Cardiometabolic Traits in Adult Twins: Heritability and BMI Impact with Age
Abstract
1. Introduction
2. Method
2.1. Study Population
2.2. Definition and Measurement of BMI, Cardiometabolic Traits, and Zygosity
2.3. Statistical Analysis
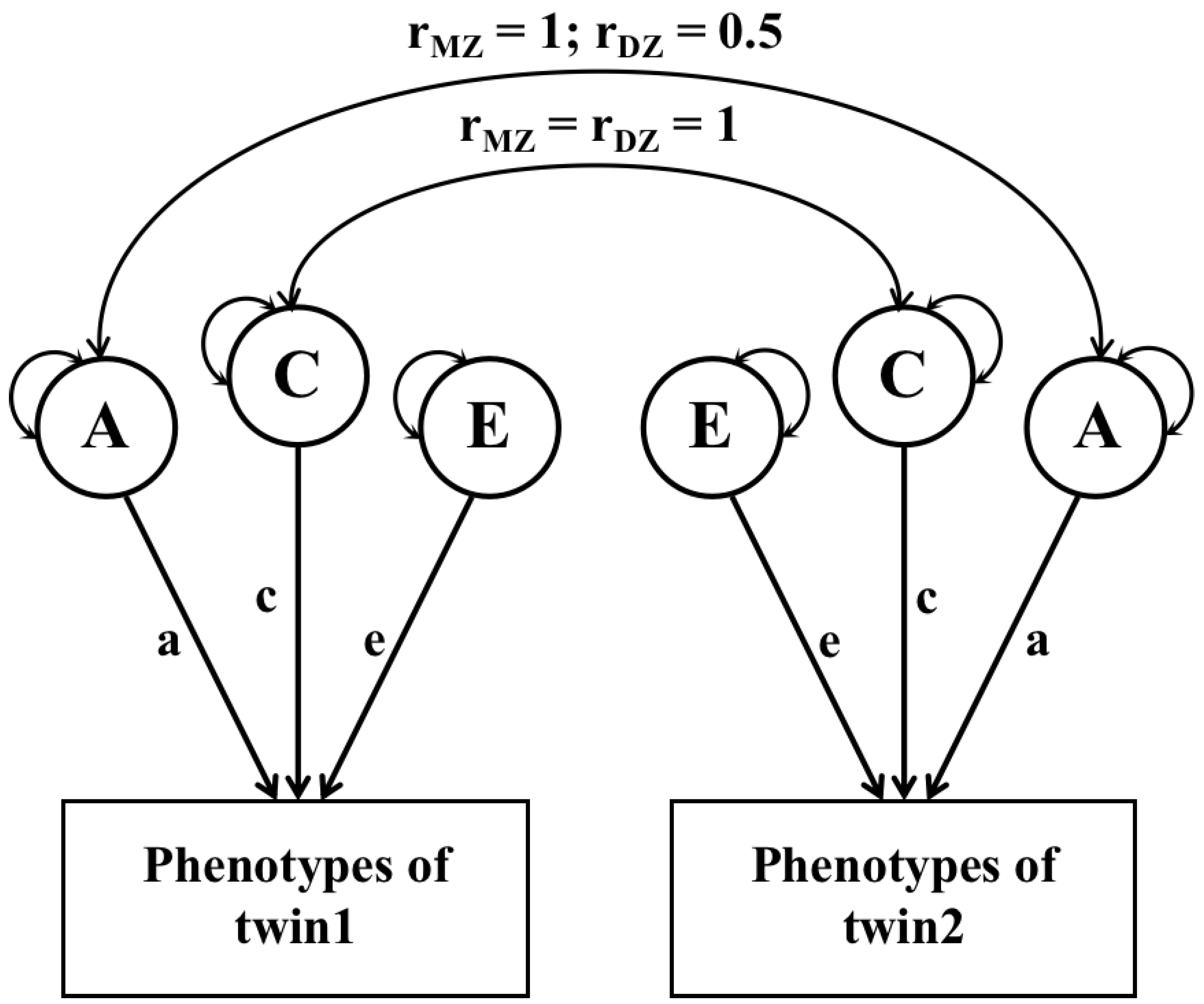
2.4. Heritability Analysis
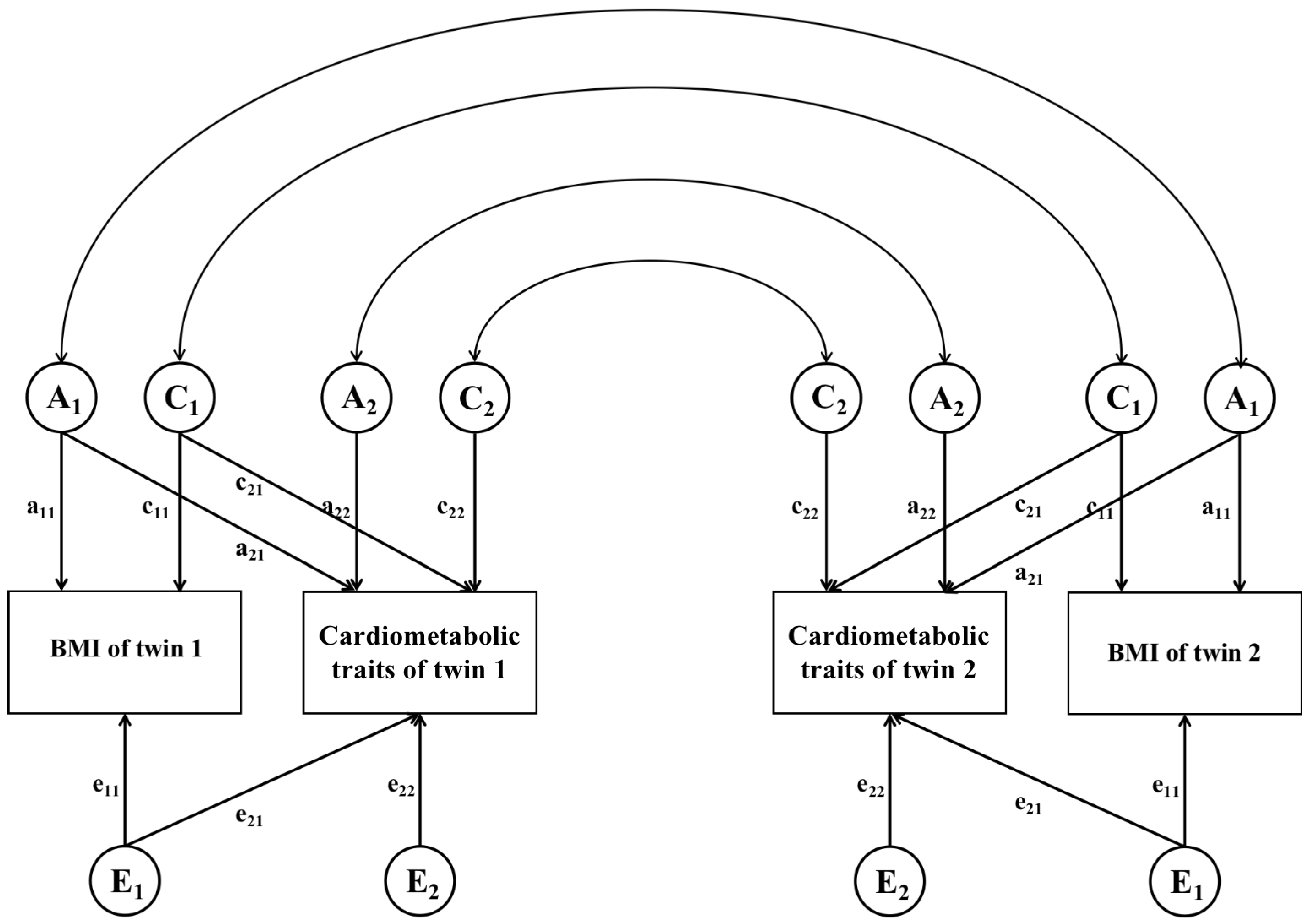
2.5. Genetic and Environmental Correlation Analysis
3. Results
3.1. Characteristics of the Study Population
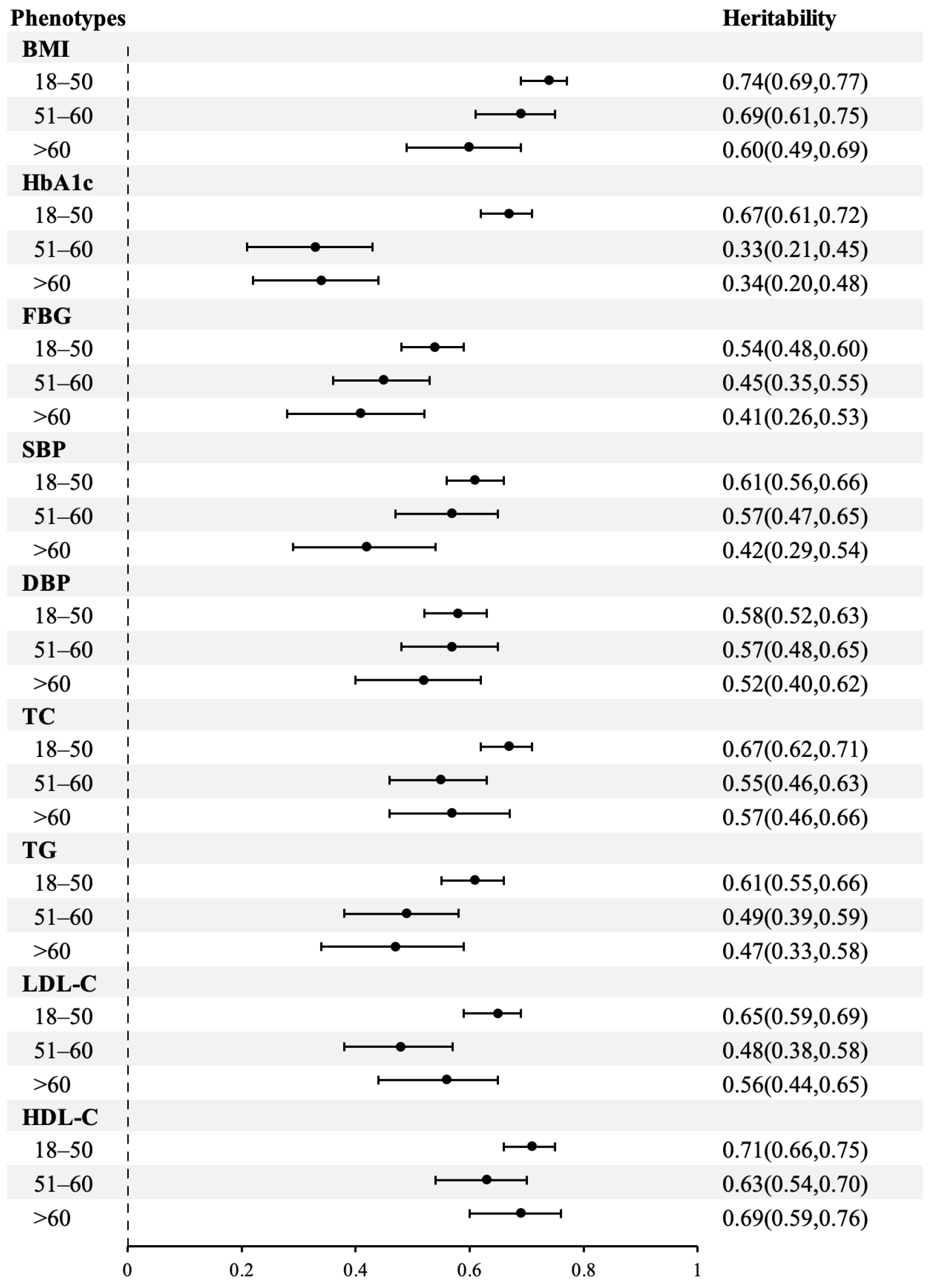
3.2. Heritability for BMI and Cardiometabolic Traits
3.3. Genetic and Environmental Factors Underlying the Correlations between BMI and Cardiometabolic Traits
4. Discussion
4.1. Heritability of BMI and Cardiometabolic Traits
4.2. Correlations of BMI and Cardiometabolic Traits
4.3. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilly, A.; Park, Y.C.; Png, G.; Barysenka, A.; Fischer, I.; Bjornland, T.; Southam, L.; Suveges, D.; Neumeyer, S.; Rayner, N.W.; et al. Whole-genome sequencing analysis of the cardiometabolic proteome. Nat. Commun. 2020, 11, 6336. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Pitt, B.; Anker, S.D.; Vincent, J.; Mujib, M.; Ahmed, A. Association of obesity and survival in systolic heart failure after acute myocardial infarction: Potential confounding by age. Eur. J. Heart Fail. 2010, 12, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Vithayathil, M.; Carter, P.; Kar, S.; Mason, A.M.; Burgess, S.; Larsson, S.C. Body size and composition and risk of site-specific cancers in the UK Biobank and large international consortia: A mendelian randomisation study. PLoS Med. 2021, 18, e1003706. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.L.; Meng, X.; Wang, C.; Dai, W.; Luo, Z.; Yin, Z.; Ju, Z.; Fu, X.; Yang, J.; Ye, Q.; et al. Histone H3K4me3 modification is a transgenerational epigenetic signal for lipid metabolism in Caenorhabditis elegans. Nat. Commun. 2022, 13, 768. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Duan, H.; Pang, Z.; Zhang, D.; Duan, H.; Hjelmborg, J.V.; Tan, Q.; Kruse, T.A.; Kyvik, K.O. Heritability of eleven metabolic phenotypes in Danish and Chinese twins: A cross-population comparison. Obesity 2013, 21, 1908–1914. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, C. Genetics of Obesity: What We Have Learned Over Decades of Research. Obesity 2021, 29, 802–820. [Google Scholar] [CrossRef]
