Abstract
This systematic review and meta-analysis provides a synthesis of the available evidence for the effects of interventions on outcome measures associated with sarcopenia in end-stage kidney disease (ESKD). Thirteen databases were searched, supplemented with internet and hand searching. Randomised controlled trials of non-pharmacological or pharmacological interventions in adults with ESKD were eligible. Trials were restricted to those which had reported measures of sarcopenia. Primary outcome measures were hand grip strength and sit-to-stand tests. Sixty-four trials were eligible (with nineteen being included in meta-analyses). Synthesised data indicated that intradialytic exercise increased hand grip strength (standardised mean difference, 0.58; 0.24 to 0.91; p = 0.0007; I2 = 40%), and sit-to-stand (STS) 60 score (mean difference, 3.74 repetitions; 2.35 to 5.14; p < 0.001; I2 = 0%). Intradialytic exercise alone, and protein supplementation alone, resulted in no statistically significant change in STS5 (−0.78 s; −1.86 to 0.30; p = 0.16; I2 = 0%), and STS30 (MD, 0.97 repetitions; −0.16 to 2.10; p = 0.09; I2 = 0%) performance, respectively. For secondary outcomes, L-carnitine and nandrolone-decanoate resulted in significant increases in muscle quantity in the dialysis population. Intradialytic exercise modifies measures of sarcopenia in the haemodialysis population; however, the majority of trials were low in quality. There is limited evidence for efficacious interventions in the peritoneal dialysis and transplant recipient populations.
1. Introduction
Sarcopenia, originally believed to be a condition related to age, is the term used to indicate a progressive reduction in muscle strength, quantity or quality, and function, and is now considered a muscle disease [1]. It is now recognised as being associated with a number of catabolic diseases. One of these diseases which can expedite changes in measures related to sarcopenia is chronic kidney disease (CKD). Sarcopenia is reported as a common comorbidity in individuals with CKD, with a prevalence of around 10% in non-dialysis-dependent individuals [2,3], and increasing up to 37% in those individuals with end-stage kidney disease [4]. The presence of sarcopenia in individuals with CKD is associated with low quality of life, major adverse cardiovascular events, and mortality [2,5]. The underlying mechanisms of sarcopenia in CKD are believed to revolve around the concomitant loss of strength and muscle mass [6]. The cause of this in the CKD population is multifactorial, and numerous, but negative protein balance, sedentary behaviour, physical inactivity, metabolic acidosis, inflammation, anorexia, and disturbed appetite regulation all play a role [3,7]. The loss of muscle mass and strength is more common in individuals with end-stage kidney disease (ESKD) compared to individuals with less advanced kidney disease [8,9].
There is currently a lack of effective interventions for the treatment of sarcopenia, particularly in the ESKD population. However, a previous clinical practice guideline has provided strong recommendations for exercise as the primary treatment of sarcopenia [10]. The evidence for other non-pharmacological interventions such as nutritional is less clear [11]. Currently, there are no specific drugs approved for the treatment of sarcopenia; however, recently there has been a growing interest in new therapeutic approaches in the CKD population [12]. Therefore, the aim of this systematic review (and meta-analysis) was to investigate the effect of non-pharmacological and pharmacological interventions on outcome measures associated with sarcopenia (as defined by the European Working Group on Sarcopenia in Older People (EWGSOP) [1]) in the ESKD population.
2. Materials and Methods
2.1. Protocol Registration
Methods were prespecified and documented in a protocol that was registered on International Prospective Register of Systematic Reviews; www.crd.york.ac.uk/PROSPERO (PROSPERO) with the identifier CRD42020199301.
2.2. Settings and Trial Population
Individuals with ESKD who have received a transplant, or are receiving dialysis (haemodialysis and peritoneal dialysis) or conservative management (for those with an estimated glomerular filtration rate <15) over the age of 18 years were included.
2.3. Intervention
Trials were considered eligible if they contained non-pharmacological (for the purpose of this review, these were defined as either containing diet, exercise, or lifestyle components) or pharmacological interventions (e.g., growth hormone, combined oestrogen-progesterone, dehydroepinadorsterone).
2.4. Comparison
Any concurrent control group who is receiving usual care could serve as the control. Control groups that receive usual care or a placebo (for dietary or pharmacological interventions), or who did not receive an intervention designed to modulate sarcopenia were included. Exercise trials that had included active control groups (e.g., stretching) were excluded, as were trials of acute interventions.
2.5. Outcome
Recently, the European Working Group on Sarcopenia in Older People (EWGSOP) published a consensus paper [1] highlighting a number of outcome measures to assess, confirm, and determine severity of sarcopenia. The outcomes in this review were chosen as a result of their inclusion in this paper. The primary outcome was muscle strength (hand grip strength (HGS) and the following sit-to-stand tests (STS), 5, 30, and 60). The secondary outcomes were muscle quality and quantity (assessed by magnetic resonance imaging (MRI), dual-energy X-ray absorptiometry (DEXA), bioelectrical impedance analysis (BIA), and computed tomography (CT) imaging), physical performance (assessed by the short physical performance battery (SPPB), the timed-up-and-go test (TUG), 400 m walk test, and gait speed), and sarcopenia health-related quality of life as assessed by the SARQoL questionnaire.
2.6. Trial Design
Trials included in this review had to have adhered to the following trial designs: parallel-group randomised controlled trials (allocation at individual or cluster levels) or crossover randomised trials.
2.7. Search Strategy
Searches were conducted to identify any relevant completed or ongoing systematic reviews using the following resources: Cochrane, PROSPERO, and the National Health Service Centre for Reviews and Dissemination (Health Technology Assessment (HTA) and Database of Abstracts of Reviews of Effects (DARE)). The following bibliographical databases and trial registers were searched for completed and ongoing trials: MEDLINE, EMBASE, CINAHL, Cochrane Central Register of Controlled Trials (CENTRAL), ClinicalTrials.gov, and the ISCRTN Registry. British Library (ETHOS), OpenGrey, and Conference Proceedings Citation Index (Web of Science™ Core Collection) were searched for unpublished data. All databases were searched from inception to 19 July 2021, and no limits on language were set. Database searches were supplemented with internet searches (e.g., Google Scholar), and contact with the Physical Activity and Wellbeing Kidney Research Study Group (in the United Kingdom). An example of a full search strategy for MEDLINE, EMBASE, and CINAHL databases is presented in Tables S1 and S2. Other databases were searched by using different combinations Wof these search terms. Search results were compiled using the web-based screening and data extraction tool Covidence (Veritas Health Innovation Ltd., Melbourne, Australia) as recommended by the Cochrane Collaboration. Duplicate citations were removed, and title and abstracts were screened independently by two reviewers against the inclusion criteria (if there was disagreement, Wthen this was settled through the use of a third reviewer). Full-text articles of trials not excluded based on title or abstracts were retrieved and assessed by two reviewers. Conference abstracts and trials included on registries only (e.g., ClinicalTrials.gov) were excluded.
2.8. Selection Criteria, Data Extraction, and Quality Appraisal
We developed, tested, and refined a structured data collection form based on the Cochrane Data Extraction Template for interventions. For each included trial, information on trial methods, participants, interventions/comparator, and outcomes was extracted and cross-checked by one reviewer (DSM). Risk of bias for each trial was assessed using the Cochrane Risk of Bias Tool across five domains. Each domain was classified as adequate, unclear, or inadequate, with risk of bias for each trial to be classified using the following criteria: (1) low risk of bias (all criteria are deemed adequate), (2) moderate risk of bias (one criterion graded as inadequate or two graded as unclear), and (3) high risk of bias (more than one criterion is deemed inadequate, or more than two are graded unclear). Funnel plots were used to visually assess publication bias in the meta-analyses performed for the primary outcome only. Formal testing for plot asymmetry would only be performed where the meta-analysis contains more than ten trials [13].
