The Benefits to Bone Health in Children and Pre-School Children with Additional Exercise Interventions: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
Objectives
2. Materials and Methods
2.1. Guidelines
2.2. Prospective Registration
2.3. Data Management
2.4. Inclusion Criteria
2.4.1. Participants
2.4.2. Primary Outcomes
2.4.3. Secondary Outcome
2.4.4. Types of Studies
2.5. Search Strategy
2.5.1. Electronic Searches
2.5.2. Searching Other Sources
2.5.3. Study Selection
2.6. Dealing with Multiple Records from the Same Cohort
2.7. Dealing with Missing Data
2.8. Data Extraction
2.9. Assessment of Methodological Quality
2.10. Assessment of Diversity and Heterogeneity
2.11. Assessment of Reporting Biases
2.12. Data Synthesis
2.13. Sensitivity and Subgroup Analysis
2.14. Assessment of the Certainty of the Body of Evidence
2.15. Deviations to Protocol
3. Results
3.1. Selection of Studies
3.2. Study Information
3.3. Intervention Information
3.4. Effect of Exercise Interventions on Dual-Energy X-ray Absorptiometry Measures
3.4.1. Bone Mineral Content
3.4.2. Areal Bone Mineral Density
3.4.3. Bone Cross-Sectional Area
3.4.4. Bone Area and Width
3.5. Effect of Exercise Interventions on Peripheral Quantitative Computed Tomography Measures
3.5.1. Volumetric Bone Mineral Content
3.5.2. Volumetric Bone Mineral Density
3.5.3. Trabecular Bone Mineral Density
3.5.4. Bone Cross-Sectional Area
3.5.5. Total Cortical Area
3.5.6. Total Cortical Thickness
3.5.7. Stress–Strain Index
3.5.8. Bone Strength Index
3.5.9. Other
3.6. Fracture Incidence
3.7. Assessment of Quality in Included Studies
3.8. Assessment of the Certainty of the Body of Evidence
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Systematic Review Search Strategy
| Search Item | Combiners | Terms |
| 1 | Problem of Interest | Fracture OR dual-energy x-ray absorptiometry OR DXA OR bone mineral density OR peripheral Quantitative Computed Tomography OR pQCT OR bone density OR trabecular area OR cortical area OR bone mineral content OR periosteal size OR endosteal size OR cortical thickness OR bone mass OR bone strength OR skeletal age assessment |
| 2 | Participants | Child* OR youth* OR juvenile OR minor OR kid |
| 3 | Participants | Physical OR activity OR exercise OR athletics OR recreation OR play OR games OR training OR gym OR resistance training OR workout OR practice |
| 4 | Exclusion | review OR meta-analysis |
| 5 | Number #1 AND #2 AND #3 NOT #4 | |
| Limitations | Clinical Trial, Humans, English, Child, Preschool Child |
Appendix B. Search Strategy Documentation
| Database | Date of Search | Search Strategy Used (Keywords & Boolean) | Search Limits or Filters (e.g., Dates, Language) | Number of Results Found | Comments |
| PUBMED | 3 May 2022 | (Child * OR youth * OR juvenile OR minor OR kid) AND (Physical OR activity OR exercise OR athletics OR recreation OR play OR games OR training OR gym OR resistance training OR workout OR practice) AND (Fracture OR dual-energy X-ray absorptiometry OR DXA OR bone mineral density OR peripheral Quantitative Computed Tomography OR pQCT OR bone density OR trabecular area OR cortical area OR bone mineral content OR periosteal size OR endosteal size OR cortical thickness OR bone mass OR bone strength OR skeletal age assessment) NOT (review OR meta-analysis) | Clinical Trial, Humans, English, Child (6–12), Preschool Child (2–5), Title/abstract | 236 | Exported to End Note |
| CINAHL (Full Text) | 3 May 2022 | (Child * OR youth * OR juvenile OR minor OR kid) AND (Physical OR activity OR exercise OR athletics OR recreation OR play OR games OR training OR gym OR resistance training OR workout OR practice) AND (Fracture OR dual-energy X-ray absorptiometry OR DXA OR bone mineral density OR peripheral Quantitative Computed Tomography OR pQCT OR bone density OR trabecular area OR cortical area OR bone mineral content OR periosteal size OR endosteal size OR cortical thickness OR bone mass OR bone strength OR skeletal age assessment) NOT (review OR meta-analysis) | Research article, Humans, Peer-reviewed, English, Child (6–12), Preschool Child (2–5), Title | 53 | Exported to End Note |
| CENTRAL | 3 May 2022 | (Child * OR youth * OR juvenile OR minor OR kid) AND (Physical OR activity OR exercise OR athletics OR recreation OR play OR games OR training OR gym OR resistance training OR workout OR practice) AND (Fracture OR dual-energy X-ray absorptiometry OR DXA OR bone mineral density OR peripheral Quantitative Computed Tomography OR pQCT OR bone density OR trabecular area OR cortical area OR bone mineral content OR periosteal size OR endosteal size OR cortical thickness OR bone mass OR bone strength OR skeletal age assessment) NOT (review OR meta-analysis) | Trial, title | 55 | Exported to End Note |
| SportsDISCUS | 3 May 2022 | (Child * OR youth * OR juvenile OR minor OR kid) AND (Physical OR activity OR exercise OR athletics OR recreation OR play OR games OR training OR gym OR resistance training OR workout OR practice) AND (Fracture OR dual-energy X-ray absorptiometry OR DXA OR bone mineral density OR peripheral Quantitative Computed Tomography OR pQCT OR bone density OR trabecular area OR cortical area OR bone mineral content OR periosteal size OR endosteal size OR cortical thickness OR bone mass OR bone strength OR skeletal age assessment) NOT (review OR meta-analysis) | Peer-reviewed, title | 40 | Exported to End Note |
| Web of Science | 3 May 2022 | (((TI = (Fracture OR dual-energy X-ray absorptiometry OR DXA OR bone mineral density OR peripheral Quantitative Computed Tomography OR pQCT OR bone density OR trabecular area OR cortical area OR bone mineral content OR periosteal size OR endosteal size OR cortical thickness OR bone mass OR bone strength OR skeletal age assessment)) AND TI = (Child * OR youth * OR juvenile OR minor OR kid)) AND TI = (Physical OR activity OR exercise OR athletics OR recreation OR play OR games OR training OR gym OR resistance training OR workout OR practice)) NOT TI = (review OR meta-analysis) | Nil | 243 | Exported to End Note |
| Key Journals | 3 May 2022 | N/A | N/A | 1 | |
| TOTAL | 628 | ||||
Appendix C. Study-by-Study Exclusion Judgements
| Study Author | Year | Title | Exclusion Reason |
| Allerton | 2008 | Physical Activity is Positively Related to Bone Mineral Density in Prepubertal Youth. | Conference abstract |
| Clark | 2007 | Vigorous physical activity at age 9 increases the risk of childhood fractures, despite increasing bone mass. | Conference abstract |
| Cole | 2010 | Physical activity is associated with increased volumetric bone density and bone strength in early childhood. | Conference abstract |
| Coster | 2014 | Increased Physical Activity during Growth Improves Muscular Development without Affecting Fracture Risk—a Four-Year Prospective Controlled Exercise Intervention Study in 2525 Children. | Conference abstract |
| Fritz | 2014 | A School-based Seven Year Exercise Intervention Program in 6–9-Year-Old Children Improve Skeletal Traits without Increasing the Fracture Risk—A Population-Based Prospective Controlled Study in 3534 Children. | Conference abstract |
| Fritz | 2015 | Increased physical activity in childhood reduces fracture risk—An 8-year intervention study in 3534 children. | Conference abstract |
| Janz | 2003 | Physical activity intensities and femoral neck strength in young children: The Iowa bone development study. | Conference abstract |
| MacKelvie | 2003 | A school-based exercise intervention elicits substantial bone health benefits: a 2-year randomized controlled trial in girls. | Conference abstract |