- van Dongen, J.; Willemsen, G.; Chen, W.M.; de Geus, E.J.; Boomsma, D.I. Heritability of metabolic syndrome traits in a large population-based sample. J. Lipid Res. 2013, 54, 2914–2923. [Google Scholar] [CrossRef]
- Liu, F.; Chang, H.C. Physiological links of circadian clock and biological clock of aging. Protein Cell 2017, 8, 477–488. [Google Scholar] [CrossRef]
- Pietilainen, K.H.; Soderlund, S.; Rissanen, A.; Nakanishi, S.; Jauhiainen, M.; Taskinen, M.R.; Kaprio, J. HDL subspecies in young adult twins: Heritability and impact of overweight. Obesity 2009, 17, 1208–1214. [Google Scholar] [CrossRef]
- Pang, Z.; Zhang, D.; Li, S.; Duan, H.; Hjelmborg, J.; Kruse, T.A.; Kyvik, K.O.; Christensen, K.; Tan, Q. Multivariate modelling of endophenotypes associated with the metabolic syndrome in Chinese twins. Diabetologia 2010, 53, 2554–2561. [Google Scholar] [CrossRef][Green Version]
- Duan, H.; Pang, Z.; Zhang, D.; Li, S.; Kruse, T.A.; Kyvik, K.O.; Christensen, K.; Tan, Q. Genetic and environmental dissections of sub-phenotypes of metabolic syndrome in the Chinese population: A twin-based heritability study. Obes. Facts 2011, 4, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Cao, W.; Lv, J.; Yu, C.; Wu, T.; Wang, S.; Meng, L.; Wang, D.; Wang, Z.; Pang, Z.; et al. The Chinese National Twin Registry: A ‘gold mine’ for scientific research. J. Intern. Med. 2019, 286, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.D.; Sheehan, N.A.; Scurrah, K.J.; Burton, P.R. Adjusting for treatment effects in studies of quantitative traits: Antihypertensive therapy and systolic blood pressure. Stat. Med. 2005, 24, 2911–2935. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gao, W.; Yu, C.; Cao, W.; Lv, J.; Wang, S.; Pang, Z.; Cong, L.; Wang, H.; Wu, X.; et al. Determination of Zygosity in Adult Chinese Twins Using the 450K Methylation Array versus Questionnaire Data. PLoS ONE 2015, 10, e0123992. [Google Scholar] [CrossRef]
- Gao, W.; Li, L.; Cao, W.; Zhan, S.; Lv, J.; Qin, Y.; Pang, Z.; Wang, S.; Chen, W.; Chen, R.; et al. Determination of zygosity by questionnaire and physical features comparison in Chinese adult twins. Twin Res. Hum. Genet. 2006, 9, 266–271. [Google Scholar] [CrossRef]
- Neale, M.C.; Hunter, M.D.; Pritikin, J.N.; Zahery, M.; Brick, T.R.; Kirkpatrick, R.M.; Estabrook, R.; Bates, T.C.; Maes, H.H.; Boker, S.M. OpenMx 2.0: Extended Structural Equation and Statistical Modeling. Psychometrika 2016, 81, 535–549. [Google Scholar] [CrossRef]