2.9. Data Synthesis
Where means and standard deviation of outcome measures were not available, they were estimated from medians and interquartile ranges [14]. Gait speed data were converted from cm/s to m/s for one trial [15], and were provided by the authors for another [16]. HGS was converted from lbs to kg for one trial [17]. Data for mid-arm muscle area (MAMA) were subtracted for one trial [18] using Web-Plot Digitizer version 4.5 [19] and 95% confidence intervals were converted to standard deviations [13]. A meta-analysis was performed for trials that reported the same outcome measures using a generic inverse variance random effects method via Review Manager (RevMan) version 5.3.26 (The Cochrane Collaboration, 2020). Primary and secondary measures of efficacy were treated as continuous data and interpreted as either difference in means or standardised mean difference dependent on the methods of measurement. Analysis was based on the final (post-intervention) values only (at last follow-up) with the exception of mean change data from two trials [15,20]. Statistical heterogeneity was interpreted using the I2 value. Data were not pooled (or subgroup analysis was considered) if I2 > 40% (this is the threshold to which heterogeneity is considered important). Separate analysis was performed for each type of population (dialysis and transplant) and each non-pharmacological and pharmacological intervention. We had prospectively planned a network meta-analysis (NMA); however, this was not possible as a result of a limited number of trials for each population reporting the same sarcopenia-associated outcome. In addition, variances between the delivered interventions within the included trials suggested that the transitivity assumption (needed for NMA) was unlikely to be met.
3. Results
3.1. Characteristics of Included Trials
Figure 1 provides a flow diagram of trial selection. Sixty-four trials were eligible for the review (Table 1, Table 2 and Table 3), with 19 trials being included in meta-analyses. Eleven conference abstracts were excluded at the full-text screening stage (due to insufficient information). There were 54 trials in the dialysis population (43 in the haemodialysis, 7 in the peritoneal dialysis, and 4 trials containing both dialysis populations) (Table 1 and Table 2). In total, 23, 20, and 8 trials tested exercise, nutritional supplement, and pharmacological interventions, respectively. Two trials tested both exercise and pharmacological interventions [15,21], and one trial tested an exercise and a nutritional intervention [22]. There were ten eligible trials in the transplant recipient population (Table 3). The most prevalent measurements of muscle strength, muscle quality/quantity, and physical performance in the ESKD population were HGS (n = 26), lean whole body mass (LBM) (n = 29), and gait speed (n = 15), respectively. There were no trials identified that included conservative management participants, and no trial reported the SARQoL questionnaire as an outcome (Table 1, Table 2 and Table 3). Twenty-nine trials (45%) reported an a priori power calculation.
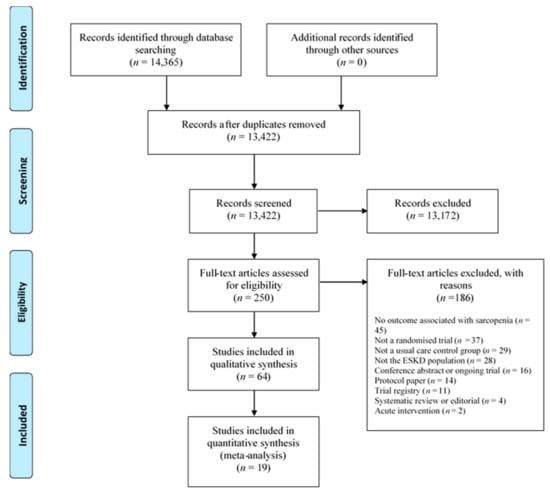
Figure 1.
Prisma flow diagram of trial selection. ESKD = end stage kidney disease.

Table 1.
Characteristics of exercise trials in the peritoneal dialysis and haemodialysis population that reported an outcome associated with sarcopenia.

Table 2.
Characteristics of trials containing either a nutritional or pharmacological intervention in the peritoneal dialysis and haemodialysis population.

Table 3.
Characteristics of trials in the transplant recipient population reporting an outcome associated with sarcopenia.
3.2. Risk of Bias
Risk of bias summaries are provided in Figure A1, Figure A2 and Figure A3. Only 10 (16%) of the included trials were rated as having an overall low risk of bias. Funnel plots are provided in Figure A4 (for the analyses presented in Figure 2, Figure 3, Figure 4 and Figure 5). There was no observation of publication bias.
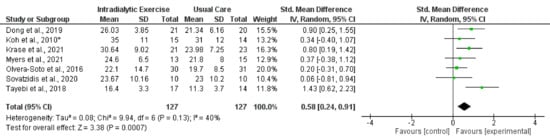
Figure 2.
Effect of intradialytic exercise on grip strength in individuals receiving haemodialysis. Data are expressed as standardised mean difference and 95% CI. * Data for exercise and control groups only [17,25,29,30,34,38,39].
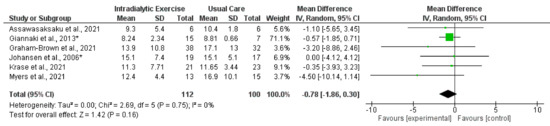
Figure 3.
Effect of intradialytic exercise on sit-to-stand test 5 (seconds) in individuals receiving haemodialysis. Data are expressed as mean difference and 95% confidence interval (CI). * Data for exercise and control groups only [14,15,16,17,21,30].
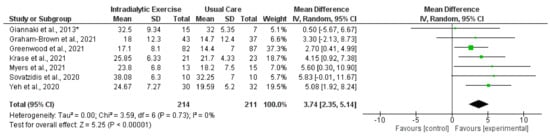
Figure 4.
Effect of intradialytic exercise on sit-to-stand test 60 (repetitions) in individuals receiving haemodialysis. Data are expressed as mean difference and 95% CI. * Data for exercise and control groups only [16,17,21,27,30,38,42].
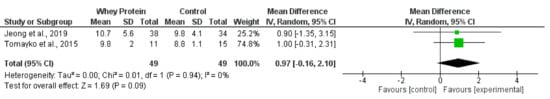
Figure 5.
Effect of whey protein supplementation on sit-to-stand test 30 (repetitions) in individuals receiving haemodialysis. Data are expressed as mean difference and 95% CI. Data for whey protein and control groups from both trials [22,66].
3.3. Muscle Strength
3.3.1. Hand Grip Strength
- Exercise Interventions
Eight trials reported measurement of HGS [17,25,29,30,34,38,39,41] following programmes of intradialytic exercise, with data available from seven trials (all except [41]). The synthesised data showed (254 participants) a statistically significant increase in HGS (standardised mean difference (SMD), 0.58; 0.24 to 0.91; p = 0.0007; I2 = 40%) (Figure 2). Four trials [26,29,35,37] reported data on HGS following exercise programmes taking place outside of dialysis, although there was considerable heterogeneity (I2 = 89%). Two of these trial reported statistically significant increases [26,35], and two reported no significant changes [29,37] (Table 4). One trial in the peritoneal dialysis population [40] reported no changes in HGS following an exercise intervention. There was significant heterogeneity (I2 = 75) between trials (28 participants) investigating the effect of programmes of exercise on HGS [71,73] in transplant recipients, with one trial reporting a significant increase [71]. A further trial showed no effect of a lifestyle intervention [70].

Table 4.
The effect of exercise programmes outside of haemodialysis treatment on grip strength.
- 2.
- Nutritional Interventions
Data from two trials (110 participants) [53,64] investigating the effect of Vitamin D (cholecalciferol) on HGS were available, but there was considerable heterogeneity between the trials (I2 = 60%). Neither trial [53,64] reported any significant change with Vitamin D. Other interventions including L-carnitine [18,67] and keto acid supplementation [58] appeared to have no effect in the dialysis population.
- 3.
- Pharmacological Interventions
Three trials reported measuring HGS following the administration of growth hormone [48,55,56] in the haemodialysis population, but the data were not suitable for meta-analysis. Individual data from two of these trials showed no statistically significant increase [48,55]. Two trials investigated the effect of anabolic steroid supplementation on HGS, one reported a significant increase [63], whilst there was no change reported in the other [20].
