The Potential Impact of Inducing a Restriction in Reimbursement Criteria on Vitamin D Supplementation in Osteoporotic Patients with or without Fractures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
2.2. Patients
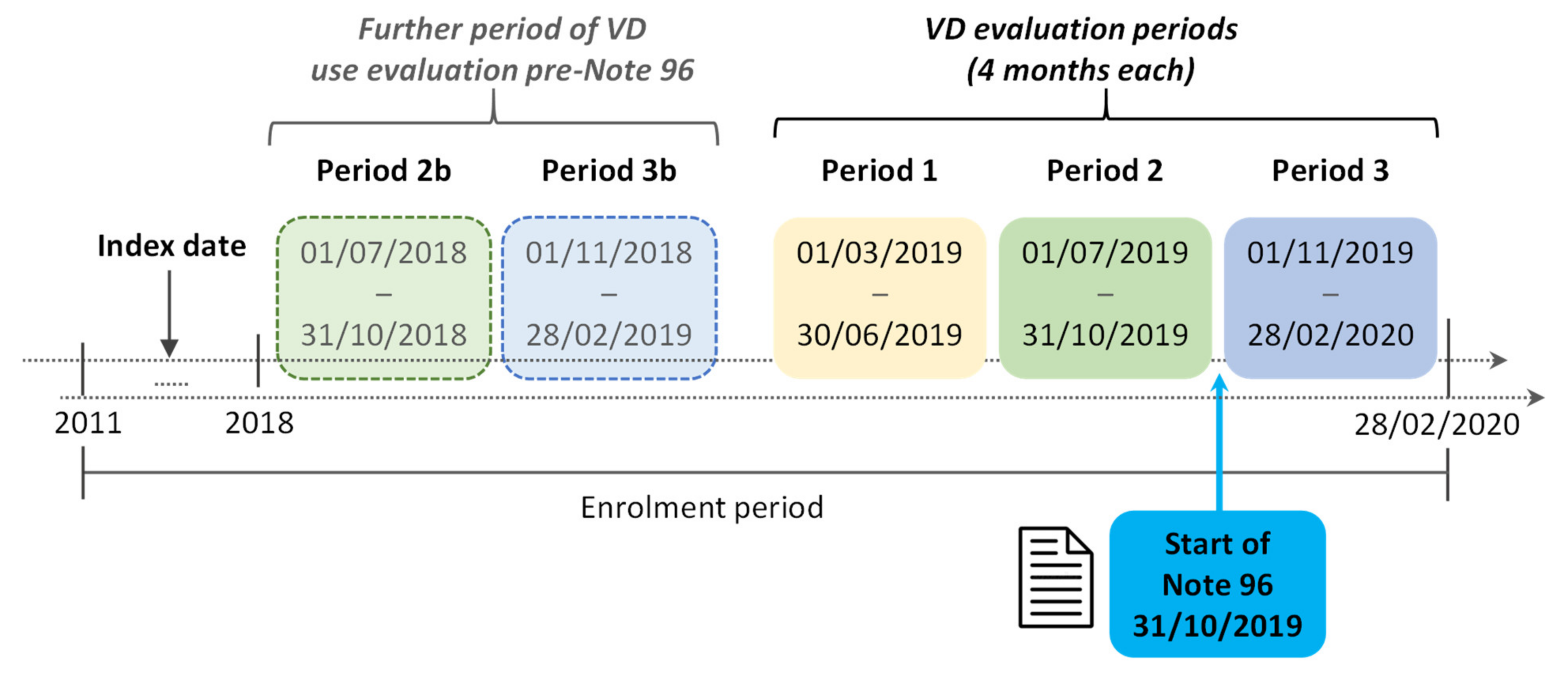
2.3. Definition of Cohorts Analyzed
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
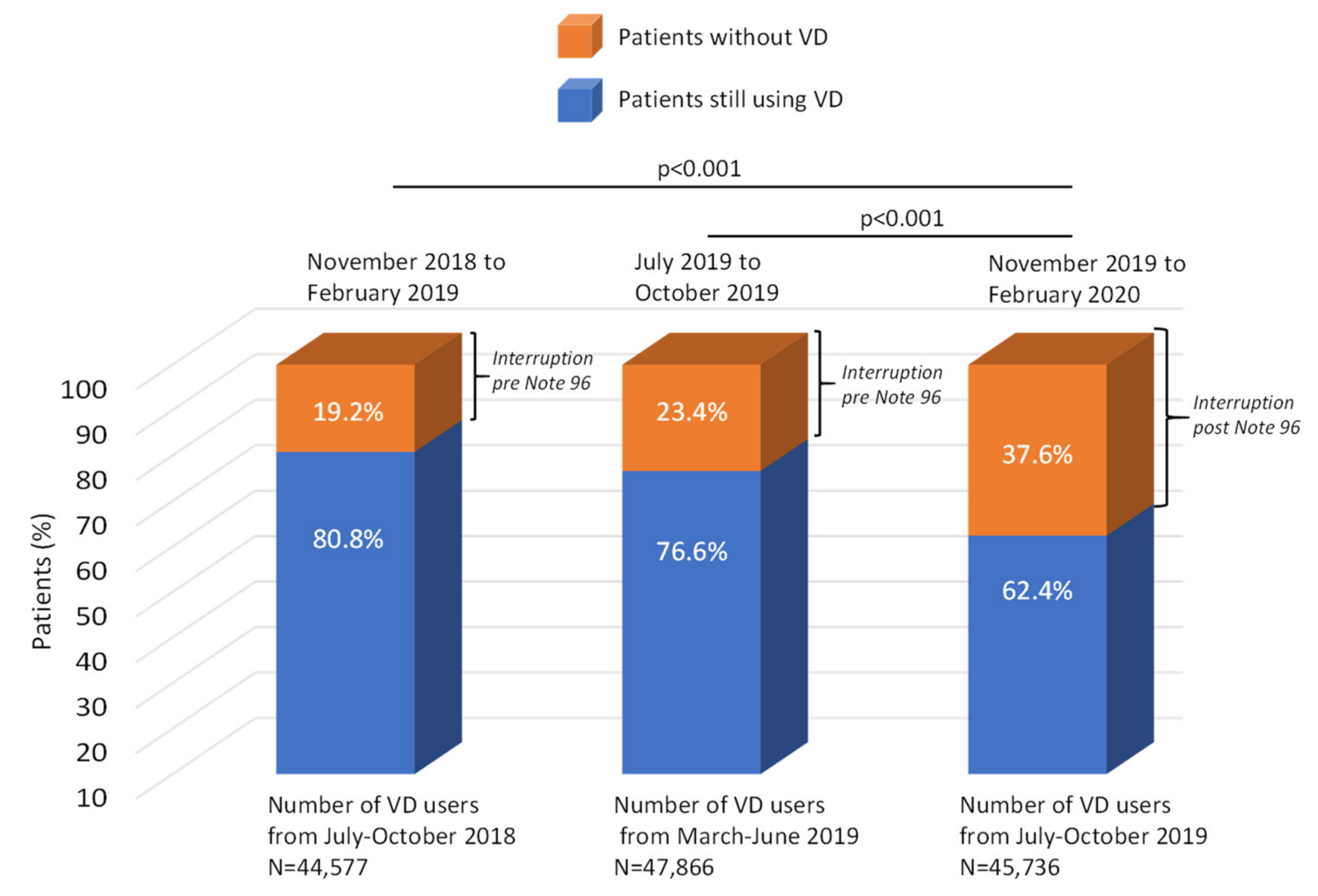
3.2. Vitamin D Interruption Rate before and after the Application of Note 96
3.3. Predictors of Vitamin D Interruption
4. Discussion
5. Study Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and Vitamin D. In Comprehensive Physiology; American Cancer Society, Wiley: New York, NY, USA, 2016; pp. 561–601. ISBN 978-0-470-65071-4. [Google Scholar]
- Hill, T.R.; Aspray, T.J. The Role of Vitamin D in Maintaining Bone Health in Older People. Ther. Adv. Musculoskelet. 2017, 9, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mithal, A.; Wahl, D.A.; Bonjour, J.-P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; El-Hajj Fuleihan, G.; Josse, R.G.; Lips, P.; Morales-Torres, J.; et al. Global Vitamin D Status and Determinants of Hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meehan, M.; Penckofer, S. The Role of Vitamin D in the Aging Adult. J. Aging Gerontol. 2014, 2, 60–71. [Google Scholar] [CrossRef]
- Wang, N.; Chen, Y.; Ji, J.; Chang, J.; Yu, S.; Yu, B. The Relationship between Serum Vitamin D and Fracture Risk in the Elderly: A Meta-Analysis. J. Orthop. Surg. Res. 2020, 15, 81. [Google Scholar] [CrossRef]
