A Healthier Smile in the Past? Dental Caries and Diet in Early Neolithic Farming Communities from Central Germany
Abstract
:1. Introduction
2. Materials and Methods
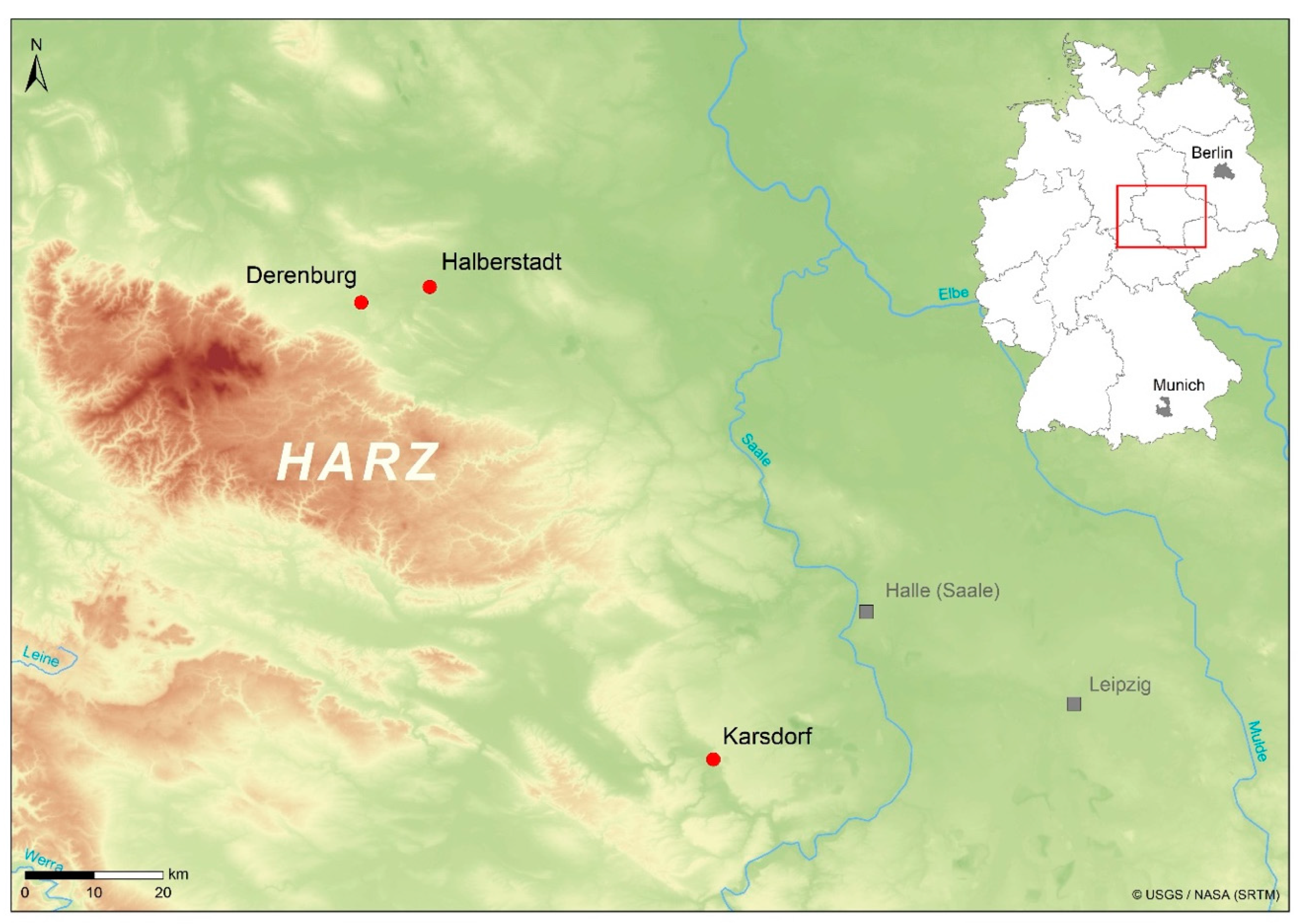
2.1. Material
2.2. Methods
2.2.1. Age and Sex Determination
2.2.2. Morphological Examination of the Teeth
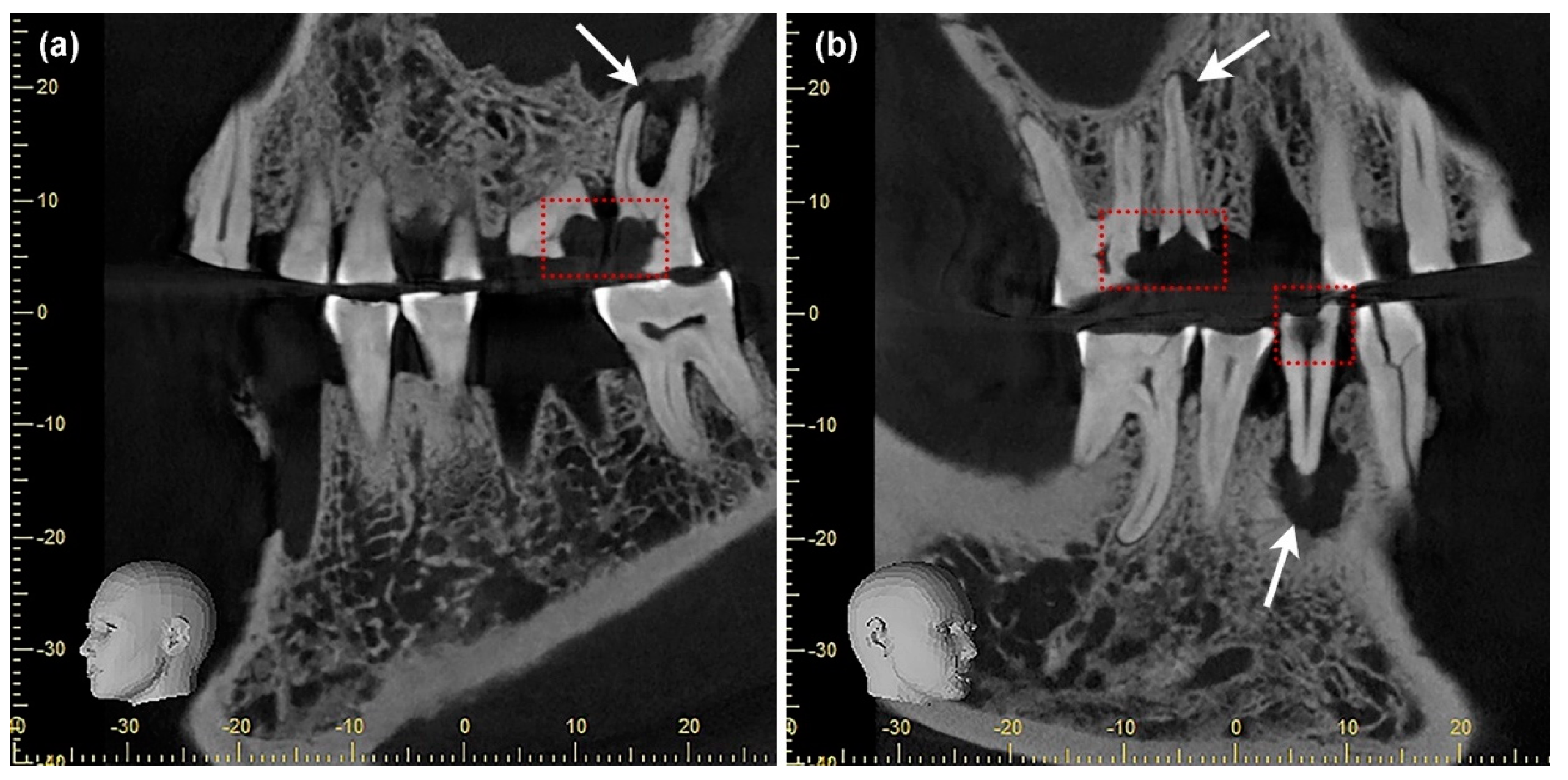
2.2.3. Digital Volume Tomography (DVT)
2.2.4. Stable Isotope Data
2.2.5. Statistical Data Analysis
3. Results
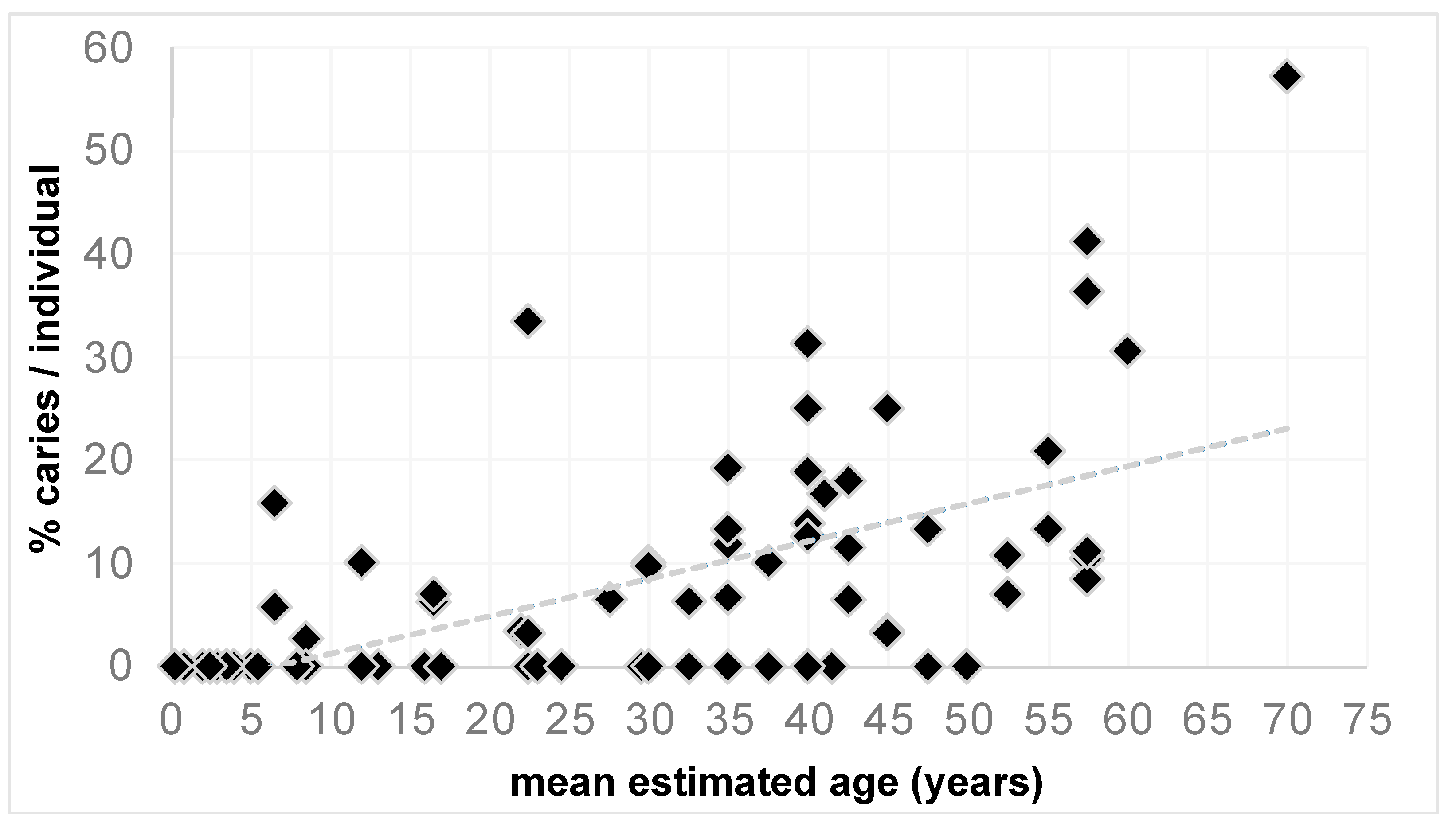
3.1. Age-Specific Differences
3.2. Sex-Related Differences
3.3. Affected Tooth Types
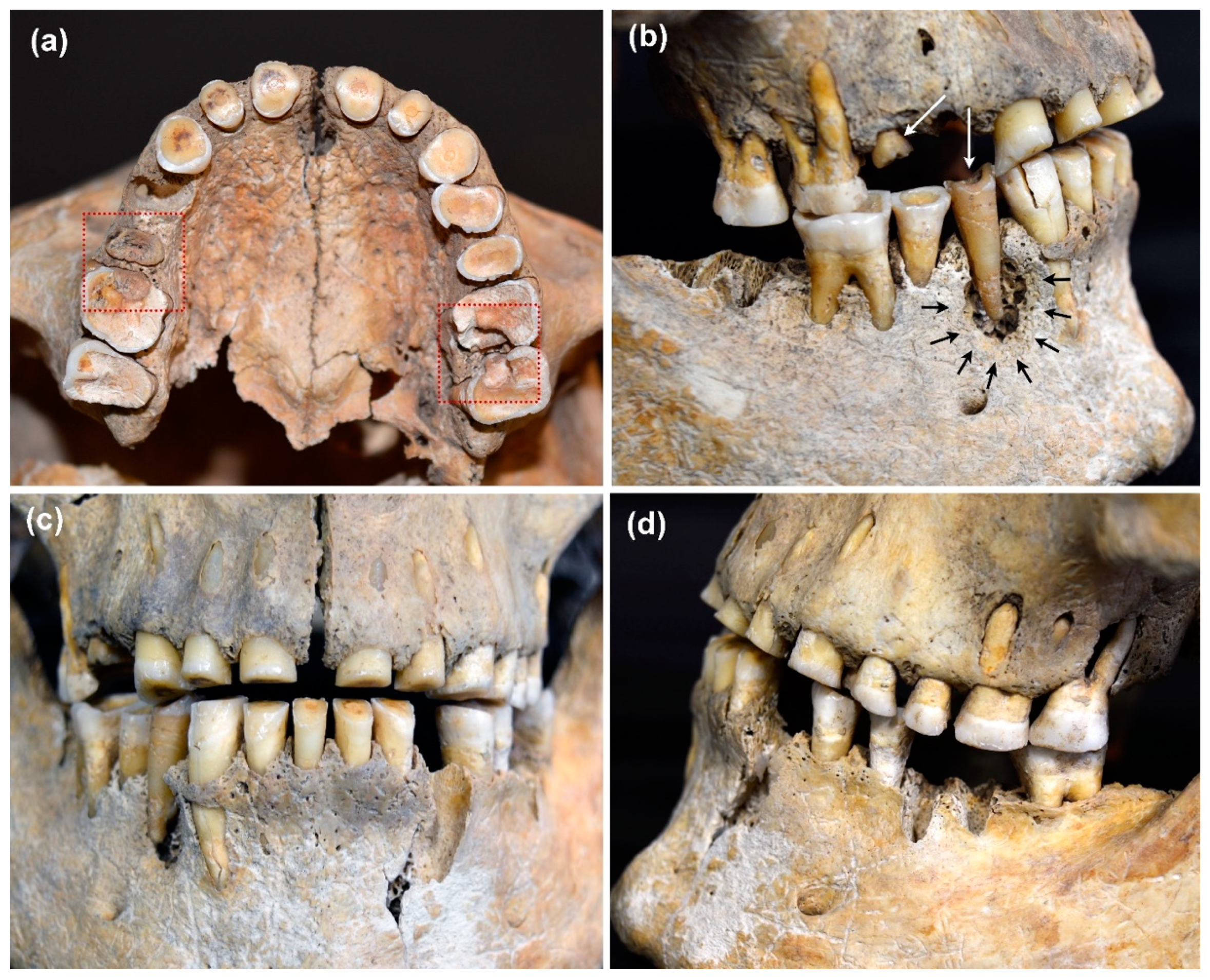
3.4. Severity of Caries Lesions
3.5. Stable Isotope Ratios and Diet
4. Discussion
4.1. The Influence of Nutrition
4.2. Age-Specific Differences and Caries Localisation
4.3. Sex-Related Differences
4.4. Influence of Dental Wear
4.5. Comparative Data from Other Neolithic Sites
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Age (Years) | Individuals | Permanent Teeth | Deciduous Teeth | AMTL |
|---|---|---|---|---|
| Derenburg | ||||
| 0–6 | 6 | 54 | 50 | 0 |
| 7–14 | 4 | 84 | 23 | 0 |
| 15–20 | 2 | 44 | 0 | 0 |
| 21–40 | 18 | 390 | 0 | 4 |
| 41–60 | 10 | 204 | 0 | 5 |
| >61 | 0 | 0 | 0 | 0 |
| total | 40 | 776 | 73 | 9 |
| Halberstadt | ||||
| 0–6 | 8 | 40 | 83 | 0 |
| 7–14 | 5 | 65 | 33 | 0 |
| 15–20 | 3 | 58 | 2 | 0 |
| 21–40 | 10 | 231 | 0 | 9 |
| 41–60 | 7 | 178 | 0 | 14 |
| >61 | 0 | 0 | 0 | 0 |
| total | 33 | 572 | 118 | 23 |
| Karsdorf | ||||
| 0–6 | 5 | 11 | 55 | 0 |
| 7–14 | 4 | 45 | 31 | 0 |
| 15–20 | 1 | 29 | 0 | 0 |
| 21–40 | 9 | 252 | 0 | 0 |
| 41–60 | 8 | 204 | 0 | 18 |
| >61 | 1 | 21 | 0 | 5 |
| total | 28 | 562 | 86 | 23 |
References
- Marcenes, W.; Kassebaum, N.J.; Bernabé, E.; Flaxman, A.; Naghavi, M.; Lopez, A.; Murray, C.J. Global burden of oral conditions in 1990–2010: A systematic analysis. J. Dent. Res. 2013, 92, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peres, M.A.; Macpherson, L.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Werneck, R.I.; Mira, M.T.; Trevilatto, P.C. A critical review: An overview of genetic influence on dental caries. Oral Dis. 2010, 16, 613–623. [Google Scholar] [CrossRef]
- Strużycka, I. The Oral Microbiome in Dental Caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef]
- Alt, K.W.; Türp, J.C.; Wächter, R. Periapical Lesions–Clinical and Anthropological Aspects. In Dental Anthropology. Fundamentals, Limits, and Prospects; Alt, K.W., Rösing, F.W., Teschler-Nicola, M., Eds.; Springer: New York, NY, USA; Vienna, Austria, 1998; pp. 247–276. [Google Scholar]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef]
- Lukacs, J.R. Dental paleopathology: Methods for reconstructing dietary patterns. In Reconstruction of Life from the Skeleton; Kennedy, M.Y.I., Kennedy, K.A.R., Eds.; Wiley: New York, NY, USA, 1989; pp. 261–286. [Google Scholar]
- Larsen, C.S. Bioarchaeology: Interpreting Behavior from the Human Skeleton, 2nd ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 67–86. [Google Scholar]
- Caselitz, P. Caries–ancient plague of humankind. In Dental Anthropology. Fundamentals, Limits, and Prospects; Alt, K.W., Rösing, F.W., Teschler-Nicola, M., Eds.; Springer: New York, NY, USA; Vienna, Austria, 1998; pp. 203–226. [Google Scholar]
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar]