- Huang, Y.; Ollikainen, M.; Sipila, P.; Mustelin, L.; Wang, X.; Su, S.; Huan, T.; Levy, D.; Wilson, J.; Snieder, H.; et al. Genetic and Environmental Effects on Gene Expression Signatures of Blood Pressure: A Transcriptome-Wide Twin Study. Hypertension 2018, 71, 457–464. [Google Scholar] [CrossRef]
- Maciejewski, D.F.; Creemers, H.E.; Lynskey, M.T.; Madden, P.A.; Heath, A.C.; Statham, D.J.; Martin, N.G.; Verweij, K.J. Overlapping genetic and environmental influences on nonsuicidal self-injury and suicidal ideation: Different outcomes, same etiology? JAMA Psychiatry 2014, 71, 699–705. [Google Scholar] [CrossRef]
- Berry, S.E.; Valdes, A.M.; Drew, D.A.; Asnicar, F.; Mazidi, M.; Wolf, J.; Capdevila, J.; Hadjigeorgiou, G.; Davies, R.; Al Khatib, H.; et al. Human postprandial responses to food and potential for precision nutrition. Nat. Med. 2020, 26, 964–973. [Google Scholar] [CrossRef]
- Silventoinen, K.; Jelenkovic, A.; Sund, R.; Yokoyama, Y.; Hur, Y.M.; Cozen, W.; Hwang, A.E.; Mack, T.M.; Honda, C.; Inui, F.; et al. Differences in genetic and environmental variation in adult BMI by sex, age, time period, and region: An individual-based pooled analysis of 40 twin cohorts. Am. J. Clin. Nutr. 2017, 106, 457–466. [Google Scholar] [CrossRef]
- Silventoinen, K.; Kaprio, J. Genetics of tracking of body mass index from birth to late middle age: Evidence from twin and family studies. Obes. Facts 2009, 2, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Dahl, A.K.; Reynolds, C.A.; Fall, T.; Magnusson, P.K.; Pedersen, N.L. Multifactorial analysis of changes in body mass index across the adult life course: A study with 65 years of follow-up. Int. J. Obes. 2014, 38, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Poveda, A.; Chen, Y.; Brandstrom, A.; Engberg, E.; Hallmans, G.; Johansson, I.; Renstrom, F.; Kurbasic, A.; Franks, P.W. The heritable basis of gene-environment interactions in cardiometabolic traits. Diabetologia 2017, 60, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Nan, C.; Guo, B.; Warner, C.; Fowler, T.; Barrett, T.; Boomsma, D.; Nelson, T.; Whitfield, K.; Beunen, G.; Thomis, M.; et al. Heritability of body mass index in pre-adolescence, young adulthood and late adulthood. Eur. J. Epidemiol. 2012, 27, 247–253. [Google Scholar] [CrossRef]