- Looker, A.C.; Mussolino, M.E. Serum 25-Hydroxyvitamin D and Hip Fracture Risk in Older U.S. White Adults. J. Bone Miner. Res. 2008, 23, 143–150. [Google Scholar] [CrossRef]
- Chapuy, M.C.; Arlot, M.E.; Duboeuf, F.; Brun, J.; Crouzet, B.; Arnaud, S.; Delmas, P.D.; Meunier, P.J. Vitamin D3 and Calcium to Prevent Hip Fractures in Elderly Women. N. Engl. J. Med. 1992, 327, 1637–1642. [Google Scholar] [CrossRef]
- Avenell, A.; Gillespie, W.J.; Gillespie, L.D.; O’Connell, D.L. Vitamin D and Vitamin D Analogues for Preventing Fractures Associated with Involutional and Post-Menopausal Osteoporosis. Cochrane Database Syst. Rev. 2005, 3, CD000227. [Google Scholar] [CrossRef]
- Carmel, A.S.; Shieh, A.; Bang, H.; Bockman, R.S. The 25(OH)D Level Needed To Maintain A Favorable Bisphosphonate Response Is ≥33 ng/Ml. Osteoporos. Int. 2012, 23, 2479–2487. [Google Scholar] [CrossRef] [Green Version]
- Adami, S.; Isaia, G.; Luisetto, G.; Minisola, S.; Sinigaglia, L.; Gentilella, R.; Agnusdei, D.; Iori, N.; Nuti, R.; ICARO Study Group. Fracture Incidence and Characterization in Patients on Osteoporosis Treatment: The ICARO Study. J. Bone Miner. Res. 2006, 21, 1565–1570. [Google Scholar] [CrossRef]
- Nurmi-Lüthje, I.; Lüthje, P.; Kaukonen, J.-P.; Kataja, M.; Kuurne, S.; Naboulsi, H.; Karjalainen, K. Post-Fracture Prescribed Calcium and Vitamin D Supplements Alone or, in Females, with Concomitant Anti-Osteoporotic Drugs Is Associated with Lower Mortality in Elderly Hip Fracture