- Hillson, S. Dental Anthropology; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Bailey, S.E.; Hublin, J.J. Dental Perspectives on Human Evolution; Springer: Dordrecht, The Netherlands, 2007. [Google Scholar]
- Maixner, F.; Turaev, D.; Cazenave-Gassiot, A.; Janko, M.; Krause-Kyora, B.; Hoopmann, M.R.; Kusebauch, U.; Sartain, M.; Guerriero, G.; O’Sullivan, N.; et al. The Iceman’s Last Meal Consisted of Fat, Wild Meat, and Cereals. Curr. Biol. 2018, 28, 2348–2355.e9. [Google Scholar] [CrossRef] [Green Version]
- Koruyucu, M.; Erdal, Y.S. Reconstruction of dietary habits in the Early Bronze Age of Anatolia through the analysis of dental caries and wear. Int. J. Osteoarchaeol. 2021, 31, 902–915. [Google Scholar] [CrossRef]
- Larsen, C.S.; Shavit, R.; Griffin, M.C. Dental caries evidence for dietary change: An archaeological context. In Advances in Dental Anthropology; Kelley, M.A., Larsen, C.S., Eds.; Wiley-Liss: New York, NY, USA, 1991; pp. 179–202. [Google Scholar]
- Nicklisch, N.; Ganslmeier, R.; Siebert, A.; Friederich, S.; Meller, H.; Alt, K.W. Holes in teeth-Dental caries in Neolithic and Early Bronze Age populations in Central Germany. Ann. Anat. 2016, 203, 90–99. [Google Scholar] [CrossRef]
- Haak, W.; Balanovsky, O.; Sanchez, J.J.; Koshel, S.; Zaporozhchenko, V.; Adler, C.J.; Der Sarkissian, C.S.; Brandt, G.; Schwarz, C.; Nicklisch, N.; et al. Ancient DNA from European early neolithic farmers reveals their near eastern affinities. PLoS Biol. 2010, 8, e1000536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, G.; Haak, W.; Adler, C.J.; Roth, C.; Szécsényi-Nagy, A.; Karimnia, S.; Möller-Rieker, S.; Meller, H.; Ganslmeier, R.; Friederich, S.; et al. Ancient DNA reveals key stages in the formation of Central European mitochondrial genetic diversity. Science 2013, 342, 257–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oelze, V.M.; Siebert, A.; Nicklisch, N.; Meller, H.; Dresely, V.; Alt, K.W. Early Neolithic diet and animal husbandry. Stable isotope evidence from three Linearbandkeramik (LBK) sites in Central Germany. J. Archaeol. Sci. 2011, 38, 270–279. [Google Scholar] [CrossRef]
- DeNiro, M.; Epstein, S. Influence of the Diet on the Distribution of Nitrogen Isotopes in Animals. Geoch. Cosmochim. Acta 1981, 48, 341–351. [Google Scholar] [CrossRef]
- Katzenberg, M.A. Stable Isotope Analysis: A Tool for Studying Past Diet, Demography, and Life History. In Biological Anthropology of the Human Skeleton; Katzenberg, M.A., Saunders, S.R., Eds.; Wiley: Hoboken, NJ, USA, 2008; pp. 411–414. [Google Scholar]
- Masclans, A.; Bickle, P.; Hamon, C. Sexual inequalities in the Early Neolithic? Exploring relationships between sexes/genders at the cemetery of Vedrovice using use-wear analysis, diet and mobility. J. Archaeol. Method Theory 2021, 28, 232–273. [Google Scholar] [CrossRef]
- Friederich, S. Frühneolithikum: Linienbandkeramik bis Gatersleben. In Früh-und Mittelneolithikum; Meller, H., Ed.; Landesamt für Denkmalpflege und Archäologie Sachsen-Anhalt: Halle (Saale), Germany, 2021; pp. 87–98. [Google Scholar]
- Ostritz, S. Naturräumliche Grundlagen der neolithischen Besiedlung im Mittelelbe-Saale-Gebiet (MESG). In Das Neolithikum im Mittelelbe-Saale-Gebiet und der Altmark; Beier, H.-J., Einicke, R., Eds.; Beier & Beran: Wilkau-Haßlau, Germany, 1994; pp. 3–6. [Google Scholar]
- Litt, T. Naturraum Mitteldeutschland im Neolithikum. In Früh-und Mittelneolithikum; Meller, H., Ed.; Landesamt für Denkmalpflege und Archäologie Sachsen-Anhalt: Halle (Saale), Germany, 2021; pp. 119–124. [Google Scholar]