| Naka | 2005 | A two-year longitudinal study on the relationship between sports activity and bone mass gain in Japanese boys and girls: Kyoto Kids Increase Density in the Skeleton Study (Kyoto KIDS Study). | Conference abstract |
| Naka | 2007 | A two-year longitudinal study on the relationship between the effective time of sports activity and bone mineral density in Japanese girls: Kyoto kids increase density in the skeleton study (Kyoto KIDS Study). | Conference abstract |
| Tamaki | 2005 | Effect of physical activity in elementary school on bone mineral density in Japanese children and adolescents: A 3-year longitudinal study. | Conference abstract |
| Tamaki | 2006 | Which element of physical activity is more important for determining peak bone mass in Japanese children and adolescents, the period, frequency, or active duration per each activity? | Conference abstract |
| Cohen | 2017 | Bone Health is Maintained, While Fat Mass is Reduced in Pre-pubertal Children with Obesity Participating in a 1-Year Family-Centered Lifestyle Intervention. | Wrong intervention (included dietary supplementation) |
| Yu | 2005 | Effects of strength training on body composition and bone mineral content in children who are obese. | Wrong intervention (included dietary supplementation) |
| Alberga | 2013 | The effects of resistance exercise training on body composition and strength in obese prepubertal children. | Wrong outcomes (did not include bone outcomes) |
| Comeras-Chueca | 2022 | Active Video Games Improve Muscular Fitness and Motor Skills in Children with Overweight or Obesity. | Wrong outcomes (did not include bone outcomes) |
| Davis | 2012 | Exercise dose and diabetes risk in overweight and obese children: a randomized controlled trial. | Wrong outcomes (did not include bone outcomes) |
| Dias | 2018 | Effect of High-Intensity Interval Training on Fitness, Fat Mass and Cardiometabolic Biomarkers in Children with Obesity: A Randomised Controlled Trial. | Wrong outcomes (did not include bone outcomes) |
| Klakk | 2013 | Effect of four additional physical education lessons on body composition in children aged 8–13 year—a prospective study during two school years. | Wrong outcomes (did not include bone outcomes) |
| Yin | 2012 | The impact of a 3-year after-school obesity prevention program in elementary school children. | Wrong outcomes (did not include bone outcomes) |
Appendix D. Study funding and conflicts of interest
| Study | Funding | Conflict of Interest |
| Alwis. 2008 | Swedish Research Council, Centre for Athletic Research, Osterlund Foundation, Kock Foundation, Region Skane Foundation. | None declared. |
| Anliker. 2012 | Swiss Federal Sports Commission Grant. | None declared. |
| Barbeau. 2007 | National Institutes of Health (NIH) Grant. | None declared. |
| Daly. 2016 | Commonwealth Education Trust, Canberra Hospital Salaried Staff Specialists. | None declared. |
| Fuchs. 2001 | National Institute of Health Grant, Division of National Institute of Arthiritis and Musculoskeletal diseases. | None declared. |
| Gutin. 2008 | National Institute of Health (NIH). | None declared. |
| Hasselstrom. 2008 | Danish Heart Foundation, National Board of Health, Danish Ministry of Health, Danish Ministry of Culture, Danish Sport Association. | None declared. |
| Larsen. 2016 | Nordea Fonden, Aase and Ejnar Danielsens Foundation, Augustinus Fonden, FIFA Medical Assessment and Research Centre, Danish Football Association, Danish Ministry of Culture. | None declared. |
| Macdonald. 2007 | British Columbia Ministry of Health, 2010 Legacies Now, BC Ministry of Tourism, Sport and Arts, Canadian Institutes of Health Research. | None declared. |
| MacKelvie. 2001 | British Columbia Health Research Foundation, Michael Smith Foundation for Health Research. | None declared. |
| McKay. 2000 | Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research. | None declared. |
| Meiring. 2014 | Carnegie Corporation, Medical Research Council of South Africa, Sugar Association of South Africa. | None declared. |
| Meyer. 2011 | Swiss Federal Office of Sports, Swiss National Science Foundation. | None declared. |
| Nogueira. 2014 | None declared. | None declared. |
| Specker. 2004 | National Institutes of Health Grant. | None declared. |
| Staiano. 2018 | American Heart Association, NORC Centre, National Institute of Diabetes and Digestive and Kidney Diseases. | None declared. |
Appendix E. Template for Intervention Description and Replication (TIDieR) Checklist Items
| Study | Materials | Procedures | Provider | Delivery | Intervention location | Dosage (Sets, Reps) | Dosage (Frequency/Week) | Dosage (Duration in Weeks) | Tailoring | Modifications | Adherence |
| Alwis. 2008 | None | Extra Activity, running, jumping, climbing to 200min/week | School teachers | Face to face | Unspecified | NR | 5/week | 2 years | No | No | N/A |
| Anliker. 2012 | None | 10 min jumping | School teachers | Face to face—class setting | Unspecified | 60–150 jumps | 2/week | 36 | No | No | N/A |
| Barbeau. 2007 | None | Skills development, MVPA, stretching, toning | Classroom teachers, assistant teachers and a research staff member | Face to face—class setting | Unspecified | NR | 5/week | 40 weeks | No | No | Students could not miss 3 sessions in a row |
| Daly. 2016 | None | General PA | Specialist Teacher from Bluearth Foundation | Face to face—class setting | Not specified | NR | 2/week | 35 weeks/year for 4 years | No | No | High |
| Fuchs. 2001 | 61cm Box | Current practice plus 20 min—3 times/week | Laboratory Researcher + 4 trained instructors | Face to Face | Gym | 100 jumps per session | 3 times/week for 20 min | 7 months | No | 50 jumps per session were started with, and slowly progressed to 100 jumps by the 5th week | N/A |
| Gutin. 2008 | None | 80 min/day, 5 days per week | Qualified Physical Trainers | Face-to-face | Gym | NR | 5/week | 3 years | No | No | 44% |
| Hasselstrom. 2008 | None | Extra PE classes—180 min/week | Qualified Physical Trainers | Face-to-face | School | NR | 2/week | 3 years | No | No | N/A |
| Larsen. 2016 (Small-sided games) | None | 3 v 3 football, basketball, floorball or other | Qualified University Trainers | Face-to-face | School | NR | 3/week | 10 months | No | No | N/A |
| Larsen. 2016 (Circuit training) | None | 50% was 30 s effort, 45 s rest, 6–10 stations. Plyos, strength, etc. 50% was games with strength, etc. | Qualified University Trainers | Face-to-face | School | NR | 3/week | 10 months | No | No | N/A |
| Macdonald. 2007 | Classroom Action Bin | Standard curriculum—2 × 40 min PE classes + 15 min per day of random activities + 9 min per day of jumping activities | Normal teachers | Face-to-face | School | NR | 5/week (school days) | 11 months dosage (14 months until follow-up) | No | No | No |
| MacKelvie. 2001 | None | School curriculum with 10–12 min diverse weight bearing activity during normal class, plus 10–12 min on an extra day. Activities included circuit training with 5 different jumping activities with GRF that ranged from 3.5–5 times body weight. Children progressed from 50 to 100 jumps per session across 10 weeks. A higher proportion of high-impact jumps were utilised in the second year of intervention | Normal teachers | Face to face | School | 50–100 jumps per session | 3/week | 20 months | No | No | No |
