Bioactive Substances of Potato Juice Reveal Synergy in Cytotoxic Activity against Cancer Cells of Digestive System Studied In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Digestion Process
2.3. GAs Content
2.4. Polyphenols Profile
2.5. In Vitro Study
2.5.1. Cell Cultures
2.5.2. Cytotoxicity Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Cytotoxicity of Individual Bioactive Compounds
3.2. Changes in Bioaccessibility of Bioactive PJ Constituents during Gastrointestinal Digestion
3.3. Cytotoxicity of Gastrointestinally-Digested Potato Juice and Products of Its Processing
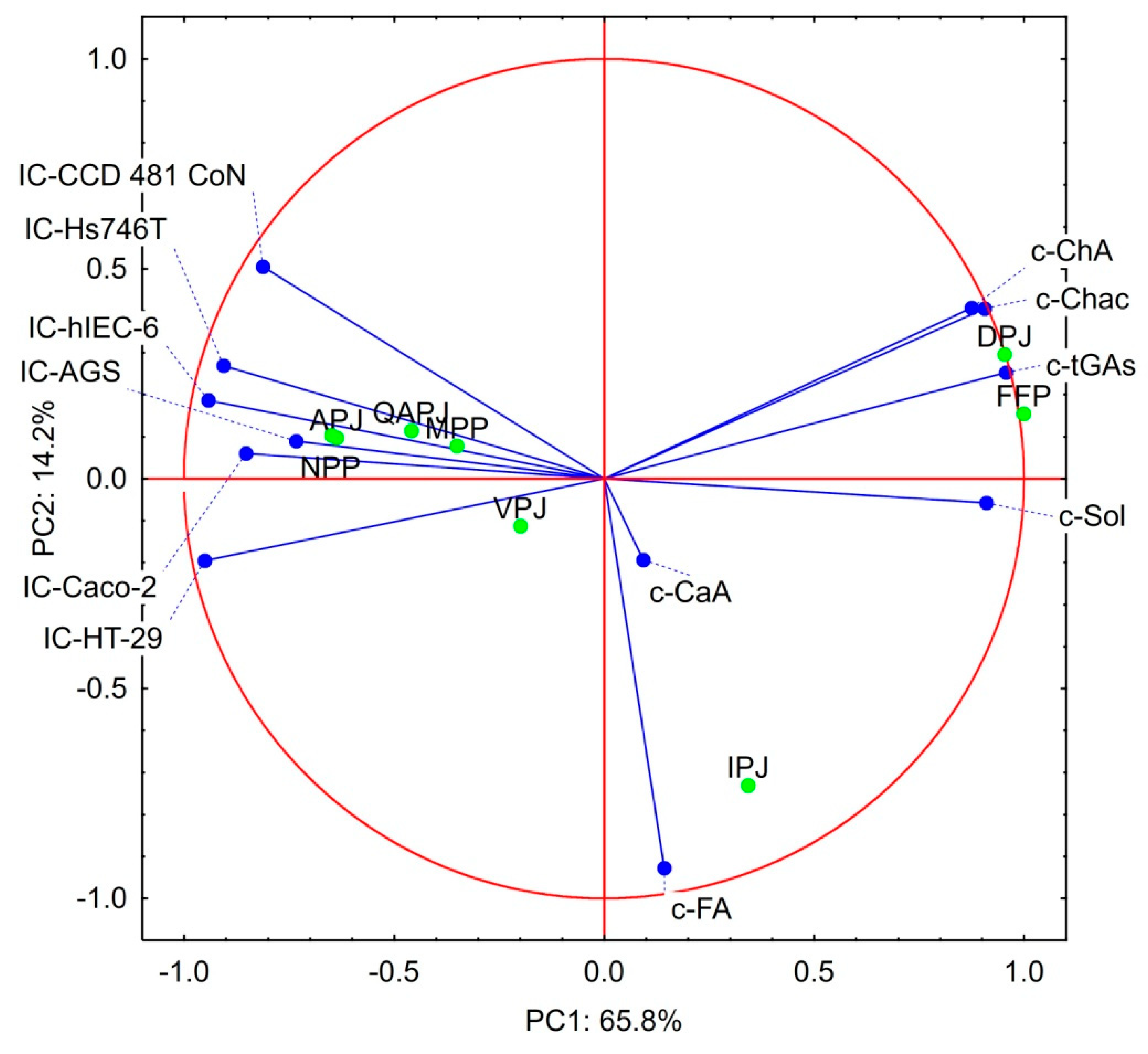
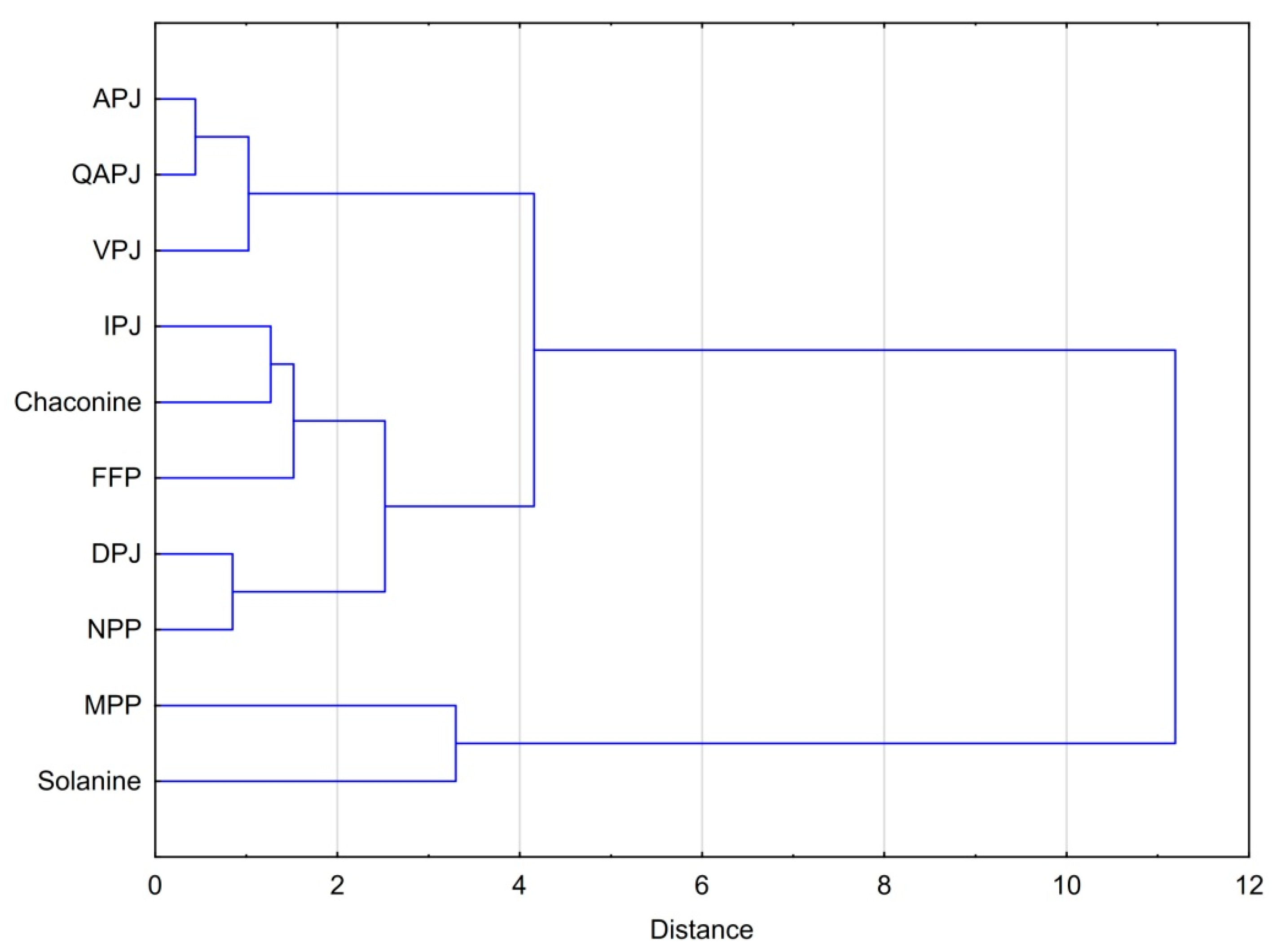
3.4. Statistical Considerations on the Bioactivity Products Studied
4. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APJ | Fresh juices from potatoes of ‘Agata’ variety |
| APJ-G | APJ after digestion in the stomach |
| APJ-GI | APJ after complete gastrointestinal digestion |
| CaA | Caffeic acid |
| ChA | Chlorogenic acid |
| DPJ | Deproteinized potato juice |
| DPJ-G | DPJ after digestion in the stomach |
| DPJ-GI | DPJ after complete gastrointestinal digestion |
| FA | Ferulic acid |
| FPP | Feed potato protein from PPZ Trzemeszno S.A. (Trzemeszno, Poland) |
| FPP-G | FPP after digestion in the stomach |
| FPP-GI | FPP after complete gastrointestinal digestion |
| GAs | Potato glycoalkaloids |
| IC50 | The half-maximal inhibitory concentration |
| IC-AGS | IC50 against AGS cell line |
| IC-Hs746T | IC50 against Hs746T cell line |
| IC-HT-29 | IC50 against HT-29 cell line |
| IC-Caco-2 | IC50 against Caco-2 cell line |
| IC- hIEC-6 | IC50 against hIEC-6 cell line |
| IC-CCD 481 CoN | IC50 against CCD 481 CoN cell line |
| IPJ | Industrial potato juice |
| IPJ-G | IPJ after digestion in the stomach |
| IPJ-GI | FPP after complete gastrointestinal digestion |
| MPP | Potato juice protein concentrate obtained by membrane processing of potato juice |
| MPP-G | MPP after digestion in the stomach |
| MPP-GI | FPP after complete gastrointestinal digestion |
| NPP | Solanic®200 from Avebe (Veendam, The Netherlands) |
| NPP-G | NPP after digestion in the stomach |
| NPP-GI | FPP after complete gastrointestinal digestion |
| PAs | Phenolic acids |
| PCA | Principal component analysis |
| PDPJPs | Post-digestion potato juice and products of its processing |
| PJ | Potato juice |
| QAPJ | Fresh juices from potatoes of the ‘Queen Anne’ variety |