- Goode, E.L.; Cherny, S.S.; Christian, J.C.; Jarvik, G.P.; de Andrade, M. Heritability of longitudinal measures of body mass index and lipid and lipoprotein levels in aging twins. Twin Res. Hum. Genet. 2007, 10, 703–711. [Google Scholar] [CrossRef]
- Elks, C.E.; den Hoed, M.; Zhao, J.H.; Sharp, S.J.; Wareham, N.J.; Loos, R.J.; Ong, K.K. Variability in the heritability of body mass index: A systematic review and meta-regression. Front. Endocrinol. 2012, 3, 29. [Google Scholar] [CrossRef]
- Min, J.; Chiu, D.T.; Wang, Y. Variation in the heritability of body mass index based on diverse twin studies: A systematic review. Obes. Rev. 2013, 14, 871–882. [Google Scholar] [CrossRef]
- Winkler, T.W.; Justice, A.E.; Graff, M.; Barata, L.; Feitosa, M.F.; Chu, S.; Czajkowski, J.; Esko, T.; Fall, T.; Kilpelainen, T.O.; et al. The Influence of Age and Sex on Genetic Associations with Adult Body Size and Shape: A Large-Scale Genome-Wide Interaction Study. PLoS Genet. 2015, 11, e1005378. [Google Scholar] [CrossRef]
- Schrempft, S.; van Jaarsveld, C.H.M.; Fisher, A.; Herle, M.; Smith, A.D.; Fildes, A.; Llewellyn, C.H. Variation in the Heritability of Child Body Mass Index by Obesogenic Home Environment. JAMA Pediatr. 2018, 172, 1153–1160. [Google Scholar] [CrossRef]
- Simonis-Bik, A.M.; Eekhoff, E.M.; Diamant, M.; Boomsma, D.I.; Heine, R.J.; Dekker, J.M.; Willemsen, G.; van Leeuwen, M.; de Geus, E.J. The heritability of HbA1c and fasting blood glucose in different measurement settings. Twin Res. Hum. Genet. 2008, 11, 597–602. [Google Scholar] [CrossRef]
- Liu, H.; Wang, W.; Zhang, C.; Xu, C.; Duan, H.; Tian, X.; Zhang, D. Heritability and Genome-Wide Association Study of Plasma Cholesterol in Chinese Adult Twins. Front. Endocrinol. 2018, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Panizzon, M.S.; Hauger, R.L.; Sailors, M.; Lyons, M.J.; Jacobson, K.C.; Murray McKenzie, R.; Rana, B.; Vasilopoulos, T.; Vuoksimaa, E.; Xian, H.; et al. A new look at the genetic and environmental coherence of metabolic syndrome components. Obesity 2015, 23, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Schousboe, K.; Visscher, P.M.; Henriksen, J.E.; Hopper, J.L.; Sorensen, T.I.; Kyvik, K.O. Twin study of genetic and environmental influences on glucose tolerance and indices of insulin sensitivity and secretion. Diabetologia 2003, 46, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Middelberg, R.P.; Martin, N.G.; Whitfield, J.B. Longitudinal genetic analysis of plasma lipids. Twin Res. Hum. Genet. 2006, 9, 550–557. [Google Scholar] [CrossRef]
