Development of a Short Questionnaire for the Screening for Vitamin D Deficiency in Italian Adults: The EVIDENCe-Q Project
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Anthropometric Measurements
2.3. Questionnaire Design and Scoring
2.4. Vitamin D Levels Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saponaro, F.; Saba, A.; Zucchi, R. An update on Vitamin D metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, S.; Porri, D.; De Giuseppe, R.; Manuelli, M.; Alessio, F.; Cena, H. The controversial role of vitamin D as an antioxidant: Results from randomised controlled trials. Nutr. Res. Rev. 2019, 32, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Biver, E.; Brennan-Speranza, T.C. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021, 9, 606–621. [Google Scholar] [CrossRef]
- Navarro, D.F.; López García-Franco, A.; Niño de Guzmán, E.; Rabassa, M.; Zamanillo Campos, R.; Pardo-Hernández, H.; Ricci-Cabello, I.; Canelo-Aybar, C.; Meneses-Echavez, J.F.; Yepes-Nuñez, J.J.; et al. Vitamin D recommendations in clinical guidelines: A systematic review, quality evaluation and analysis of potential predictors. Int. J. Clin. Pract. 2021, 75, e14805. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am. J. Clin. Nutr. 2004, 79, 362–371, Erratum in: Am. J. Clin. Nutr. 2004, 79, 890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesareo, R.; Attanasio, R.; Caputo, M.; Castello, R.; Chiodini, I.; Falchetti, A.; Guglielmi, R.; Papini, E.; Santonati, A.; Scillitani, A.; et al. Italian Association of Clinical Endocrinologists (AME) and Italian Chapter of the American Association of Clinical Endocrinologists (AACE) Position Statement: Clinical management of Vitamin D deficiency in adults. Nutrients 2018, 10, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbar, M.R.; Wibowo, A.; Pranata, R.; Setiabudiawan, B. Low serum 25-hydroxyvitamin D (Vitamin D) level is associated with susceptibility to COVID-19, severity, and mortality: A systematic review and meta-analysis. Front. Nutr. 2021, 8, 660420. [Google Scholar] [CrossRef]
- Draft Vitamin D and Health Report. Scientific Consultation: 22 July to 23 September 2015. Available online: https://www.gov.uk/government/consultations/consultation-on-draft-sacn-vitamin-d-and-health-report (accessed on 8 January 2020).
- Hajhashemy, Z.; Foshati, S.; Saneei, P. Relationship between abdominal obesity (based on waist circumference) and serum vitamin D levels: A systematic review and meta-analysis of epidemiologic studies. Nutr. Rev. 2021, 80, 1105–1117. [Google Scholar] [CrossRef]
- Carrelli, A.; Bucovsky, M.; Horst, R.; Cremers, S.; Zhang, C.; Bessler, M.; Schrope, B.; Evanko, J.; Blanco, J.; Silverberg, S.J.; et al. Vitamin D storage in adipose tissue of obese and normal weight women. J. Bone Miner. Res. 2017, 32, 237–242. [Google Scholar] [CrossRef] [Green Version]
- Christensen, R.; Lorenzen, J.K.; Svith, C.R.; Bartels, E.M.; Melanson, E.L.; Saris, W.H.; Tremblay, A.; Astrup, A. Effect of calcium from dairy and dietary supplements on faecal fat excretion: A meta-analysis of randomized controlled trials. Obes. Rev. 2009, 10, 475–486. [Google Scholar] [CrossRef]
- Soares, M.J.; Chan She Ping-Delfos, W.; Ghanbari, M.H. Calcium and vitamin D for obesity: A review of randomized controlled trials. Eur. J. Clin. Nutr. 2011, 65, 994–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Q.; Sergeev, I.N. Calcium and vitamin D in obesity. Nutr. Res. Rev. 2012, 25, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Berisha, A.T.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; Dowling, K.G.; Škrabáková, Z.; Gonzalez-Gross, M.; Valtueña, J.; de Henauw, S.; Moreno, L.; Damsgaard, C.T.; Michaelsen, K.F.; Mølgaard, C.; et al. Vitamin D deficiency in Europe: Pandemic? Am. J. Clin. Nutr. 2016, 103, 1033–1044. [Google Scholar] [CrossRef] [Green Version]
- Balachandar, R.; Pullakhandam, R.; Kulkarni, B.; Sachdev, H.S. Relative Efficacy of Vitamin D2 and Vitamin D3 in improving Vitamin D status: Systematic review and meta-Analysis. Nutrients 2021, 13, 3328. [Google Scholar] [CrossRef]
- Sanò, M.; Dutto, P.; D’Anna, S.; Rognoni, C. Can a different formulation of Vitamin D3 allow savings? An analysis from an italian regional perspective. Health Serv. Res. Manag. Epidemiol. 2019, 6, 2333392819861881. [Google Scholar] [CrossRef] [Green Version]
- Agenzia Italiana del Farmaco. Nota 96. Available online: https://www.aifa.gov.it/Nota-96 (accessed on 1 October 2019).