| QAPJ-G | QAPJ after digestion in the stomach |
| QAPJ-GI | FPP after complete gastrointestinal digestion |
| VPJ | Fresh juices from potatoes of the ‘Vivaldi’ variety |
| VPJ-G | VPJ after digestion in the stomach |
| VPJ-GI | FPP after complete gastrointestinal digestion |
References
- Grommers, H.E.; van der Krogt, D.A. Potato Starch: Production, Modifications and Uses. In Starch; Bemiller, J.N., Whistler, R.L., Eds.; Academic Press: London, UK, 2009; pp. 511–539. [Google Scholar]
- Nowak, J.; Lasik, M.; Miskiewicz, T.; Czarnecki, Z. Biodegradation of high temperature wastewater from potato starch industry. In Proceedings of the Waste Management and the Environment; Lmorza, D., Brebbia, C.A., Sales, D., Popov, V., Eds.; WIT Press Southampton: Boston, MA, USA, 2002; pp. 655–663. [Google Scholar]
- Løkra, S.; Strætkvern, K. Industrial Proteins from Potato Juice. A Review. Food 2009, 3, 88–95. [Google Scholar]
- Waglay, A.; Karboune, S.; Alli, I. Potato protein isolates: Recovery and characterization of their properties. Food Chem. 2014, 142, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Jeżowski, P.; Polcyn, K.; Tomkowiak, A.; Rybicka, I.; Radzikowska, D. Technological and antioxidant properties of proteins obtained from waste potato juice. Open Life Sci. 2020, 15, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.; Olejnik, A.; Białas, W.; Rybicka, I.; Zielińska-Dawidziak, M.; Siger, A.; Kubiak, P.; Lewandowicz, G. The Nutritional Value and Biological Activity of Concentrated Protein Fraction of Potato Juice. Nutrients 2019, 11, 1523. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.; Olejnik, A.; Świtek, S.; Bzducha-Wróbel, A.; Kubiak, P.; Kujawska, M.; Lewandowicz, G. Bioactive compounds of potato (Solanum tuberosum L.) juice: From industry waste to food and medical applications. Crit. Rev. Plant Sci. 2022, 41, 52–89. [Google Scholar] [CrossRef]
- Hellmann, H.; Goyer, A.; Navarre, D. Antioxidants in Potatoes: A Functional View on One of the Major Food Crops Worldwide. Molecules 2021, 26, 2446. [Google Scholar] [CrossRef]
- Singh Pannu, J.; Kapoor, R.K.; Yadav, R. Comparative antibiotic potential of different varieties of potato tubers. Int. J. Pharm. Sci. Res. 2014, 5, 5393. [Google Scholar] [CrossRef]
- Visvanathan, R.; Jayathilake, C.; Jayawardana, B.; Liyanage, R. Health-beneficial properties of potato and compounds of interest. J. Sci. Food Agric. 2016, 96, 4850–4860. [Google Scholar] [CrossRef]
