Nutrition Patterns of Polish Esports Players
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Survey Tools
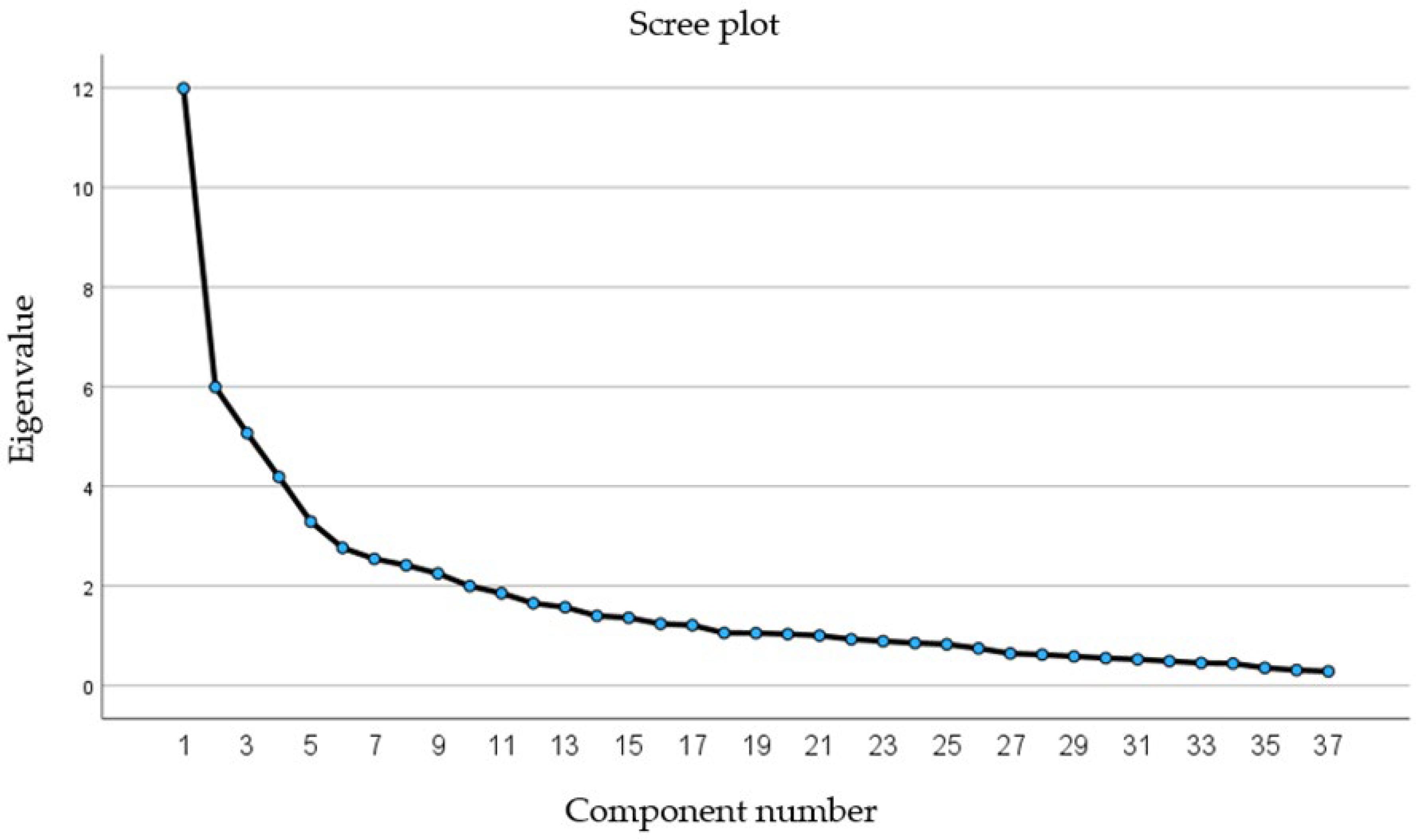
2.2. Derivation of Dietary Patterns
2.3. Statistical and Data Analyses
3. Results
3.1. Dietary Habits of the Study Subjects
3.2. Food Frequency Questionnaires
3.3. Dietary Pattern Characterization
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Dietary Habits | Responses | Percent |
|---|---|---|
| Number of meals | 1 meal | 0.00 |
| 2 meals | 13.91 | |
| 3 meals | 40.87 | |
| 4 meals | 33.91 | |
| 5 meals and more | 11.30 | |
| The frequency of eating between meals | Never | 5.22 |
| 1–3 times a month | 6.09 | |
| Once a week | 13.04 | |
| Several times a week | 36.52 | |
| Once a day | 22.61 | |
| Several times a day | 16.52 | |
| The regularity of eating meals | No | 41.74 |
| Yes, but only some | 47.83 | |
| Yes, all | 10.43 | |
| Eating the last meal before going to bed | 2–3 h before bedtime | 31.30 |
| Less than 2 h | 11.30 | |
| There is no rule | 57.39 | |
| The composition of the snacks consumed | Fruits | 41.74 |
| Vegetables | 9.57 | |
| Unsweetened milk drinks and desserts, e.g., yoghurts, cottage cheese, milk | 18.26 | |
| Sweetened milk drinks and desserts (homogenized cheese, sweetened milk drinks, flavored milk) | 21.74 | |
| Sweet snacks, e.g., candies, cookies, cakes, chocolate bars, muesli bars, wafers | 39.13 | |
| Salty snacks, e.g., crackers, sticks, chips, chips | 28.70 | |
| Nuts, almonds, seeds, pips | 12.17 | |
| Other products | 9.57 | |
| Use of fast-food products (instant soups, ready-made sauces, ready meals, etc.) | Never | 26.09 |
| Almost every day | 5.22 | |
| Often, i.e., several times a week | 8.70 | |
| Sometimes, i.e., 5 or more times a month | 33.91 | |
| Rarely, i.e., 3–4 times a month | 26.09 | |
| The type of milk and milk drinks consumed | Standard fat (full fat) | 66.09 |
| Low fat | 27.83 | |
| Fat free | 0.87 | |
| I don’t eat dairy products | 5.22 | |
| Culinary techniques used to prepare meat dishes | Boiled | 45.22 |
| Braised | 15.65 | |
| Grilled | 30.43 | |
| Baked | 53.04 | |
| Fried | 80.87 | |
| I don’t eat meat | 0.87 | |
| The type of fat used to fry food | I do not use any frying fat | 6.96 |
| I use various fats | 19.13 | |
| I use vegetable oil (including olive oil) | 47.83 | |
| I use a margarine | 3.48 | |
| I use a butter | 20.00 | |
| I use lard | 2.61 | |
| The use of fat as a spread | I do not use any fat to spread my bread | 21.55 |
| I use various fats | 9.48 | |
| I use a mayonnaise | 1.72 | |
| I use a margarine | 5.17 | |
| I use a butter | 56.90 | |
| I use a mix butter with margarine | 2.59 | |
| I use lard | 2.59 | |
| Sweetening hot drinks (e.g., tea, coffee, cocoa) | No | 20.87 |
| Yes, I sweeten it with one teaspoon of sugar (or honey) | 22.61 | |
| Yes, I sweeten it with two or more teaspoons of sugar (or honey) | 52.17 | |
| Yes, I use sweeteners (low-energy sweeteners) | 4.35 | |
| The use of salt in dishes and sandwiches at the table | No | 47.83 |
| Yes, but only sometimes | 40.00 | |
| Yes, I add salt in most of the dishes | 12.17 | |
| Daily fluid intake | Less than 1 L (less than 4 glasses) | 0.00 |
| About 1.5 L (about 6 glasses) | 26.96 | |
| About 2 L (about 8 glasses) | 35.65 | |
| More than 2 L (more than 8 glasses) | 37.39 | |
| The type of water drunk | I don’t drink water | 0.87 |
| I drink non-sparkling water | 69.57 | |
| I drink sparkling water | 24.35 | |
| I drink flavored water | 5.22 |