Patients: A Prospective Analysis. Drugs Aging 2009, 26, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Nurmi-Lüthje, I.; Sund, R.; Juntunen, M.; Lüthje, P. Post-Hip Fracture Use of Prescribed Calcium plus Vitamin D or Vitamin D Supplements and Antiosteoporotic Drugs Is Associated with Lower Mortality: A Nationwide Study in Finland. J. Bone Miner. Res. 2011, 26, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Eastell, R.; Rosen, C.J.; Black, D.M.; Cheung, A.M.; Murad, M.H.; Shoback, D. Pharmacological Management of Osteoporosis in Postmenopausal Women: An Endocrine Society* Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2019, 104, 1595–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuti, R.; Brandi, M.L.; Checchia, G.; Di Munno, O.; Dominguez, L.; Falaschi, P.; Fiore, C.E.; Iolascon, G.; Maggi, S.; Michieli, R.; et al. Guidelines for the Management of Osteoporosis and Fragility Fractures. Intern. Emerg. Med. 2019, 14, 85–102. [Google Scholar] [CrossRef] [Green Version]
- Adami, S.; Romagnoli, E.; Carnevale, V.; Scillitani, A.; Giusti, A.; Rossini, M.; Gatti, D.; Nuti, R.; Minisola, S. Guidelines on prevention and treatment of vitamin D deficiency. Reumatismo 2011, 63, 129–147. [Google Scholar] [CrossRef]
- Sunyecz, J.A. The Use of Calcium and Vitamin D in the Management of Osteoporosis. Ther. Clin. Risk Manag. 2008, 4, 827–836. [Google Scholar] [CrossRef] [Green Version]
- AIFA Notes. Available online: https://aifa.gov.it/note-aifa (accessed on 12 September 2021).