- Nicklisch, N. Spurensuche an Skeletten. Paläodemografische und epidemiologische Untersuchungen an neolithischen und frühbronzezeitlichen Bestattungen aus dem Mittelelbe-Saale-Gebiet im Kontext populationsdynamischer Prozesse. In Forschungsberichte des Landesmuseums für Vorgeschichte Halle; Landesamt für Denkmalpflege und Archäologie Sachsen-Anhalt: Halle (Saale), Germany, 2017. [Google Scholar]
- Fritsch, B.; Claßen, E.; Müller, U.; Dresely, V. Die linienbandkeramischen Gräberfelder von Derenburg "Meerenstieg II" und Halberstadt “Sonntagsfeld”, Lkr. Harz. Jahresschr. Mitteldt. Vorgesch. 2011, 92, 25–229. [Google Scholar]
- Behnke, H.J. Erste Siedler der Linienbandkeramik in der Karsdorfer Feldflur. Ergebnisse der Grabungen im Jahr 2005. Arch. Sachsen-Anhalt 2007, 5, 18–33. [Google Scholar]
- White, T.D.; Folkens, P.A. Human Osteology, 2nd ed.; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Buikstra, J.E.; Ubelaker, D.H. Standards for Data Collection from Human Skeletal Remains. In Proceedings of a Seminar at the Field Museum of Natural History; Arkansas Archeological Survey: Fayetteville, NC, USA, 1994. [Google Scholar]
- Meindl, R.S.; Lovejoy, C.O. Ectocranial suture closure: A revised method for determination of skeletal age at death based on the lateral-anterior sutures. Am. J. Phys. Anthropol. 1985, 68, 57–66. [Google Scholar] [CrossRef]
- Lovejoy, C.O.; Meindel, R.S.; Pryzbeck, T.R.; Mensforth, R.P. Chronological metamorphosis of the auricular surface of the ilium: A new method for the determination of adult skeletal age and death. Am. J. Phys. Anthropol. 1985, 68, 15–28. [Google Scholar] [CrossRef]
- Buckberry, J.L.; Chamberlain, A.T. Age estimation from the auricular surface of the Ilium: A Revised Method. Am. J. Phys. Anthropol. 2002, 119, 231–239. [Google Scholar] [CrossRef]
- Brooks, S.T.; Suchey, J.M. Skeletal age determination based on the os pubis: A comparison of the Acsádi-Nemeskéri and Suchey-Brooks methods. J. Hum. Evol. 1990, 5, 227–238. [Google Scholar] [CrossRef]
- Ubelaker, D.H. Human Skeletal Remains: Excavation, Analysis, Interpretation, 2nd ed.; Taraxacum: Washington, DC, USA, 1998. [Google Scholar]
- Stloukal, M.; Hanáková, H. Die Länge der Längsknochen altslavischer Bevölkerungen unter besonderer Berücksichtigung von Wachstumsfragen. HOMO 1978, 29, 53–69. [Google Scholar]
- Ferembach, D.; Schwidetzky, I.; Stloukal, M. Recommendations for age and sex diagnoses of skeletons. J. Hum. Evol. 1980, 9, 517–549. [Google Scholar] [CrossRef]
- Phenice, T.W. A newly developed visual method of sexing the os pubis. Am. J. Phys. Anthropol. 1969, 30, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Murail, P.; Bruzek, J.; Houët, F.; Cunha, E. DSP: A tool for probabilistic sexdiagnosis using worldwide variability in hip-bone measurements. Bull. Mem. Soc. Anthropol. Paris 2005, 17, 167–176. [Google Scholar] [CrossRef]
- Knussmann, R. Vergleichende Biologie des Menschen. In Lehrbuch der Anthropologie und Humangenetik; Fischer: Stuttgart, Germany, 1996; p. 169. [Google Scholar]
- Alt, K.W.; Türp, J.C. Roll call: Thirty-two white horses on a red field. The advantages of the FDI two-digit system of designating teeth. In Dental Anthropology. Fundamentals, Limits, and Prospects; Alt, K.W., Rösing, F.W., Teschler-Nicola, M., Eds.; Springer: New York, NY, USA; Vienna, Austria, 1998; pp. 41–55. [Google Scholar]