- Kujawska, M.; Olejnik, A.; Lewandowicz, G.; Kowalczewski, P.; Forjasz, R.; Jodynis-Liebert, J. Spray-Dried Potato Juice as a Potential Functional Food Component with Gastrointestinal Protective Effects. Nutrients 2018, 10, 259. [Google Scholar] [CrossRef]
- Kenny, O.M.; McCarthy, C.M.; Brunton, N.P.; Hossain, M.B.; Rai, D.K.; Collins, S.G.; Jones, P.W.; Maguire, A.R.; O’Brien, N.M. Anti-inflammatory properties of potato glycoalkaloids in stimulated Jurkat and Raw 264.7 mouse macrophages. Life Sci. 2013, 92, 775–782. [Google Scholar] [CrossRef]
- Elizalde-Romero, C.A.; Montoya-Inzunza, L.A.; Contreras-Angulo, L.A.; Heredia, J.B.; Gutiérrez-Grijalva, E.P. Solanum Fruits: Phytochemicals, Bioaccessibility and Bioavailability, and Their Relationship with Their Health-Promoting Effects. Front. Nutr. 2021, 8, 790582. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic compounds in potato ( Solanum tuberosum L.) peel and their health-promoting activities. Int. J. Food Sci. Technol. 2019, 55, 2273–2281. [Google Scholar] [CrossRef]
- Ku, S.K.; Sung, S.H.; Choung, J.J.; Choi, J.-S.; Shin, Y.K.; Kim, J.W. Anti-obesity and anti-diabetic effects of a standardized potato extract in ob/ob mice. Exp. Ther. Med. 2016, 12, 354–364. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spielmann, J.; Kluge, H.; Stangl, G.I.; Eder, K. Hypolipidaemic effects of potato protein and fish protein in pigs. J. Anim. Physiol. Anim. Nutr. 2009, 93, 400–409. [Google Scholar] [CrossRef]
- Chrubasik, S.; Torda, T.; Madisch, A. Efficacy and tolerability of potato juice in dyspeptic patients: A pilot study. Phytomedicine 2006, 13, 11–15. [Google Scholar] [CrossRef]
- Vlachojannis, J.E.; Cameron, M.; Chrubasik, S. Medicinal use of potato-derived products: A systematic review. Phytother. Res. 2009, 24, 159–162. [Google Scholar] [CrossRef]
- Bártová, V.; Bárta, J.; Jarošová, M. Antifungal and antimicrobial proteins and peptides of potato (Solanum tuberosum L.) tubers and their applications. Appl. Microbiol. Biotechnol. 2019, 103, 5533–5547. [Google Scholar] [CrossRef]
- Burlingame, B.; Mouillé, B.; Charrondière, R. Nutrients, bioactive non-nutrients and anti-nutrients in potatoes. J. Food Compos. Anal. 2009, 22, 494–502. [Google Scholar] [CrossRef]
- European Commission Document L:2002:050:TOC. Off. J. Eur. Communities L 50 2002, 45, 93. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2002:050:TOC (accessed on 12 November 2022).