3.3.2. Sit-to-Stand
- Exercise Interventions
Synthesised data from six trials (212 participants) [14,15,16,17,21,30] indicated that intradialytic exercise resulted in no statistically significant change in STS5 score (mean difference (MD), −0.78 s; 95% confidence interval, −1.86 to 0.30; p = 0.16; I2 = 0%) (Figure 3). For STS60 score, intradialytic exercise (data from seven trials (425 participants) [16,17,21,27,30,38,42]) resulted in a statistically significant increase (MD, 3.74 repetitions; 2.35 to 5.14; p < 0.0001; I2 = 0%) (Figure 4). A further trial in 296 dialysis participants showed that a programme of home-based walking significantly increased STS5 score compared to a control group [31]. For the peritoneal dialysis population, one trial [23] reported no statistically significant change in STS30 following a programme of exercise. Data from two trials (62 participants) [69,71] was available in the transplant population investigating the effect of programmes of exercise on STS60; however, there was considerable statistical heterogeneity between trials (I2 = 83%). Individually both trials reported statistically significant increases in STS60 (only for the resistance group in one trial [69]).
- 2.
- Nutritional Interventions
Synthesised data from two trials [22,66] in the haemodialysis population (98 participants) indicated that oral whey protein supplementation resulted in no statistically significant change in STS30 score (MD, 0.97 repetitions; −0.16 to 2.10; p = 0.09; I2 = 0%) (Figure 5). There was no significant effect of pomegranate extract [68] or beta-hydroxy-beta-methylbutyrate [49] supplementation on STS30.
- 3.
- Pharmacological Intervention
One trial reported a lack of effect of anabolic steroids on STS5 [15].
3.4. Muscle Quality/Quantity
3.4.1. Exercise Interventions
Data from four trials [14,15,21,32] reported measurement of LBM using DEXA following intradialytic exercise. Synthesised data from three trials [14,15,21] (70 participants) reported a non-statistically significant effect (MD, 0.63 kg; −3.46 to 4.72; p < 0.76; I2 = 0%) (Figure 6). Mean change data for mid-thigh cross-sectional area (MT-CSA) ([21,24] and fat-free mass (FFM)) [25,28] were available from two trials each (which included programmes of intradialytic exercise); respectively, there was considerable heterogeneity between trials (I2 = 57% for MT-CSA, and I2 = 54% for FFM); neither outcome was meta-analysed. No significant changes for either of these outcomes were reported in these trials. Four trials involving programmes of exercise in the transplant recipient reported measurement of LBM [72,73,74,76], with data available from two trials [73,74] (107 participants) for synthesis, although there was considerable heterogeneity (I2 = 78%); resultantly, a meta-analysis was not performed. One trial reported a statistically significant increase [73], whilst another reported no difference between the intervention and control groups [74]. Trials involving lifestyle interventions of nutrition counselling and exercise/physical activity programmes [70,77] reported lack of effects on MAMA [70], LBM, [70,77], or FFM [70].
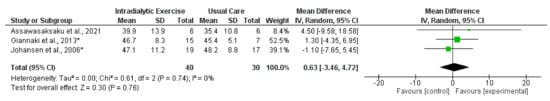
Figure 6.
Effect of intradialytic exercise on lean whole body mass (kg) measured by DEXA in individuals receiving haemodialysis. Data are expressed as mean difference and 95% CI. * Data for exercise and control groups only [14,15,21].
3.4.2. Nutritional Interventions
Synthesised data from two trials [18,43] including 108 haemodialysis participants indicated that L-carnitine supplementation significantly increased MAMA (MD, 3.10 cm2; 0.92 to 5.28; p = 0.005; I2 = 0%) (Figure 7). One of these trials [18] also reported data for LBM, skeletal muscle mass, and appendicular lean mass (ALM) with no statistically significant change in these outcomes following L-carnitine supplementation. Synthesised data from two trials [22,66] in the haemodialysis population (98 participants) indicated that oral whey protein supplementation resulted in no statistically significant effect on LBM (MD, −1.55 kg; −4.25 to 1.14; p = 0.26; I2 = 0%) (Figure S1). Data were reported on LBM from trials investigating a number of heterogeneous nutritional interventions (see Table 2). Individual results from these trials reported statistically significant increases in LBM following water-soluble vitamin supplementation [44], amino acid supplementation [54], and creatine supplementation [60]. Other trials reported data for ALM [49], MAMA [44], and FFM [45,51,62] and individually reported no significant changes (see Table 2 for interventions). For the peritoneal dialysis population, data from three trials were available reporting on the effect of protein supplementation on mid-arm muscle circumference (MAMC) [50,61,65]; there was heterogeneity between trials (p = 45%). One trial reported a statistically significant increase in MAMC (along with LBM) [65], whilst there was no change for this variable in the other two trials [50,61].
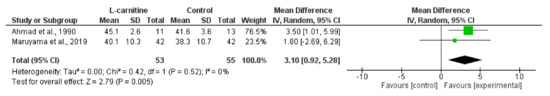
Figure 7.
Effect of L-carnitine supplementation on mid-arm muscle area in individuals receiving haemodialysis. Data are expressed as mean difference and 95% CI [18,43].
3.4.3. Pharmacological Interventions
Synthesised mean change data from two trials [15,20] investigating the effect of nandrolone decanoate (an anabolic steroid) on LBM showed a statistically significant increase (MD, 3.10 kg; 2.12 to 4.08; p < 0.044; I2 = 0%) (Figure 8). One of these trials [15] also reported a significant increase in MT-CSA, and another has shown an increase in FFM following oxymetholone [63]. Mean change data were available for LBM from three trials [48,52,57] investigating the effect of growth hormone. There was considerable heterogeneity between trials (I2 = 75%). Two trials reported significant increases in LBM following growth hormone injections compared to placebo [48,52]. In two trials investigating the effect of early steroid withdrawal in transplant recipients there was no effect of this on LBM [75,78].
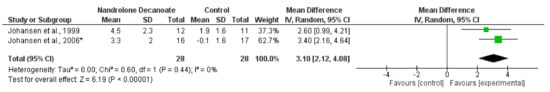
Figure 8.
Effect of nandrolone decanoate on lean whole body mass (mean change data) measured by DEXA in haemodialysis patients. Data are expressed as mean difference and 95% CI. * Data for nandrolone decanoate and control groups only [15,20].
3.5. Physical Performance
3.5.1. Gait Speed
- Exercise Interventions
Eight trials [14,15,16,21,25,27,33,41] reported measurement of gait speed [16]. Data were available for synthesis from five trials (364 participants) [15,16,25,27,33]; there was a significant increase in gait speed following intradialytic exercise (SMD, 0.24; 0.03 to 0.44; p = 0.03; I2 = 0%) (Figure 9). In transplant recipients, one trial [70] found no effect of a lifestyle intervention on gait speed.
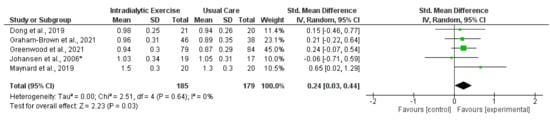
Figure 9.
Effect of intradialytic exercise on gait speed (m/s) in individuals receiving haemodialysis. Data are expressed as standardised mean difference and 95% CI. * Data for exercise and control groups only [15,16,25,27,33].
- 2.
- Nutritional Interventions
Synthesised data from two trials [22,66] (98 participants) indicated that oral whey protein resulted in no significant effect on gait speed (MD, 0.08 m/s; −0.02 to 0.18; p = 0.12; I2 = 0%) (Figure 10). Two trials showed no effect of creatine supplementation [60] or beta-hydroxy-beta-methylbutyrate supplementation [49] on gait speed.
