kidney Int. Rep. 2017, 2, 261–270. [Google Scholar] [CrossRef]
- Ben-Porat, T.; Weiss-Sadan, A.; Rottenstreich, A.; Sherf-Dagan, S.; Schweiger, C.; Yosef-Levi, I.M.; Weiner, D.; Azulay, O.; Sakran, N.; Harari, R.; et al. Nutritional Management for Chronic Kidney Disease Patients Who Undergo Bariatric Surgery: A Narrative Review. Adv. Nutr. 2019, 10, 122–132. [Google Scholar] [CrossRef]
- Leal, A.A.; Faintuch, J.; Morais, A.A.; Noe, J.A.; Bertollo, D.M.; Morais, R.C.; Cabrini, D. Bioimpedance analysis: Should it be used in morbid obesity? Am. J. Hum. Biol. 2011, 23, 420–422. [Google Scholar] [CrossRef]
- Lightner, A.L.; Lau, J.; Obayashi, P.; Birge, K.; Melcher, M.L. Potential nutritional conflicts in bariatric and renal transplant patients. Obes. Surg. 2011, 21, 1965–1970. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Apovian, C.; Brethauer, S.; Garvey, W.T.; Joffe, A.M.; Kim, J.; Kushner, R.F.; Lindquist, R.; Pessah-Pollack, R.; Seger, J.; et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedure—2019 update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists. Surg. Obes. Relat. Dis. 2020, 16, 175–247. [Google Scholar] [CrossRef]
- Huckfeldt, P.J.; Frenier, C.; Pajewski, N.M.; Espeland, M.; Peters, A.; Casanova, R.; Pi-Sunyer, X.; Cheskin, L.; Goldman, D.P. Associations of Intensive Lifestyle Intervention in Type 2 Diabetes with Health Care Use, Spending, and Disability: An Ancillary Study of the Look AHEAD Study. JAMA Netw. Open 2020, 3, e2025488. [Google Scholar] [CrossRef]
- Cupisti, A.; Bolasco, P. Keto-analogues and essential aminoacids and other supplements in the conservative management of chronic kidney disease. Panminerva Med. 2017, 59, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Brunori, G.; Di Iorio, B.R.; D’Alessandro, C.; Pasticci, F.; Cosola, C.; Bellizzi, V.; Bolasco, P.; Capitanini, A.; Fantuzzi, A.L.; et al. Nutritional treatment of advanced CKD: Twenty consensus statements. J. Nephrol. 2018, 31, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, M.; Safaei, A.; Tolouian, A.; Tolouian, R.; Ebrahimzadeh Attari, V.; Jalili, M. Hypophosphatemia after hemodialysis and its association with some clinical complications in patients with chronic kidney disease. Casp. J. Intern. Med. 2022, 13, 527–532. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76 (Suppl. 1), S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J. Phosphorus homeostasis in normal health and in chronic kidney disease patients with special emphasis on dietary phosphorus intake. Semin. Dial. 2007, 20, 295–301. [Google Scholar] [CrossRef]
- Kaczkan, M.; Bienias, A.; Małgorzewicz, S. Realization of low phosphate diet and hidden sources of phosphorus. Forum Nefrol. 2018, 11, 15–23. [Google Scholar]
- Chan, W. Chronic Kidney Disease and Nutrition Support. Nutr. Clin. Pract. 2021, 36, 312–330. [Google Scholar] [CrossRef]
- Paccou, J.; Caiazzo, R.; Lespessailles, E.; Cortet, B. Bariatric Surgery and Osteoporosis. Calcif. Tissue Int. 2022, 110, 576–591. [Google Scholar] [CrossRef]
- Ermer, T.; Eckardt, K.U.; Aronson, P.S.; Knauf, F. Oxalate, inflammasome, and progression of kidney disease. Curr. Opin. Nephrol. Hypertens. 2016, 25, 363–371. [Google Scholar] [CrossRef]
- Watanabe, R. Hyperkalemia in chronic kidney disease. Rev. Assoc. Med. Bras. 2020, 66 (Suppl. 1), s31–s36. [Google Scholar] [CrossRef]
- Dhondup, T.; Qian, Q. Electrolyte and Acid-Base Disorders in Chronic Kidney Disease and End-Stage Kidney Failure. Blood Purif. 2017, 43, 179–188. [Google Scholar] [CrossRef]
- Sumida, K.; Yamagata, K.; Kovesdy, C.P. Constipation in CKD. Kidney Int. Rep. 2019, 5, 121–134. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Treatment of Abnormalities of Potassium Homeostasis in CKD. Adv. Chronic. Kidney Dis. 2017, 24, 319–324. [Google Scholar] [CrossRef]
- Vervloet, M.G.; van Ballegooijen, A.J. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int. 2018, 93, 1060–1072. [Google Scholar] [CrossRef]
- Kuchanowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Tabele Składu i Wartości Odżywczej Żywności; PZWL Wydawnictwo: Lekarskie, Poland, 2020. [Google Scholar]
- US Department of Agriculture. Agricular Research Service. Available online: https://fdc.nal.usda.gov/ (accessed on 20 December 2022).