- Middelberg, R.P.; Martin, N.G.; Whitfield, J.B. A longitudinal genetic study of plasma lipids in adolescent twins. Twin Res. Hum. Genet. 2007, 10, 127–135. [Google Scholar] [CrossRef][Green Version]
- Chen, T.J.; Ji, C.Y.; Hu, Y.H. Genetic and environmental influences on serum lipids and the effects of puberty: A Chinese twin study. Acta Paediatr. 2009, 98, 1029–1036. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.H.; Jung, Y.; Lee, Y.; Yoon, J.H.; Choi, J.Y.; Hwang, C.Y.; Son, Y.H.; Park, S.S.; Hwang, G.S.; et al. FABP3-mediated membrane lipid saturation alters fluidity and induces ER stress in skeletal muscle with aging. Nat. Commun. 2020, 11, 5661. [Google Scholar] [CrossRef]
- Vinck, W.J.; Fagard, R.H.; Loos, R.; Vlietinck, R. The impact of genetic and environmental influences on blood pressure variance across age-groups. J. Hypertens. 2001, 19, 1007–1013. [Google Scholar] [CrossRef]
- Menni, C.; Mangino, M.; Zhang, F.; Clement, G.; Snieder, H.; Padmanabhan, S.; Spector, T.D. Heritability analyses show visit-to-visit blood pressure variability reflects different pathological phenotypes in younger and older adults: Evidence from UK twins. J. Hypertens. 2013, 31, 2356–2361. [Google Scholar] [CrossRef]
- Li, X.; Tan, H.; Zhou, S.; Hu, S.; Zhang, T.; Li, Y.; Dou, Q.; Lai, Z.; Chen, F. Renin-angiotensin-aldosterone system gene polymorphisms in gestational hypertension and preeclampsia: A case-control gene-association study. Sci. Rep. 2016, 6, 38030. [Google Scholar] [CrossRef]
- Simino, J.; Shi, G.; Bis, J.C.; Chasman, D.I.; Ehret, G.B.; Gu, X.; Guo, X.; Hwang, S.J.; Sijbrands, E.; Smith, A.V.; et al. Gene-age interactions in blood pressure regulation: A large-scale investigation with the CHARGE, Global BPgen, and ICBP Consortia. Am. J. Hum. Genet. 2014, 95, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, D.; Pang, Z.; Jiang, W.; Wang, S.; Li, S.; von Bornemann Hjelmborg, J.; Tan, Q. Multivariate modeling of body mass index, pulse pressure, systolic and diastolic blood pressure in Chinese twins. Twin Res. Hum. Genet. 2015, 18, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Gao, W.; Cao, W.; Lv, J.; Yu, C.; Wang, S.; Zhou, B.; Pang, Z.; Cong, L.; Wang, H.; et al. Associations of Body Composition Measurements with Serum Lipid, Glucose and Insulin Profile: A Chinese Twin Study. PLoS ONE 2015, 10, e0140595. [Google Scholar] [CrossRef]