- Smith, D. Potato glycoalkaloids: Some unanswered questions. Trends Food Sci. Technol. 1996, 7, 126–131. [Google Scholar] [CrossRef]
- Hassan, S.H.; Gul, S.; Zahra, H.S.; Maryam, A.; Shakir, H.A.; Khan, M.; Irfan, M. Alpha Solanine: A Novel Natural Bioactive Molecule with Anticancer Effects in Multiple Human Malignancies. Nutr. Cancer 2020, 73, 1541–1552. [Google Scholar] [CrossRef]
- Lee, K.-R.; Kozukue, N.; Han, J.-S.; Park, J.-H.; Chang, E.-Y.; Baek, E.-J.; Chang, J.-S.; Friedman, M. Glycoalkaloids and Metabolites Inhibit the Growth of Human Colon (HT29) and Liver (HepG2) Cancer Cells. J. Agric. Food Chem. 2004, 52, 2832–2839. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Lee, K.-R.; Kim, H.-J.; Lee, I.-S.; Kozukue, N. Anticarcinogenic Effects of Glycoalkaloids from Potatoes against Human Cervical, Liver, Lymphoma, and Stomach Cancer Cells. J. Agric. Food Chem. 2005, 53, 6162–6169. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-K.; Shih, Y.-W.; Chien, T.-T.C.; Fang, L.-H.; Huang, H.-C.; Chen, P.-S. ALPHA.-Solanine Inhibits Human Melanoma Cell Migration and Invasion by Reducing Matrix Metalloproteinase-2/9 Activities. Biol. Pharm. Bull. 2010, 33, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Mohsenikia, M.; Alizadeh, A.M.; Khodayari, S.; Khodayari, H.; Kouhpayeh, S.A.; Karimi, A.; Zamani, M.; Azizian, S.; Mohagheghi, M.A. The protective and therapeutic effects of alpha-solanine on mice breast cancer. Eur. J. Pharmacol. 2013, 718, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Zhong, W.; Deng, Z.; Lai, C.; Chu, J.; Jiao, G.; Liu, J.; Zhou, Q. Inhibition of prostate cancer growth by solanine requires the suppression of cell cycle proteins and the activation of ROS/P38 signaling pathway. Cancer Med. 2016, 5, 3214–3222. [Google Scholar] [CrossRef] [PubMed]
- Mensinga, T.T.; Sips, A.J.A.M.; Rompelberg, C.J.M.; van Twillert, K.; Meulenbelt, J.; van den Top, H.J.; van Egmond, H.P. Potato glycoalkaloids and adverse effects in humans: An ascending dose study. Regul. Toxicol. Pharmacol. 2005, 41, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Lu, Y.; Bowman, L.L.; Qian, Y.; Castranova, V.; Ding, M. Inhibition of Activator Protein-1, NF-κB, and MAPKs and Induction of Phase 2 Detoxifying Enzyme Activity by Chlorogenic Acid. J. Biol. Chem. 2005, 280, 27888–27895. [Google Scholar] [CrossRef]
- Huang, C.; Ma, W.-Y.; Ryan, C.A.; Dong, Z. Proteinase inhibitors I and II from potatoes specifically block UV-induced activator protein-1 activation through a pathway that is independent of extracellular signal-regulated kinases, c-Jun N-terminal kinases, and P38 kinase. Proc. Natl. Acad. Sci. USA 1997, 94, 11957–11962. [Google Scholar] [CrossRef]