- Silva, M.; Neves, J.; Borges-Canha, M.; Mendes, A.; Fonseca, M.J.; Mendonca, F.; Ferreira, M.J.; Salazar, D.; Pedro, J.; Guerreiro, V.; et al. Higher magnesium levels are associated with better glycaemic control and diabetes remission post-bariatric surgery. BMC Endocr. Disord. 2022, 22, 303. [Google Scholar] [CrossRef]
- Johansson, K.; Svensson, P.A.; Söderling, J.; Peltonen, M.; Neovius, M.; Carlsson, L.M.S.; Sjöholm, K. Long-term risk of anaemia after bariatric surgery: Results from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2021, 9, 515–524. [Google Scholar] [CrossRef]
- Portolés, J.; Martín, L.; Broseta, J.J.; Cases, A. Anemia in Chronic Kidney Disease: From Pathophysiology and Current Treatments, to Future Agents. Front. Med. 2021, 8, 642296. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron Balance and the Role of Hepcidin in Chronic Kidney Disease. Semin. Nephrol. 2016, 36, 87–93. [Google Scholar] [CrossRef]
- Vinke, J.S.J.; Francke, M.I.; Eisenga, M.F.; Hesselink, D.A.; de Borst, M.H. Iron deficiency after kidney transplantation. Nephrol. Dial. Transpl. 2021, 36, 1976–1985. [Google Scholar] [CrossRef]
- Colucci, S.; Pagani, A.; Pettinato, M.; Artuso, I.; Nai, A.; Camaschella, C.; Silvestri, L. The immunophilin FKBP12 inhibits hepcidin expression by binding the BMP type I receptor ALK2 in hepatocytes. Blood 2017, 130, 2111–2120. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Chen, J.C.; Tsai, Y.C.; Chen, T.W. Low-dose ferrous bisglycinate chelate supplementation in chronic kidney disease and hemodialysis patients. J. Chin. Med. Assoc. 2022, 85, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Więcek, A.; Dębska-Ślizień, A.; Durlik, M.; Małyszko, J.; Nieszporek, T.; Nowicki, M.; Rutkowski, B.; Stompór, T. Management of anaemia in patients with chronic kidney disease. A Polish Society of Nephrology position statement. Forum Nefrol. 2015, 8, 110–121. [Google Scholar]
- Batchelor, E.K.; Kapitsinou, P.; Pergola, P.E.; Kovesdy, C.P.; Jalal, D.I. Iron Deficiency in Chronic Kidney Disease: Updates on Pathophysiology, Diagnosis, and Treatment. J. Am. Soc. Nephrol. 2020, 31, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Vynckier, A.K.; Ceulemans, D.; Vanheule, G.; De Mulder, P.; Van Den Driessche, M.; Devlieger, R. Periconceptional Folate Supplementation in Women after Bariatric Surgery—A Narrative Review. Nutrients 2021, 13, 1557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Matherly, L.H.; Goldman, I.D. Membrane transporters and folate homeostasis: Intestinal absorption and transport into systemic compartments and tissues. Expert Rev. Mol. Med. 2009, 11, e4. [Google Scholar] [CrossRef]
- Majumder, S.; Soriano, J.; Louie Cruz, A.; Dasanu, C.A. Vitamin B12 deficiency in patients undergoing bariatric surgery: Preventive strategies and key recommendations. Surg. Obes. Relat. Dis. 2013, 9, 1013–1019. [Google Scholar] [CrossRef]
- Guéant, J.L.; Guéant-Rodriguez, R.M.; Alpers, D.H. Vitamin B12 absorption and malabsorption. Vitam. Horm. 2022, 119, 241–274. [Google Scholar] [CrossRef]