- Alt, K.W. Karies in Vergangenheit und Gegenwart. Zur Epidemiologie einer “Volksseuche”. In Pein und Plagen. Aspekte Einer Historischen Epidemiologie; Kemkes-Grottenthaler, A., Henke, W., Eds.; Edition Archaea: Gelsenkirchen, Germany, 2001; pp. 156–213. [Google Scholar]
- Hillson, S. Recording dental caries in archaeological human remains. Int. J. Osteoarchaeol. 2001, 11, 249–289. [Google Scholar] [CrossRef]
- Machiulskiene, V.; Campus, G.; Carvalho, J.C.; Dige, I.; Ekstrand, K.R.; Jablonski-Momeni, A.; Maltz, M.; Manton, D.J.; Martignon, S.; Martinez-Mier, E.A.; et al. Terminology of Dental Caries and Dental Caries Management: Consensus Report of a Workshop Organized by ORCA and Cariology Research Group of IADR. Caries Res. 2020, 54, 7–14. [Google Scholar] [CrossRef]
- Hillson, S. Teeth; Cambridge University Press: Cambridge, UK, 2005; p. 295. [Google Scholar]
- Richards, M.P.; Hedges, R.E.M. Stable Isotope Evidence for Similarities in the Types of Marine Foods Used by Late Mesolithic Humans at Sites along the Atlantic Coast of Europe. J. Archaeol. Sci. 1999, 26, 717–722. [Google Scholar] [CrossRef]
- Ambrose, S.H. Preparation and Characterization of Bone and Tooth Collagen for Isotopic Analysis. J. Archaeol. Sci. 1990, 17, 431–451. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 11 August 2021).
- Beug, H.-J. Vegetationsgeschichtliche Untersuchungen über die Besiedlung im Unteren Eichsfeld, Landkreis Göttingen, vom frühen Neolithikum bis zum Mittelalter. Neue Ausgr. Forsch. Niedersachs. 1992, 20, 261–339. [Google Scholar]
- Drucker, D.G.; Bridault, A.; Hobson, K.A.; Szuma, E.; Bocherens, H. Can carbon-13 in large herbivores reflect the canopy effect in temperate and boreal ecosystems? Evidence from modern and ancient ungulates. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 266, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Burger, J.; Kirchner, M.; Bramanti, B.; Haak, W.; Thomas, M.G. Absence of the lactase-persistence-associated allele in early Neolithic Europeans. Proc. Natl. Acad. Sci. USA 2007, 104, 3736–3741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fogel, M.L.; Tuross, N.; Owsley, D.W. Nitrogen isotope tracers of human lactation in modern and archaeological populations. In Annual Report of the Director: Geophysical Laboratory; Carnegie Institution: Washington, DC, USA, 1989; Volume 88, pp. 111–117. [Google Scholar]
- Moynihan, P.; Petersen, P.E. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004, 7, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Halvorsrud, K.; Lewney, J.; Craig, D.; Moynihan, P.J. Effects of Starch on Oral Health: Systematic Review to Inform WHO Guideline. J. Dent. Res. 2019, 98, 46–53. [Google Scholar] [CrossRef]
- Adler, C.J.; Dobney, K.; Weyric, L.S.; Kaidonis, J.; Walker, A.W.; Haak, H.; Corey, J.A.; Bradshaw, C.J.A.; Townsend, G.; Sołtysiak, A.; et al. Sequencing ancient calcified dental plaque shows changes in oral micro-biota with dietary shifts of the Neolithic and Industrial revolutions. Nat. Genet. 2013, 45, 450–455. [Google Scholar] [CrossRef] [Green Version]
- Alt, K.W.; Nicklisch, N.; Held, P.; Meyer, C.; Rossbach, A.; Burwinkel, M. Zähne als Gesundheits- und Mortalitätsrisiko. In Traumatologische und pathologische Veränderungen an prähistorischen und historischen Skelettreste. Diagnose, Ursachen und Kontext; Piek, J., Terberger, T., Eds.; Verlag Marie Leidorf: Rahden/Westf., Germany, 2008; pp. 25–42. [Google Scholar]