- Pouvreau, L.; Gruppen, H.; Piersma, S.R.; van den Broek, L.A.M.; van Koningsveld, G.A.; Voragen, A.G.J. Relative Abundance and Inhibitory Distribution of Protease Inhibitors in Potato Juice from cv. Elkana. J. Agric. Food Chem. 2001, 49, 2864–2874. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Różańska, M.; Makowska, A.; Jezowski, P.; Kubiak, P. Production of wheat bread with spray-dried potato juice: Influence on dough and bread characteristics. Food Sci. Technol. Int. 2018, 25, 223–232. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Lewandowicz, G.; Krzywdzińska-Bartkowiak, M.; Piątek, M.; Baranowska, H.M.; Białas, W.; Jeziorna, M.; Kubiak, P. Finely comminuted frankfurters fortified with potato juice—Quality and structure. J. Food Eng. 2015, 167, 183–188. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Lewandowicz, G.; Makowska, A.; Knoll, I.; Błaszczak, W.; Białas, W.; Kubiak, P. Pasta Fortified with Potato Juice: Structure, Quality, and Consumer Acceptance. J. Food Sci. 2015, 80, S1377–S1382. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, H.M.; Masewicz, L.; Kowalczewski, P.; Lewandowicz, G.; Piątek, M.; Kubiak, P. Water properties in pâtés enriched with potato juice. Eur. Food Res. Technol. 2017, 244, 387–393. [Google Scholar] [CrossRef]
- Olejnik, A.; Rychlik, J.; Kidoń, M.; Czapski, J.; Kowalska, K.; Juzwa, W.; Olkowicz, M.; Dembczyński, R.; Moyer, M.P. Antioxidant effects of gastrointestinal digested purple carrot extract on the human cells of colonic mucosa. Food Chem. 2016, 190, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.; Zembrzuska, J.; Drożdżyńska, A.; Smarzyński, K.; Radzikowska, D.; Kieliszek, M.; Jeżowski, P.; Sawinska, Z. Influence of potato variety on polyphenol profile composition and glycoalcaloid contents of potato juice. Open Chem. 2021, 19, 1216–1223. [Google Scholar] [CrossRef]
- Cybulska, I.; Zembrzuska, J.; Brudecki, G.; Thomsen, M.H. Optimizing methods to characterize caffeic, ferulic, and chlorogenic acids in Salicornia sinuspersica and Salicornia bigelovii extracts by tandem mass spectrometry (LC-MS/MS). BioResources 2021, 16, 5508–5523. [Google Scholar] [CrossRef]
- Olejnik, A.; Olkowicz, M.; Kowalska, K.; Rychlik, J.; Dembczyński, R.; Myszka, K.; Juzwa, W.; Białas, W.; Moyer, M.P. Gastrointestinal digested Sambucus nigra L. fruit extract protects in vitro cultured human colon cells against oxidative stress. Food Chem. 2016, 197, 648–657. [Google Scholar] [CrossRef]
- Langkilde, S.; Schrøder, M.; Stewart, D.; Meyer, O.; Conner, S.; Davies, H.; Poulsen, M. Acute Toxicity of High Doses of the Glycoalkaloids, α-Solanine and α-Chaconine, in the Syrian Golden Hamster. J. Agric. Food Chem. 2008, 56, 8753–8760. [Google Scholar] [CrossRef]