| McKay. 2000 | None | School curriculum with 10–30 min within each PE class + one extra day—minimum 10 min loading which focused on jumping. 10 DBL tuck jumps before each class. Bench jumping and circuit training were added as the intervention progressed | Classroom teachers | Face to face | School | 10 tuck jumps to start the class + exercise intervention | 3/week | 8 months | No | No | N/A |
| Meiring. 2014 | None | School curriculum + 2 × 45 min sessions—Exercise circuit with 5 activities | Classroom teachers or after school care teachers | Face-to-face | Sports field | 45 min | 2/week | 20 weeks | No | No | N/A |
| Meyer. 2011 | None | School curriculum + 2 additional PE classes (5 total)—At least 10 min of jumping activities. Additionally, included short activity breaks in the middle of normal class, plus students had physical activity homework to complete | School teachers | Face to face | School + home | NR | 5/week | 9 months | No | No | N/A |
| Nogueira. 2014 | None | School curriculum + 10 min of continuous high-intensity movements of medium to high impact at various speeds—jumps, hops, squats, lunges, handstands, cartwheels | Trained Instructor | Face to face | School | NR | 3/week | 9 months | No | No | N/A |
| Specker. 2004 | None | 30 min of moderate vigorous activity, which focused on jumping, hopping, skipping on every school day | Childcare teachers | Face to face | Childcare/School | NR | 5/week | 12 months | No | No | 72% |
| Staiano. 2018 | Kinect and XBOX 360 gaming console, 24-week XBOX live subscription and 4 exercise games | 3 times weekly playing exergames on XBOX—10 min per session in Week 1, increasing by 10 min each session each week, sustaining at 60 min per session after Week 6 | Parent/Guardian | Face to face | Home | NR | 3/week | 24 weeks | No | No | 94.40% |
Appendix F. Dual X-ray Absorptiometry
| Outcome | Timepoint (Weeks) | Study | Girls (Baseline)- Mean (SD), n | Girls (Follow-up)- Mean (SD), n | Boys (Baseline)- Mean (SD), n | Boys (Follow-up)- Mean (SD), n | All (Baseline)- Mean (SD), n | All (Follow-up)- Mean (SD), n | ||||||
| INT | CON | INT | CON | INT | CON | INT | CON | INT | CON | INT | CON | |||
| BMC (Whole Body) | 20 | Meiring. 2014 | 778.40 (164.00), 12 | 753.70 (103.60), 10 | 822.60 (195.50), 12 | 792.90 (116.70), 10 | ||||||||
| BMC (Whole Body) | 32 | McKay. 2005 | 1054.00 (97.00), 51 | 1047.00 (169.00), 73 | 1146.70 (NR), 51 | 1153.00 (NR), 73 | ||||||||
| BMC (Whole Body) | 36 | Nogueira. 2014 | 1523.67 (225.67), 12 | 1561.67 (87.44), 6 | 1732.80 (314.97), 12 | 1701.50 (117.21), 6 | ||||||||
| BMC (Whole Body) | 40 | Barbeau. 2007 | 1260.00 (310.00), 118 | 1230.00 (280.00), 83 | 1460.00 (360.00), 118 | 1400.00 (330.00), 83 | ||||||||
| BMC (Whole Body) | 52 | Alwis. 2008 | 991.00 (84.00), 80 | 980.00 (74.00), 57 | 1135.00 (NR), 78 | 1127.00 (NR), 54 | ||||||||
| BMC (Whole Body) | 60 | Macdonald. 2008 | 1040.30 (213.20), 142 | 1003.50 (159.10), 55 | 1253.20 (263.60), 142 | 1216.30 (213.80), 55 | 1078.60 (193.10), 151 | 1098.10 (178.10), 62 | 1260.30 (237.60), 151 | 1259.90 (206.60), 62 | ||||
| BMC (Whole Body) | 104 | Linden. 2006 | 939.00 (153.00), 49 | 933.00 (181.00), 50 | 1245.00 (240.00), 49 | 1204.00 (245.00), 50 | ||||||||
| BMC (Whole Body) | 156 | Meyer. 2013 | 732.00 (223.00), 149 | 750 (257.00), 65 | 1196.00 (458.00), 149 | 1153.00 (424.00), 65 | ||||||||
| BMC (Whole Body) | 208 | Daly. 2016 | 886.00 (101.00), 192 | 909.00 (114.00), 170 | 1362.00 (NR), 94 | 1364.00 (NR), 69 | 933.00 (116.00), 206 | 956.00 (117.00), 159 | 1342.00 (NR), 97 | 1365.00 (NR), 76 | ||||
| BMC (Whole Body) | 364 | Fritz. 2016b | 614.00 (132.00), 72 | 607.00 (142.00), 45 | 1828.00 (NR), 72 | 1745.00 (NR), 45 | 646.00 (151.00), 100 | 671.00 (144.00), 47 | 1986.00 (NR), 100 | 2005.00 (NR), 47 | ||||
| BMC (Whole Body) | 416 | Coster. 2016 | 609.00 (132.00) | 612.00 (145.00), 39 | 1986.00 (365.00), 65 | 1840.00 (384.00), 39 | 650.00 (151.00), 93 | 681.00 (140.00), 37 | 2210.00 (513.00), 93 | 2292.00 (473.00), 37 | ||||
| BMC (Total Hip) | 20 | Meiring. 2014 | 17.60 (4.90), 12 | 16.30 (2.90), 10 | 18.70 (5.50), 12 | 16.50 (3.10), 10 | ||||||||
| BMC (Total Hip) | 156 | Meyer. 2013 | 14.37 (5.59), 149 | 14.72 (5.69), 65 | 24.98 (10.80), 149 | 23.53 (8.37), 65 | ||||||||
| BMC (Total Hip) | 364 | Gunter. 2008 | 10.60 (2.96), 14 | 10.10 (1.37), 8 | 31.26 (5.4), 11 | 30.15 (3.38), 7 | 11.64 (2.33), 19 | 10.89 (2.53), 16 | 39.93 (9.56), 18 | 39.31 (9.93), 13 | ||||
| BMC (Radius) | 20 | Meiring. 2014 | 3.60 (0.80), 12 | 3.40 (0.50), 10 | 3.80 (0.80), 12 | 3.60 (0.60), 10 | ||||||||
| BMC (Ulna) | 20 | Meiring. 2014 | 2.50 (0.60), 12 | 2.30 (0.40), 10 | 2.70 (0.60), 12 | 2.50 (0.40), 10 | ||||||||
| BMC (Forearm) | 156 | Hasselstrom. 2008 | 1.75 (0.26), 135 | 1.77 (0.29), 76 | 2.14 (0.34), 135 | 2.12 (0.40), 76 | 1.92 (0.30), 135 | 1.97 (0.31), 62 | 2.33 (0.38), 135 | 2.35 (0.45), 62 | ||||
| BMC (Calcaneus) | 156 | Hasselstrom. 2008 | 0.77 (0.23), 135 | 0.77 (0.21), 76 | 1.26 (0.27), 135 | 1.29 (0.25), 76 | 0.75 (0.24), 135 | 0.8 (0.23), 62 | 1.38 (0.25), 135 | 1.35 (0.26), 62 | ||||
| BMC (Leg) | 104 | Linden. 2006 | 283.90 (60.70), 49 | 282.70 (72.50), 50 | 430.30 (105.10), 49 | 409.80 (106.60), 50 | ||||||||
| BMC (Inter- trochanteric) | 32 | McKay. 2005 | 10.60 (2.20), 51 | 10.20 (2.20), 73 | 12.10 (NR), 51 | 11.40 (NR), 73 | ||||||||
| BMC (Greater trochanter) | 32 | McKay. 2005 | 2.90 (0.90), 51 | 2.70 (0.80), 73 | 3.50 (NR), 51 | 3.25 (NR), 73 | ||||||||
| BMC (Greater trochanter) | 364 | Gunter. 2008 | 2.47 (0.84), 14 | 2.13 (0.54), 8 | 8.31 (1.42), 11 | 7.49 (1.05), 7 | 2.54 (0.68), 19 | 2.24 (0.59), 16 | 11.11 (2.62), 18 | 10.91 (2.54), 13 | ||||
| BMC (Proximal Femur) | 32 | McKay. 2005 | 16.20 (3.40), 51 | 15.60 (3.20), 73 | 18.50 (NR), 51 | 17.50 (NR), 73 | ||||||||
| BMC (Proximal Femur) | 60 | Macdonald. 2008 | 15.20 (3.50), 142 | 14.40 (2.90), 55 | 19.00 (4.50), 142 | 18.40 (4.00), 55 | 16.10 (3.40), 151 | 16.00 (3.00), 62 | 19.60 (4.50), 151 | 19.60 (3.90), 62 | ||||
| BMC (Femoral Neck) | 20 | Meiring. 2014 | 2.90 (0.50), 12 | 2.70 (0.40), 10 | 3.00 (0.50), 12 | 2.70 (0.3), 10 | ||||||||
| BMC (Femoral Neck) | 28 | Fuchs. 2001 | 1.84 (0.07), 45 | 1.82 (0.06), 44 | 2.00 (0.07), 45 | 1.89 (0.06), 44 | ||||||||
| BMC (Femoral Neck) | 32 | McKay. 2005 | 2.70 (0.40), 51 | 2.60 (0.40), 73 | 2.89 (NR), 51 | 2.80 (NR), 73 | ||||||||
| BMC (Femoral Neck) | 36 | Nogueira. 2014 | 2.77 (0.45), 12 | 3.05 (0.28), 6 | 3.11 (0.56), 12 | 3.24 (0.39), 6 | ||||||||
| BMC (Femoral Neck) | 52 | Alwis. 2008 | 2.90 (0.60), 80 | 2.8 (0.50), 57 | 3.13 (NR), 76 | 3.08 (NR), 51 | ||||||||
| BMC (Femoral Neck) | 60 | Macdonald. 2008 | 2.52 (0.42), 142 | 2.47 (0.38), 55 | 2.89 (0.52), 142 | 2.80 (0.50), 55 | 2.79 (0.43), 151 | 2.83 (0.41), 62 | 3.08 (0.48), 151 | 3.10 (0.48), 62 | ||||
| BMC (Femoral Neck) | 104 | Linden. 2006 | 2.60 (0.60), 49 | 2.70 (0.60), 50 | 3.30 (0.60), 49 | 3.20 (0.60), 50 | ||||||||
| BMC (Femoral Neck) | 156 | Meyer. 2013 | 2.33 (0.77), 149 | 2.39 (0.78), 65 | 3.54 (0.92), 65 | 3.67 (1.03), 149 | ||||||||
| BMC (Femoral Neck) | 364 | Gunter. 2008 | 1.59 (0.37), 14 | 1.50 (0.37), 8 | 4.18 (0.53), 11 | 4.21 (0.53), 7 | 1.89 (0.38), 19 | 1.79 (0.40), 16 | 4.92 (0.88), 18 | 4.72 (0.93), 13 | ||||
| BMC (Femoral Neck) | 364 | Fritz. 2016b | 2.50 (0.50), 72 | 2.60 (0.60), 45 | 4.90 (NR), 72 | 4.60 (NR), 45 | 2.80 (0.60), 100 | 2.80 (0.50), 47 | 5.10 (NR), 100 | 5.20 (NR), 47 | ||||
| BMC (Femoral Neck) | 364 | Fritz. 2016c | 2.70 (0.60), 172 | 2.70 (0.60), 92 | 5.10 (NR), 172 | 4.90 (NR), 92 | ||||||||