| Food Items | The Frequency of Consumption of Food Products—the Percentage Distribution of the Response | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Mineral water | 4.29 | 2.15 | 5.58 | 10.73 | 15.88 | 21.46 | 39.91 |
| Hot beverages (tea, coffee, herb or fruit infusions) | 6.87 | 9.44 | 12.02 | 17.17 | 22.32 | 28.76 | 3.43 |
| Spices | 12.88 | 3.86 | 14.16 | 26.18 | 23.61 | 15.45 | 3.86 |
| Table water, spring water | 30.04 | 4.29 | 5.58 | 13.73 | 10.30 | 11.59 | 24.46 |
| Fruits | 3.86 | 8.15 | 20.60 | 34.33 | 19.31 | 12.45 | 1.29 |
| Vegetables | 4.72 | 6.87 | 16.74 | 39.48 | 21.03 | 9.87 | 1.29 |
| Milk (flavored milks, cocoa, coffee with milk) | 6.87 | 9.01 | 15.88 | 31.76 | 24.03 | 11.16 | 1.29 |
| White meat dishes (chicken, turkey, rabbit) | 4.72 | 7.73 | 21.46 | 50.64 | 13.73 | 0.00 | 1.72 |
| Eggs | 6.44 | 10.73 | 27.47 | 37.77 | 14.16 | 3.00 | 0.43 |
| Potatoes (excluding fries and chips) | 7.73 | 14.59 | 23.18 | 45.92 | 7.73 | 0.43 | 0.43 |
| Olive oil and rapeseed oils | 43.35 | 17.17 | 17.17 | 18.03 | 3.43 | 0.43 | 0.43 |
| Fruit juices | 11.59 | 18.45 | 25.75 | 29.18 | 6.44 | 7.30 | 1.29 |
| Fermented milk drinks (yoghurt, kefir) | 18.88 | 20.17 | 24.46 | 24.46 | 8.58 | 2.58 | 0.86 |
| Buckwheats, other whole grains, cereals, whole grain pasta | 14.16 | 26.18 | 29.61 | 21.89 | 5.15 | 2.58 | 0.43 |
| Whole grain bread | 34.33 | 20.60 | 12.88 | 21.89 | 5.15 | 4.72 | 0.43 |
| Cottage cheese (homogenized cheese, cheese deserts) | 29.18 | 30.04 | 19.74 | 14.16 | 5.58 | 0.86 | 0.43 |
| Fishes | 24.89 | 28.76 | 31.33 | 12.45 | 2.15 | 0.00 | 0.43 |
| Nuts, pumpkin seeds, sunflower seeds and other oil seeds | 27.47 | 34.76 | 18.45 | 12.02 | 6.44 | 0.43 | 0.43 |
| Legumes or dishes from legume seeds (beans, peas, lens, soya) | 37.77 | 30.04 | 17.60 | 12.02 | 2.15 | 0.43 | 0.00 |
| Vegetable or vegetable-fruit juices | 48.50 | 24.89 | 12.45 | 9.44 | 2.58 | 2.15 | 0.00 |
| Vegetable canned, marinated or pickled vegetable | 57.08 | 21.89 | 13.30 | 5.58 | 1.29 | 0.43 | 0.43 |
| Food Items | The Frequency of Consumption of Food Products—The Percentage Distribution of the Response | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Light bread (wheat, rye, mixed wheat and rye bread, toast, rolls, rogals) | 1.72 | 3.86 | 10.30 | 17.60 | 27.47 | 36.05 | 3.00 |
| Fried food (meat tor flour) | 3.00 | 5.58 | 15.88 | 52.79 | 17.60 | 3.86 | 1.29 |
| Meats, pork sausages | 6.44 | 8.58 | 17.60 | 39.48 | 16.31 | 10.73 | 0.86 |
| Cheese (molded cheese, blue cheese) | 7.73 | 11.59 | 22.32 | 31.76 | 18.45 | 7.73 | 0.43 |
| White rice, standard pasta, fine groats (manna, cuscus) | 2.15 | 10.30 | 30.90 | 42.49 | 9.44 | 4.29 | 0.43 |
| Sweets (cookies, cakes, chocolate bars, ‘muesli’ types and other confectionery products) | 6.01 | 15.45 | 23.61 | 37.34 | 11.16 | 6.01 | 0.43 |
| Sweet carbonated or non-carbonated beverages (coca-cola, pepsi, sprite, fanta, lemonade) | 11.59 | 19.74 | 22.32 | 26.61 | 8.15 | 7.73 | 3.86 |
| Meat, sausage, poultry and veal | 12.45 | 18.45 | 23.18 | 32.62 | 9.01 | 3.86 | 0.43 |
| Fast food (fries, hamburgers, pizza, chips) | 6.87 | 33.48 | 31.33 | 23.18 | 3.43 | 1.29 | 0.43 |
| Red meat dishes (pork, beef, veal, lamb, venison) | 16.31 | 31.33 | 24.03 | 22.75 | 3.86 | 0.43 | 1.29 |
| Energy drinks (red bull, shot, black horse and others) | 25.32 | 24.03 | 24.03 | 15.88 | 6.01 | 3.43 | 1.29 |
| Margarine or mixes with butter and margarine | 46.35 | 19.74 | 10.30 | 13.30 | 5.15 | 4.72 | 0.43 |
| Lard/coconut oil | 47.64 | 20.17 | 9.44 | 16.31 | 4.29 | 1.29 | 0.86 |
| Alcohol drinks | 37.34 | 37.77 | 16.31 | 6.44 | 1.29 | 0.86 | 0.00 |