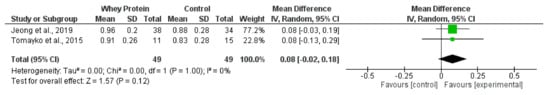
Figure 10.
Effect of whey protein supplementation on gait speed (m/s) in individuals receiving haemodialysis. Data are expressed as mean difference and 95% CI. Data for whey protein and control groups from both trials [22,66].
- 3.
- Pharmacological Interventions
Data (which were unsuitable for meta-analysis) were reported for two trials investigating the effect of human growth hormone [48,55]. Only one trial reported a significant increase in gait speed following the administration of growth hormone [55]. Another trial found a lack of effect following anabolic steroid supplementation [15].
3.5.2. Timed-Up-and-Go and Short Physical Performance Battery
- Exercise Intervention
Synthesised data from two trials (69 haemodialysis participants) [29,33] for TUG reported no significant effect (MD, −1.05 s; −2.12 to 0.02; p = 0.06; I2 = 0%) (Figure 11) following intradialytic exercise. Moreover, a supervised programme of exercise performed on non-dialysis days significantly improved TUG [26]. Programmes of home-based walking [29,36] and intradialytic exercise [16] did not significantly improve SPPB [16,36] or TUG [29]. In contrast, one trial [23] in the peritoneal dialysis population and another in transplant recipients [71] demonstrated significant increases in TUG following programmes of exercise.
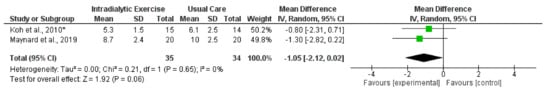
Figure 11.
Effect of intradialytic exercise on timed-up-and-go score (s) in individuals receiving haemodialysis. Data are expressed as mean difference and 95% CI. * Data for exercise and control groups only [29,33].
- 2.
- Nutritional Intervention
Synthesised data from two trials [22,66] (98 participants) indicated that oral whey protein resulted in no change in TUG (MD, −0.54 s; −1.33 to 0.25; p = 0.18; I2 = 0%) (Figure S2).
4. Discussion
This is the first review that has aimed to synthesise the effect of non-pharmacological and pharmacological interventions for sarcopenia outcomes (using the most up-to-date and widely accepted definition [1]) in the ESKD population. The main findings of this review were that intradialytic exercise significantly improved measures of muscle strength (HGS and STS60) and physical performance as measured by gait speed. However, the majority of trials included in the review were considered to be at high risk of bias. There was some evidence that programmes of exercise in transplant recipients may improve STS scores. The evidence for nutritional and pharmacological interventions was less clear, with some tentative evidence that L-carnitine and nandrolone decanoate may have favourable effects on muscle quantity (MAMA and LBM, respectively) in individuals receiving haemodialysis. There was a lack of evidence for efficacious interventions to treat sarcopenia in the transplant and peritoneal dialysis population, and there were no included trials in those individuals with ESKD receiving conservative management.
A recent systematic review exploring the effect of exercise interventions on objective physical function in the ESKD population [79] reported that the majority of included trials reported a significant improvement in STS and HGS, although unlike the present review they were not able to perform a meta-analysis for these outcomes. This is in agreement with another review [80] that demonstrated that exercise training in the haemodialysis population was able to increase muscle strength. Our review confirms that exercise is efficacious at modifying outcomes associated with sarcopenia; however, the evidence for pharmacological and nutritional interventions is less clear. This review included trials with a number of heterogeneous nutritional and pharmacological interventions with a lack of evidence for their efficacy on measures of sarcopenia. However, this is with the exception of synthesised data for L-carnitine and nandrolone-decanoate showing modifications to MAMA and LBM. However, it is unclear whether changes to these outcomes would translate to improvement in muscle strength and function.
Sarcopenia is highly prevalent in CKD [3], particularly for those with the advanced stages of the disease (ESKD) [6]. It is associated with hard endpoints including cardiovascular events and mortality [2,5]. With prevalence of ESKD projected to increase [81], identifying effective interventions for the treatment of sarcopenia is particularly relevant. Therefore, the finding of this review, that intradialytic exercise improves HGS and gait speed, has clinical significance. A low walk (gait) speed has been shown to be associated with mortality in 752 individuals receiving dialysis [82], with a walk speed of >0.6 m/s associated with greater survival [82]. Another study [83] has also reported that both low gait speed and HGS are predictors of cardiovascular events and all-cause mortality in individuals receiving haemodialysis [83]. This supports the recent shift from low muscle mass to low muscle strength as a key characteristic for the diagnosis of sarcopenia [1], as low muscle strength appears to be better at predicting outcomes [3,84]. Furthermore, muscle strength (STS and HGS) can be easily evaluated in the clinical setting (outpatient clinics and dialysis units, etc.). The evidence from this review that intradialytic exercise increases muscle strength, coupled with recent RCT data [16] (that this mode of exercise improves cardiovascular health and is safe), suggests that the methods of implementation should be considered as outlined in the recent Clinical Practice Guideline for Exercise and Lifestyle in CKD [85].
It is believed that increasing protein intake may be an effective countermeasure to sarcopenia for individuals with CKD. This is highlighted by the recommendation of increased intake (compared to the general population) for individuals with ESKD in the updated KDOQI Clinical Practice Guideline for Nutrition in CKD [86]. However, the present review found limited current RCT evidence for the efficacy of protein supplementation for sarcopenia in CKD, a point that has recently been highlighted by others [6]. Protein without an adequate exercise stimulus often provides little benefit, although notably the largest RCT to date in the ESKD population investigating the combined effect of exercise and protein supplementation found no effect on muscle strength or function [22]. This review identified a limited number of trials in the peritoneal dialysis and transplant recipient population. Given the positive effects that we have seen for exercise interventions (particularly for muscle strength in the haemodialysis population), it would be prudent to test these in future RCTs involving other ESKD populations. A recent review article [6] has highlighted a number of pharmacological interventions as having the potential to mitigate sarcopenia in the CKD population. However, this review found no evidence for the benefit of pharmacological interventions on muscle strength. There was some indication from synthesised data that nandrolone-decanoate increases LBM and individual data from two trials show that growth hormone may improve LBM. Whether these changes may improve outcome is unlikely. A previous trial of nandrolone decanoate in individuals with rheumatoid arthritis found an increase in LBM but no accompanying change in muscle strength [87]. Properly powered (<50% of the included trials reported an a priori sample size calculation) trials are required to test both the efficacy and safety of pharmacological and nutritional interventions in the ESKD population. This should enable a wide range of evidence-based therapeutics to be available in line with a personalised medicine approach to tackling sarcopenia. Lastly, although we have shown that exercise programmes may be an effective countermeasure to sarcopenia in the ESKD population, there remains a lack of evidence for these interventions on associated hard endpoints such as cardiovascular events and mortality. Despite the inclusion of 64 trials in the review, only a small number of these were able to be included in meta-analyses (with only fifteen trials being included in analyses for the primary outcome (muscle strength)) and the majority were assessed as having a high risk of bias.
5. Conclusions
Currently, exercise appears to be the strongest therapeutic intervention for sarcopenia in the end-stage kidney disease population. There is a lack of proven efficacy for nutritional and pharmacological interventions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14091817/s1, Table S1: Full search strategy for MEDLINE and CINAHL databases; Table S2: Full search strategy for EMBASE databases; Figure S1: Effect of whey protein supplementation on lean body mass (kg); Figure S2: Effect of whey protein supplementation on timed-up-and-go in individuals receiving haemodialysis.