| BMC (Femoral Neck) | 416 | Coster. 2016 | 2.50 (0.60), 65 | 2.60 (0.60), 39 | 5.20 (0.90), 65 | 4.80 (1.10), 39 | 2.80 (0.60), 93 | 2.90 (0.50), 37 | 5.60 (1.10), 93 | 5.80 (1.00), 37 | ||||
| BMC (L3) | 52 | Alwis. 2008 | 5.30 (1.10), 80 | 5.60 (1.00), 57 | 6.02 (NR), 76 | 6.18 (NR), 51 | ||||||||
| BMC (L3) | 104 | Linden. 2006 | 5.00 (1.00), 49 | 5.30 (1.20), 50 | 6.70 (1.60), 49 | 6.30 (1.40), 50 | ||||||||
| BMC (Lumbar Spine) | 20 | Meiring. 2014 | 23.10 (4.60), 12 | 23.40 (4.60), 10 | 24.30 (6.20), 12 | 24.40 (4.70), 10 | ||||||||
| BMC (Lumbar Spine) | 28 | Fuchs. 2001 | 20.10 (0.51), 45 | 20.39 (0.55), 44 | 22.06 (0.57), 45 | 21.64 (0.58), 44 | ||||||||
| BMC (Lumbar Spine) | 32 | McKay. 2005 | 24.60 (5.10), 51 | 24.30 (4.40), 73 | 27.20 (NR), 51 | 27.10 (NR), 73 | ||||||||
| BMC (Lumbar Spine) | 36 | Nogueira. 2014 | 20.40 (4.20), 12 | 21.10 (3.00), 6 | 24.80 (7.20), 12 | 23.20 (3.60), 6 | ||||||||
| BMC (Lumbar Spine) | 60 | Macdonald. 2008 | 24.00 (6.10), 142 | 23.20 (4.90), 55 | 30.80 (8.40), 142 | 29.6 (7.00), 55 | 23.10 (4.90), 151 | 23.60 (4.30), 62 | 27.30 (6.40), 151 | 27.20 (5.10), 62 | ||||
| BMC (Lumbar Spine) | 104 | Linden. 2006 | 15.20 (3.10), 49 | 15.50 (3.30), 50 | 19.70 (4.90), 49 | 19.00 (4.00), 50 | ||||||||
| BMC (Femoral Neck) | 156 | Meyer. 2013 | 22.26 (6.10), 149 | 22.99 (7.09), 65 | 39.07 (16.09), 149 | 37.86 (14.99), 65 | ||||||||
| BMC (Lumbar Spine) | 364 | Fritz. 2016b | 82.40 (19.10), 72 | 77.00 (17.60), 45 | 242.6 (NR), 72 | 227.80 (NR), 45 | 84.80 (21.40), 100 | 85.20 (17.50), 47 | 232.50 (NR), 100 | 232.80 (NR), 47 | ||||
| BMC (Lumbar Spine) | 364 | Fritz. 2016c | 83.80 (20.50), 172 | 81.30 (17.90), 92 | 236.70 (NR), 172 | 230.40 (NR), 92 | ||||||||
| BMC (Lumbar Spine) | 416 | Coster. 2016 | 82.60 (19.30), 65 | 77.70 (17.40), 39 | 266.30 (50.60), 65 | 245.5 (50.80), 39 | 85.60 (21.60), 93 | 86.70 (17.30), 37 | 261.00, 69.60), 93 | 267.50 (65.10), 37 | ||||
| Bone Area (Whole Body) | 32 | McKay. 2005 | 1232.00 (178.00), 51 | 1228.00 (153.00), 73 | 1308.70 (NR), 51 | 1323.60 (NR), 73 | ||||||||
| Bone Area (Femoral Neck) | 32 | McKay. 2005 | 4.00 (0.40), 51 | 4.00 (0.30), 73 | 4.15 (NR), 51 | 4.16 (NR), 73 | ||||||||
| Bone Area (Greater trochanter) | 32 | McKay. 2005 | 5.30 (1.20), 51 | 5.10 (1.10), 73 | 6.06 (NR), 51 | 5.83 (NR), 73 | ||||||||
| Bone Area (Inter-trochanteric) | 32 | McKay. 2005 | 13.95 (1.90), 51 | 13.60 (1.60), 73 | 15.15 (NR), 51 | 14.50 (NR), 73 | ||||||||
| Bone Area (Femur) | 32 | McKay. 2005 | 23.30 (3.00), 51 | 22.70 (2.60), 73 | 25.40 (NR), 51 | 24.50 (NR), 73 | ||||||||
| Bone Area (Lumbar Spine) | 32 | McKay. 2005 | 39.20 (4.50), 51 | 38.50 (4.40), 73 | 41.50 (NR), 51 | 40.90 (NR), 73 | ||||||||
| Bone Area (Forearm) | 156 | Hasselstrom. 2008 | 6.28 (0.71), 135 | 6.31 (0.72), 76 | 7.42 (0.82), 135 | 7.29 (0.84), 76 | 6.66 (0.68), 135 | 6.78 (0.59), 62 | 7.49 (0.82), 135 | 7.58 (0.84), 62 | ||||
| Bone Area (Calcaneus) | 156 | Hasselstrom. 2008 | 2.44 (0.64), 135 | 2.43 (0.54), 76 | 3.08 (0.44), 135 | 3.11 (0.43), 76 | 2.38 (0.67), 135 | 2.53 (0.61), 62 | 3.38 (0.40), 135 | 3.38 (0.44), 62 | ||||
| CSA (Femoral Neck) | 28 | Fuchs. 2001 | 2.99 (0.08), 45 | 2.89 (0.06), 44 | 3.13 (0.08), 45 | 2.96 (0.07), 44 | ||||||||
| CSA (Femoral Neck) | 52 | Alwis. 2008 | 1.02 (0.2), 80 | 1.0 (0.17), 57 | 1.10 (NR), 73 | 1.09 (NR), 48 | ||||||||
| CSA (Femoral Neck) | 416 | Coster. 2016 | 3.50 (0.40), 65 | 3.60 (0.50), 39 | 5.00 (0.40), 65 | 4.90 (0.60), 39 | 3.60 (0.40), 93 | 3.60 (0.30), 37 | 5.40 (0.50), 93 | 5.40 (0.60), 37 | ||||
| CSA (Lumbar Spine) | 28 | Fuchs. 2001 | 36.43 (0.67), 45 | 37.25 (0.68), 44 | 38.44 (0.68), 45 | 38.91 (0.66), 44 | ||||||||
| CSA (Lumbar Spine) | 416 | Coster. 2016 | 27.50 (3.20), 65 | 27.90 (3.60), 39 | 50.00 (5.20), 65 | 48.30 (5.50), 39 | 29.10 (3.50), 93 | 29.90 (3.40), 37 | 54.50 (7.80), 93 | 55.50 (7.10), 37 | ||||
| Width (L3) | 104 | Linden. 2006 | 2.86 (0.24), 49 | 2.91 (0.27), 50 | 3.14 (0.28), 49 | 3.08 (0.26), 50 | ||||||||
| Width (Femoral Neck) | 104 | Linden. 2006 | 2.43 (0.24), 49 | 2.48 (0.32), 50 | 2.76 (0.32), 49 | 2.67 (0.27), 50 | ||||||||
| aBMD (Whole Body) | 32 | McKay. 2005 | 0.78 (0.01), 63 | 0.81 (0.01), 81 | 0.81 (0.01), 63 | 0.82 (0.01), 81 | ||||||||
| aBMD (Whole Body) | 36 | Nogueira. 2014 | 0.71 (0.06), 12 | 0.73 (0.02), 6 | 0.76 (0.08), 12 | 0.74 (0.04), 6 | ||||||||
| aBMD (Whole Body) | 40 | Barbeau. 2007 | 0.89 (0.07), 118 | 0.88 (0.06), 83 | 0.94 (0.08), 118 | 0.92 (0.07), 83 | ||||||||
| aBMD (Whole Body) | 104 | Linden. 2006 | 0.84 (0.04), 49 | 0.84 (0.05), 50 | 0.90 (0.05), 49 | 0.89 (0.06), 50 | ||||||||
| aBMD (Whole Body) | 156 | Meyer. 2013 | 0.65 (0.10), 149 | 0.65 (0.11), 65 | 0.79 (0.13), 149 | 0.78 (0.13), 65 | ||||||||
| aBMD (Whole Body) | 364 | Fritz. 2016b | 0.68 (0.05), 72 | 0.68 (0.05), 45 | 0.98 (NR), 72 | 0.95 (NR), 45 | 0.69 (0.05), 100 | 0.70 (0.06), 47 | 0.98 (NR), 100 | 0.99 (NR), 47 | ||||
| aBMD (Whole Body) | 416 | Coster. 2016 | 0.68 (0.05), 65 | 0.68 (0.05), 39 | 1.02 (0.08), 65 | 0.98 (0.09), 39 | 0.69 (0.05), 93 | 0.7 (0.05), 37 | 1.03 (0.11), 93 | 1.05 (0.1), 37 | ||||
| aBMD (Forearm) | 156 | Hasselstrom. 2008 | 0.28 (0.03), 135 | 0.28 (0.03), 76 | 0.29 (0.03), 135 | 0.29 (0.04), 76 | 0.29 (0.03), 135 | 0.29 (0.03), 62 | 0.31 (0.04), 135 | 0.31 (0.04), 62 | ||||
| aBMD (Calcaneus) | 156 | Hasselstrom. 2008 | 0.32 (0.05), 135 | 0.32 (0.05), 76 | 0.41 (0.06), 135 | 0.41 (0.06), 76 | 0.32 (0.04), 135 | 0.31 (0.04), 62 | 0.41 (0.05), 135 | 0.4 (0.06), 62 | ||||
| aBMD (Leg) | 104 | Linden. 2006 | 0.76 (0.06), 49 | 0.76 (0.07), 50 | 0.88 (0.08), 49 | 0.85 (0.09), 50 | ||||||||
| aBMD (Femur) | 32 | McKay. 2005 | 0.63 (0.01), 63 | 0.66 (0.01), 81 | 0.66 (0.01), 63 | 0.68 (0.01), 81 | ||||||||
| aBMD (Greater Trochanter) | 32 | McKay. 2005 | 0.52 (0.01), 63 | 0.53 (0.01), 81 | 0.54 (0.01), 63 | 0.55 (0.01), 81 | ||||||||
| aBMD (Hip) | 156 | Meyer | 0.68 (0.09), 149 | 0.69 (0.10), 65 | 0.83 (0.15), 149 | 0.83 (0.14), 65 | ||||||||
| aBMD (Femoral Neck) | 28 | Fuchs. 2001 | 0.61 (0.01), 45 | 0.62 (0.01), 44 | 0.64 (0.01), 45 | 0.64 (0.01), 44 | ||||||||
| aBMD (Femoral Neck) | 32 | McKay. 2005 | 0.62 (0.01), 63 | 0.64 (0.01), 81 | 0.64 (0.01), 63 | 0.66 (0.01), 81 | ||||||||
| aBMD (Femoral Neck) | 36 | Nogueira. 2014 | 0.67 (0.08), 12 | 0.71 (0.06), 6 | 0.74 (0.10), 12 | 0.76 (0.08), 6 | ||||||||
| aBMD (Femoral Neck) | 104 | Linden. 2006 | 0.72 (0.09), 49 | 0.71 (0.10), 50 | 0.80 (0.08), 49 | 0.79 (0.09), 50 | ||||||||
| aBMD (Femoral Neck) | 156 | Meyer. 2013 | 0.65 (0.09), 149 | 0.66 (0.10), 65 | 0.78 (0.12), 149 | 0.78 (0.13), 65 | ||||||||
| aBMD (Femoral Neck) | 364 | Fritz. 2016b | 0.71 (0.09), 72 | 0.70 (0.09), 45 | 1.01 (NR), 72 | 0.96 (NR), 45 | 0.78 (0.11), 100 | 0.78 (0.12), 47 | 1.00 (NR), 100 | 1.01 (NR), 47 | ||||
| aBMD (Femoral Neck) | 364 | Fritz. 2016c | 0.75 (0.10), 172 | 0.74 (0.11), 92 | 1.00 (NR), 172 | 0.99 (NR), 92 | ||||||||
| aBMD (Femoral Neck) | 416 | Coster. 2016 | 0.71 (0.1), 65 | 0.70 (0.09), 39 | 1.04 (0.13), 65 | 0.98 (0.14), 39 | 0.78 (0.11), 93 | 0.79 (0.12), 37 | 1.03 (0.14), 93 | 1.07 (0.15), 37 | ||||
| aBMD (L3) | 104 | Linden. 2006 | 0.70 (0.08), 49 | 0.71 (0.08), 50 | 0.77 (0.09), 49 | 0.76 (0.09), 50 | ||||||||
| aBMD (Lumbar Spine) | 32 | McKay. 2005 | 0.57 (0.01), 63 | 0.58 (0.01) | 0.58 (0.01), 63 | 0.59 (0.01), 81 | ||||||||
| aBMD (Lumbar Spine) | 36 | Nogueira. 2014 | 0.65 (0.09), 12 | 0.69 (0.07), 6 | 0.72 (0.12), 12 | 0.73 (0.06), 6 | ||||||||
| aBMD (Lumbar Spine) | 156 | Meyer | 0.59 (0.10), 149 | 0.60 (0.11), 65 | 0.78 (0.16), 149 | 0.77 (0.17), 65 | ||||||||