- Zoellner, H. Dental infection and vascular disease. Semin. Thromb. Hemost. 2011, 37, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Rustemeyer, J.; Bremerich, A. Necessity of surgical dental foci treatment prior to organ transplantation and heart valve replacement. Clin. Oral Investig. 2007, 11, 171–174. [Google Scholar] [CrossRef]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef]
- Bennike, P. Palaeopathology of Danish Skeletons; Akademisk Forlag: Copenhagen, Denmark, 1985. [Google Scholar]
- Lukacs, J.R. Sex differences in dental caries rates with the origin of agriculture in South Asia. Curr. Anthropol. 1996, 37, 147–153. [Google Scholar] [CrossRef]
- Lukacs, J.R.; Largaespada, L.L. Explaining sex differences in dental caries prevalence: Saliva, hormones, and “life-history” etiologies. Am. J. Hum. Biol. 2008, 18, 540–555. [Google Scholar] [CrossRef]
- Peterson, J. Woman’s Share in Neolithic Society: A View from the Southern Levant. Near East. Archaeol. 2016, 79, 132–139. [Google Scholar] [CrossRef]
- Vacca-Smith, A.M.; van Wuyckhouuse, B.C.; Tabak, L.A.; Bowen, W.H. The effect of milk and casein proteins on the adherence of streptococcus mutans to saliva-coated hydroxyapatite. Arch. Oral Biol. 1994, 39, 1063–1069. [Google Scholar] [CrossRef]
- Kashket, S.; DePaola, D.P. Cheese consumption and the development and progression of dental caries. Nutr. Rev. 2002, 60, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Aimutis, W.R. Bioactive properties of milk proteins with particular focus on anticariogenesis. J. Nutr. 2004, 134, S989–S995. [Google Scholar] [CrossRef] [Green Version]
- Lukacs, J.R. Sex differences in dental caries experience: Clinical evidence, complex etiology. Clin. Oral Investig. 2011, 15, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Bergdahl, M. Salivary flow and oral complaints in adult dental patients. Community Dent. Oral Epidemiol. 2000, 28, 59–66. [Google Scholar] [CrossRef]
- Dodds, M.W.J.; Johnson, D.; Yeh, C.-K. Health benefits of saliva: A review. J. Dent. 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Laine, M.; Tenovuo, J.; Lehtonen, O.P.; Ojanotko-Harri, A.; Vilja, P.; Tuohimaa, P. Pregnancy-related changes in human whole saliva. Arch. Oral Biol. 1988, 33, 913–917. [Google Scholar] [CrossRef]
- Salvolini, E.; Di Giorgio, R.; Curatola, A.; Mazzanti, L.; Fratto, G. Biochemical modifications of human whole saliva induced by pregnancy. Br. J. Obstet. Gynaecol. 1998, 195, 656–660. [Google Scholar] [CrossRef]
- Christensen, K.; Gaist, D.; Jeune, B.; Vaupel, J.W. A tooth per child? Lancet 1998, 352, 204–210. [Google Scholar] [CrossRef]
- Laine, M.A. Effect of pregnancy on periodontal and dental health. Acta Odontol. Scand. 2002, 60, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.L.; Ickovics, J.R.; Yaffee, R.A. Parity and untreated dental caries in US women. J. Dent. Res. 2010, 89, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.C.; Ungar, P.S. Gross dental wear and dental microwear in historical perspective. In Dental Anthropology. Fundamentals, Limits, and Prospects; Alt, K.W., Rösing, F.W., Teschler-Nicola, M., Eds.; Springer: New York, NY, USA; Vienna, Austria, 1998; pp. 349–386. [Google Scholar]
- Alt, K.W.; Rossbach, A. Nothing in Nature Is as Consistent as Change. In Comparative Dental Morphology; Koppe, T., Meyer, G., Alt, K.W., Eds.; Karger: Basel, Switzerland, 2009; Volume 3, pp. 190–196. [Google Scholar]