| Powder soups or ready soups (can, jar, concentrated) not including frozen soups | 48.93 | 28.33 | 11.59 | 7.73 | 3.43 | 0.00 | 0.00 |
| Canned meat | 68.24 | 20.60 | 4.72 | 4.29 | 1.72 | 0.43 | 0.00 |
References
- Nagorsky, E.; Wiemeyer, J. The structure of performance and training in esports. PLoS ONE 2020, 15, e0237584. [Google Scholar] [CrossRef] [PubMed]
- Mangeloja, E. Economics of Esports. Electron. J. Bus. Ethics Organ. Stud. 2019, 24, 34–42. [Google Scholar]
- Giakoni-Ramírez, F.; Merellano-Navarro, E.; Duclos-Bastías, D. Professional Esports Players: Motivation and Physical Activity Levels. Int. J. Environ. Res. Public Health 2022, 19, 2256. [Google Scholar] [CrossRef]
- Pluss, M.A.; Bennett, K.J.M.; Novak, A.R.; Panchuk, D.; Coutts, A.J.; Fransen, J. Esports: The Chess of the 21st Century. Front. Psychol. 2019, 10, 156. [Google Scholar] [CrossRef]
- Hilvoorde, I.V.; Pot, N. Embodiment and fundamental motor skills in eSports. Sport Ethics Philos. 2016, 10, 14–27. [Google Scholar] [CrossRef]
- Baumann, A.; Mentzoni, R.A.; Erevik, E.; Pallesen, S. A qualitative study on Norwegian esports students’ sleep, nutritional and physical activity habits and the link to health and performance. Int. J. Esports 2022, 1. [Google Scholar]
- Huth, C. Nutritional behaviour of (Non-) eSports players—A comparative study. Qual. Sport 2021, 7, 38–44. [Google Scholar] [CrossRef]
- Rudolf, K.; Bickmann, P.; Froböse, I.; Tholl, C.; Wechsler, K.; Grieben, C. Demographics and Health Behavior of Video Game and eSports Players in Germany: The eSports Study 2019. Int. J. Environ. Res. Public Health 2020, 17, 1870. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Huo, Y.; Kelly, S.; Leung, J.; Tisdale, C.; Gullo, M. The impact of eSports and online video gaming on lifestyle behaviours in youth: A systematic review. Comput. Hum. Behav. 2022, 126, 106974. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Herbeth, B.; Samara, A.; Stathopoulou, M.; Siest, G.; Visvikis-Siest, S. Alcohol Consumption, Beverage Preference, and Diet in Middle-Aged Men from the STANISLAS Study. J. Nutr. Metab. 2012, 2012, 987243. [Google Scholar] [CrossRef] [PubMed]
- Jeżewska-Zychowicz, M.; Gawęcki, J.; Wądołowska, L.; Czarnocińska, J.; Galiński, G.; Kołłajtis-Dołowy, A.; Roszkowski, W.; Wawrzyniak, A.; Przybyłowicz, K.; Krusińska, B.; et al. Kwestionariusz Do Badania Poglądów i Zwyczajów Żywieniowych Oraz Procedura Opracowania Danych; Wydawnictwo Komitetu Nauki o Żywieniu Człowieka Polskiej Akademii Nauk: Warszawa, Poland, 2014; pp. 21–33. [Google Scholar] [CrossRef]
- Stark, A. An historical review of the Harvard and the National Cancer Institute Food Frequency Questionnaires: Their similarities, differences, and their limitations in assessment of food intake. Ecol. Food Nutr. 2002, 41, 35–74. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; National Center for Health Statistics (NHANES). Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx (accessed on 15 October 2022).
- Alvin, R.C. Factor analysis. In Methods of Multivariate Analysis; John Wiley and Sons, Inc.: New York, NY, USA, 2002; pp. 408–450. [Google Scholar]
- Hair, J.F.; Black, W.C.; Babin, B.J. Factor analysis. In Multivariate Data Analysis: A Global Perspective; Pearson Education: London, UK, 2010; Chapter 3. [Google Scholar]