- Musella, M.; Berardi, G.; Vitiello, A.; Dayan, D.; Schiavone, V.; Franzese, A.; Abu-Abeid, A. Vitamin D Deficiency in Patients with Morbid Obesity before and after Metabolic Bariatric Surgery. Nutrients 2022, 14, 3319. [Google Scholar] [CrossRef]
- Christodoulou, M.; Aspray, T.J.; Schoenmakers, I. Vitamin D Supplementation for Patients with Chronic Kidney Disease: A Systematic Review and Meta-analyses of Trials Investigating the Response to Supplementation and an Overview of Guidelines. Calcif. Tissue Int. 2021, 109, 157–178. [Google Scholar] [CrossRef]
- Hammoud, D.; El Haddad, B.; Abdallah, J. Hypercalcaemia secondary to hypervitaminosis a in a patient with chronic renal failure. West Indian Med. J. 2014, 63, 105–108. [Google Scholar] [CrossRef][Green Version]
- Gernand, A.D. The upper level: Examining the risk of excess micronutrient intake in pregnancy from antenatal supplements. Ann. N. Y. Acad. Sci. 2019, 1444, 22–34. [Google Scholar] [CrossRef]
- Tabesh, M.R.; Maleklou, F.; Ejtehadi, F.; Alizadeh, Z. Nutrition, Physical Activity, and Prescription of Supplements in Pre- and Post-bariatric Surgery Patients: A Practical Guideline. Obes. Surg. 2019, 29, 3385–3400. [Google Scholar] [CrossRef]
- Fouque, D.; Vennegoor, M.; ter Wee, P.; Wanner, C.; Basci, A.; Canaud, B.; Haage, P.; Konner, K.; Kooman, J.; Martin-Malo, A. EBPG guideline on nutrition. Nephrol. Dial. Transpl. 2007, 22 (Suppl. 2), ii45–ii87. [Google Scholar] [CrossRef]
- Wagner, S.; Merkling, T.; Metzger, M.; Bankir, L.; Laville, M.; Frimat, L.; Combe, C.; Jacquelinet, C.; Fouque, D.; Massy, Z.A.; et al. Water intake and progression of chronic kidney disease: The CKD-REIN cohort study. Nephrol. Dial. Transpl. 2022, 37, 730–739. [Google Scholar] [CrossRef]
- Scarpellini, E.; Arts, J.; Karamanolis, G.; Laurenius, A.; Siquini, W.; Suzuki, H.; Ukleja, A.; Van Beek, A.; Vanuytsel, T.; Bor, S.; et al. International consensus on the diagnosis and management of dumping syndrome. Nat. Rev. Endocrinol. 2020, 16, 448–466. [Google Scholar] [CrossRef]
- Van Beek, A.P.; Emous, M.; Laville, M.; Tack, J. Dumping syndrome after esophageal, gastric or bariatric surgery: Pathophysiology, diagnosis, and management. Obes. Rev. 2017, 18, 68–85. [Google Scholar] [CrossRef]
- Van Furth, A.M.; de Heide, L.J.M.; Emous, M.; Veeger, N.; van Beek, A.P. Dumping Syndrome and Postbariatric Hypoglycemia: Supporting Evidence for a Common Etiology. Surg. Obes. Relat. Dis. 2021, 17, 1912–1918. [Google Scholar] [CrossRef]
- Sheehan, A.; Patti, M.E. Hypoglycemia after upper Gastrointestinal Surgery: Clinical Approach to Assessment, Diagnosis, and Treatment. Diabetes Metab. Syndr. Obes. 2020, 13, 4469–4482. [Google Scholar] [CrossRef]
| Reference | Health Condition | Sample Size | Exposure | Outcomes |
|---|---|---|---|---|
| CKD stages 1–5 (without dialysis) | ||||
| Navanethan et al. 2009 [58] | CKD stage 3 | 25 | BS | GFR improvement at 6 and 12 months after surgery (from 47.9 to 61.6 mL/min/1.73 m2), a decrease in blood pressure was observed |
| Imam et al. 2017 [59] | CKD stages 3–4 group 1 group 2 | 714 714 | SG/RYGB control | Bariatric surgery, especially the RYGB procedure, improves GFR for up to 3 years |