- Sangeeta; Kaur, J.; Rani, P. Solanine and Chaconine. In Handbook of Plant and Animal Toxins in Food; CRC Press: Boca Raton, FL, USA, 2022; pp. 73–96. [Google Scholar]
- Lasik, M.; Nowak, J.; Krzywonos, M.; Cibis, E. Impact of batch, repeated-batch (with cell recycle and medium replacement) and continuous processes on the course and efficiency of aerobic thermophilic biodegradation of potato processing wastewater. Bioresour. Technol. 2010, 101, 3444–3451. [Google Scholar] [CrossRef]
- Laus, M.C.; Klip, G.; Giuseppin, M.L.F. Improved Extraction and Sample Cleanup of Tri-glycoalkaloids α-Solanine and α-Chaconine in Non-denatured Potato Protein Isolates. Food Anal. Methods 2016, 10, 845–853. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 94391. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef] [PubMed]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Mishra, T.; Raigond, P.; Thakur, N.; Dutt, S.; Singh, B. Recent Updates on Healthy Phytoconstituents in Potato: A Nutritional Depository. Potato Res. 2020, 63, 323–343. [Google Scholar] [CrossRef]
- Sun, Y.; Jiang, L.; Wei, D. Partial characterization, in vitro antioxidant and antiproliferative activities of patatin purified from potato fruit juice. Food Funct. 2013, 4, 1502–1511. [Google Scholar] [CrossRef]
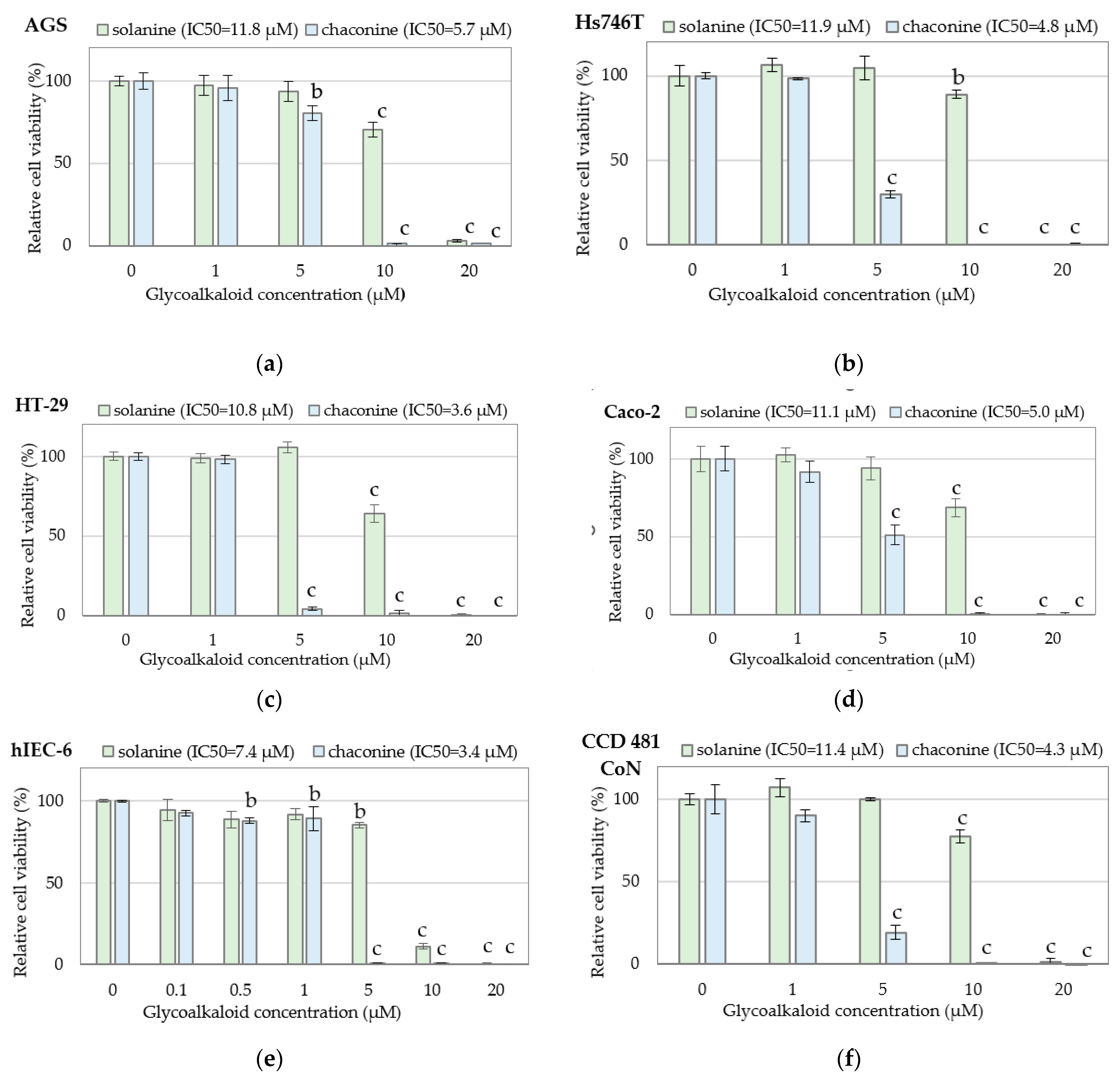
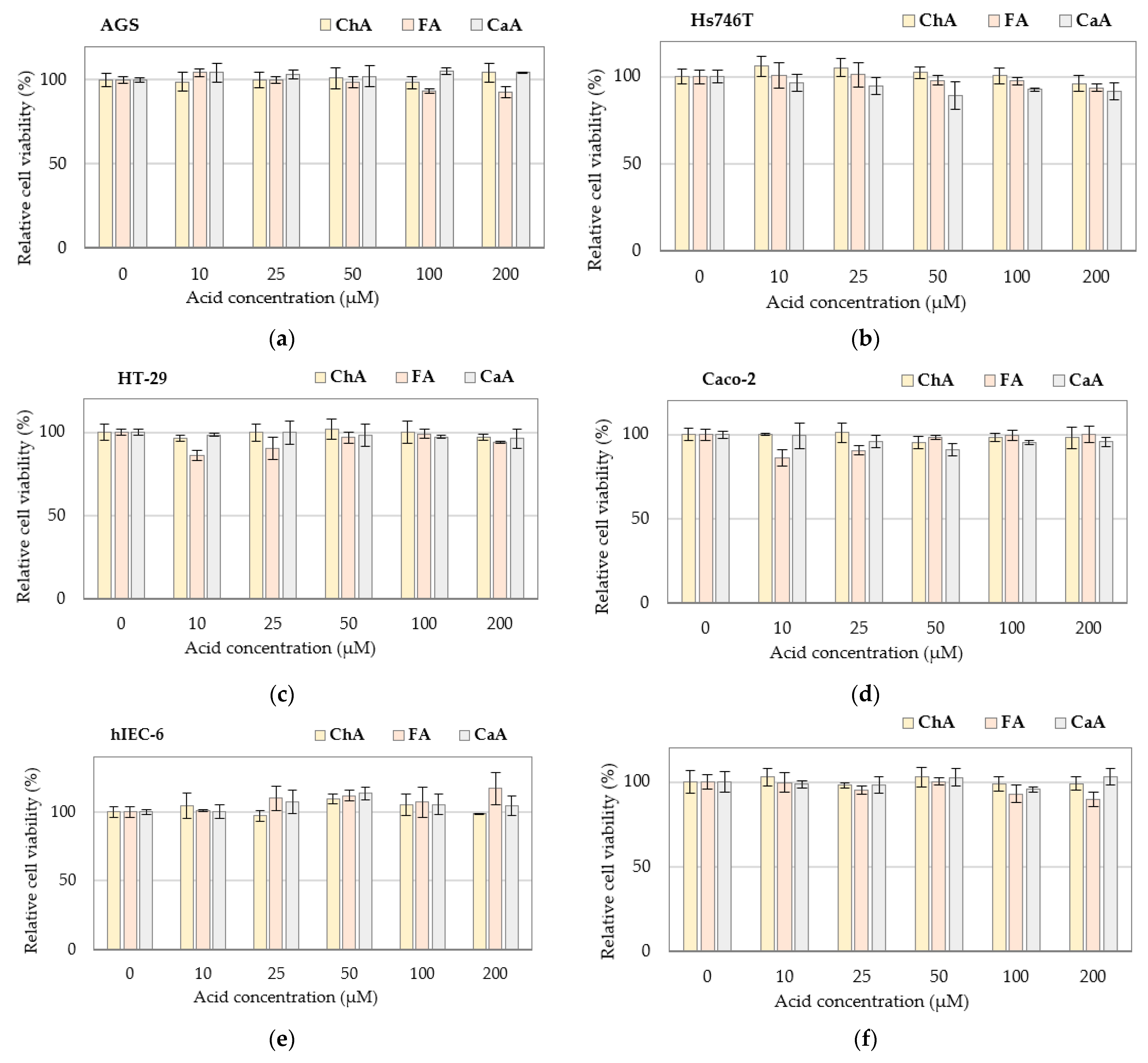
| Cell Line | Solanine (μg/mL) | Chaconine (μg/mL) |
|---|---|---|
| AGS | 10.26 ± 0.12 bc | 4.87 ± 0.29 c |
| Hs746T | 10.36 ± 0.49 c | 4.06 ± 0.03 abc |
| HT-29 | 9.37 ± 0.13 b | 3.04 ± 0.85 ab |
| Caco-2 | 9.64 ± 0.28 bc | 4.28 ± 0.06 bc |
| hIEC-6 | 6.44 ± 0.28 a | 2.92 ± 0.55 a |
| CCD 481 CoN | 9.90 ± 0.46 bc | 3.67 ± 0.45 abc |
| Product | Glycoalkaloids (μg/g) | Phenolic Acids (μg/g) | ||||
|---|---|---|---|---|---|---|
| Solanine | Chaconine | Total GAs | CaA | FA | ChA | |
| APJ | 3.11 ± 0.26 aA | 0.33 ± 0.07 aA | 3.44 ± 0.33 | n.d. | 1.92 ± 0.23 aC | 0.48 ± 0.06 aA |
| APJ-G | 3.26 ± 0.61 A | 1.18 ± 0.61 A | 4.44 ± 1.23 | n.d. | 0.07 ± 0.05 A | 0.56 ± 0.04 A |
| APJ-GI | 3.35 ± 0.62 A | 0.57 ± 0.05 A | 3.92 ± 0.68 | n.d. | 0.60 ± 0.01 B | 0.62 ± 0.12 A |
| QAPJ | 34.0 ± 2.8 aA | 25.3 ± 0.8 aA | 59.3 ± 3.7 | n.d. | 0.83 ± 0.01 aB | 0.46 ± 0.04 aA |
| QAPJ-G | 26.3 ± 0.2 A | 18.2 ± 4.2 A | 44.5 ± 4.3 | n.d. | 0.78 ± 0.01 A | 0.58 ± 0.02 B |
| QAPJ-GI | 25.5 ± 10.5 A | 17.4 ± 9.4 A | 42.9 ± 20.0 | n.d. | 0.90 ± 0.00 C | 0.68 ± 0.01 B |
| VPJ | 105 ± 6 aB | 146 ± 5 aC | 251 ± 11 | 0.80 ± 0.02 cA | 0.58 ± 0.01 aB | 0.23 ± 0.01 aA |
| VPJ-G | 109 ± 3 B | 124 ± 4 B | 233 ± 7 | 0.70 ± 0.10 A | 0.54 ± 0.01 A | 0.50 ± 0.12 B |
| VPJ-GI | 71.8 ± 1.9 A | 80.6 ± 2.4 A | 152 ± 4 | n.d. | 0.78 ± 0.00 C | 0.64 ± 0.01 B |