- Adami, S.; Romagnoli, E.; Carnevale, V.; Scillitani, A.; Giusti, A.; Rossini, M.; Gatti, D.; Nuti, R.; Minisola, S. Linee guida su prevenzione e trattamento dell’ipovitaminosi D. Reumatismo 2011, 63, 129–147. [Google Scholar]
- Google Forms. Available online: https://www.google.com/forms/about/ (accessed on 1 October 2019).
- Google Privacy Policy. Available online: https://policies.google.com/privacy?hl=en-US (accessed on 1 October 2019).
- Ministero della Salute. Linee di Indirizzo Sull’attività Fisica per le Differenti Fasce D’età e con Riferimento a Situazioni Fisiologiche e Fisiopatologiche e a Sottogruppi Specifici di Popolazione. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_2828_allegato.pdf (accessed on 1 October 2019).
- Faid, F.; Nikolic, M.; Milesevic, J.; Zekovic, M.; Kadvan, A.; Gurinovic, M.; Glibetic, M. Assessment of vitamin D intake among Libyan women–adaptation and validation of specific food frequency questionnaire. Libyan J. Med. 2018, 13, 1502028. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Douglas, C.S.; Thomas, J.J.; Johnson, E.C.; Barcal, J.N.; Heller, J.E.; Hollis, B.W.; Halliday, T.M. Validation of a Vitamin D specific questionnaire to determine Vitamin D status in athletes. Nutrients 2019, 11, 2732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bärebring, L.; Amberntsson, A.; Winkvist, A.; Augustin, H. Validation of dietary Vitamin D intake from two food frequency questionnaires, using food records and the biomarker 25-hydroxyvitamin D among pregnant women. Nutrients 2018, 10, 745. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, A.; Tsugawa, N.; Mizuno, K.; Ogasawara, H.; Watanabe, Y.; Tanaka, K. A simple questionnaire for the prediction of vitamin D deficiency in Japanese adults (Vitaimn D Deficiency questionnaire for Japanese: VDDQ-J). J. Bone Miner. Metab. 2019, 37, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Le Moigno, J.M.; Annweiler, G.; Karras, S.N.; Llewellyn, D.J.; Riou, J.; Annweiler, C.; SOCOS group. Clinical identification of older adults with hypovitaminosis D: Feasibility, acceptability and accuracy of the ‘Vitamin D Status Diagnosticator’ in primary care. J. Steroid Biochem. Mol. Biol. 2020, 197, 105523. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US), Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Wolff, D.L.; Waldorff, F.B.; von Plessen, C.; Mogensen, C.B.; Sørensen, T.L.; Houlind, K.C.; Bogh, S.B.; Rubin, K.H. Rate and predictors for non-attendance of patients undergoing hospital outpatient treatment for chronic diseases: A register-based cohort study. BMC Health Serv. Res. 2019, 19, 386. [Google Scholar] [CrossRef] [PubMed]
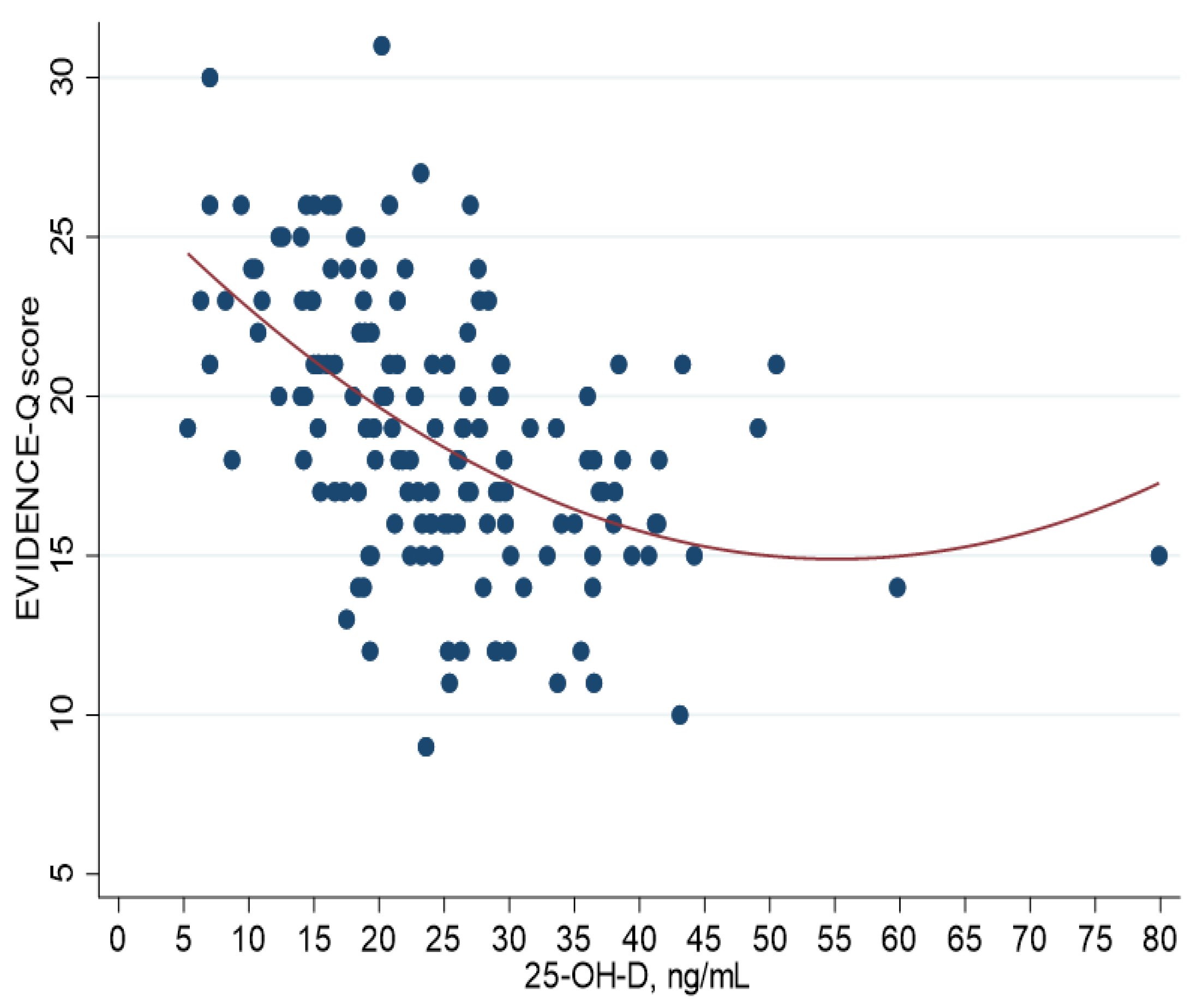
| Total (n = 150) | Men (n = 38) | Women (n = 112) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Min–Max | Mean (SD) | Median | Min–Max | Mean (SD) | Median | Min–Max | p | |
| Age (years) | 55.3 (19.5) | 57.5 | 19–90 | 59.1 (19.16) | 60.5 | 20–89 | 54 (19.53) | 55 | 19–90 | 0.162 |
| Weight (kg) | 73.5 (17.59) | 70 | 42–125.3 | 85 (16.62) | 82.5 | 58–120 | 69.6 (16.21) | 66 | 42–125.3 | <0.01 |
| Height (m) | 1.7 (0.08) | 1.6 | 1.5–1.9 | 1.7 (0.06) | 1.8 | 1.6–1.9 | 1.6 (0.06) | 1.6 | 1.5–1.8 | <0.01 |
| BMI (Kg/m2) | 26.9 (5.78) | 26.1 | 17.5–46.1 | 28.1 (5.78) | 27.3 | 19.4–40.6 | 26.5 (5.75) | 25.6 | 17.5–46.1 | 0.147 |
| WC (cm) | 91.9 (15.51) | 90 | 61.5–134 | 100.4 (13.8) | 101 | 73–125 | 89.1 (15.06) | 89 | 61.5–134 | <0.01 |
| 25-OH-D (ng/mL) | 24.6 (10.74) | 23.3 | 5.3–79.9 | 22.4 (8.71) | 22.2 | 7–38.4 | 25.4 (11.28) | 24 | 5.3–79.9 | 0.129 |
| EVIDENCE-Q score | 18.9 (4.21) | 19 | 9–31 | 18.5 (4.99) | 18.5 | 9–31 | 19.1 (3.93) | 19 | 10–27 | 0.479 |
| EVIDENCE-Q Score | Mean (SD) | Median | Min–Max | p-Value |
|---|---|---|---|---|
| Severe deficiency (25-OH-D < 10 ng/mL) | 23.3 (3.99) | 23 | 18–30 | 0.0001 |
| Deficiency (10 ≤ 25-OH-D < 30 ng/mL) | 19.4 (4.14) | 20 | 9–31 | |
| Adequate (25-OH-D ≥ 30 ng/mL) | 16.2 (2.83) | 16 | 10–21 |
| Severe Deficiency (25-OH-D < 10 ng/mL) | Deficiency (25-OH-D < 20 ng/mL) | Insufficiency (25-OH-D < 30 ng/mL) | |
|---|---|---|---|