| aBMD (Total Spine) | 28 | Fuchs. 2001 | 0.55 (0.01), 45 | 0.54 (0.01), 44 | 0.57 (0.01), 45 | 0.55 (0.01), 44 | ||||||||
| aBMD (Total Spine) | 104 | Linden. 2006 | 0.69 (0.08), 49 | 0.70 (0.08), 50 | 0.76 (0.09), 49 | 0.75 (0.08), 50 | ||||||||
| aBMD (Total Spine) | 364 | Fritz. 2016c | 0.68 (0.06), 72 | 0.69 (0.07), 45 | 0.99 (NR), 72 | 0.96 (NR), 45 | 0.68 (0.07), 100 | 0.69 (0.06), 47 | 0.93 (NR), 100 | 0.92 (NR), 47 | ||||
| aBMD (Total Spine) | 364 | Fritz. 2016c | 0.68 (0.06), 172 | 0.69 (0.06), 92 | 0.96 (NR), 172 | 0.94 (NR),92 | ||||||||
| aBMD (Total Spine) | 416 | Coster. 2016 | 0.68 (0.06), 65 | 0.69 (0.07), 39 | 1.05 (0.12), 65 | 0.99 (0.11), 39 | 0.68 (0.07), 93 | 0.70 (0.06), 37 | 0.98 (0.13), 93 | 1.00 (0.12), 37 | ||||
| Legend: INT = Intervention group, CON = Control group, BMC = Bone mineral Content, L3 = Third Lumbar Vertebrae, CSA = Cross-sectional Area, aBMD = areal bone mineral density. NR = not reported. Notes: Data from Detter. 2013, Detter. 2014, Gutin. 1999, Gutin. 2008, Larsen. 2016, Lofgren. 2011, MacKelvie. 2004, Meyer. 2011, Specker. 2004 and Staiano. 2018 were not included as they did not provide raw follow-up values. Data from Fuchs. 2002 was not presented as it represents a subsample of Fuchs 2001 at the same time-point. | ||||||||||||||
Appendix G
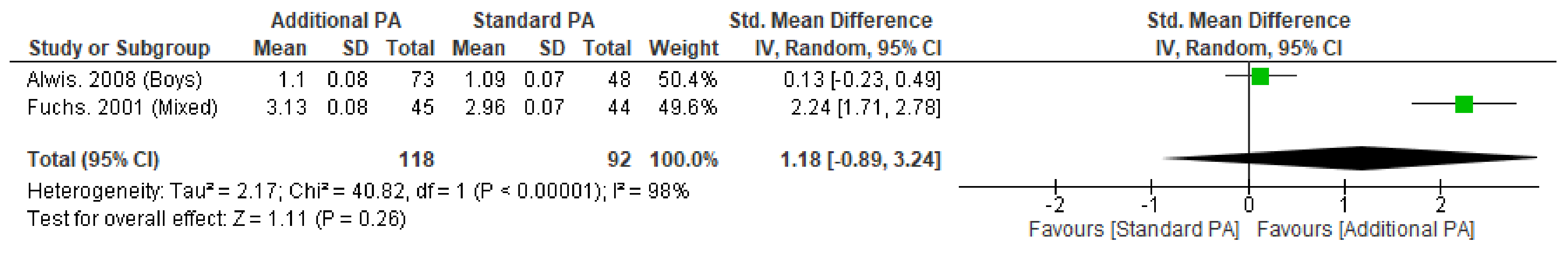
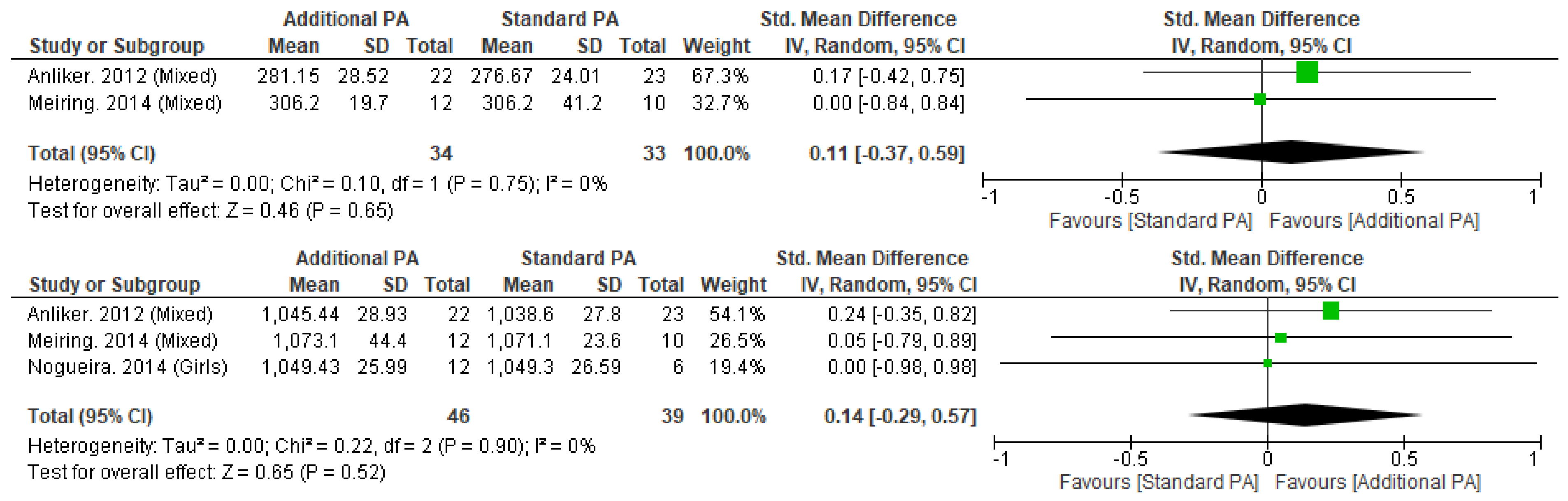
Appendix H. Peripheral Quantitative Computed Tomography
| Outcome | Timepoint (Weeks) | Study | Girls (Baseline)- Mean (SD), n | Girls (Follow-up)- Mean (SD), n | Boys (Baseline)- Mean (SD), n | Boys (Follow-up)- Mean (SD), n | All (Baseline)- Mean (SD), n | All (Follow-up)- Mean (SD), n | ||||||
| INT | CON | INT | CON | INT | CON | INT | CON | INT | CON | INT | CON | |||
| vBMC (Tibia 4%) | 36 | Anliker. 2012 | 2.05 (0.45), 22 | 2.19 (0.37), 23 | 2.26 (0.52), 22 | 2.35 (0.35), 23 | ||||||||
| vBMC (Tibia 4%) | 36 | Nogueira. 2014 | 176.16 (28.16), 12 | 199.38 (19.19), 6 | 186.28 (21.67), 12 | 197.21 (19.76), 6 | ||||||||
| vBMC (Tibia 14%) | 36 | Anliker. 2012 | 1.53 (0.35), 22 | 1.56 (0.20), 23 | 1.66 (0.36), 22 | 1.68 (0.24), 23 | ||||||||
| vBMC (Tibia 38%) | 36 | Anliker. 2012 | 2.10 (0.43), 22 | 2.19 (0.30), 23 | 2.27 (0.48), 22 | 2.35 (0.35), 23 | ||||||||
| vBMC (Tibia 66%) | 36 | Anliker. 2012 | 2.34 (0.49), 22 | 2.38 (0.49), 23 | 2.55 (0.53), 22 | 2.58 (0.41), 23 | ||||||||
| vBMC (Radius 4%) | 36 | Nogueira. 2014 | 41.20 (6.65), 12 | 48.40 (10.91), 6 | 52.66 (11.17), 12 | 50.41 (5.160), 6 | ||||||||
| vBMD (Tibia 4%) | 20 | Meiring. 2014 | 304.60 (22.10), 12 | 319.60 (46.70), 10 | 306.20 (19.70), 12 | 306.20 (41.20), 10 | ||||||||
| vBMD (Tibia 4%) | 36 | Anliker. 2012 | 277.33 (28.89), 22 | 275.76 (25.78), 23 | 281.15 (28.52), 22 | 276.67 (24.01), 23 | ||||||||
| vBMD (Tibia 8%) | 60 | Macdonald. 2007 | 298.20 (36.40), 126 | 292.80 (32.30), 63 | 310.30 (41.40), 126 | 300.90 (34.60), 136 | 303.50 (30.40), 136 | 311.60 (32.20), 60 | 305.60 (29.90), 136 | 309.80 (34.00), 60 | ||||
| vBMD (Tibia 14%) | 36 | Anliker. 2012 | 978.39 (23.97), 22 | 964.78 (24.70), 23 | 984.40 (23.97), 22 | 972.43 (28.16), 23 | ||||||||
| vBMD (Tibia 38%) | 20 | Meiring. 2014 | 1059.80 (51.40), 12 | 1071.10 (27.00), 10 | 1073.10 (44.40), 12 | 1071,10 (23.60), 10 | ||||||||
| vBMD (Tibia 38%) | 36 | Nogueira. 2014 | 1032.96 (34.67), 12 | 1046.33 (25.57), 6 | 1049.43 (25.99), 12 | 1049.30 (26.59), 6 | ||||||||
| vBMD (Tibia 38%) | 36 | Anliker. 2012 | 1041.94 (29.04), 22 | 1032.58 (26.22), 23 | 1045.44 (28.93), 22 | 1038.60 (27.80), 23 | ||||||||
| vBMD (Tibia 50%) | 60 | Macdonald. 2007 | 1057.20 (33.70), 134 | 1058.60 (29.90), 64 | 1073.40 (35.80), 134 | 1067.00 (32.80), 64 | 1047.50 (35.80), 142 | 1046.00 (30.60), 64 | 1047.30 (35.70), 142 | 1046.00 (35.80), 64 | ||||
| vBMD (Tibia 66%) | 36 | Anliker. 2012 | 1018.85 (22.48), 22 | 1006.00 (20.42), 23 | 1027.21 (24.82), 22 | 1012.78 (23.63), 23 | ||||||||
| vBMD (Tibia 66%) | 208 | Daly. 2016 | 1013.00 (34.00), 192 | 1016.00 (37.00), 170 | 1048.00 (NR), 94 | 1046.00 (NR), 69 | 1004.00 (36.00), 206 | 1015.00 (36.00), 159 | 1027.00 (NR), 97 | 1027.00 (NR), 76 | ||||
| vBMD (Radius 38%) | 36 | Nogueira 2014 | 992.41 (42.25), 12 | 1004.74 (34.50), 6 | 1019.50 (32.61), 12 | 1008.04 (25.94), 6 | ||||||||
| Trabecular BMD (Tibia 4%) | 20 | Meiring. 2014 | 270.20 (29.20), 12 | 291.40 (59.10), 10 | 277.20 (24.60), 12 | 264.10 (54.10), 10 | ||||||||
| Trabecular BMD (Tibia 4%) | 36 | Nogueira 2014 | 232.64 (26.05), 12 | 243.05 (10.15), 6 | 230.38 (26.63), 12 | 233.60 (10.74), 6 | ||||||||
| Trabecular BMD (Radius 4%) | 36 | Nogueira 2014 | 218.32 (29.85), 12 | 219.56 (19.12), 6 | 230.23 (22.75), 12 | 224.08 (23.39), 6 | ||||||||
| Total Bone CSA (Tibia 4%) | 20 | Meiring. 2014 | 802.00 (136.90), 12 | 738.50 (86.80), 10 | 847.80 (146.30), 12 | 741.30 (99.70), 10 | ||||||||
| Total Bone CSA (Tibia 4%) | 36 | Anliker. 2012 | 734.07 (128.55), 22 | 794.13 (126.25), 23 | 798.10 (137.15), 22 | 851.35 (115.98), 23 | ||||||||
| Total Bone CSA (Tibia 8%) | 60 | Macdonald. 2007 | 509.70 (74.70), 126 | 503.40 (80.00), 63 | 561.80 (77.60), 126 | 569.10 (90.90), 63 | 552.40 (88.20), 136 | 548.10 (88.10), 60 | 626.20 (100.60), 136 | 625.30 (94.70), 60 | ||||
| Total Bone CSA (Tibia 14%) | 36 | Anliker. 2012 | 327.57 (64.04), 22 | 353.48 (51.90), 23 | 356.60 (66.71), 22 | 382.02 (52.85), 23 | ||||||||
| Total Bone CSA (Tibia 38%) | 20 | Meiring. 2014 | 294.90 (48.80), 12 | 269.20 (19.00), 10 | 304.10 (48.20), 12 | 279.40 (21.60), 10 | ||||||||
| Total Bone CSA (Tibia 38%) | 36 | Anliker. 2012 | 278.57 (56.89), 22 | 286.37 (38.35), 23 | 297.59 (57.45), 22 | 304.75 (43.72), 23 | ||||||||
| Total Bone CSA (Tibia 50%) | 60 | Macdonald. 2007 | 322.10 (51.60), 134 | 312.40 (53.70), 64 | 352.40 (54.20), 134 | 345.70 (56.90), 64 | 335.50 (54.00), 142 | 340.50 (49.90), 64 | 374.10 (62.10), 142 | 376.80 (58.60), 64 | ||||
| Total Bone CSA (Tibia 66%) | 36 | Anliker. 2012 | 422.56 (98.46), 22 | 426.30 (53.77), 23 | 441.48 (102.22), 22 | 446.86 (61.95), 23 | ||||||||
| Total Bone CSA (Tibia 66%) | 208 | Daly. 2016 | 335.00 (51.00), 192 | 339.00 (58.00), 170 | 432.00 (NR), 94 | 459.00 (NR), 69 | 354.00 (61.00), 206 | 343.00 (58.00), 159 | 440.00 (NR), 97 | 450.00 (NR), 76 | ||||
| Total Cortical Area (Tibia 38%) | 20 | Meiring. 2014 | 165.70 (26.10), 12 | 160.90 (17.50), 10 | 170.20 (25.00), 12 | 170.10 (17.20), 10 | ||||||||
| Total Cortical Area (Tibia 50%) | 60 | Macdonald. 2007 | 211.00 (33.10), 134 | 205.20 (36.50), 64 | 236.50 (37.50), 134 | 232.20 (41.60), 64 | 219.10 (36.00), 142 | 221.40 (30.60), 64 | 249.30 (42.80), 142 | 249.10 (36.60), 64 | ||||
| Total Cortical Area (Tibia 66%) | 208 | Daly. 2016 | 150.00 (20.00), 192 | 154.00 (21.00), 170 | 232.00 (NR), 94 | 228.00 (NR), 69 | 145.00 (22.00), 206 | 154.00 (22.00), 159 | 237.00 (NR), 97 | 232.00 (NR), 76 | ||||
| Total Cortical Thickness (Tibia 38%) | 20 | Meiring. 2014 | 3.40 (0.20), 12 | 3.30 (0.10), 10 | 3.40 (0.30), 12 | 3.40 (0.10), 10 | ||||||||
| Total Cortical Thickness (Tibia 66%) | 208 | Daly. 2016 | 2.69 (0.37), 192 | 2.75 (0.33), 170 | 3.94 (NR), 94 | 3.75 (NR), 69 | 2.71 (0.41), 206 | 2.72 (0.41), 159 | 4.00 (NR), 97 | 3.83 (NR), 76 | ||||
| SSIPol (Tibia 14%) | 36 | Anliker. 2012 | 781.82 (248.63), 22 | 827.60 (161.14), 23 | 892.35 (270.53), 22 | 933.32 (190.72), 23 | ||||||||
| SSIPol (Tibia 38%) | 20 | Meiring. 2014 | 840.90 (180.70), 12 | 761.60 (77.30), 10 | 888.70 (187.10), 12 | 808.50 (99.00), 10 | ||||||||