- Newby, P.K.; Tucker, K.L. Empirically derived eating patterns using factor or cluster analysis: A review. Nutr. Rev. 2004, 62, 177–203. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinilla, F. Brain foods: The effects of nutrients on brain function. Nat. Rev. Neurosci. 2008, 9, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Szot, M.; Karpęcka-Gałka, E.; Dróżdż, R.; Frączek, B. Can Nutrients and Dietary Supplements Potentially Improve Cognitive Performance Also in Esports? Healthcare 2022, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand, B.; Scheers, N.; Rasmussen, M.K.; Young, J.F.; Ross, A.B.; Landberg, R. Brain foods-the role of diet in brain performance and health. Nutr. Rev. 2021, 79, 693–708. [Google Scholar] [CrossRef]
- Himmelstein, D.; Liu, Y.; Shapiro, J.L. An exploration of mental skills among competitive league of legend players. Int. J. Gaming Comput.-Mediat. Simul. (IJGCMS) 2017, 9, 1–21. [Google Scholar] [CrossRef]
- Pederaza-Ramirez, I.; Musculus, L.; Raab, M.; Laborde, S. Setting the scientific stage for esports psychology: A systematic review. Int. Rev. Sport Exerc. Psychol. 2020, 13, 319–352. [Google Scholar] [CrossRef]
- Ding, Y.; Hu, X.; Li, J.; Ye, J.; Wang, F.; Zhang, D. What Makes a Champion: The Behavioral and Neural Correlates of Expertise in Multiplayer Online Battle Arena Games. Int. J. Hum. Comput. Interact. 2018, 34, 682–694. [Google Scholar] [CrossRef]
- Toth, A.J.; Kowal, M.; Campbell, M.J. The Color-Word Stroop Task Does Not Differentiate Cognitive Inhibition Ability among Esports Gamers of Varying Expertise. Front. Psychol. 2019, 10, 2852. [Google Scholar] [CrossRef]
- Li, X.; Huang, L.; Li, B.; Wang, H.; Han, C. Time for a true display of skill: Top players in League of Legends have better executive control. Acta Psychol. 2020, 204, 103007. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Barbagallo, M.; Muñoz-Garcia, M.; Godos, J.; Martinez-Gonzalez, M.A. Dietary Patterns and Cognitive Decline: Key features for prevention. Curr. Pharm. Des. 2019, 25, 2428–2442. [Google Scholar] [CrossRef] [PubMed]
- Féart, C.; Samieri, C.; Barberger-Gateau, P. Mediterranean diet and cognitive function in older adults. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 14–18. [Google Scholar] [CrossRef]
- Moustafa, B.; Trifan, G.; Isasi, C.R.; Lipton, R.B.; Sotres-Alvarez, D.; Cai, J.; Tarraf, W.; Stickel, A.; Mattei, J.; Talavera, G.A.; et al. Association of Mediterranean Diet With Cognitive Decline Among Diverse Hispanic or Latino Adults From the Hispanic Community Health Study/Study of Latinos. JAMA Netw. Open 2022, 5, e2221982. [Google Scholar] [CrossRef] [PubMed]
- Mamalaki, E.; Charisis, S.; Anastasiou, C.A.; Ntanasi, E.; Georgiadi, K.; Balomenos, V.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; et al. The Longitudinal Association of Lifestyle with Cognitive Health and Dementia Risk: Findings from the HELIAD Study. Nutrients 2022, 14, 2818. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, F.; Graziano, M.; Hagnäs, M.; Frittitta, L.; Tumminia, A. Influence of the Mediterranean and Ketogenic Diets on Cognitive Status and Decline: A Narrative Review. Nutrients 2020, 12, 1019. [Google Scholar] [CrossRef] [PubMed]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Millerm, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef]
- Ushula, T.W.; Mamun, A.; Darssan, D.; Wang, W.Y.S.; Williams, G.M.; Whiting, S.J.; Najman, J.M. Dietary patterns and the risks of metabolic syndrome and insulin resistance among young adults: Evidence from a longitudinal study. Clin. Nutr. 2022, 41, 1523–1531. [Google Scholar] [CrossRef]
- Deshmukh-Taskar, P.R.; O’Neil, C.E.; Nicklas, T.A.; Yang, S.J.; Liu, Y.; Gustat, J.; Berenson, G.S. Dietary patterns associated with metabolic syndrome, sociodemographic and lifestyle factors in young adults: The Bogalusa Heart Study. Public Health Nutr. 2009, 12, 2493–2503. [Google Scholar] [CrossRef]