- Nota 79. Gazzetta Ufficiale Della Repubblica Italiana. Serie Generale–n.75. 30 Marzo. 2017. Available online: https://www.gazzettaufficiale.it/eli/id/2017/03/30/17A02253/sg (accessed on 30 January 2020).
- Nota 96. Available online: https://aifa.gov.it/nota-96 (accessed on 12 September 2021).
- Monitoraggio Delle Note AIFA. Available online: https://aifa.gov.it/monitoraggio-note-aifa (accessed on 12 September 2021).
- Fischer, V.; Haffner-Luntzer, M.; Amling, M.; Ignatius, A. Calcium and Vitamin D in Bone Fracture Healing and Post-Traumatic Bone Turnover. Eur. Cells Mater. 2018, 35, 365–385. [Google Scholar] [CrossRef]
- Bove, M.; Colia, A.L.; Dimonte, S.; Trabace, L. Increase in Vitamin D Prescriptions in a Southern Italy Region over 2011–2015 Period. Pharmadvances 2021, 3, 467. [Google Scholar] [CrossRef]
- Cesareo, R.; Attanasio, R.; Caputo, M.; Castello, R.; Chiodini, I.; Falchetti, A.; Guglielmi, R.; Papini, E.; Santonati, A.; Scillitani, A.; et al. Italian Association of Clinical Endocrinologists (AME) and Italian Chapter of the American Association of Clinical Endocrinologists (AACE) Position Statement: Clinical Management of Vitamin D Deficiency in Adults. Nutrients 2018, 10, 546. [Google Scholar] [CrossRef] [Green Version]
- Vitamina D e Osteoporosi L’appropriatezza Non è Un Miraggio. AboutPharma 2021, 185, 78–79.
- Adami, S.; Giannini, S.; Bianchi, G.; Sinigaglia, L.; Di Munno, O.; Fiore, C.E.; Minisola, S.; Rossini, M. Vitamin D Status and Response to Treatment in Post-Menopausal Osteoporosis. Osteoporos. Int. 2009, 20, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Esposti, L.D.; Girardi, A.; Saragoni, S.; Sella, S.; Andretta, M.; Rossini, M.; Giannini, S. Use of Antiosteoporotic Drugs and Calcium/Vitamin D in Patients with Fragility Fractures: Impact on Re-Fracture and Mortality Risk. Endocrine 2019, 64, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bouillon, R.; Ebeling, P.R.; Lazaretti-Castro, M.; Marcocci, C.; Rizzoli, R.; Sempos, C.T.; Bilezikian, J.P. Controversies in Vitamin D: Summary Statement From an International Conference. J. Clin. Endocrinol. Metab. 2019, 104, 234–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degli Esposti, L.; Saragoni, S.; Perrone, V.; Sella, S.; Andretta, M.; Rossini, M.; Giannini, S. Economic Burden of Osteoporotic Patients with Fracture: Effect of Treatment With or Without Calcium/Vitamin D Supplements. NDS 2020, 12, 21–30. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Overall Patients (n = 94,505) | Cohort Period 1 (n = 47,866) | Cohort Period 2 (n = 45,736) | p-Value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 69.4 ± 9.5 | 68.8 ± 9.5 | 68.8 ± 9.5 | 1.000 |
| Female, n (%) | 86,278 (91.3) | 45,557 (95.2) | 43,453 (95.0) | 0.235 |
| Comorbidities, n (%) | ||||
| Hypertension | 56,878 (60.2) | 28,282 (59.1) | 27,109 (59.3) | 0.561 |
| Diabetes | 12,041 (12.7) | 5453 (11.4) | 5246 (11.5) | 0.708 |
| Rheumatoid arthritis | 1307 (1.4) | 833 (1.7) | 803 (1.8) | 0.857 |
| Dyslipidemia | 27,060 (28.6) | 14,143 (29.5) | 13,528 (29.6) | 0.916 |
| Ischemic heart disease | 731 (0.8) | 315 (0.7) | 301 (0.7) | 0.999 |
| Cardiac dysrhythmias | 3553 (3.8) | 1649 (3.4) | 1600 (3.5) | 0.656 |