| SSIPol (Tibia 38%) | 36 | Anliker. 2012 | 819.54 (239.80), 22 | 859.78 (174.23), 23 | 921.72 (269.10), 22 | 950.26 (205.84), 23 | ||||||||
| SSIPol (Tibia 50%) | 60 | Macdonald. 2007 | 954.40 (218.80), 134 | 915.50 (221.30), 64 | 1124.30 (251.50), 134 | 1093.50 (264.50), 64 | 1006.10 (241.40), 142 | 1029.80 (211.60), 64 | 1204.20 (295.50), 142 | 1208.00 (250.40), 64 | ||||
| SSIPol (Tibia 66%) | 36 | Anliker. 2012 | 1290.15 (421.74), 22 | 1282.57 (251.26), 23 | 1419.45 (481.47), 22 | 1417.32 (303.63), 23 | ||||||||
| SSIPol (Tibia 66%) | 208 | Daly. 2016 | 905.00 (170.00), 192 | 943.00 (204.00), 170 | 1454.00 (NR), 94 | 1543.00 (NR), 69 | 974.00 (210.00), 206 | 937.00 (200.00), 159 | 1481.00 (NR), 97 | 1495.00 (NR), 76 | ||||
| Bone Strength Index (Tibia 4%) | 20 | Meiring. 2014 | 7503.60 (1753.10), 12 | 7685.40 (2470.60), 10 | 7978.20 (1688.10), 12 | 7095.60 (2270.70), 10 | ||||||||
| Bone Strength Index (Tibia 8%) | 60 | Macdonald. 2007 | 4562.30 (1178.70), 126 | 4351.20 (1136.00), 63 | 5470.20 (1524.40), 126 | 5193.20 (1383.60), 63 | 5087.50 (1074.20), 136 | 5322.70 (1136.70), 60 | 5855.50 (1254.50), 136 | 6005.40 (1290.10), 60 | ||||
| Periosteal Circumference (Tibia 38%) | 20 | Meiring. 2014 | 59.40 (3.30), 12 | 59.60 (1.80), 10 | 60.70 (3.80), 12 | 60.20 (1.60), 10 | ||||||||
| Endosteal Circumference (Tibia 38%) | 20 | Meiring. 2014 | 38.30 (2.70), 12 | 38.80 (1.10), 10 | 39.10 (3.00), 12 | 39.20 (1.00), 10 | ||||||||
Appendix I

Appendix J

Appendix K

Appendix L
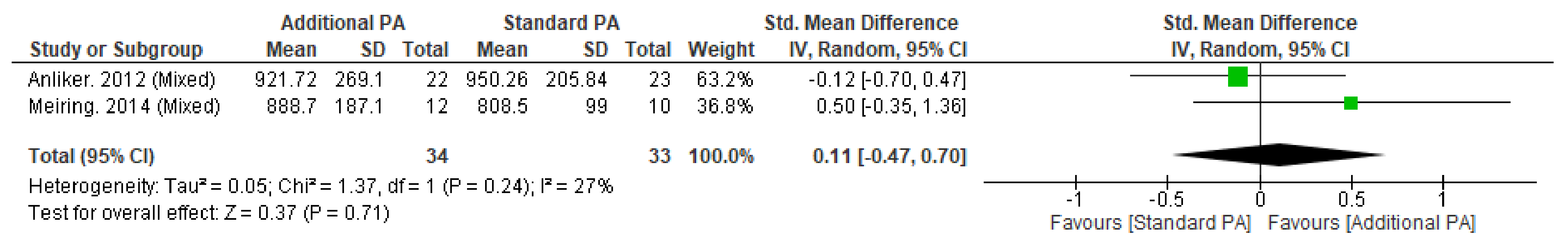
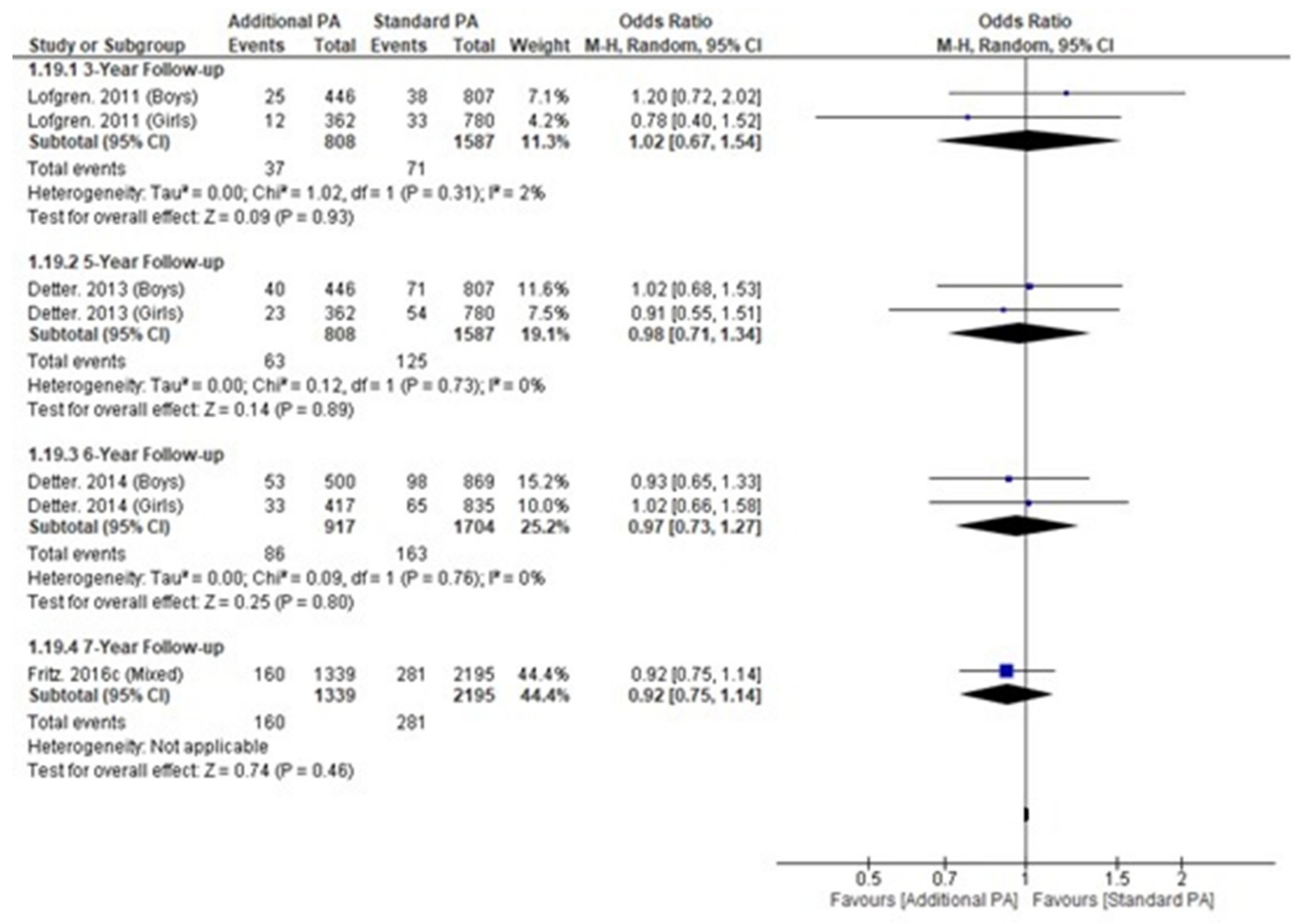
Appendix M. Fracture Incidence within the Malmo POP Trial
| Timepoint (Years) | Study | Girls (INT) | Girls (CONT) | Boys (INT) | Boys (CONT) | All (INT) | All (CONT) | ||||||
| Fractures (n) | Sample (n) | Fractures (n) | Sample (n) | Fractures (n) | Sample (n) | Fractures (n) | Sample (n) | Fractures (n) | Sample (n) | Fractures (n) | Sample (n) | ||
| 3 | Lofgren. 2011 | 12 | 362 | 33 | 780 | 25 | 446 | 38 | 807 | ||||
| 5 | Detter. 2013 | 23 | 362 | 54 | 780 | 40 | 446 | 71 | 807 | ||||
| 6 | Detter. 2014 | 33 | 417 | 65 | 835 | 53 | 500 | 98 | 869 | ||||
| 7 | Fritz. 2016c | 160 | 1339 | 281 | 2195 | ||||||||
Appendix N. Risk of Bias Assessment
| Study | Risk of Bias Arising from the Randomisation Process | Risk of Bias Arising from the Timing of Identification or Recruitment of Participants in a Cluster-Randomised Trial | Risk of Bias Due to Deviations from the Intended Interventions (Effect of Assignment to Intervention) | Risk of Bias Due to Deviations from the Intended Interventions (Effect of Adhering to Intervention) | Risk of Bias Due to Missing Outcome Data | Risk of Bias in Measurement of the Outcome | Risk of Bias in Selection of the Reported Result | Overall Risk of Bias |
| Alwis. 2008 | High | Low | High | High | Low | Low | Low | High |
| Anliker. 2012 | Low | Low | High | High | Low | Low | Low | High |
| Barbeau. 2007 | Low | Low | High | High | Low | Low | Low | High |
| Daly. 2016 | Low | Low | High | Low | Low | Low | Low | High |
| Fuchs. 2001 | Low | Low | High | High | Low | Low | Low | High |
| Gutin. 2008 | Low | Low | High | High | Low | Low | Low | High |
| Hasselstrom. 2008 | High | Low | High | High | Low | Low | Low | High |
| Larsen. 2016 | Low | Low | High | High | Low | Low | Low | High |
| Macdonald. 2007 | Low | Low | High | High | Low | Low | Low | High |
| MacKelvie. 2001 | Low | Low | High | High | Low | Low | Low | High |
| McKay. 2000 | Low | Low | High | High | Low | Low | Low | High |
| Meiring. 2014 | Low | Low | High | High | Low | Low | Low | High |
| Meyer. 2011 | Low | Low | High | High | Low | Low | Low | High |
| Nogueira. 2014 | Low | Low | High | High | High | Low | Low | High |
| Specker. 2004 | Low | Low | High | High | Low | Low | Low | High |
| Staiano. 2018 | Low | Low | High | High | Low | Low | Low | High |
| Legend: INT = Intervention group, CON = Control group, vBMC = Volumetric bone mineral Content, CSA = Cross-sectional Area, vBMD = volumetric bone mineral, density, SSIPol = polar stress strain index. | ||||||||
References
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed]
- Gunter, K.B.; Almstedt, H.C.; Janz, K.F. Physical activity in childhood may be the key to optimizing lifespan skeletal health. Exerc. Sport Sci. Rev. 2012, 40, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Behringer, M.; Gruetzner, S.; McCourt, M.; Mester, J. Effects of weight-bearing activities on bone mineral content and density in children and adolescents: A meta-analysis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014, 29, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Nikander, R.; Sievänen, H.; Heinonen, A.; Daly, R.M.; Uusi-Rasi, K.; Kannus, P. Targeted exercise against osteoporosis: A systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010, 8, 47. [Google Scholar] [CrossRef]
- Nogueira, R.C.; Weeks, B.K.; Beck, B.R. Exercise to improve pediatric bone and fat: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 610–621. [Google Scholar] [CrossRef]
- Tan, V.P.; Macdonald, H.M.; Kim, S.; Nettlefold, L.; Gabel, L.; Ashe, M.C.; McKay, H.A. Influence of physical activity on bone strength in children and adolescents: A systematic review and narrative synthesis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014, 29, 2161–2181. [Google Scholar] [CrossRef]
- Golden, N.H.; Abrams, S.A.; Nutrition, C.O.; Daniels, S.R.; Abrams, S.A.; Corkins, M.R.; de Ferranti, S.D.; Golden, N.H.; Magge, S.N.; Schwarzenberg, S.J. Optimizing Bone Health in Children and Adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef]
- Hart, N.H.; Newton, R.U.; Tan, J.; Rantalainen, T.; Chivers, P.; Siafarikas, A.; Nimphius, S. Biological basis of bone strength: Anatomy, physiology and measurement. J. Musculoskelet. Neuronal Interact. 2020, 20, 347–371. [Google Scholar]
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Australian Institute of Health and Welfare. Australia’s Children; Australian Institute of Health and Welfare: Canberra, Australia, 2020. [Google Scholar]
- Jenkins, M.; Nimphius, S.; Hart, N.H.; Chivers, P.; Rantalainen, T.; Rueter, K.; Borland, M.L.; McIntyre, F.; Stannage, K.; Siafarikas, A. Appendicular fracture epidemiology of children and adolescents: A 10-year case review in Western Australia (2005 to 2015). Arch Osteoporos 2018, 13, 63. [Google Scholar] [CrossRef]
- Ireland, A.; Rittweger, J. Exercise for osteoporosis: How to navigate between overeagerness and defeatism. J. Musculoskelet. Neuronal Interact. 2017, 17, 155–161. [Google Scholar]
- Macdonald, H.M.; Kontulainen, S.A.; Khan, K.M.; McKay, H.A. Is a school-based physical activity intervention effective for increasing tibial bone strength in boys and girls? J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2007, 22, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, H.M.; Kontulainen, S.A.; Petit, M.A.; Beck, T.J.; Khan, K.M.; McKay, H.A. Does a novel school-based physical activity model benefit femoral neck bone strength in pre- and early pubertal children? Osteoporos. Int. 2008, 19, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Alwis, G.; Linden, C.; Stenevi-Lundgren, S.; Ahlborg, H.G.; Besjakov, J.; Gardsell, P.; Karlsson, M.K. A one-year exercise intervention program in pre-pubertal girls does not influence hip structure. BMC Musculoskelet. Disord. 2008, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Coster, M.E.; Rosengren, B.E.; Karlsson, C.; Dencker, M.; Karlsson, M.K. Effects of an 8-year childhood physical activity intervention on musculoskeletal gains and fracture risk. Bone 2016, 93, 139–145. [Google Scholar] [CrossRef]