| IPJ | 675 ± 21 bcB | 101 ± 13 aA | 776 ± 28 | 0.47 ± 0.12 bA | 10.9 ± 1.7 bB | 0.49 ± 0.07 aA |
| IPJ-G | 770 ± 83 B | 180 ± 25 B | 859 ± 102 | 4.80 ± 5.80 A | 13.1 ± 0.3 B | 0.36 ± 0.07 A |
| IPJ-GI | 351 ± 4 A | 78.4 ± 7.8 A | 429 ± 12 | n.d. | 3.44 ± 0.12 A | 0.51 ± 0.04 A |
| DPJ | 984 ± 131 dB | 1621 ± 269 bB | 2605 ± 400 | 0.15 ± 0.01 aA | 0.51 ± 0.05 aB | 2.70 ± 0.14 bB |
| DPJ-G | 1001 ± 165 B | 1739 ± 156 B | 2740 ± 320 | 0.61 ± 0.06 B | 0.28 ± 0.01 A | 6.13 ± 0.04 C |
| DPJ-GI | 353 ± 43 A | 557 ± 12 A | 910 ± 55 | n.d. | 0.70 ± 0.03 C | 0.40 ± 0.01 A |
| FPP | 753 ± 178 cA | 1477 ± 469 bA | 2230 ± 646 | 0.72 ± 0.01 cA | 0.18 ± 0.00 aB | 3.50 ± 0.50 cB |
| FPP-G | 699 ± 38 A | 1479 ± 12 A | 2178 ± 49 | 0.65 ± 0.01 A | 0.08 ± 0.04 A | 5.12 ± 0.11 C |
| FPP-GI | 528 ± 37 A | 1051 ± 67 A | 1579 ± 104 | n.d. | 0.12 ± 0.02 A | 0.72 ± 0.01 A |
| MPP | 500 ± 43 bA | 3.63 ± 1.00 aA | 504 ± 44 | 0.75 ± 0.01 cA | 0.26 ± 0.01 aA | 0.42 ± 0.03 aB |
| MPP-G | 508 ± 16 A | 4.35 ± 2.90 A | 602 ± 19 | 0.77 ± 0.01 A | 0.22 ± 0.05 A | 0.19 ± 0.03 A |
| MPP-GI | 495 ± 248 A | 6.30 ± 3.10 A | 501 ± 251 | n.d. | 0.64 ± 0.01 B | 0.68 ± 0.01 C |
| NPP | 43.1 ± 1.0 aA | 23.2 ± 4.8 aA | 66.3 ± 5.9 | 0.69 ± 0.06 cA | 0.14 ± 0.02 aA | 0.44 ± 0.02 aA |
| NPP-G | 156 ± 6 B | 19.9 ± 2.7 A | 176 ± 9 | 0.86 ± 0.09 A | 0.73 ± 0.04 C | 0.76 ± 0.02 B |
| NPP-GI | 37.5 ± 21.8 A | 21.1 ± 10.9 A | 58.6 ± 32.7 | n.d. | 0.65 ± 0.03 B | 0.76 ± 0.01 B |
| Correlation | r | p | Equation |
|---|---|---|---|
| Solanine content in the raw material (S) vs. solanine content after gastric digestion (SG) | 0.98988 | <0.0001 | SG = 0.98232 × S + 28.765 |
| (S) vs. solanine content after gastrointestinal digestion (SGI) | 0.85662 | 0.0066 | SGI = 0.48459 × S + 48.034 |
| Chaconine content in the raw material (Ch) vs. chaconine content after gastric digestion (ChG) | 0.99831 | <0.0001 | ChG = 1.0375 × Ch + 5.1128 |
| (Ch) vs. chaconine content after gastrointestinal digestion (ChGI) | 0.91727 | 0.0013 | ChGI = 0.50155 × Ch + 13.958 |
| Cell Line | PJ and Products of its Processing Subjected to the Gastric (-G) Digestion | |||||||
|---|---|---|---|---|---|---|---|---|
| APJ-G | QAPJ-G | VPJ-G | IPJ-G | DPJ-G | FPP-G | MPP-G | NPP-G | |
| AGS | 14.39 ± 0.51 a | 9.20 ± 0.22 c | 11.40 ± 0.05 e | 1.90 ± 0.13 f | 0.061 ± 0.001 h | 1.00 ± 0.12 g | 11.40 ± 0.05 b | 2.74 ± 0.13 d |
| Hs746T | 30.49 ± 0.03b | 30.68 ± 2.57 b | 11.45 ± 0.09 d | 4.86 ± 0.07 e | 2.57 ± 0.07 e | 3.01 ± 0.02 e | 20.73 ± 0.84 c | 36.49 ± 1.42 a |
| Cell Line | PJ and Products of its Processing Subjected to the Gastrointestinal (-GI) Digestion | |||||||
| APJ-GI | QAPJ-GI | VPJ-GI | IPJ-GI | DPJ-GI | FPP-GI | MPP-GI | NPP-GI | |