| Prasad et al. 2020 [60] | CKD stages 1–3 and 5 | 13 | SG/RYGB | Significant decrease in protein and albumin excretion rate 6 months after surgery, and no significant changes in GFR |
| Kassam et al. 2020 [50] | ESRD without dialysis CKD stages 1–4 | 198 45 | SG | The lasting effect on weight loss, reduction in comorbidities in both groups; improvement of renal function in patients with stage 3 CKD |
| ESRD—dialysis | ||||
| Sheetz et al. 2020 [61] | group 1 ESRD and BS group 2 ESRD | 1597 4750 | group 1 BS | BS was associated with lower all-cause mortality and increased incidence of kidney transplantation |
| Kidney transplantation | ||||
| Cohen et al. 2019 [62] | KTRs CKD before KT | 21 43 | BS | BS before kidney transplantation was associated with a lower risk of graft failure than with BS after organ transplantation—disturbed tacrolimus levels among KTRs after BS |
| Schindel et al. 2019 [63] | KTRs BS group control group | 30 50 | SG/RYGB | Improvement of renal function, graft survival, and obesity-related comorbidities among kidney transplant recipients who underwent bariatric surgery |
| Products | Potassium Content [mg of K per 100 g of Product] | |
|---|---|---|
| Vegetables | ||
| High potassium content | potatoes | 420 |
| brussels sprouts | 416 | |
| broccoli | 385 | |
| green peas | 353 | |
| celery | 320 | |
| Lower potassium content | onion | 121 |
| cucumber | 125 | |
| butter lettuce | 134 | |
| Chinese cabbage | 150 | |
| green beans | 211 | |
| Fruit | ||
| High potassium content | avocado | 600 |
| banana | 395 | |
| kiwi | 290 | |
| grapefruit | 277 | |
| apricots | 275 | |
| Lower potassium content | blueberry | 77 |
| cranberries | 80 | |
| pear | 118 | |
| watermelon | 130 | |
| strawberries | 133 | |
| apple | 134 | |
| Nuts, seeds, dried fruit | ||
| High potassium content | dried apricots | 1666 |
| dried figs | 938 | |
| pumpkin seeds | 810 | |
| prunes | 804 | |
| cashew nuts | 660 | |
| Lower potassium content | dried cranberries | 49 |
| sesame | 387 | |
| pecans | 360 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potrykus, M.; Czaja-Stolc, S.; Małgorzewicz, S.; Proczko-Stepaniak, M.; Dębska-Ślizień, A. Diet Management of Patients with Chronic Kidney Disease in Bariatric Surgery. Nutrients 2023, 15, 165. https://doi.org/10.3390/nu15010165
Potrykus M, Czaja-Stolc S, Małgorzewicz S, Proczko-Stepaniak M, Dębska-Ślizień A. Diet Management of Patients with Chronic Kidney Disease in Bariatric Surgery. Nutrients. 2023; 15(1):165. https://doi.org/10.3390/nu15010165
Chicago/Turabian StylePotrykus, Marta, Sylwia Czaja-Stolc, Sylwia Małgorzewicz, Monika Proczko-Stepaniak, and Alicja Dębska-Ślizień. 2023. "Diet Management of Patients with Chronic Kidney Disease in Bariatric Surgery" Nutrients 15, no. 1: 165. https://doi.org/10.3390/nu15010165
APA StylePotrykus, M., Czaja-Stolc, S., Małgorzewicz, S., Proczko-Stepaniak, M., & Dębska-Ślizień, A. (2023). Diet Management of Patients with Chronic Kidney Disease in Bariatric Surgery. Nutrients, 15(1), 165. https://doi.org/10.3390/nu15010165