- Sperber, G.H. Dental Wear: Attrition, Erosion, and Abrasion-A Palaeo-Odontological Approach. Dent. J. 2017, 5, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mays, S. The Archaeology of Human Bones; Routledge: London, UK; New York, NY, USA, 1998. [Google Scholar]
- Nasse, D. Betrachtung Abnormaler Abrasionsmuster Prähistorischer Bevölkerungen aus Sachsen-Anhalt. Master’s Thesis, Johannes-Gutenberg-University, Mainz, Germany, 2011. [Google Scholar]
- Alt, K.W.; Pichler, S.L. Artificial modifications of human teeth. In Dental Anthropology. Fundamentals, Limits, and Prospects; Alt, K.W., Rösing, F.W., Teschler-Nicola, M., Eds.; Springer: New York, NY, USA; Vienna, Austria, 1998; pp. 387–415. [Google Scholar]
- Molnar, P. Dental wear and oral pathology: Possible evidence and consequences of habitual use of teeth in a Swedish Neolithic sample. Am. J. Phys. Anthropol. 2008, 136, 423–431. [Google Scholar] [CrossRef]
- Bach, A. Neolithische Populationen im Mittelelbe-Saale-Gebiet. Zur Anthropologie des Neolithikums unter Besonderer Berücksichtigung der Bandkeramiker; Weimarer Monographien zur Ur-und Frühgeschichte: Weimar, Germany, 1978. [Google Scholar]
- Penser, E. Stomatologische Untersuchungen an Erwachsenen Neolithikern aus Dem Mittelelbe-Saale-Gebiet. Ph.D. Thesis, Ludwig-Maximilians-University, Munich, Germany, 1985. [Google Scholar]
- Haschen, S. Stomatologische Untersuchungen an der Linienbandkeramischen Bevölkerung von Wandersleben, Kreis Gotha. Ph.D. Thesis, Friedrich-Schiller-University, Jena, Germany, 1991. [Google Scholar]
- Baum, N. Aiterhofen-Ödmühle. Paläodontologie eines bandkeramischen Gräberfeldes in Niederbayern. Prähist. Zeitschr. 1989, 65, 157–202. [Google Scholar]
- Tiefenböck, B.; Teschler-Nicola, M. Teil II: Anthropologie. In Das Linearbandkeramische Gräberfeld von Kleinhadersdorf; Horjes, B., Ed.; Mitteilungen der Prähistorischen Kommission, Österreichische Akademie der Wissenschaften: Wien, Austria, 2013; Volume 82, pp. 382–392. [Google Scholar]
- Carli-Thiele, P. Spuren von Mangelerkrankungen An Steinzeitlichen Kinderskeletten; Fortschritte in der Palaeopathologie und Osteoarchaeologie; Erich Goltze: Göttingen, Germany, 1996. [Google Scholar]
- Knipper, C. Kohlenstoff-und Stickstoffanalysen an bandkeramischen Bestattungen vom “Viesenhäuser Hof” bei Stuttgart-Mühlhausen: Implikationen zur Ernährungsrekonstruktion, Geschlechtsspezifik und Siedlungsdynamik. In Der Zahn der Zeit; Meyer, C., Held, P., Knipper, C., Nicklisch, N., Eds.; Landesamt für Denkmalpflege und Archäologie Sachsen-Anhalt: Halle (Saale), Germany, 2020; pp. 211–225. [Google Scholar]
- Bach, A.; Bach, H. Zur Anthropologie der Schnurkeramiker. In Beiträge zur Kultur und Anthropologie der Mitteldeutschen Schnurkeramiker II; Bach, A., Bach, H., Gall, W., Feustel, R., Teichert, M., Eds.; Alt-Thüringen: Weimar, Germany, 1975; Volume 13, pp. 43–107. [Google Scholar]
- Holtfreter, J. Zur Anthropologie der Aunjetitzer des Mittelelbe-Saale-Gebietes. In Paläanthropologie im Mittelelbe-Saale-Werra-Gebiet; Feustel, R., Ed.; Weimarer Monographien zur Ur-und Frühgeschichte: Weimar, Germany, 1989; Volume 23, pp. 105–132. [Google Scholar]
- Münster, A.; Knipper, C.; Oelze, V.M.; Nicklisch, N.; Stecher, M.; Schlenker, B.; Ganslmeier, R.; Fragata, M.; Friederich, S.; Dresely, V.; et al. 4000 years of human dietary evolution in central Germany, from the first farmers to the first elites. PLoS ONE 2018, 13, e0194862. [Google Scholar] [CrossRef]