- Hare-Bruun, H.; Togo, P.; Andersen, L.B.; Heitmann, B.L. Adult food intake patterns are related to adult and childhood socioeconomic status. J. Nutr. 2011, 141, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Olinto, M.T.A.; Willett, W.C.; Gigante, D.P.; Victora, C.G. Sociodemographic and lifestyle characteristics in relation to dietary patterns among young Brazilian adults. Public Health Nutr. 2011, 14, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Morinaka, T.; Wozniewicz, M.; Jeszka, J.; Bajerska, J.; Nowaczyk, P.; Sone, Y. Westernization of dietary patterns among young Japanese and Polish females—A comparison study. Ann. Agric. Environ. Med. 2013, 20, 122–130. [Google Scholar] [PubMed]
- Hasegawa, Y.; Chen, S.Y.; Sheng, L.; Jena, P.K.; Kalanetra, K.M.; Mills, D.A.; Wan, Y.Y.; Slupsky, C.M. Long-term effects of western diet consumption in male and female mice. Sci. Rep. 2020, 10, 14686. [Google Scholar] [CrossRef] [PubMed]
- López-Taboada, I.; González-Pardo, H.; Conejo, N.M. Western Diet: Implications for Brain Function and Behavior. Front. Psychol. 2020, 11, 564413. [Google Scholar] [CrossRef]
- Parrott, M.; Shatenstein, B.; Ferland, G.; Morais, J.A.; Belleville, S.; Kergoat, M.J.; Payette, H.; Greenwood, C.E. The influence of empirically derived dietary patterns on cognitive function in independent older adults depends on income and education: The NuAge Study. FASEB J. 2011, 25, 97.1. [Google Scholar] [CrossRef]
- Parrott, M.D.; Shatenstein, B.; Ferland, G.; Payette, H.; Morais, J.A.; Belleville, S.; Kergoat, M.J.; Gaudreau, P.; Greenwood, C.E. Relationship between diet quality and cognition depends on socioeconomic position in healthy older adults. J. Nutr. 2013, 143, 1767–1773. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Davidson, T.L. Western diet consumption and cognitive impairment: Links to hippocampal dysfunction and obesity. Physol. Behav. 2011, 103, 59–68. [Google Scholar] [CrossRef]
- Attuquayefio, T.; Stevenson, R.J.; Boakes, R.A.; Oaten, M.J.; Yeomans, M.R.; Mahmut, M.; Francis, H.M. A high-fat high-sugar diet predicts poorer hippocampal-related memory and a reduced ability to suppress wanting under satiety. J. Exp. Psychol. Anim. Learn Cogn. 2016, 42, 415–428. [Google Scholar] [CrossRef]
- Krusińska, B.; Hawrysz, I.; Słowińska, M.A.; Wądołowska, L.; Biernacki, M.; Czerwińska, A.; Gołota, J.J. Dietary patterns and breast or lung cancer risk: A pooled analysis of 2 case-control studies in north-eastern Poland. Adv. Clin. Exp. Med. 2017, 26, 1367–1375. [Google Scholar] [CrossRef]
- Gajda, R.; Jeżewska-Zychowicz, M.; Raczkowska, E. Differences in Dietary Patterns among the Polish Elderly: A Challenge for Public Health. Nutrients 2021, 13, 3966. [Google Scholar] [CrossRef] [PubMed]
- Crichton, G.E.; Elias, M.F.; Dore, G.A.; Robbins, M.A. Relation between dairy food intake and cognitive function: The Maine-Syracuse Longitudinal Study. Int. Dairy J. 2012, 22, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Goulart, J.; Aitken, L.; Siddiqui, S.; Cardenas, J.; Cuevas, M.; Riechman, S.; Beathard, K. Nutrition, Vision, and Cognition in Sport: E-Sport Gaming Athletes. Curr. Dev. Nutr. 2022, 6, 789. [Google Scholar] [CrossRef]
- Mancini, E.; Beglinger, C.; Drewe, J.; Zanchi, D.; Lang, U.E.; Borgwardt, S. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine 2017, 34, 26–37. [Google Scholar] [CrossRef]