- Zeng, Y.; He, H.; Zhang, L.; Zhu, W.; Shen, H.; Yan, Y.J.; Deng, H.W. GWA-based pleiotropic analysis identified potential SNPs and genes related to type 2 diabetes and obesity. J. Hum. Genet. 2021, 66, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Snieder, H.; Li, L.; Cao, W.; Zhan, S.; Lv, J.; Gao, W.; Wang, X.; Ding, X.; Hu, Y. Genetic and environmental influences on blood pressure and body mass index in Han Chinese: A twin study. Hypertens. Res. 2011, 34, 173–179. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Tian, X.; Duan, H.; Xu, C.; Zhang, D. Bivariate genome-wide association study (GWAS) of body mass index and blood pressure phenotypes in northern Chinese twins. PLoS ONE 2021, 16, e0246436. [Google Scholar] [CrossRef] [PubMed]
- Basseri, S.; Lhotak, S.; Fullerton, M.D.; Palanivel, R.; Jiang, H.; Lynn, E.G.; Ford, R.J.; Maclean, K.N.; Steinberg, G.R.; Austin, R.C. Loss of TDAG51 results in mature-onset obesity, hepatic steatosis, and insulin resistance by regulating lipogenesis. Diabetes 2013, 62, 158–169. [Google Scholar] [CrossRef]
- Tarnoki, A.D.; Tarnoki, D.L.; Bogl, L.H.; Medda, E.; Fagnani, C.; Nistico, L.; Stazi, M.A.; Brescianini, S.; Lucatelli, P.; Boatta, E.; et al. Association of body mass index with arterial stiffness and blood pressure components: A twin study. Atherosclerosis 2013, 229, 388–395. [Google Scholar] [CrossRef]
- Fumagalli, C.; Maurizi, N.; Day, S.M.; Ashley, E.A.; Michels, M.; Colan, S.D.; Jacoby, D.; Marchionni, N.; Vincent-Tompkins, J.; Ho, C.Y.; et al. Association of Obesity With Adverse Long-Term Outcomes in Hypertrophic Cardiomyopathy. JAMA Cardiol. 2020, 5, 65–72. [Google Scholar] [CrossRef]
- Liu, R.; Brickman, W.J.; Christoffel, K.K.; Liu, X.; Wang, G.; Arguelles, L.; Zhang, S.; Zimmerman, D.; Wang, B.; Xu, X.; et al. Association of adiposity trajectories with insulin sensitivity and glycemic deterioration: A longitudinal study of rural Chinese twin adults. Diabetes Care 2012, 35, 1506–1512. [Google Scholar] [CrossRef][Green Version]