Author Contributions
Conceptualisation, D.S.M. and J.O.B.; methodology, D.S.M., T.B., T.J.W., R.E.B., K.J., L.A.B., A.T., K.A.R. and A.W.J.; formal analysis, D.S.M. and T.B.; draft writing—original draft preparation, D.S.M. and A.W.J.; writing—review and editing, D.S.M., T.B., T.J.W., R.E.B., K.J., L.A.B., A.T., K.A.R., E.L.W., M.P.M.G.-B., A.W.J. and J.O.B.; supervision, J.O.B.; project administration, D.S.M. All authors contributed substantially to the work reported. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available upon a request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
Risk of bias for included exercise, nutrition, and pharmacological intervention trials in the dialysis population, and included transplant trials (assessed using the Cochrane Risk of Bias tool). Unclear risk of bias is indicated by “?”, low risk of bias “+”, high risk of bias “-”.
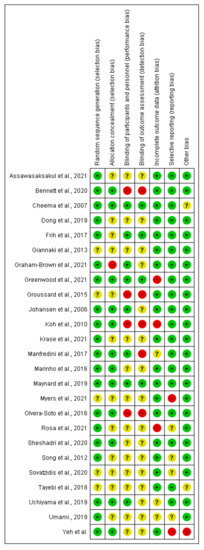
Figure A1.
Exercise trials in the dialysis population [14,15,16,17,21,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42].
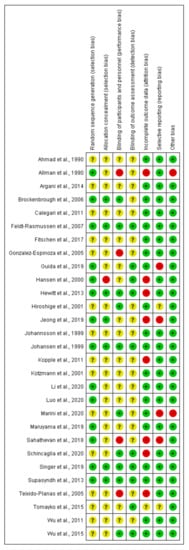
Figure A2.
Nutrition and pharmacological trials in the dialysis population [18,20,22,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68].
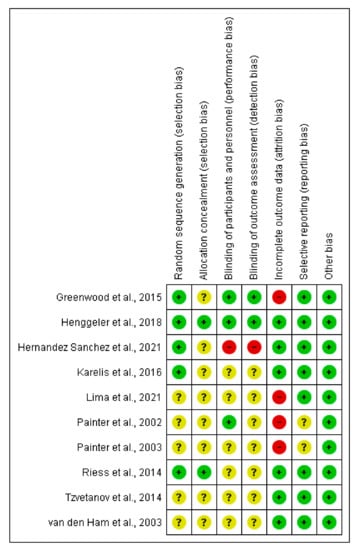
Figure A3.
Transplant trials [69,70,71,72,73,74,75,76,77,78].
Appendix B
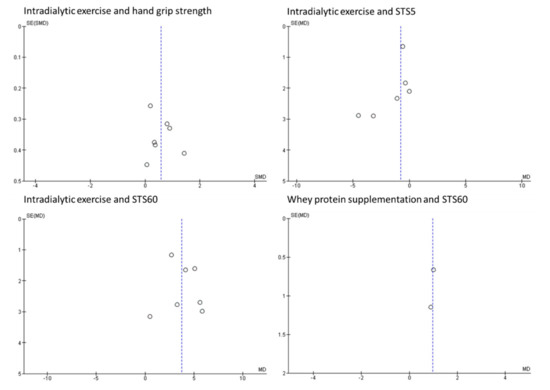
Figure A4.
Funnel plots for primary outcomes. SE = standard error, MD = mean difference.
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.A.; Cordeiro, A.C.; Avesani, C.M.; Carrero, J.J.; Lindholm, B.; Amparo, F.C.; Amodeo, C.; Cuppari, L.; Kamimura, M.A. Sarcopenia in chronic kidney disease on conservative therapy: Prevalence and association with mortality. Nephrol. Dial. Transplant. 2015, 30, 1718–1725. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Miksza, J.; Yates, T.; Lightfoot, C.J.; Baker, L.A.; Watson, E.L.; Zaccardi, F.; Smith, A.C. Association of sarcopenia with mortality and end-stage renal disease in those with chronic kidney disease: A UK Biobank study. J. Cachexia Sarcopenia Muscle 2021, 12, 586–598. [Google Scholar] [CrossRef]
- Kim, J.-K.; Choi, S.R.; Choi, M.J.; Kim, S.G.; Lee, Y.K.; Noh, J.W.; Kim, H.J.; Song, Y.R. Prevalence of and factors associated with sarcopenia in elderly patients with end-stage renal disease. Clin. Nutr. 2014, 33, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Sanchez-Niño, M.D. Sarcopenia in CKD: A roadmap from basic pathogenetic mechanisms to clinical trials. Clin. Kidney J. 2019, 12, 110–112. [Google Scholar] [CrossRef]
- Sabatino, A.; Cuppari, L.; Stenvinkel, P.; Lindholm, B.; Avesani, C.M. Sarcopenia in chronic kidney disease: What have we learned so far? J. Nephrol. 2021, 34, 1347–1372. [Google Scholar] [CrossRef]
- Wang, X.H.; Mitch, W.E. Mechanisms of muscle wasting in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, C.W.; Selby, N.M.; Sigrist, M.; Pearce, L.E.; Mercer, T.H.; Naish, P.F. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol. Dial. Transplant. 2006, 21, 2210–2216. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Nixon, D.G.D.; Richler-Potts, D.; Neale, J.; Song, Y.; Smith, A.C. Identification of the most clinically useful skeletal muscle mass indices pertinent to sarcopenia and physical performance in chronic kidney disease. Nephrology 2020, 25, 467–474. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International clinical practice guidelines for sarcopenia (ICFSR): Screening, diagnosis and management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Lozano-Montoya, I.; Correa-Pérez, A.; Abraha, I.; Soiza, R.L.; Cherubini, A.; O’Mahony, D.; Cruz-Jentoft, A.J. Nonpharmacological interventions to treat physical frailty and sarcopenia in older patients: A systematic overview–the SeNATOR Project ONTOP Series. Clin. Interv. Aging 2017, 12, 721. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Cheung, W.W. MicroRNA as novel exercise mimetic for muscle wasting in CKD. J. Am. Soc. Nephrol. 2017, 28, 2557–2559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Assawasaksakul, N.; Sirichana, W.; Joosri, W.; Kulaputana, O.; Eksakulkla, S.; Ketanun, C.; Kittiskulnam, P.; Chantadisai, M.; Takkavatakarn, K.; Susantitaphong, P.; et al. Effects of intradialytic cycling exercise on daily physical activity, physical fitness, body composition, and clinical parameters in high-volume online hemodiafiltration patients: A pilot randomized-controlled trial. Int. Urol. Nephrol. 2021, 53, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Johansen, K.L.; Painter, P.L.; Sakkas, G.K.; Gordon, P.; Doyle, J.; Shubert, T. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: A randomized, controlled trial. J. Am. Soc. Nephrol. 2006, 17, 2307–2314. [Google Scholar] [CrossRef] [PubMed]
- Graham-Brown, M.P.; March, D.S.; Young, R.; Highton, P.J.; Young, H.M.; Churchward, D.R.; Dungey, M.; Stensel, D.J.; Bishop, N.C.; Brunskill, N.J.; et al. A randomized controlled trial to investigate the effects of intra-dialytic cycling on left ventricular mass. Kidney Int. 2021, 99, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Chan, K.; Chen, Y.; Lit, Y.; Patti, A.; Massaband, P.; Kiratli, B.J.; Tamura, M.; Chertow, G.M.; Rabkin, R. Effect of a Home-Based Exercise Program on Indices of Physical Function and Quality of Life in Elderly Maintenance Hemodialysis Patients. Kidney Blood Press. Res. 2021, 46, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Maruyama, N.; Higuchi, T.; Nagura, C.; Takashima, H.; Kitai, M.; Utsunomiya, K.; Tei, R.; Furukawa, T.; Yamazaki, T.; et al. Efficacy of L-carnitine supplementation for improving lean body mass and physical function in patients on hemodialysis: A randomized controlled trial. Eur. J. Clin. Nutr. 2019, 73, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A. WebPlotDigitizer. 2021. Available online: https://automeris.io/WebPlotDigitizer/ (accessed on 29 January 2022).