- Shukitt-Hale, B.; Miller, M.; Chu, Y.F.; Lyle, B.J.; Joseph, J.A. Coffee, but not caffeine, has positive effects on cognition and psychomotor behavior in aging. Age 2013, 35, 2183–2192. [Google Scholar] [CrossRef]
- Panza, F.; Solfrizzi, V.; Barulli, M.R.; Bonfiglio, C.; Guerra, V.; Osella, A.; Seripa, D.; Sabbà, C.; Pilotto, A.; Logroscino, G. Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: A systematic review. J. Nutr. Health Aging 2015, 19, 313–328. [Google Scholar] [CrossRef]
- Gardener, S.L.; Rainey-Smith, S.R. The Role of Nutrition in Cognitive Function and Brain Ageing in the Elderly. Curr. Nutr. Rep. 2018, 7, 139–149. [Google Scholar] [CrossRef]
- Carrillo, J.Á.; Zafrilla, M.P.; Marhuenda, J. Cognitive Function and Consumption of Fruit and Vegetable Polyphenols in a Young Population: Is There a Relationship? Foods 2019, 8, 507. [Google Scholar] [CrossRef]
- Lamport, D.J.; Lawton, C.L.; Merat, N.; Jamson, H.; Myrissa, K.; Hofman, D.; Chadwick, H.K.; Quadt, F.; Wightman, J.D.; Dye, L. Concord grape juice, cognitive function, and driving performance: A 12-wk, placebo-controlled, randomized crossover trial in mothers of preteen children. Am. J. Clin. Nutr. 2016, 103, 775–783. [Google Scholar] [CrossRef]
- Essaw, E.; Moses, M.O.; Afrifa, D.; Acheampong, I.K.; Mensah, W.; Owusu, L. Physical activity patterns and dietary habits of undergraduate students. Balt. J. Health Phys. Act. 2019, 11, 115–123. [Google Scholar] [CrossRef]
- Halder, S.; Anand, U.; Nandy, S.; Oleksak, P.; Qusti, S.; Alshammari, E.M.; El-Saber Batiha, G.; Koshy, E.P.; Dey, A. Herbal drugs and natural bioactive products as potential therapeutics: A review on pro-cognitives and brain boosters perspectives. Saudi Pharm J. 2021, 29, 879–907. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Iuliano, L.; Praticò, D. Extra-virgin olive oil ameliorates cognition and neuropathology of the 3xTg mice: Role of autophagy. Ann. Clin. Transl. Neurol. 2017, 4, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Bunner, A.E.; Agarwal, U. Saturated and trans fats and dementia: A systematic review. Neurobiol. Aging 2014, 35, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Karpęcka, E.; Frączek, B. Macronutrients and water—Do they matter in the context of cognitive performance in athletes? Balt. J. Health Phys. Act. 2020, 12, 114–124. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Verberne, L.; De Irala, J.; Ruíz-Canela, M.; Toledo, E.; Serra-Majem, L.; Martínez-González, M.A. Dietary fat intake and the risk of depression: The SUN Project. PLoS ONE 2011, 6, e16268. [Google Scholar] [CrossRef]
- Slavin, J. Whole grains and human health. Nutr. Res. Rev. 2004, 17, 99–110. [Google Scholar] [CrossRef]
- Hawkins, M.A.W.; Keirns, N.G.; Helms, Z. Carbohydrates and cognitive function. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 302–307. [Google Scholar] [CrossRef]
- Jarosz, M. Human Nutrition Recommendations for Polish Population; IZZ: Warsaw, Poland, 2017. (In Polish) [Google Scholar]
- Deep-Dive: The Eating and Drinking Habits of Consumers While Playing and Viewing Games. Available online: https://newzoo.com/insights/articles/the-eating-and-drinking-habits-of-consumers-while-playing-and-viewing-games (accessed on 13 November 2022).
- Mente, A.; O’Donnell, M.J.; Rangarajan, S.; McQueen, M.J.; Poirier, P.; Wielgosz, A.; Morrison, H.; Li, W.; Wang, X.; Di, C.; et al. PURE Investigators. Association of urinary sodium and potassium excretion with blood pressure. N. Engl. J. Med. 2014, 371, 601–611. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline: Sodium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012.