- Song, Y.M.; Sung, J.; Lee, K. Associations Between Adiposity and Metabolic Syndrome Over Time: The Healthy Twin Study. Metab. Syndr. Relat. Disord. 2017, 15, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Fagard, R.H.; Loos, R.J.; Beunen, G.; Derom, C.; Vlietinck, R. Influence of chorionicity on the heritability estimates of blood pressure: A study in twins. J. Hypertens. 2003, 21, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, W.; Li, Z.; Xu, C.; Tian, X.; Zhang, D. Heritability and genome-wide association study of blood pressure in Chinese adult twins. Mol. Genet. Genom. Med. 2021, 9, e1828. [Google Scholar] [CrossRef] [PubMed]


| Total | 18 to 50 Years | 51 to 60 Years | >60 Years | |
|---|---|---|---|---|
| N | 2842 | 1684 | 702 | 456 |
| Age, years * | 48 (39–55) | 42 (34–47) | 55 (52–57) | 65 (62–69) |
| Female, n (%) | 978 (34.4) | 632 (37.5) | 230 (32.8) | 114 (25.0) |
| MZ, n (%) | 1690 (59.4) | 942 (55.9) | 438 (62.4) | 306 (67.1) |
| BMI (kg/m2) * | 24.6 (22.3–26.9) | 25.0 (22.5–27.5) | 24.4 (22.4–26.7) | 23.5 (21.5–25.6) |
| HbA1c (%) | 5.8 ± 1.2 | 5.6 ± 1.1 | 6.1 ± 1.4 | 6.1 ± 1.3 |
| FBG (mmol/L) | 5.8 ± 2.1 | 5.6 ± 1.8 | 6.3 ± 2.6 | 6.1 ± 2.1 |
| SBP (mmHg) | 133.8 ± 22.2 | 127.8 ± 19.5 | 138.9 ± 22.3 | 148.2 ± 23.2 |
| DBP (mmHg) | 82.2 ± 13.8 | 80.3 ± 13.6 | 85.1 ± 13.8 | 85.0 ± 13.4 |
| TC (mmol/L) | 4.9 ± 1.0 | 4.8 ± 1.0 | 5.1 ± 1.1 | 4.8 ± 0.9 |
| TG (mmol/L) | 1.9 ± 2.4 | 2.0 ± 2.5 | 1.9 ± 2.6 | 1.5 ± 1.1 |
| LDL (mmol/L) | 2.6 ± 0.8 | 2.5 ± 0.8 | 2.7 ± 0.8 | 2.5 ± 0.8 |
| HDL (mmol/L) | 1.3 ± 0.4 | 1.3 ± 0.3 | 1.3 ± 0.4 | 1.4 ± 0.4 |
| Hypertension, n (%) | 519 (18.3) | 180 (10.7) | 171 (24.3) | 168 (36.8) |
| T2DM, n (%) | 225 (7.9) | 70 (4.2) | 99 (14.1) | 56 (12.2) |
| CHD, n (%) | 61 (2.1) | 15 (0.9) | 28 (4.0) | 18 (3.9) |
| Use of antihypertensive medication, n (%) | 433 (15.2) | 107 (6.4) | 152 (21.7) | 174 (38.2) |
| Use of glucoregulatory medicine, n (%) | 199 (7.0) | 60 (3.6) | 77 (11.0) | 62 (13.6) |
| Use of lipid medicine, n (%) | 21 (0.7) | 7 (0.4) | 9 (1.2) | 5 (1.1) |
| Variance Component | |||
|---|---|---|---|
| Phenotypes | h2 | c2 | e2 |
| BMI | 0.72 (0.69, 0.75) | 0.00 (0.00, 0.00) | 0.28 (0.25, 0.31) |
| HbA1c | 0.30 (0.09, 0.54) | 0.20 (0.00, 0.40) | 0.50 (0.44, 0.55) |
| FBG | 0.49 (0.44, 0.54) | 0.00 (0.00, 0.00) | 0.51 (0.46, 0.56) |
| SBP | 0.39 (0.20, 0.59) | 0.17 (0.00, 0.35) | 0.44 (0.40, 0.49) |
| DBP | 0.39 (0.21, 0.59) | 0.19 (0.00, 0.36) | 0.42 (0.38, 0.47) |