- Johansen, K.L.; Mulligan, K.; Schambelan, M. Anabolic effects of nandrolone decanoate in patients receiving dialysis: A randomized controlled trial. JAMA 1999, 281, 1275–1281. [Google Scholar] [CrossRef]
- Giannaki, C.D.; Sakkas, G.K.; Karatzaferi, C.; Hadjigeorgiou, G.M.; Lavdas, E.; Kyriakides, T.; Koutedakis, Y.; Stefanidis, I. Effect of exercise training and dopamine agonists in patients with uremic restless legs syndrome: A six-month randomized, partially double-blind, placebo-controlled comparative study. BMC Nephrol. 2013, 14, 194. [Google Scholar] [CrossRef]
- Jeong, J.H.; Biruete, A.; Tomayko, E.J.; Wu, P.T.; Fitschen, P.; Chung, H.R.; Ali, M.; McAuley, E.; Fernhall, B.; Phillips, S.A.; et al. Results from the randomized controlled IHOPE trial suggest no effects of oral protein supplementation and exercise training on physical function in hemodialysis patients. Kidney Int. 2019, 96, 777–786. [Google Scholar] [CrossRef]
- Bennett, P.N.; Hussein, W.F.; Matthews, K.; West, M.; Smith, E.; Reiterman, M.; Alagadan, G.; Shragge, B.; Patel, J.; Schiller, B.M. An exercise program for peritoneal dialysis patients in the United States: A feasibility study. Kidney Med. 2020, 2, 267–275. [Google Scholar] [CrossRef]
- Cheema, B.; Abas, H.; Smith, B.; O’Sullivan, A.; Chan, M.; Patwardhan, A.; Kelly, J.; Gillin, A.; Pang, G.; Lloyd, B.; et al. Progressive exercise for anabolism in kidney disease (PEAK): A randomized, controlled trial of resistance training during hemodialysis. J. Am. Soc. Nephrol. 2007, 18, 1594–1601. [Google Scholar] [CrossRef]
- Dong, Z.-J.; Zhang, H.-L.; Yin, L.-X. Effects of intradialytic resistance exercise on systemic inflammation in maintenance hemodialysis patients with sarcopenia: A randomized controlled trial. Int. Urol. Nephrol. 2019, 51, 1415–1424. [Google Scholar] [CrossRef]
- Frih, B.; Jaafar, H.; Mkacher, W.; Ben Salah, Z.; Hammami, M.; Frih, A. The effect of interdialytic combined resistance and aerobic exercise training on health related outcomes in chronic hemodialysis patients: The Tunisian randomized controlled study. Front. Physiol. 2017, 8, 288. [Google Scholar] [CrossRef]
- Greenwood, S.A.; Koufaki, P.; Macdonald, J.H.; Bulley, C.; Bhandari, S.; O Burton, J.; Dasgupta, I.; Farrington, K.; Ford, I.; A Kalra, P.; et al. Exercise programme to improve quality of life for patients with end-stage kidney disease receiving haemodialysis: The PEDAL RCT. Health Technol. Assess. 2021, 25, 1. [Google Scholar] [CrossRef]
- Groussard, C.; Rouchon-Isnard, M.; Coutard, C.; Romain, F.; Malardé, L.; Lemoine-Morel, S.; Martin, B.; Pereira, B.; Boisseau, N. Beneficial effects of an intradialytic cycling training program in patients with end-stage kidney disease. Appl. Physiol. Nutr. Metab. 2015, 40, 550–556. [Google Scholar] [CrossRef]
- Koh, K.P.; Fassett, R.G.; Sharman, J.; Coombes, J.; Williams, A. Effect of intradialytic versus home-based aerobic exercise training on physical function and vascular parameters in hemodialysis patients: A randomized pilot study. Am. J. Kidney Dis. 2010, 55, 88–99. [Google Scholar] [CrossRef]
- Krase, A.A.; Terzis, G.; Giannaki, C.D.; Stasinaki, A.N.; Wilkinson, T.J.; Smith, A.C.; Zorz, C.; Karatzaferi, C.; Stefanidis, I.; Sakkas, G.K. Seven months of aerobic intradialytic exercise training can prevent muscle loss in haemodialysis patients: An ultrasonography study. Int. Urol. Nephrol. 2021, 54, 447–456. [Google Scholar] [CrossRef]
- Manfredini, F.; Mallamaci, F.; D’Arrigo, G.; Baggetta, R.; Bolignano, D.; Torino, C.; Lamberti, N.; Bertoli, S.; Ciurlino, D.; Rocca-Rey, L.; et al. Exercise in patients on dialysis: A multicenter, randomized clinical trial. J. Am. Soc. Nephrol. 2017, 28, 1259–1268. [Google Scholar] [CrossRef]
- Marinho, S.M.S.d.A.; Mafra, D.; Pelletier, S.; Hage, V.; Teuma, C.; Laville, M.; Eduardo, J.C.C.; Fouque, D. In hemodialysis patients, intradialytic resistance exercise improves osteoblast function: A pilot study. J. Ren. Nutr. 2016, 26, 341–345. [Google Scholar] [CrossRef]
- Maynard, L.G.; De Menezes, D.L.; Lião, N.S.; De Jesus, E.M.; Andrade, N.L.S.; Santos, J.C.D.; Júnior, W.M.D.S.; Bastos, K.D.A.; Filho, J.A.S.B. Effects of exercise training combined with virtual reality in functionality and health-related quality of life of patients on hemodialysis. Games Health J. 2019, 8, 339–348. [Google Scholar] [CrossRef]
- Olvera-Soto, M.G.; Valdez-Ortiz, R.; Alvarenga, J.C.L.; Espinosa-Cuevas, M.D.L. Effect of resistance exercises on the indicators of muscle reserves and handgrip strength in adult patients on hemodialysis. J. Ren. Nutr. 2016, 26, 53–60. [Google Scholar] [CrossRef]
- Rosa, T.S.; Corrêa, H.L.; Deus, L.A.; Stone, W.; Reis, A.L.; Gadelha, A.B.; de Araújo, T.B.; Junior, P.R.S.; Moraes, M.R.; Silva, J.A.B.; et al. Effects of dynamic and isometric resistance training protocols on metabolic profile in hemodialysis patients: A randomized controlled trial. Appl. Physiol. Nutr. Metab. 2021, 46, 1029–1037. [Google Scholar] [CrossRef]
- Sheshadri, A.; Kittiskulnam, P.; Lazar, A.A.; Johansen, K.L. A walking intervention to increase weekly steps in dialysis patients: A pilot randomized controlled trial. Am. J. Kidney Dis. 2020, 75, 488–496. [Google Scholar] [CrossRef]
- Song, W.-J.; Sohng, K.-Y. Effects of progressive resistance training on body composition, physical fitness and quality of life of patients on hemodialysis. J. Korean Acad. Nurs. 2012, 42, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Sovatzidis, A.; Chatzinikolaou, A.; Fatouros, I.; Panagoutsos, S.; Draganidis, D.; Nikolaidou, E.; Avloniti, A.; Michailidis, Y.; Mantzouridis, I.; Batrakoulis, A.; et al. Intradialytic cardiovascular exercise training alters redox status, reduces inflammation and improves physical performance in patients with chronic kidney disease. Antioxidants 2020, 9, 868. [Google Scholar] [CrossRef]
- Tayebi, M.; Ramezani, A.; Kashef, M. The effect of intradialytic isometric resistance training on muscle capacity and serum albumin levels in hemodialysis patients. Nephro-Urol. Mon. 2018, 10, e65081. [Google Scholar] [CrossRef]
- Uchiyama, K.; Washida, N.; Morimoto, K.; Muraoka, K.; Kasai, T.; Yamaki, K.; Miyashita, K.; Wakino, S.; Itoh, H. Home-based aerobic exercise and resistance training in peritoneal dialysis patients: A randomized controlled trial. Sci. Rep. 2019, 9, 2632. [Google Scholar] [CrossRef] [PubMed]
- Umami, V.; Tedjasukmana, D.; Setiati, S. The effect of intradialytic exercise twice a week on the physical capacity, inflammation, and nutritional status of dialysis patients: A randomized controlled trial. Hemodial. Int. 2019, 23, 486–493. [Google Scholar]