| Food Items | M | SD |
|---|---|---|
| Mineral water | 5.56 | 1.65 |
| Hot beverages (tea, coffee, herb or fruit infusions) | 4.39 | 1.63 |
| Spices | 4.06 | 1.63 |
| Table water, spring water | 4.03 | 2.39 |
| Fruits | 4.00 | 1.30 |
| Vegetables | 4.00 | 1.26 |
| Milk (flavored milks, cocoa, coffee with milk) | 3.96 | 1.40 |
| White meat dishes (chicken, turkey, rabbit) | 3.68 | 1.07 |
| Eggs | 3.53 | 1.17 |
| Potatoes (excluding fries and chips) | 3.34 | 1.10 |
| Olive oil and rapeseed oils | 3.33 | 1.43 |
| Fruit juices | 3.27 | 1.41 |
| Fermented milk drinks (yoghurt, kefir) | 2.95 | 1.39 |
| Buckwheats, other whole grains, cereals, whole grain pasta | 2.87 | 1.24 |
| Whole grain bread | 2.59 | 1.53 |
| Cottage cheese (homogenized cheese, cheese deserts) | 2.41 | 1.28 |
| Fishes | 2.40 | 1.10 |
| Nuts, pumpkin seeds, sunflower seeds and other oil seeds | 2.38 | 1.25 |
| Legumes or dishes from legume seeds (beans, peas, lens, soya) | 2.12 | 1.13 |
| Vegetable or vegetable-fruit juices | 1.99 | 1.25 |
| Vegetable canned, marinated or pickled vegetable | 1.75 | 1.08 |
| Food Items | M | SD |
|---|---|---|
| Light bread (wheat, rye, mixed wheat and rye bread, toast, rolls, rogals) | 4.85 | 1.29 |
| Fried food (meat tor flour) | 3.93 | 1.05 |
| Meats, pork sausages | 3.86 | 1.33 |
| Cheese (molded cheese, blue cheese) | 3.67 | 1.35 |
| White rice, standard pasta, fine groats (manna, cuscus) | 3.61 | 1.04 |
| Sweets (cookies, cakes, chocolate bars, ‘muesli’ types and other confectionery products) | 3.52 | 1.25 |
| Sweet carbonated or non-carbonated beverages (coca-cola, pepsi, sprite, fanta, lemonade) | 3.39 | 1.56 |
| Meat, sausage, poultry and veal | 3.21 | 1.32 |
| Fast food (fries, hamburgers, pizza, chips) | 2.88 | 1.07 |
| Red meat dishes (pork, beef, veal, lamb, venison) | 2.73 | 1.24 |
| Energy drinks (red bull, shot, black horse and others) | 2.69 | 1.44 |
| Margarine or mixes with butter and margarine | 2.27 | 1.54 |
| Lard/coconut oil | 2.17 | 1.42 |
| Alcohol drinks | 1.99 | 1.03 |
| Powder soups or ready soups (can, jar, concentrated) not including frozen soups | 1.88 | 1.10 |
| Canned meat | 1.52 | 0.95 |
| Dietary Patterns (n = 233) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Almost Healthy | Western Fast Food | High-Processed Food, Meat and Confectionery | Sweet | Fat-diary Products | Vegetable-Fruit | Spices and Additives | Fats | Cereal | |
| Variance explained (%) | 9.47 | 9.22 | 7.14 | 5.81 | 5.56 | 5.34 | 4.61 | 4.08 | 3.73 |
| Food and food products | Factor loadings | ||||||||
| White rice, standard pasta, fine groats (manna, cuscus) | 0.663 | −0.001 | −0.097 | 0.040 | 0.048 | 0.027 | 0.056 | −0.035 | 0.107 |
| Whole grain bread | 0.645 | 0.133 | 0.027 | −0.062 | 0.156 | −0.035 | −0.147 | 0.066 | 0.265 |
| White meat dishes (chicken, turkey, rabbit) | 0.644 | 0.065 | 0.293 | 0.172 | −0.049 | 0.051 | 0.174 | −0.019 | −0.187 |
| Fishes | 0.531 | 0.344 | 0.204 | −0.073 | −0.078 | 0.049 | 0.081 | 0.034 | 0.127 |
| Nuts, pumpkin seeds, sunflower seeds and other oil seeds | 0.487 | 0.213 | −0.028 | −0.094 | −0.092 | 0.142 | 0.123 | 0.313 | 0.145 |
| Legumes or dishes from legume seeds (beans, peas, lens, soya) | 0.476 | 0.414 | 0.136 | −0.153 | 0.150 | 0.145 | 0.051 | 0.092 | 0.179 |
| Vegetables | 0.435 | 0.112 | 0.130 | −0.105 | 0.017 | 0.426 | 0.351 | −0.069 | 0.119 |
| Eggs | 0.403 | 0.082 | 0.149 | 0.002 | 0.286 | −0.007 | 0.196 | 0.263 | −0.080 |
| Canned meat | 0.258 | 0.680 | 0.103 | 0.142 | 0.132 | 0.006 | −0.015 | 0.101 | 0.111 |
| Vegetable canned, marinated or pickled vegetable | 0.261 | 0.679 | 0.172 | −0.094 | 0.154 | 0.028 | 0.101 | 0.124 | 0.071 |
| Vegetable or vegetable-fruit juices | −0.091 | 0.662 | 0.153 | 0.012 | −0.004 | 0.316 | 0.004 | 0.011 | 0.190 |