| TC | 0.63 (0.59, 0.67) | 0.00 (0.00, 0.00) | 0.37 (0.33, 0.41) |
| TG | 0.58 (0.53, 0.62) | 0.00 (0.00, 0.00) | 0.42 (0.38, 0.47) |
| LDL-C | 0.60 (0.55, 0.64) | 0.00 (0.00, 0.00) | 0.40 (0.36, 0.45) |
| HDL-C | 0.69 (0.65, 0.72) | 0.00 (0.00, 0.00) | 0.31 (0.28, 0.35) |
| Correlations | Rph | Ra | Re | Pa | Pe |
|---|---|---|---|---|---|
| BMI&HbA1c | 0.16 (0.11, 0.21) | 0.23 (0.10, 0.44) | 0.14 (0.08, 0.19) | 0.60 (0.35, 0.76) | 0.40 (0.24, 0.65) |
| BMI&FBG | 0.16 (0.12, 0.21) | 0.18 (0.09, 0.30) | 0.17 (0.11, 0.23) | 0.59 (0.37, 0.74) | 0.41 (0.26, 0.63) |
| BMI&SBP | 0.21 (0.17, 0.26) | 0.31 (0.19, 0.50) | 0.17 (0.12, 0.22) | 0.64 (0.49, 0.75) | 0.36 (0.25, 0.51) |
| BMI&DBP | 0.27 (0.23, 0.32) | 0.39 (0.27, 0.55) | 0.20 (0.15, 0.26) | 0.69 (0.58, 0.77) | 0.31 (0.23, 0.42) |
| BMI&TC | 0.16 (0.11, 0.20) | 0.19 (0.10, 0.29) | 0.13 (0.07, 0.19) | 0.69 (0.50, 0.83) | 0.31 (0.17, 0.50) |
| BMI&TG | 0.33 (0.29, 0.38) | 0.37 (0.28, 0.48) | 0.31 (0.24, 0.39) | 0.66 (0.56, 0.74) | 0.34 (0.26, 0.44) |
| BMI&LDL-C | 0.14 (0.09, 0.18) | 0.14 (0.06, 0.23) | 0.13 (0.07, 0.21) | 0.65 (0.39, 0.82) | 0.35 (0.18, 0.61) |
| BMI&HDL-C | −0.27 (−0.31, −0.22) | −0.34 (−0.44, −0.26) | −0.15 (−0.21, −0.09) | 0.80 (0.71, 0.88) | 0.20 (0.12, 0.29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, X.; Wu, Z.; Cao, W.; Lv, J.; Yu, C.; Huang, T.; Sun, D.; Liao, C.; Pang, Y.; Pang, Z.; et al. Cardiometabolic Traits in Adult Twins: Heritability and BMI Impact with Age. Nutrients 2023, 15, 164. https://doi.org/10.3390/nu15010164
Hong X, Wu Z, Cao W, Lv J, Yu C, Huang T, Sun D, Liao C, Pang Y, Pang Z, et al. Cardiometabolic Traits in Adult Twins: Heritability and BMI Impact with Age. Nutrients. 2023; 15(1):164. https://doi.org/10.3390/nu15010164
Chicago/Turabian StyleHong, Xuanming, Zhiyu Wu, Weihua Cao, Jun Lv, Canqing Yu, Tao Huang, Dianjianyi Sun, Chunxiao Liao, Yuanjie Pang, Zengchang Pang, and et al. 2023. "Cardiometabolic Traits in Adult Twins: Heritability and BMI Impact with Age" Nutrients 15, no. 1: 164. https://doi.org/10.3390/nu15010164
APA StyleHong, X., Wu, Z., Cao, W., Lv, J., Yu, C., Huang, T., Sun, D., Liao, C., Pang, Y., Pang, Z., Cong, L., Wang, H., Wu, X., Liu, Y., Gao, W., & Li, L. (2023). Cardiometabolic Traits in Adult Twins: Heritability and BMI Impact with Age. Nutrients, 15(1), 164. https://doi.org/10.3390/nu15010164