| Heart failure | 292 (0.3) | 114 (0.2) | 104 (0.2) | 0.733 |
| Stroke | 873 (0.9) | 367 (0.8) | 326 (0.7) | 0.336 |
| Dementia | 69 (0.1) | 26 (0.1) | 27 (0.1) | 0.762 |
| Schizophrenic disorders | 227 (0.2) | 118 (0.2) | 104 (0.2) | 0.548 |
| COPD | 12,127 (12.8) | 6195 (12.9) | 5970 (13.1) | 0.614 |
| Co-treatments, n (%) | ||||
| Corticosteroids for systemic use | 13,792 (14.6) | 7365 (15.4) | 7073 (15.5) | 0.741 |
| Platelet aggregation inhibitors excl. heparin | 22,957 (24.3) | 11,334 (23.7) | 10,888 (23.8) | 0.647 |
| VKA/direct factor Xa inhibitors | 2774 (2.9) | 1259 (2.6) | 1207 (2.6) | 0.933 |
| Analgesics | 7482 (7.9) | 3836 (8.0) | 3642 (8.0) | 0.774 |
| Antiepileptics | 5429 (5.7) | 2696 (5.6) | 2603 (5.7) | 0.696 |
| Antipsychotics | 1336 (1.4) | 572 (1.2) | 522 (1.1) | 0.445 |
| Proton pump inhibitors | 42,367 (44.8) | 22,177 (46.3) | 21,326 (46.6) | 0.362 |
| VD Treatment Pre-Note 96 | VD Treatment Post-Note 96 | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Period | Period 1 VD Users | Period 2 VD Users | Period 2 VD Non-Users | Period 2 VD Users | Period 3 VD Users | Period 3 VD Non-Users | |
| Patient classification | Total number of VD users in March–June 2019 | % of patients still using VD in July–October 2019 | % of patients not using VD in July–October 2019 | Number of VD users in July–October 2019 | % of patients still using VD after Note 96 introduction (November 2019–February 2020) | % of patients not using VD after Note 96 introduction (November 2019–February 2020) | |
| Patients without vertebral or femur fractures | 46,454 | 76.6 | 23.4 | 44,334 | 62.2 | 37.8 | <0.001 |
| Patients with vertebral or femur fractures | 1412 | 76.1 | 23.9 | 1402 | 67.1 | 32.9 | <0.001 |
| Age distribution | |||||||
| 50–59 | 8288 | 74.3 | 25.7 | 7877 | 60.0 | 40.0 | <0.001 |
| 60–69 | 17,106 | 77.5 | 22.5 | 16,303 | 63.5 | 36.5 | <0.001 |
| 70–79 | 16,500 | 77.7 | 22.3 | 15,807 | 63.3 | 36.7 | <0.001 |
| 80–89 | 5643 | 74.5 | 25.5 | 5423 | 60.3 | 39.7 | <0.001 |
| 90+ | 329 | 69.6 | 30.4 | 326 | 52.8 | 47.2 | <0.001 |
| Covariates | OR | 95% CI | p-Value | |
|---|---|---|---|---|
| Age | 1.000 | 0.999 | 1.002 | 0.692 |
| Gender (ref. female) | 1.355 | 1.272 | 1.444 | <0.001 |
| Hypertension (ref. absence) | 0.989 | 0.958 | 1.021 | 0.488 |
| Diabetes (ref. absence) | 1.019 | 0.973 | 1.068 | 0.418 |
| Rheumatoid arthritis (ref. absence) | 0.816 | 0.728 | 0.915 | 0.001 |
| Dyslipidemia (ref. absence) | 0.916 | 0.886 | 0.947 | <0.001 |
| Ischemic heart disease (ref. absence) | 1.046 | 0.866 | 1.264 | 0.638 |
| Cardiac dysrhythmias (ref. absence) | 1.026 | 0.946 | 1.113 | 0.536 |
| Heart failure (ref. absence) | 1.004 | 0.747 | 1.350 | 0.978 |
| Stroke (ref. absence) | 1.206 | 1.024 | 1.420 | <0.05 |
| Dementia (ref. absence) | 1.257 | 0.708 | 2.233 | 0.435 |
| Schizophrenic disorders (ref. absence) | 1.024 | 0.764 | 1.371 | 0.876 |
| COPD (ref. absence) | 0.963 | 0.922 | 1.006 | 0.088 |
| Corticosteroids for systemic use (ref. absence) | 0.962 | 0.923 | 1.004 | 0.073 |
| Platelet aggregation inhibitors excl. heparin (ref. absence) | 0.983 | 0.946 | 1.021 | 0.377 |
| VKA/direct factor Xa inhibitors (ref. absence) | 1.142 | 1.043 | 1.252 | <0.01 |