- Yeh, M.-L.; Wang, M.-H.; Hsu, C.-C.; Liu, Y.-M. Twelve-week intradialytic cycling exercise improves physical functional performance with gain in muscle strength and endurance: A randomized controlled trial. Clin. Rehabil. 2020, 34, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Robertson, H.T.; Golper, T.A.; Wolfson, M.; Kurtin, P.; Katz, L.A.; Hirschberg, R.; Nicora, R.; Ashbrook, D.W.; Kopple, J.D. Multicenter trial of L-carnitine in maintenance hemodialysis patients. II. Clinical and biochemical effects. Kidney Int. 1990, 38, 912–918. [Google Scholar] [CrossRef]
- Allman, M.A.; Stewart, P.M.; Tiller, D.J.; Horvath, J.S.; Duggin, G.G.; Truswell, A.S. Energy supplementation and the nutritional status of hemodialysis patients. Am. J. Clin. Nutr. 1990, 51, 558–562. [Google Scholar] [CrossRef]
- Argani, H.; Mahdavi, R.; Ghorbani-Haghjo, A.; Razzaghi, R.; Nikniaz, L.; Gaemmaghami, S.J. Effects of zinc supplementation on serum zinc and leptin levels, BMI, and body composition in hemodialysis patients. J. Trace Elem. Med. Biol. 2014, 28, 35–38. [Google Scholar] [CrossRef]
- Brockenbrough, A.T.; Dittrich, M.O.; Page, S.T.; Smith, T.; Stivelman, J.C.; Bremner, W.J. Transdermal androgen therapy to augment EPO in the treatment of anemia of chronic renal disease. Am. J. Kidney Dis. 2016, 47, 251–262. [Google Scholar] [CrossRef]
- Calegari, A.; Barros, E.G.; Veronese, F.V.; Thomé, F.S. Malnourished patients on hemodialysis improve after receiving a nutritional intervention. J. Bras. de Nefrol. 2011, 33, 394–401. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, B.; Lange, M.; Sulowicz, W.; Gafter, U.; Lai, K.N.; Wiedemann, J.; Christiansen, J.S.; El Nahas, M. Growth hormone treatment during hemodialysis in a randomized trial improves nutrition, quality of life, and cardiovascular risk. J. Am. Soc. Nephrol. 2007, 18, 2161–2171. [Google Scholar] [CrossRef]
- Fitschen, P.J.; Biruete, A.; Jeong, J.H.; Wilund, K.R. Efficacy of beta-hydroxy-beta-methylbutyrate supplementation in maintenance hemodialysis patients. Hemodial. Int. 2017, 21, 107–116. [Google Scholar] [CrossRef]
- González-Espinoza, L.; Gutiérrez-Chávez, J.; del Campo, F.M.; Martínez-Ramírez, H.R.; Cortés-Sanabria, L.; Rojas-Campos, E.; Cueto-Manzano, A.M. Randomized, open label, controlled clinical trial of oral administration of an egg albumin-based protein supplement to patients on continuous ambulatory peritoneal dialysis. Perit. Dial. Int. 2005, 25, 173–180. [Google Scholar] [CrossRef]
- Guida, B.; Parolisi, S.; Coco, M.; Ruoppo, T.; Veccia, R.; di Maro, M.; Trio, R.; Memoli, A.; Cataldi, M. The impact of a nutritional intervention based on egg white for phosphorus control in hemodialyis patients. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 45–50. [Google Scholar] [CrossRef]
- Hansen, T.; Gram, J.; Jensen, P.B.; Kristiansen, J.H.; Ekelund, B.; Christiansen, J.S.; Pedersen, F.B. Influence of growth hormone on whole body and regional soft tissue composition in adult patients on hemodialysis. A double-blind, randomized, placebo-controlled study. Clin. Nephrol. 2000, 53, 99–107. [Google Scholar]
- Hewitt, N.A.; O’Connor, A.A.; O’Shaughnessy, D.V.; Elder, G.J. Effects of cholecalciferol on functional, biochemical, vascular, and quality of life outcomes in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Hiroshige, K.; Sonta, T.; Suda, T.; Kanegae, K.; Ohtani, A. Oral supplementation of branched-chain amino acid improves nutritional status in elderly patients on chronic haemodialysis. Nephrol. Dial. Transplant. 2001, 16, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Johannsson, G.; Bengtsson, B.-Å.; Ahlmén, J. Double-blind, placebo-controlled study of growth hormone treatment in elderly patients undergoing chronic hemodialysis: Anabolic effect and functional improvement. Am. J. Kidney Dis. 1999, 33, 709–717. [Google Scholar] [CrossRef]
- Kopple, J.D.; Cheung, A.K.; Christiansen, J.S.; Djurhuus, C.B.; El Nahas, M.; Feldt-Rasmussen, B.; Mitch, W.E.; Wanner, C.; Göthberg, M.; Ikizler, T.A. OPPORTUNITY™: A large-scale randomized clinical trial of growth hormone in hemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 4095–4103. [Google Scholar] [CrossRef]
- Kotzmann, H.; Yilmaz, N.; Lercher, P.; Riedl, M.; Schmidt, A.; Schuster, E.; Kreuzer, S.; Geyer, G.; Frisch, H.; Hörl, W.H.; et al. Differential effects of growth hormone therapy in malnourished hemodialysis patients. Kidney Int. 2001, 60, 1578–1585. [Google Scholar] [CrossRef]
- Li, H.-L.; Li, H.; Cao, Y.F.; Qi, Y.; Wang, W.Q.; Liu, S.Q.; Yang, C.D.; Yu, X.Y.; Xu, T.; Zhu, Y.; et al. Effects of keto acid supplements on Chinese patients receiving maintenance hemodialysis: A prospective, randomized, controlled, single-center clinical study. Chin. Med. J. 2020, 133, 9. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, Y.; Zhang, Y.; Xiang, J.; Wu, Q. Effect of nurse-led food exchange intervention for patients undergoing peritoneal dialysis. Clin. Nephrol. 2020, 93, 140–148. [Google Scholar] [CrossRef]
- Marini, A.C.B.; Motobu, R.D.; Freitas, A.T.; Mota, J.F.; Wall, B.T.; Pichard, C.; Laviano, A.; Pimentel, G.D. Short-Term Creatine Supplementation May Alleviate the Malnutrition-Inflammation Score and Lean Body Mass Loss in Hemodialysis Patients: A Pilot Randomized Placebo-Controlled Trial. J. Parenter. Enter. Nutr. 2020, 44, 815–822. [Google Scholar] [CrossRef]
- Sahathevan, S.; Se, C.-H.; Ng, S.; Khor, B.-H.; Chinna, K.; Goh, B.L.; Gafor, H.A.; Bavanandan, S.; Ahmad, G.; Karupaiah, T. Clinical efficacy and feasibility of whey protein isolates supplementation in malnourished peritoneal dialysis patients: A multicenter, parallel, open-label randomized controlled trial. Clin. Nutr. ESPEN 2018, 25, 68–77. [Google Scholar] [CrossRef]
- Schincaglia, R.M.; Cuppari, L.; Neri, H.F.; Cintra, D.E.; Sant’Ana, M.R.; Mota, J.F. Effects of baru almond oil (Dipteryx alata Vog.) supplementation on body composition, inflammation, oxidative stress, lipid profile, and plasma fatty acids of hemodialysis patients: A randomized, double-blind, placebo-controlled clinical trial. Complement. Ther. Med. 2020, 52, 102479. [Google Scholar] [CrossRef]
- Supasyndh, O.; Satirapoj, B.; Aramwit, P.; Viroonudomphol, D.; Chaiprasert, A.; Thanachatwej, V.; Vanichakarn, S.; Kopple, J.D. Effect of oral anabolic steroid on muscle strength and muscle growth in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2013, 8, 271–279. [Google Scholar] [CrossRef]