- Detter, F.; Rosengren, B.E.; Dencker, M.; Lorentzon, M.; Nilsson, J.; Karlsson, M.K. A 6-year exercise program improves skeletal traits without affecting fracture risk: A prospective controlled study in 2621 children. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2014, 29, 1325–1336. [Google Scholar] [CrossRef]
- Detter, F.T.; Rosengren, B.E.; Dencker, M.; Nilsson, J.; Karlsson, M.K. A 5-year exercise program in pre- and peripubertal children improves bone mass and bone size without affecting fracture risk. Calcif. Tissue Int. 2013, 92, 385–393. [Google Scholar] [CrossRef]
- Fritz, J.; Cöster, M.E.; Nilsson, J.; Rosengren, B.E.; Dencker, M.; Karlsson, M.K. The associations of physical activity with fracture risk--a 7-year prospective controlled intervention study in 3534 children. Osteoporos. Int. 2016, 27, 915–922. [Google Scholar] [CrossRef]
- Fritz, J.; Cöster, M.E.; Stenevi-Lundgren, S.; Nilsson, J.; Dencker, M.; Rosengren, B.E.; Karlsson, M.K. A 5-year exercise program in children improves muscle strength without affecting fracture risk. Eur. J. Appl. Physiol. 2016, 116, 707–715. [Google Scholar] [CrossRef]
- Fritz, J.; Rosengren, B.E.; Dencker, M.; Karlsson, C.; Karlsson, M.K. A seven-year physical activity intervention for children increased gains in bone mass and muscle strength. Acta Paediatr. 2016, 105, 1216–1224. [Google Scholar] [CrossRef]
- Linden, C.; Ahlborg, H.G.; Besjakov, J.; Gardsell, P.; Karlsson, M.K. A school curriculum-based exercise program increases bone mineral accrual and bone size in prepubertal girls: Two-year data from the pediatric osteoporosis prevention (POP) study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2006, 21, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Löfgren, B.; Detter, F.; Dencker, M.; Stenevi-Lundgren, S.; Nilsson, J.; Karlsson, M.K. Influence of a 3-year exercise intervention program on fracture risk, bone mass, and bone size in prepubertal children. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2011, 26, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Mackelvie, K.J.; McKay, H.A.; Khan, K.M.; Crocker, P.R. A school-based exercise intervention augments bone mineral accrual in early pubertal girls. J. Pediatr. 2001, 139, 501–508. [Google Scholar] [CrossRef] [PubMed]
- MacKelvie, K.J.; Petit, M.A.; Khan, K.M.; Beck, T.J.; McKay, H.A. Bone mass and structure are enhanced following a 2-year randomized controlled trial of exercise in prepubertal boys. Bone 2004, 34, 755–764. [Google Scholar] [CrossRef] [PubMed]
- McKay, H.A.; MacLean, L.; Petit, M.; MacKelvie-O’Brien, K.; Janssen, P.; Beck, T.; Khan, K.M. ‘Bounce at the Bell’: A novel program of short bouts of exercise improves proximal femur bone mass in early pubertal children. Br. J. Sports Med. 2005, 39, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, R.K.; Bauer, J.J.; Snow, C.M. Jumping improves hip and lumbar spine bone mass in prepubescent children: A randomized controlled trial. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2001, 16, 148–156. [Google Scholar] [CrossRef]
- Fuchs, R.K.; Snow, C.M. Gains in hip bone mass from high-impact training are maintained: A randomized controlled trial in children. J. Pediatr. 2002, 141, 357–362. [Google Scholar] [CrossRef]
- Gunter, K.; Baxter-Jones, A.D.; Mirwald, R.L.; Almstedt, H.; Fuchs, R.K.; Durski, S.; Snow, C. Impact exercise increases BMC during growth: An 8-year longitudinal study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2008, 23, 986–993. [Google Scholar] [CrossRef]
- Meyer, U.; Ernst, D.; Zahner, L.; Schindler, C.; Puder, J.J.; Kraenzlin, M.; Rizzoli, R.; Kriemler, S. 3-Year follow-up results of bone mineral content and density after a school-based physical activity randomized intervention trial. Bone 2013, 55, 16–22. [Google Scholar] [CrossRef]
- Meyer, U.; Romann, M.; Zahner, L.; Schindler, C.; Puder, J.J.; Kraenzlin, M.; Rizzoli, R.; Kriemler, S. Effect of a general school-based physical activity intervention on bone mineral content and density: A cluster-randomized controlled trial. Bone 2011, 48, 792–797. [Google Scholar] [CrossRef]
- Gutin, B.; Owens, S.; Okuyama, T.; Riggs, S.; Ferguson, M.; Litaker, M. Effect of physical training and its cessation on percent fat and bone density of children with obesity. Obes. Res. 1999, 7, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Gutin, B.; Yin, Z.; Johnson, M.; Barbeau, P. Preliminary findings of the effect of a 3-year after-school physical activity intervention on fitness and body fat: The Medical College of Georgia Fitkid Project. Int. J. Pediatr. Obes. 2008, 3 (Suppl. 1), 3–9. [Google Scholar] [CrossRef]
- Larsen, M.N.; Nielsen, C.M.; Helge, E.W.; Madsen, M.; Manniche, V.; Hansen, L.; Hansen, P.R.; Bangsbo, J.; Krustrup, P. Positive effects on bone mineralisation and muscular fitness after 10 months of intense school-based physical training for children aged 8-10 years: The FIT FIRST randomised controlled trial. Br. J. Sports Med. 2018, 52, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Specker, B.; Binkley, T. Increased periosteal circumference remains present 12 months after an exercise intervention in preschool children. Bone 2004, 35, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- Staiano, A.E.; Beyl, R.A.; Guan, W.; Hendrick, C.A.; Hsia, D.S.; Newton, R.L., Jr. Home-based exergaming among children with overweight and obesity: A randomized clinical trial. Pediatr. Obes. 2018, 13, 724–733. [Google Scholar] [CrossRef]
- The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Eds.; Cochrane: Chichester, UK, 2022; Available online: https://training.cochrane.org/handbook/current (accessed on 1 May 2022).
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348, g1687. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Murphy, M.; Travers, M.J.; Gibson, W.; Chivers, P.; Debenham, J.; Docking, S.; Rio, E. The rate of improvement of pain and function in mid-portion Achilles tendinopathy with loading protocols: A Systematic Review and Longitudinal Meta-Analysis. Sports Med. 2018, 48, 1875–1891. [Google Scholar] [CrossRef]
- Murphy, M.C.; Travers, M.J.; Chivers, P.; Debenham, J.R.; Docking, S.I.; Rio, E.K.; Gibson, W. Efficacy of heavy eccentric calf training for treating mid-portion Achilles tendinopathy: A systematic review and meta-analysis. Br. J. Sports Med. 2019, 53, 1070–1077. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Dechartres, A.; Trinquart, L.; Boutron, I.; Ravaud, P. Influence of trial sample size on treatment effect estimates: Meta-epidemiological study. BMJ 2013, 346, f2304. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.A.; Dempsey, A.R.; Mackie, K.; King, D.; Hecimovich, M.; Murphy, M.C. Do Sideline Tests of Vestibular and Oculomotor Function Accurately Diagnose Sports-Related Concussion in Adults? A Systematic Review and Meta-analysis. Am. J. Sports Med. 2021, 50, 3635465211027946. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.; George, H.; Naqi, M.; Owen, P.; Chivers, P.; Hart, N.H. Musculoskeletal injury epidemiology in law enforcement and firefighter recruits during physical training: A systematic review. BMJ Open Sport Exerc. Med. 2022. epub ahead of print. [Google Scholar] [CrossRef]
- GRADE Working Group. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach; Schünemann, H., Brożek, J., Guyatt, G., Oxman, A., Eds.; GRADE: Hamilton, ON, USA, 2013; Available online: https://gradepro.org/ (accessed on 1 May 2022).