| Archeological Sites | N Excavated Individuals | N Adults | N Subadults | N Individuals with Teeth | N Adults with Teeth | N Subadults with Teeth |
|---|---|---|---|---|---|---|
| DEB | 47 | 32 | 15 | 40 | 28 | 12 |
| HBS | 38 | 18 | 20 | 33 | 17 | 16 |
| KAR | 31 | 20 | 11 | 28 | 18 | 10 |
| Total | 116 | 70 | 46 | 101 | 63 | 38 |
| Site | Adults | Subadults | ||
|---|---|---|---|---|
| Individuals | CF % | Individuals | CF % | |
| DEB | 18/28 | 64.3 | 2/12 | 16.7 |
| HBS | 12/17 | 70.6 | 2/16 | 12.5 |
| KAR | 13/18 | 72.2 | 3/10 | 30.0 |
| total | 43/63 | 68.3 | 7/38 | 18.4 |
| Teeth | CE % | Teeth | CE % | |
| DEB | 52/594 | 8.8 | 4/255 | 1.6 |
| HBS | 49/409 | 12.0 | 4/281 | 1.4 |
| KAR | 52/477 | 10.9 | 5/171 | 2.9 |
| total | 153/1480 | 10.3 | 13/707 | 1.8 |
| Adults with AMTL | ||||
| AMTL by individuals | % | AMTL by alveolar sockets | % | |
| DEB | 5/28 | 17.9 | 9/625 | 1.4 |
| HBS | 3/17 | 17.6 | 23/453 | 5.1 |
| KAR | 6/18 | 33.3 | 23/517 | 4.4 |
| total | 14/63 | 22.2 | 55/1595 | 3.4 |
| AMTL + caries by individuals | % | AMTL + caries by alveolar sockets | % | |
| DEB | 19/28 | 67.9 | 61/625 | 9.8 |
| HBS | 12/17 | 70.6 | 72/453 | 15.9 |
| KAR | 14/18 | 77.8 | 75/517 | 14.5 |
| total | 45/63 | 71.4 | 208/1595 | 13.0 |
| Age (Years) | Indidviduals | % | Permanent Teeth/Alveolar Sockets | % | Deciduous Teeth | % |
|---|---|---|---|---|---|---|
| Caries (CF) | Caries by teeth (CE) | |||||
| 0–6 | 0/19 | 0.0 | 0/105 | 0.0 | 0/188 | 0.0 |
| 7–14 | 5/13 | 38.5 | 2/194 | 1.0 | 8/87 | 9.2 |
| 15–20 | 2/6 | 33.3 | 3/131 | 2.3 | 0/2 | 0.0 |
| 21–40 | 22/37 | 59.5 | 75/873 | 8.6 | --- | --- |
| 41–60 | 20/25 | 80.0 | 66/586 | 11.3 | --- | --- |
| >61 | 1/1 | 100 | 12/21 | 57.1 | --- | --- |
| AMTL | AMTL by alveolar sockets | |||||
| 21–40 | 3/37 | 8.1 | 13/917 | 1.4 | --- | --- |
| 41–60 | 10/25 | 40.0 | 37/652 | 5.7 | --- | --- |
| >61 | 1/1 | 100 | 5/26 | 19.2 | --- | --- |
| all adults | 14/63 | 22.2 | 55/1595 | 3.4 | --- | --- |
| AMTL + caries | AMTL + caries by alveolar sockets | |||||
| 21–40 | 22/37 | 59.5 | 88/917 | 9.6 | --- | --- |
| 41–60 | 22/25 | 84.0 | 103/652 | 15.8 | --- | --- |
| >61 | 1/1 | 100 | 17/26 | 65.4 | --- | --- |
| all adults | 45/63 | 71.4 | 208/1595 | 13.0 | --- | --- |
| Site | Male | % | Female | % | Male | % | Female | % |
|---|---|---|---|---|---|---|---|---|
| Caries by individuals (CF) | Caries by teeth (CE) | |||||||
| DEB | 4/10 | 40.0 | 13/16 | 81.3 | 13/221 | 5.9 | 38/365 | 10.4 |
| HBS | 6/6 | 100 | 6/11 | 54.5 | 24/175 | 13.7 | 27/234 | 11.5 |
| KAR | 7/11 | 63.6 | 5/6 | 83.3 | 14/285 | 4.9 | 35/162 | 21.6 |
| Total | 17/27 | 63.0 | 24/33 | 72.7 | 51/682 | 7.5 | 100/761 | 13.1 |
| AMTL by individuals | AMTL by alveolar sockets | |||||||
| DEB | 0/10 | 0.0 | 5/16 | 31.2 | 0/224 | 0.0 | 9/393 | 2.3 |
| HBS | 1/6 | 16.7 | 2/11 | 18.2 | 6/185 | 3.2 | 17/268 | 6.3 |
| KAR | 3/11 | 27.3 | 3/6 | 50.0 | 12/313 | 3.8 | 11/174 | 6.3 |
| Total | 4/27 | 14.8 | 10/33 | 30.3 | 18/722 | 2.5 | 37/835 | 4.4 |
| AMTL + caries by individuals | AMTL + caries by alveolar sockets | |||||||
| DEB | 4/10 | 40.0 | 14/16 | 87.5 | 13/224 | 5.8 | 47/393 | 12.0 |
| HBS | 6/6 | 100 | 6/11 | 54.5 | 28/185 | 15.1 | 44/268 | 16.4 |
| KAR | 8/11 | 72.7 | 5/6 | 83.3 | 26/313 | 8.3 | 46/174 | 26.4 |
| Total | 18/27 | 66.7 | 25/33 | 75.7 | 67/722 | 9.3 | 137/835 | 16.4 |