| Optimal operating slope | 1 | 1 | 1 |
| Optimal cut-off | 23 | 21 | 20 |
| Optimal sensitivity | 0.625 | 0.6182 | 0.5214 |
| Optimal specificity | 0.8028 | 0.8 | 0.8788 |
| Clinical information statistic | 0.4278 | 0.4182 | 0.4002 |
| Area under the ROC Curve | 0.8129 | 0.7702 | 0.7682 |
| SE of Area (Hanley) | 0.0697 | 0.0423 | 0.0418 |
| Sample size | 150 | 150 | 150 |
| (25-OH-D) | EVIDENCE-Q Score Cut-Off | |
|---|---|---|
| n (%) | n (%) | |
| Severe deficiency < 10 ng/mL | 8 (5.3) | 33 (22) |
| Deficiency < 20 ng/mL a | 55 (36.7) | 53 (35.3) |
| Insufficiency < 30 ng/mL | 117 (78) | 65 (43.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Giuseppe, R.; Tomasinelli, C.E.; Cena, H.; Braschi, V.; Giampieri, F.; Preatoni, G.; Centofanti, D.; Princis, M.P.; Bartoletti, E.; Biino, G. Development of a Short Questionnaire for the Screening for Vitamin D Deficiency in Italian Adults: The EVIDENCe-Q Project. Nutrients 2022, 14, 1772. https://doi.org/10.3390/nu14091772
De Giuseppe R, Tomasinelli CE, Cena H, Braschi V, Giampieri F, Preatoni G, Centofanti D, Princis MP, Bartoletti E, Biino G. Development of a Short Questionnaire for the Screening for Vitamin D Deficiency in Italian Adults: The EVIDENCe-Q Project. Nutrients. 2022; 14(9):1772. https://doi.org/10.3390/nu14091772
Chicago/Turabian StyleDe Giuseppe, Rachele, Chiara Elena Tomasinelli, Hellas Cena, Valentina Braschi, Francesca Giampieri, Giorgia Preatoni, Domenico Centofanti, Maria Pilar Princis, Emanuele Bartoletti, and Ginevra Biino. 2022. "Development of a Short Questionnaire for the Screening for Vitamin D Deficiency in Italian Adults: The EVIDENCe-Q Project" Nutrients 14, no. 9: 1772. https://doi.org/10.3390/nu14091772
APA StyleDe Giuseppe, R., Tomasinelli, C. E., Cena, H., Braschi, V., Giampieri, F., Preatoni, G., Centofanti, D., Princis, M. P., Bartoletti, E., & Biino, G. (2022). Development of a Short Questionnaire for the Screening for Vitamin D Deficiency in Italian Adults: The EVIDENCe-Q Project. Nutrients, 14(9), 1772. https://doi.org/10.3390/nu14091772