- Singer, R.; Chacko, B.; Talaulikar, G.; Karpe, K.; Walters, G. Placebo-controlled, randomized clinical trial of high-dose cholecalciferol in renal dialysis patients: Effect on muscle strength and quality of life. Clin. Kidney J. 2019, 12, 281–287. [Google Scholar] [CrossRef]
- Teixidó–Planas, J.; Ortíz, A.; Coronel, F.; Montenegro, J.; López-Menchero, R.; Ortíz, R.; Gómez, C.; Doñate, T. Oral protein-energy supplements in peritoneal dialysis: A multicenter study. Perit. Dial. Int. 2005, 25, 163–172. [Google Scholar] [CrossRef]
- Tomayko, E.J.; Kistler, B.M.; Fitschen, P.J.; Wilund, K.R. Intradialytic protein supplementation reduces inflammation and improves physical function in maintenance hemodialysis patients. J. Ren. Nutr. 2015, 25, 276–283. [Google Scholar] [CrossRef]
- Wu, H.-L.; Tseng, C.C.; Wu, A.B.; Wang, M.C. Effect of Oral L-Carnitine Supplementation on the Nutritional Status and Muscle Strength of Patients with Chronic Peritoneal Dialysis. Nutr. Sci. J. 2011, 36, 19–26. [Google Scholar]
- Wu, P.-T.; Fitschen, P.J.; Kistler, B.M.; Jeong, J.H.; Chung, H.R.; Aviram, M.; Phillips, S.A.; Fernhall, B.; Wilund, K.R. Effects of pomegranate extract supplementation on cardiovascular risk factors and physical function in hemodialysis patients. J. Med. Food 2015, 18, 941–949. [Google Scholar] [CrossRef]
- Greenwood, S.A.; Koufaki, P.; Mercer, T.H.; Rush, R.; O’Connor, E.; Tuffnell, R.; Lindup, H.; Haggis, L.; Dew, T.; Abdulnassir, L.; et al. Aerobic or resistance training and pulse wave velocity in kidney transplant recipients: A 12-week pilot randomized controlled trial (the Exercise in Renal Transplant [ExeRT] Trial). Am. J. Kidney Dis. 2015, 66, 689–698. [Google Scholar] [CrossRef]
- Henggeler, C.K.; Plank, L.D.; Ryan, K.J.; Gilchrist, E.L.; Casas, J.M.; Lloyd, L.E.; Mash, L.E.; McLellan, S.L.; Robb, J.M.; Collins, M.G. A randomized controlled trial of an intensive nutrition intervention versus standard nutrition care to avoid excess weight gain after kidney transplantation: The INTENT trial. J. Ren. Nutr. 2018, 28, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Hernández Sánchez, S.; Carrero, J.J.; Morales, J.S.; Ruiz, J.R. Effects of a resistance training program in kidney transplant recipients: A randomized controlled trial. Scand. J. Med. Sci. Sports 2021, 31, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Karelis, A.D.; Hébert, M.-J.; Rabasa-Lhoret, R.; Räkel, A. Impact of resistance training on factors involved in the development of new-onset diabetes after transplantation in renal transplant recipients: An open randomized pilot study. Can. J. Diabetes 2016, 40, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.S.; de Campos, A.S.; Neto, O.D.F.; Ferreira, T.C.; Amorim, C.E.; Stone, W.J.; Prestes, J.; Garcia, A.M.; Urtado, C.B. Effects of combined resistance plus aerobic training on body composition, muscle strength, aerobic capacity, and renal function in kidney transplantation subjects. J. Strength Cond. Res. 2021, 35, 3243–3250. [Google Scholar] [CrossRef]
- Painter, P.L.; Hector, L.; Ray, K.; Lynes, L.; Dibble, S.; Paul, S.M.; Tomlanovich, S.L.; Ascher, N.L. A randomized trial of exercise training after renal transplantation. Transplantation 2002, 74, 42–48. [Google Scholar] [CrossRef]
- Painter, P.L.; Topp, K.; Krasnoff, J.; Adey, D.; Strasner, A.; Tomlanovich, S.; Stock, P. Health-related fitness and quality of life following steroid withdrawal in renal transplant recipients. Kidney Int. 2003, 63, 2309–2316. [Google Scholar] [CrossRef]
- Riess, K.J.; Haykowsky, M.; Lawrance, R.; Tomczak, C.R.; Welsh, R.; Lewanczuk, R.; Tymchak, W.; Haennel, R.G.; Gourishankar, S. Exercise training improves aerobic capacity, muscle strength, and quality of life in renal transplant recipients. Appl. Physiol. Nutr. Metab. 2014, 39, 566–571. [Google Scholar] [CrossRef]
- Tzvetanov, I.; West-Thielke, P.; D’Amico, G.; Johnsen, M.; Ladik, A.; Hachaj, G.; Grazman, M.; Heller, R.; Fernhall, B.; Daviglus, M.; et al. A novel and personalized rehabilitation program for obese kidney transplant recipients. In Transplantation Proceedings; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- van den Ham, E.C.; Kooman, J.P.; Christiaans, M.H.; van Hooff, J.P. The influence of early steroid withdrawal on body composition and bone mineral density in renal transplantation patients. Transpl. Int. 2003, 16, 82–87. [Google Scholar]
- Clarkson, M.J.; Bennett, P.N.; Fraser, S.F.; Warmington, S.A. Exercise interventions for improving objective physical function in patients with end-stage kidney disease on dialysis: A systematic review and meta-analysis. Am. J. Physiol. Ren. Physiol. 2019, 316, F856–F872. [Google Scholar] [CrossRef]
- Heiwe, S.; Jacobson, S.H. Exercise training in adults with CKD: A systematic review and meta-analysis. Am. J. Kidney Dis. 2014, 64, 383–393. [Google Scholar] [CrossRef]
- McCullough, K.P.; Morgenstern, H.; Saran, R.; Herman, W.H.; Robinson, B.M. Projecting ESRD incidence and prevalence in the United States through 2030. J. Am. Soc. Nephrol. 2019, 30, 127–135. [Google Scholar] [CrossRef]
- Kutner, N.G.; Zhang, R.; Huang, Y.; Painter, P. Gait speed and mortality, hospitalization, and functional status change among hemodialysis patients: A US renal data system special study. Am. J. Kidney Dis. 2015, 66, 297–304. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, J.S.; Jung, S.-W.; Hwang, H.S.; Moon, J.-Y.; Jeong, K.-H.; Lee, S.-H.; Lee, S.-Y.; Ko, G.J.; Lee, D.-Y.; et al. Gait speed and handgrip strength as predictors of all-cause mortality and cardiovascular events in hemodialysis patients. BMC Nephrol. 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A., Jr.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Baker, L.A.; March, D.S.; Wilkinson, T.J.; Billany, R.E.; Bishop, N.C.; Castle, E.M.; Chilcot, J.; Davies, M.D.; Graham-Brown, M.P.M.; Greenwood, S.A.; et al. Clinical practice guideline exercise and lifestyle in chronic kidney disease. BMC Nephrol. 2022, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.D.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.N.; Ghaddar, S.; Goldstein-Fuchs, D.J.; et al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef] [PubMed]
- Lemmey, A.B.; Elamanchi, S.R.; Marcora, S.M.; Casanova, F.; Maddison, P.J. Efficacy of nandrolone decanoate in treating rheumatoid cachexia in male rheumatoid arthritis patients. In Innovative Rheumatology; IntechOpen: London, UK, 2013. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).