Abstract
Phytonutrients comprise many different chemicals, including carotenoids, indoles, glucosinolates, organosulfur compounds, phytosterols, polyphenols, and saponins. This review focuses on the human healthcare benefits of seven phytochemical families and highlights the significant potential contribution of phytonutrients in the prevention and management of pathologies and symptoms in the field of family health. The structure and function of these phytochemical families and their dietary sources are presented, along with an overview of their potential activities across different health and therapeutic targets. This evaluation has enabled complementary effects of the different families of phytonutrients in the same area of health to be recognized.
1. Introduction
Phytochemicals are bioactive compounds generated from secondary plant metabolism in response to environmental changes [1,2]. Phytochemicals function as attractants for pollination or act as protectants against insect and pest attacks or exposure to various stresses, such as ultraviolet light [1,2]. In addition, phytochemicals contribute to the color, flavor, and aroma of plants and are recognized as having potential value in nutrition and human health. In fact, they are typically found in our diet through the intake of fruits, vegetables, whole grains, nuts, beans, herbs, tea, and coffee [3].
Numerous epidemiological studies have shown that high intakes of plant products are correlated with lower risks of chronic diseases and mortality, suggesting key protective roles of antioxidants [4,5]. In addition to the antioxidative vitamins C and E, plant-based diets provide numerous phytochemicals, also known as phytonutrients, that may contribute to the maintenance of good health, not only through their antioxidant activity, but also as anti-inflammatory and anticarcinogenic agents [6,7]. Phytonutrients comprise many different chemicals, including carotenoids, indoles, glucosinolates, organosulfur compounds, phytosterols, polyphenols, and saponins. The intake of phytonutrients among European populations appears to be highly variable [8], suggesting that theiy are benefits for consumers with a high adherence to World Health Organization dietary recommendations. The high variability of phytonutrient intake is related to the seasonal availability and affordability of healthy plant products. Our previous study estimated the levels of seven phytonutrients (phenolic acids, flavonoids, tannins, anthocyanins, carotenoids, organosulfur compounds, and caffeine) in a well-balanced French diet that met the requirements for macro- and micronutrients to better identify any gaps in target phytonutrient intakes and recommend personalized nutritional strategies for maintaining good health [9].
Our present review focuses on healthcare targets for which benefits exist through an enrichment of the diet with at least one of the seven phytochemical families. Potential activities are presented on specific human healthcare targets. The first part of this review presents the structure and function of the seven phytochemical families and their dietary sources, while the second part describes their potential activities across different healthcare goals.
2. Materials and Methods
An analysis of the scientific literature listed on PubMed up to December 2021 was undertaken using keywords related to the most common therapeutic indications in the following healthcare areas: digestive health, stress and sleep, immunity and ear, nose, and throat (ENT) diseases, vitality and cognition, and bones and joints (Table 1).

Table 1.
Search terms/keywords used for PubMed literature search.
The details of a number of publications selected by phytochemicals and therapeutic area are presented in Table 2.

Table 2.
Details of selected publications.
We cross-referenced each keyword with each phytonutrient by filtering only the scientific literature on clinical studies. This search identified 23,830 articles. A second filter to eliminate duplicates, studies outside the scope of the search, or those not dealing specifically with phytonutrients resulted in the selection of 1012 publications. An in-depth analysis of these articles allowed us to identify 125 publications specifically addressing the therapeutic value of phytonutrients in the selected therapeutic areas and based on a robust experimental methodology. A total of 74 articles were included in this review, with all others being discarded for lack of robustness or significance in the experimental results (Figure 1). This review presents the analysis of the identified articles.
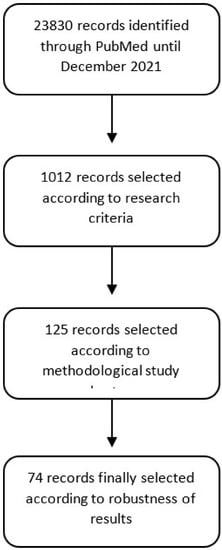
Figure 1.
Literature search methodology.
3. Results
3.1. Phytonutrients
More than 10,000 phytonutrients have been identified in dietary plants [10,11,12]. Their concentrations differ greatly between species and cultivars, and also vary according to environmental conditions (light, soil, etc.), agricultural modes (fertilization and irrigation), storage, processing, and home uses [13]. A representation of the main families and chemical structures of phytonutrients found in dietary plants is shown in Figure 2 and Figure 3. The properties of phytonutrients allow them to play a role in aspects of metabolic syndrome and associated mechanisms, notably inflammation and oxidation [14]. Experimental studies in cells or in animals have deciphered their mechanistic actions as being antioxidant, anti-inflammatory, antimicrobial, and anticancer in nature [15,16,17,18,19].