- Slade, S.C.; Dionne, C.E.; Underwood, M.; Buchbinder, R. Consensus on Exercise Reporting Template (CERT): Explanation and Elaboration Statement. Br. J. Sports Med. 2016, 50, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Anliker, E.; Dick, C.; Rawer, R.; Toigo, M. Effects of jumping exercise on maximum ground reaction force and bone in 8- to 12-year-old boys and girls: A 9-month randomized controlled trial. J. Musculoskelet. Neuronal Interact. 2012, 12, 56–67. [Google Scholar]
- Barbeau, P.; Johnson, M.H.; Howe, C.A.; Allison, J.; Davis, C.L.; Gutin, B.; Lemmon, C.R. Ten months of exercise improves general and visceral adiposity, bone, and fitness in black girls. Obesity 2007, 15, 2077–2085. [Google Scholar] [CrossRef]
- Daly, R.M.; Ducher, G.; Hill, B.; Telford, R.M.; Eser, P.; Naughton, G.; Seibel, M.J.; Telford, R.D. Effects of a Specialist-Led, School Physical Education Program on Bone Mass, Structure, and Strength in Primary School Children: A 4-Year Cluster Randomized Controlled Trial. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016, 31, 289–298. [Google Scholar] [CrossRef]
- Hasselstrøm, H.A.; Karlsson, M.K.; Hansen, S.E.; Grønfeldt, V.; Froberg, K.; Andersen, L.B. A 3-year physical activity intervention program increases the gain in bone mineral and bone width in prepubertal girls but not boys: The prospective copenhagen school child interventions study (CoSCIS). Calcif. Tissue Int. 2008, 83, 243–250. [Google Scholar] [CrossRef]
- McKay, H.A.; Petit, M.A.; Schutz, R.W.; Prior, J.C.; Barr, S.I.; Khan, K.M. Augmented trochanteric bone mineral density after modified physical education classes: A randomized school-based exercise intervention study in prepubescent and early pubescent children. J. Pediatr. 2000, 136, 156–162. [Google Scholar] [CrossRef]
- Meiring, R.M.; Micklesfield, L.K.; Avidon, I.; McVeigh, J.A. Osteogenic effects of a physical activity intervention in South African black children. J. Musculoskelet. Neuronal Interact. 2014, 14, 276–285. [Google Scholar]
- Nogueira, R.C.; Weeks, B.K.; Beck, B.R. An in-school exercise intervention to enhance bone and reduce fat in girls: The CAPO Kids trial. Bone 2014, 68, 92–99. [Google Scholar] [CrossRef] [PubMed]
- McCaskie, C.J.; Sim, M.; Newton, R.U.; Heasman, J.; Rogalski, B.; Hart, N.H. Characterising lower-body musculoskeletal morphology and whole-body composition of elite female and male Australian Football players. BMC Sports Sci. Med. Rehabil. 2022, 14, 168. [Google Scholar] [CrossRef]
- Hui, S.L.; Gao, S.; Zhou, X.H.; Johnston, C.C., Jr.; Lu, Y.; Glüer, C.C.; Grampp, S.; Genant, H. Universal standardization of bone density measurements: A method with optimal properties for calibration among several instruments. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 1997, 12, 1463–1470. [Google Scholar] [CrossRef]
- Hart, N.H.; Nimphius, S.; Weber, J.; Spiteri, T.; Rantalainen, T.; Dobbin, M.; Newton, R.U. Musculoskeletal Asymmetry in Football Athletes: A Product of Limb Function over Time. Med. Sci. Sports Exerc. 2016, 48, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.H.; Hung, V.W.; Cheuk, K.Y.; Chau, W.W.; Tsoi, K.K.; Wong, R.M.; Chow, S.K.; Lam, T.P.; Yung, P.S.; Law, S.W.; et al. Best Performance Parameters of HR-pQCT to Predict Fragility Fracture: Systematic Review and Meta-Analysis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2021, 36, 2381–2398. [Google Scholar] [CrossRef] [PubMed]
- Mikolajewicz, N.; Bishop, N.; Burghardt, A.J.; Folkestad, L.; Hall, A.; Kozloff, K.M.; Lukey, P.T.; Molloy-Bland, M.; Morin, S.N.; Offiah, A.C.; et al. HR-pQCT Measures of Bone Microarchitecture Predict Fracture: Systematic Review and Meta-Analysis. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2020, 35, 446–459. [Google Scholar] [CrossRef]
- Travers, M.J.; Murphy, M.C.; Debenham, J.R.; Chivers, P.; Bulsara, M.K.; Bagg, M.K.; Palsson, T.S.; Gibson, W. Should this systematic review and meta-analysis change my practice? Part 1: Exploring treatment effect and trustworthiness. Br. J. Sports Med. 2019, 53, 1488–1492. [Google Scholar] [CrossRef]
- Travers, M.J.; Murphy, M.C.; Debenham, J.R.; Chivers, P.; Bulsara, M.K.; Bagg, M.K.; Palsson, T.S.; Gibson, W. Should this systematic review and meta-analysis change my practice? Part 2: Exploring the role of the comparator, diversity, risk of bias and confidence. Br. J. Sports Med. 2019, 53, 1493–1497. [Google Scholar] [CrossRef]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- Micheli, L.J.; Fehlandt, A.F., Jr. Overuse injuries to tendons and apophyses in children and adolescents. Clin. Sports Med. 1992, 11, 713–726. [Google Scholar] [CrossRef]


| Study | Location | Design | Population | Sample Size (n) | Ages | Intervention Type | Group | Age, Mean (SD) Years | Height, Mean (SD) cm | Weight, Mean (SD) kg |
|---|---|---|---|---|---|---|---|---|---|---|
| Alwis. 2008 | Sweden | Cluster RCT | Healthy | 103 | Children | Specific impact loading | INT | 7.8 (0.6) | 129.4 (6.5) | 28.4 (5.8) |
| Standard physical activity | CON | 8.0 (0.6) | 130.1 (6.7) | 27.6 (5.4) | ||||||
| Anliker. 2012 | Switzerland | Cluster RCT | Healthy | 45 | Children | Specific impact loading | INT | 10.5 (1.2) | 140 (12.0) | 34.6 (7.7) |
| Standard physical activity | CON | 10.8 (1.1) | 143 (7.0) | 34 (5.7) | ||||||
| Barbeau. 2007 | United States of America | RCT | Healthy | 201 | Children | General Physical Activity | INT | 9.5 (NR) | NR | NR |
| Standard physical activity | CON | 9.5 (NR) | NR | NR | ||||||
| Daly. 2016 | Australia | Cluster RCT | Healthy | 727 | Children | General Physical Activity | INT (Girls) | 8.1 (0.3) | 128.4 (5.5) | 28.6 (5.9) |
| General Physical Activity | INT (Boys) | 8.1 (0.4) | 130.4 (5.5) | 28.9 (5.2) | ||||||
| Standard physical activity | CON (Girls) | 8.1 (0.4) | 129 (5.3) | 28.9 (5.7) | ||||||
| Standard physical activity | CON (Boys) | 8.2 (0.3) | 129.9 (5.8) | 28.7 (5.3) | ||||||
| Fuchs. 2001 | United States of America | RCT | Healthy | 89 | Children | Specific impact loading | INT | 7.5 (0.2) | 125.1 (1.3) | 27.1 (0.8) |
| Standard physical activity | CON | 7.6 (0.2) | 126.8 (1.2) | 28.0 (1.0) | ||||||
| Gutin. 2008 | United States of America | Cluster RCT | Healthy | 617 | Children | General Physical Activity | INT | NR | NR | NR |
| Standard physical activity | CON | NR | NR | NR | ||||||
| Hasselstrom. 2008 | Denmark | Non-RCT | Healthy | 704 | Children | General Physical Activity | INT (Girls) | 6.7 (0.3) | 121.5 (4.9) | 23.6 (3.2) |
| General Physical Activity | INT (Boys) | 6.8 (0.4) | 124.2 (4.5) | 24.4 (3.0) | ||||||
| Standard physical activity | CON (Girls) | 6.7 (0.4) | 122.5 (4.6) | 23.9 (3.8) | ||||||
| Standard physical activity | CON (Boys) | 6.8 (0.4) | 123.6 (5.2) | 24.7 (3.6) | ||||||
| Larsen. 2016 | Denmark | Cluster RCT | Healthy | 295 | Children | Team Impact Sport | INT A (Girls) | 9.3 (0.3) | 137.6 (7.1) | 33.0 (8.2) |
| Team Impact Sport | INT A (Boys) | 9.3 (0.4) | 139.2 (6.4) | 32.6 (5.4) | ||||||
| Specific impact loading | INT B (Girls) | 9.3 (0.3) | 136 (5.7) | 32.2 (6.7) | ||||||
| Specific impact loading | INT B (Boys) | 9.2 (0.4) | 138.7 (5.5) | 32.2 (7.3) | ||||||
| Standard physical activity | CON (Girls) | 9.4 (0.3) | 138.7 (6.5) | 33.5 (6.7) | ||||||
| Standard physical activity | CON (Boys) | 9.3 (0.3) | 137.9 (5.3) | 31.7 (5.2) | ||||||
| Macdonald. 2007 | Canada | Cluster RCT | Healthy | 410 | Children | Specific impact loading | INT (Girls) | 10.2 (0.6) | 141.5 (7.5) | 36.3 (8.4) |
| Specific impact loading | INT (Boys) | 10.2 (0.6) | 141.5 (7.2) | 37.2 (9.3) | ||||||
| Standard physical activity | CON (Girls) | 10.3 (0.5) | 140.2 (7.5) | 35.2 (8.7) | ||||||
| Standard physical activity | CON (Boys) | 10.3 (0.6) | 141.2 (6.8) | 39.7 (9.6) | ||||||
| MacKelvie. 2001 | Canada | Cluster RCT | Healthy | 198 | Children | Specific impact loading | INT (Pre-pubertal Girls) | 10.0 (0.6) | 138.6 (7.6) | 31.2 (6.1) |
| Specific impact loading | INT (Early-pubertal Girls) | 10.4 (0.7) | 143.8 (7.7) | 39.1 (8.3) | ||||||
| Specific impact loading | INT (Boys) | 10.2 (0.6) | 140.6 (6.0) | 35.5 (8.3) | ||||||
| Standard physical activity | CON (Pre-pubertal Girls) | 10.1 (0.5) | 137.3 (6.2) | 31.1 (5.6) | ||||||
| Standard physical activity | CON (Early-pubertal Girls) | 10.5 (0.6) | 145.6 (6.4) | 41.3 (8.3) | ||||||
| Standard physical activity | CON (Boys) | 10.3 (0.7) | 141.8 (7.1) | 36.6 (10.1) | ||||||
| McKay. 2000 | Canada | Cluster RCT | Healthy | 144 | Children | Specific impact loading | INT | NR | 133.9 (0.7) | 30.5 (0.8) |
| Standard physical activity | CON | NR | 135.1 (1.1) | 30.8 (1.0) | ||||||
| Meiring. 2014 | South Africa | Cluster RCT | Healthy | 22 | Children | Standard physical activity | CON | 9.3 (0.9) | 135.1 (8.2) | 30.6 (4.7) |
| Specific impact loading | INT | 9.7 (1.2) | 135.9 (8.7) | 30.0 (5.1) | ||||||
| Meyer. 2011 | Switzerland | Cluster RCT | Healthy | 291 | Children | Specific impact loading | INT | 8.8 (2.1) | 133.3 (13.1) | 30.7 (8.7) |
| Standard physical activity | CON | 8.8 (2.2) | 134.2 (14.2) | 30.4 (9.8) | ||||||
| Nogueira. 2014 | Australia | Cluster RCT | Healthy | 138 | Children | Specific impact loading | INT | 10.5 (0.6) | 144.2 (6.7) | 39.3 (9.4) |
| Standard physical activity | CON | 10.7 (0.6) | 142.5 (7.1) | 37.2 (7.2) | ||||||
| Specker. 2004 | United States of America | RCT | Healthy | 161 | Preschool Children | Specific impact loading | INT | 3.8 (0.5) | 100.6 (6.1) | 16.3 (2.2) |
| Standard physical activity | CON | 4.0 (0.6) | 102.4 (5.4) | 16.9 (2.3) | ||||||
| Staiano. 2018 | United States of America | RCT | Overweight/Obese | 46 | Children | Specific impact loading | INT | NR | NR | NR |
| Standard physical activity | CON | NR | NR | NR | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCaskie, C.; Siafarikas, A.; Cochrane Wilkie, J.; Sutton, V.; Chivers, P.; Hart, N.H.; Murphy, M.C. The Benefits to Bone Health in Children and Pre-School Children with Additional Exercise Interventions: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 127. https://doi.org/10.3390/nu15010127
McCaskie C, Siafarikas A, Cochrane Wilkie J, Sutton V, Chivers P, Hart NH, Murphy MC. The Benefits to Bone Health in Children and Pre-School Children with Additional Exercise Interventions: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(1):127. https://doi.org/10.3390/nu15010127
Chicago/Turabian StyleMcCaskie, Callum, Aris Siafarikas, Jodie Cochrane Wilkie, Vanessa Sutton, Paola Chivers, Nicolas H. Hart, and Myles C. Murphy. 2023. "The Benefits to Bone Health in Children and Pre-School Children with Additional Exercise Interventions: A Systematic Review and Meta-Analysis" Nutrients 15, no. 1: 127. https://doi.org/10.3390/nu15010127
APA StyleMcCaskie, C., Siafarikas, A., Cochrane Wilkie, J., Sutton, V., Chivers, P., Hart, N. H., & Murphy, M. C. (2023). The Benefits to Bone Health in Children and Pre-School Children with Additional Exercise Interventions: A Systematic Review and Meta-Analysis. Nutrients, 15(1), 127. https://doi.org/10.3390/nu15010127