| HT-29 | 7.16 ± 0.03 b | 6.60 ± 0.22 b | 5.97 ± 0.33 b | 4.90 ± 0.12 c | 0.087 ± 0.02 e | 0.25 ± 0.03 d | 5.11 ± 0.20 c | 9.19 ± 1.56 a |
| Caco-2 | 11.34 ± 0.33 b | 5.24 ± 0.14 d | 8.22 ± 0.87 c | 4.70 ± 0.13 d | 1.75 ± 0.16 e | 1.84 ± 0.12 e | 12.23 ± 0.15 b | 14.23 ± 0.25 a |
| hIEC-6 | 12.92 ± 0.06 b | 14.95 ± 0.13 b | 7.61 ± 0.08 c | 5.94 ± 0.08 c | 2.19 ± 0.14 d | 3.04 ± 0.15 d | 15.04 ± 0.10 b | 16.07 ± 0.96 a |
| CCD 481 CoN | 11.34 ± 0.31 b | 10.58 ± 0.58 b | 7.93 ± 0.53 c | 7.57 ± 0.05 c | 3.06 ± 0.09 d | 2.23 ± 0.06 d | 10.92 ± 0.21 b | 14.72 ± 0.96 a |
| Cell Line | PJ and Products of Its Processing Subjected to the Gastric (-G) Digestion | |||||||
|---|---|---|---|---|---|---|---|---|
| APJ-G | QAPJ-G | VPJ-G | IPJ-G | DPJ-G | FPP-G | MPP-G | NPP-G | |
| AGS | 0.06 | 0.41 | 1.35 | 1.63 | 0.17 | 2.17 | 6.86 | 0.48 |
| Hs746T | 0.14 | 1.37 | 2.67 | 4.17 | 7.03 | 6.56 | 12.48 | 6.42 |
| Cell Line | PJ and Products of Its Processing Subjected to the Gastrointestinal (-GI) Digestion | |||||||
| APJ-GI | QAPJ-GI | VPJ-GI | IPJ-GI | DPJ-GI | FPP-GI | MPP-GI | NPP-GI | |
| HT-29 | 0.03 | 0.28 | 0.91 | 2.10 | 0.08 | 0.40 | 2.56 | 0.54 |
| Caco-2 | 0.04 | 0.22 | 1.25 | 2.02 | 1.59 | 2.91 | 5.88 | 0.83 |
| hIEC-6 | 0.05 | 0.64 | 1.16 | 2.55 | 1.99 | 4.0 | 7.541 | 0.94 |
| CCD 481 CoN | 0.04 | 0.45 | 1.21 | 3.25 | 2.78 | 3.52 | 5.47 | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczewski, P.Ł.; Olejnik, A.; Wieczorek, M.N.; Zembrzuska, J.; Kowalska, K.; Lewandowicz, J.; Lewandowicz, G. Bioactive Substances of Potato Juice Reveal Synergy in Cytotoxic Activity against Cancer Cells of Digestive System Studied In Vitro. Nutrients 2023, 15, 114. https://doi.org/10.3390/nu15010114
Kowalczewski PŁ, Olejnik A, Wieczorek MN, Zembrzuska J, Kowalska K, Lewandowicz J, Lewandowicz G. Bioactive Substances of Potato Juice Reveal Synergy in Cytotoxic Activity against Cancer Cells of Digestive System Studied In Vitro. Nutrients. 2023; 15(1):114. https://doi.org/10.3390/nu15010114
Chicago/Turabian StyleKowalczewski, Przemysław Łukasz, Anna Olejnik, Martyna Natalia Wieczorek, Joanna Zembrzuska, Katarzyna Kowalska, Jacek Lewandowicz, and Grażyna Lewandowicz. 2023. "Bioactive Substances of Potato Juice Reveal Synergy in Cytotoxic Activity against Cancer Cells of Digestive System Studied In Vitro" Nutrients 15, no. 1: 114. https://doi.org/10.3390/nu15010114
APA StyleKowalczewski, P. Ł., Olejnik, A., Wieczorek, M. N., Zembrzuska, J., Kowalska, K., Lewandowicz, J., & Lewandowicz, G. (2023). Bioactive Substances of Potato Juice Reveal Synergy in Cytotoxic Activity against Cancer Cells of Digestive System Studied In Vitro. Nutrients, 15(1), 114. https://doi.org/10.3390/nu15010114