| Powder soups or ready soups (can, jar, concentrated) not including frozen soups | 0.106 | 0.598 | 0.083 | 0.273 | 0.137 | 0.014 | −0.099 | 0.194 | −0.011 |
| Fast food (fries, hamburgers, pizza, chips) | 0.058 | 0.522 | 0.099 | 0.388 | −0.156 | −0.152 | −0.079 | −0.048 | −0.098 |
| Alcohol drinks | 0.097 | 0.402 | −0.136 | 0.152 | −0.002 | 0.007 | 0.164 | 0.091 | −0.071 |
| Red meat dishes (pork, beef, veal, lamb, venison) | 0.355 | 0.368 | 0.338 | 0.122 | 0.003 | 0.139 | −0.104 | −0.108 | −0.205 |
| Meats, pork sausages | 0.070 | 0.137 | 0.739 | 0.060 | 0.207 | 0.067 | 0.072 | 0.172 | −0.038 |
| Meat, sausage, poultry and veal | 0.224 | 0.193 | 0.636 | 0.043 | 0.135 | 0.076 | 0.141 | −0.045 | 0.073 |
| Light bread (wheat, rye, mixed wheat and rye bread, toast, rolls, rogals) | −0.098 | −0.038 | 0.621 | −0.067 | 0.155 | 0.300 | −0.187 | 0.169 | 0.073 |
| Fried food (meat tor flour) | 0.145 | 0.182 | 0.480 | 0.276 | −0.004 | −0.076 | 0.152 | 0.106 | 0.001 |
| Sweet carbonated or non-carbonated beverages (coca-cola, pepsi, sprite, fanta, lemonade) | −0.163 | 0.147 | 0.165 | 0.812 | −0.095 | 0.075 | 0.074 | 0.128 | −0.070 |
| Energy drinks (red bull, shot, black horse and others) | −0.034 | 0.250 | −0.088 | 0.774 | 0.179 | 0.012 | −0.090 | 0.021 | 0.110 |
| Sweets (cookies, cakes, chocolate bars, ‘muesli’ types and other confectionery products) | 0.150 | −0.041 | 0.409 | 0.503 | 0.053 | 0.066 | 0.035 | −0.024 | 0.030 |
| Milk (flavored milks, cocoa, coffee with milk) | −0.016 | −0.068 | 0.130 | 0.036 | 0.751 | 0.054 | −0.009 | 0.097 | 0.056 |
| Fermented milk drinks (yoghurt, kefir) | 0.182 | 0.260 | 0.099 | 0.003 | 0.661 | 0.208 | 0.069 | −0.228 | 0.045 |
| Cheese (molded cheese, blue cheese) | −0.081 | 0.185 | 0.335 | 0.074 | 0.515 | 0.077 | 0.216 | 0.183 | 0.098 |
| Cottage cheese (homogenized cheese, cheese deserts) | 0.461 | 0.316 | 0.159 | −0.007 | 0.468 | 0.010 | 0.068 | 0.105 | −0.013 |
| Hot beverages (tea, coffee, herb or fruit infusions) | 0.184 | −0.274 | −0.141 | 0.174 | 0.374 | 0.660 | 0.100 | 0.256 | −0.167 |
| Fruit juices | −0.143 | 0.367 | 0.200 | 0.214 | 0.055 | 0.626 | 0.145 | 0.042 | 0.043 |
| Fruits | 0.374 | 0.103 | 0.225 | −0.187 | 0.051 | 0.608 | 0.089 | −0.095 | 0.208 |
| Potatoes (excluding fries and chips) | 0.063 | 0.094 | 0.211 | 0.057 | 0.186 | 0.348 | 0.075 | 0.295 | 0.219 |
| Spices | 0.002 | 0.010 | −0.026 | 0.001 | 0.121 | 0.226 | 0.864 | −0.067 | −0.097 |
| Olive oil and rapeseed oils | 0.345 | −0.005 | 0.219 | −0.004 | 0.045 | −0.005 | 0.615 | 0.121 | 0.086 |
| Margarine or mixes with butter and margarine | 0.017 | 0.289 | 0.271 | 0.063 | 0.048 | 0.142 | −0.126 | 0.758 | −0.132 |
| Lard/coconut oil | 0.249 | 0.168 | 0.057 | 0.207 | 0.064 | −0.122 | 0.219 | 0.534 | 0.429 |
| Buckwheats, other whole grains, cereals, whole grain pasta | 0.268 | 0.096 | 0.028 | 0.019 | 0.083 | 0.172 | −0.067 | −0.029 | 0.822 |
| Mineral water | 0.053 | −0.029 | 0.068 | −0.073 | 0.019 | 0.016 | −0.021 | 0.018 | 0.035 |
| Table water, spring water | −0.011 | 0.116 | 0.123 | −0.059 | −0.015 | 0.011 | 0.088 | 0.002 | 0.057 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szot, M.; Frączek, B.; Tyrała, F. Nutrition Patterns of Polish Esports Players. Nutrients 2023, 15, 149. https://doi.org/10.3390/nu15010149
Szot M, Frączek B, Tyrała F. Nutrition Patterns of Polish Esports Players. Nutrients. 2023; 15(1):149. https://doi.org/10.3390/nu15010149
Chicago/Turabian StyleSzot, Monika, Barbara Frączek, and Florentyna Tyrała. 2023. "Nutrition Patterns of Polish Esports Players" Nutrients 15, no. 1: 149. https://doi.org/10.3390/nu15010149
APA StyleSzot, M., Frączek, B., & Tyrała, F. (2023). Nutrition Patterns of Polish Esports Players. Nutrients, 15(1), 149. https://doi.org/10.3390/nu15010149