| Analgesics (ref. absence) | 1.000 | 0.948 | 1.056 | 0.990 |
| Antiepileptics (ref. absence) | 0.937 | 0.880 | 0.999 | <0.05 |
| Antipsychotics (ref. absence) | 1.168 | 1.024 | 1.333 | <0.05 |
| Proton pump inhibitors (ref. absence) | 0.911 | 0.883 | 0.940 | <0.001 |
| Previous fractures (ref. absence) | 0.905 | 0.831 | 0.985 | <0.05 |
| Cohort | ||||
| Vitamin D treated in P1 | 1.000 | |||
| Vitamin D treated in P2 | 1.979 | 1.924 | 2.036 | <0.001 |
| Osteoporosis Treatment Pre-Note 96 | Osteoporosis Treatment Post-Note 96 | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Period | Period 1 Osteoporosis Treatment | Period 2 Osteoporosis Treatment | Period 2 No Osteoporosis Treatment | Period 2 Osteoporosis Treatment | Period 3 Osteoporosis Treatment | Period 3 No osteoporosis Treatment | |
| Patient classification | Number of osteoporosis treatment users in March–June 2019 | % of patients still using osteoporosis treatments in July–October 2019 | % of patients without osteoporosis treatments in July–October 2019 | Number of osteoporosis treatment users in July–October 2019 | % of patients still using osteoporosis treatments after Note 96 introduction (November 2019–February 2020) | % of patients without osteoporosis treatments after Note 96 introduction (November 2019–February 2020) | |
| Patients with osteoporosis treatment | 31,089 | 77.3 | 22.7 | 29,578 | 79.6 | 20.4 | <0.001 |
| Patients without vertebral or femur fractures | 30,241 | 77.4 | 22.6 | 28,768 | 79.6 | 20.4 | <0.001 |
| Patients with vertebral or femur fractures | 848 | 74.8 | 25.2 | 810 | 79.1 | 20.9 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposti, L.D.; Perrone, V.; Sella, S.; Arcidiacono, G.; Bertoldo, F.; Giustina, A.; Minisola, S.; Napoli, N.; Passeri, G.; Rossini, M.; et al. The Potential Impact of Inducing a Restriction in Reimbursement Criteria on Vitamin D Supplementation in Osteoporotic Patients with or without Fractures. Nutrients 2022, 14, 1877. https://doi.org/10.3390/nu14091877
Esposti LD, Perrone V, Sella S, Arcidiacono G, Bertoldo F, Giustina A, Minisola S, Napoli N, Passeri G, Rossini M, et al. The Potential Impact of Inducing a Restriction in Reimbursement Criteria on Vitamin D Supplementation in Osteoporotic Patients with or without Fractures. Nutrients. 2022; 14(9):1877. https://doi.org/10.3390/nu14091877
Chicago/Turabian StyleEsposti, Luca Degli, Valentina Perrone, Stefania Sella, Gaetano Arcidiacono, Francesco Bertoldo, Andrea Giustina, Salvatore Minisola, Nicola Napoli, Giovanni Passeri, Maurizio Rossini, and et al. 2022. "The Potential Impact of Inducing a Restriction in Reimbursement Criteria on Vitamin D Supplementation in Osteoporotic Patients with or without Fractures" Nutrients 14, no. 9: 1877. https://doi.org/10.3390/nu14091877
APA StyleEsposti, L. D., Perrone, V., Sella, S., Arcidiacono, G., Bertoldo, F., Giustina, A., Minisola, S., Napoli, N., Passeri, G., Rossini, M., & Giannini, S., on behalf of the LHU Study Group. (2022). The Potential Impact of Inducing a Restriction in Reimbursement Criteria on Vitamin D Supplementation in Osteoporotic Patients with or without Fractures. Nutrients, 14(9), 1877. https://doi.org/10.3390/nu14091877