| Right Jaw | Left Jaw | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper Jaw | 18 | 17 | 16 | 15 | 14 | 13 | 12 | 11 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 |
| N teeth | 31 | 49 | 49 | 49 | 49 | 51 | 44 | 42 | 45 | 47 | 47 | 47 | 51 | 48 | 48 | 27 |
| N affected | 4 | 7 | 11 | 10 | 8 | 4 | 2 | 1 | 1 | 1 | 2 | 2 | 6 | 11 | 16 | 5 |
| % affected | 12.9 | 14.3 | 22.4 | 20.4 | 16.3 | 7.8 | 4.5 | 2.4 | 2.2 | 2.1 | 4.3 | 4.3 | 11.8 | 22.9 | 33.3 | 18.5 |
| Lower Jaw | 48 | 47 | 46 | 45 | 44 | 43 | 42 | 41 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 |
| N teeth | 35 | 50 | 49 | 51 | 48 | 51 | 47 | 45 | 40 | 47 | 49 | 51 | 53 | 49 | 51 | 40 |
| N affected | 6 | 7 | 7 | 5 | 2 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 4 | 9 | 9 | 8 |
| % affected | 17.1 | 14.0 | 14.3 | 9.8 | 4.2 | 2.0 | 0.0 | 2.2 | 0.0 | 2.1 | 2.0 | 2.0 | 7.5 | 18.4 | 17.6 | 20.0 |
| Grade | DEB | HBS | KAR | Total | ||||
|---|---|---|---|---|---|---|---|---|
| N Teeth | % | N Teeth | % | N Teeth | % | N Teeth | % | |
| 1 | 11 | 21.2 | 20 | 40.8 | 19 | 36.5 | 50 | 32.7 |
| 2 | 24 | 46.2 | 9 | 18.4 | 9 | 17.3 | 42 | 27.5 |
| 3 | 3 | 5.8 | 7 | 14.3 | 10 | 19.2 | 20 | 13.1 |
| 4 | 13 | 25.0 | 3 | 6.1 | 3 | 5.8 | 19 | 12.4 |
| 5 | 1 | 1.9 | 10 | 20.4 | 11 | 21.2 | 22 | 14.4 |
| Total | 52 | 100 | 49 | 100 | 52 | 100 | 153 | 100 |
| Period/Site | CF % | CE % | References | ||
|---|---|---|---|---|---|
| Subadult | Adult | Subadult | Adult | ||
| DEB | 26.7 | 64.3/69.9 ** | 1.6 | 8.8/9.8 ** | this study |
| HBS | 12.5 | 70.6/70.6 ** | 1.4 | 12.0/15.9 ** | this study |
| KAR | 30.0 | 72.2/77.8 ** | 2.9 | 10.9/14.5 ** | this study |
| All sites | 18.4 | 68.3/71.4 ** | 1.2/2.9 * | 10.3/13.0 ** | this study |
| BK/Wandersleben (MES) | --- | 63.8 | --- | 14.4 | [85] ** |
| BK/Wandersleben (MES) | 12.5 | --- | 3.2/0.0 * | --- | [88] |
| BK/Sondershausen (MES) | --- | 69.0 | --- | 11.8 | [83] ** |
| BK/collection | --- | 58.1 | --- | 11.3 | [84] |
| LBK/Aiterhofen (SB) | 36.8 | --- | 5.4/2.8 * | --- | [88] |
| LBK/Aiterhofen (SB) | --- | 37.0 | --- | 9.2 | [86] |
| LBK/Kleinhadersdorf (LA) | --- | 60.7 | 4.9/2.0 * | 7.3 | [87] |
| MN/collection (MES) | 8.0 | 44.0 | 1.3 | 4.9 | [17] |
| LN/collection (MES) | 9.8 | 38.3 | 0.9 | 5.5 | [17] |
| LN/collection (MES) | --- | 36.4 | --- | 6.0 | [90] ** |
| EBA/collection (MES) | 11.4 | 35.6 | 0.9 | 5.8 | [17] |
| EBA/collection (MES) | --- | 38.3 | --- | 6.9 | [91] ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicklisch, N.; Oelze, V.M.; Schierz, O.; Meller, H.; Alt, K.W. A Healthier Smile in the Past? Dental Caries and Diet in Early Neolithic Farming Communities from Central Germany. Nutrients 2022, 14, 1831. https://doi.org/10.3390/nu14091831
Nicklisch N, Oelze VM, Schierz O, Meller H, Alt KW. A Healthier Smile in the Past? Dental Caries and Diet in Early Neolithic Farming Communities from Central Germany. Nutrients. 2022; 14(9):1831. https://doi.org/10.3390/nu14091831
Chicago/Turabian StyleNicklisch, Nicole, Vicky M. Oelze, Oliver Schierz, Harald Meller, and Kurt W. Alt. 2022. "A Healthier Smile in the Past? Dental Caries and Diet in Early Neolithic Farming Communities from Central Germany" Nutrients 14, no. 9: 1831. https://doi.org/10.3390/nu14091831
APA StyleNicklisch, N., Oelze, V. M., Schierz, O., Meller, H., & Alt, K. W. (2022). A Healthier Smile in the Past? Dental Caries and Diet in Early Neolithic Farming Communities from Central Germany. Nutrients, 14(9), 1831. https://doi.org/10.3390/nu14091831