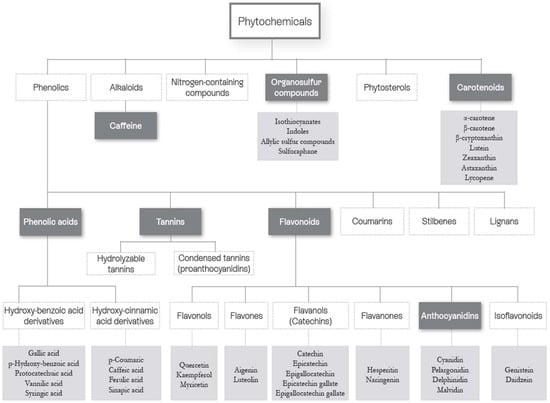
Figure 2.
Classification of the main phytonutrient families (modified from [6]). Dark grey: Phytonutrients Families. Light grey: Phytonutrients examples.
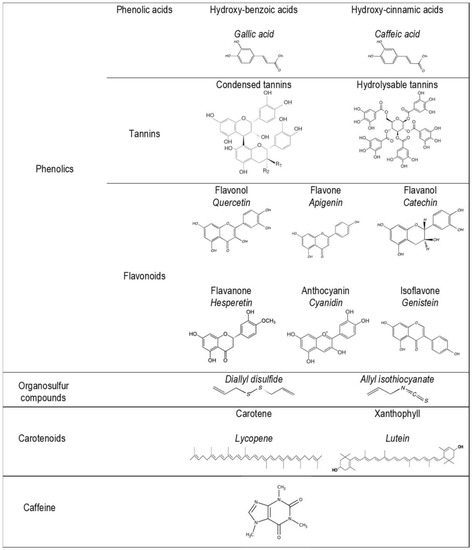
Figure 3.
Main phytonutrient family chemical structures.
3.1.1. Phenolic Acids
Phenolic acids, or phenolcarboxylic acids, belong to the polyphenol family and are among the most widely distributed plant non-flavonoid phenolic compounds [20]. They have at least one carboxylic function and one phenolic hydroxyl [21].
This category includes hydroxybenzoic acid and its derivatives (gallic acid, vanillic acid, parahydroxybenzoic acid, syringic acid, and protocatechic acid), as well as cinnamic acid and its derivatives (ferulic, paracoumaric, caffeic, and sinapic acids) [22].
Phenolic acids are found in many foods such as artichoke, cereals, wheat flours, onions, coffee, kiwis, berries, apples, and citrus fruits [11,23]. In addition to dietary sources, phenolic acids can also be derived from the colonic microflora’s secondary metabolism of other types of polyphenols [20].
3.1.2. Flavonoids
Flavonoids belong to the polyphenol family and include flavonols, flavones, flavanols, flavanones and isoflavonoids [21,24]. Anthocyanins are also part of the flavonoid family, but these are discussed in a separate paragraph in light of their specificities and therapeutic value. Flavonoids have a generic structure composed of two aromatic rings linked by three carbons: C6-C3-C6, a chain often closed in an oxygenated heterocycle called a C-ring [25]. The differences in the generic structure of the heterocyclic C-ring classify them as flavonols, flavones, flavanols, flavanones, anthocyanidins, or isoflavonoids [25]. Flavonoids are found in many plants and, as universal pigments of the yellow, red, and purple colors, are the molecules that give plants their ‘colorful hues’. When they are not directly visible, flavonoids contribute to coloring through their role as co-pigments. This is the case of colorless flavones and flavonols that co-pigment and protect anthocyanosides. Flavonols are the flavonoids most widely found in foods, with quercetin and kaempferol being the main representatives of this group [11].
Flavonoids are present in a very wide variety of plants, albeit in relatively low concentrations [11,24]. The main sources of flavonoids are tea, onions, and apples, but they are found in many other colored plants [26]. Flavanones are found in tomatoes, citrus fruits, and herbs. Flavanols are found in olives, onions, cabbage, and lettuce. Flavones are found in celery and olives. Pears, red wine, and tea are good sources of flavanols. Finally, isoflavones are mainly found in soy products [12,26,27].
3.1.3. Anthocyanins
Anthocyanins are a subfamily of flavonoids and are derived from the general metabolism of flavonoids [21,24].
The most common anthocyanins are cyanidin, pelargonidin, delphinidin, and malvidin, and they are most commonly found in red-, pink-, blue-, or purple-colored fruits and vegetables [28]. The color of anthocyanins varies from orange to purple. By increasing the degree of hydroxylation, the absorbance wavelength is increased from orange-colored pelargonidin to purple delphinidin.
Anthocyanins are particularly concentrated in cherries, berries (such as blackcurrants, elderberries, and blueberries), and plums. They are also present in root vegetables such as beets and radishes, red onion bulbs, and in drinks such as fruit juices and red wine. Anthocyanins are also found in eggplant and red cabbage [12,29,30,31].
3.1.4. Tannins
Like flavonoids, tannins belong to the family of phenolic compounds. They differ in structure and biogenetic origin and are subdivided into two categories: condensed tannins and hydrolyzable tannins [32]. Condensed tannins, also known as catechins or proanthocyanidins, are oligomers or polymers of flavanols comprising units of flavan-3-ols linked together by carbon–carbon bonds of type 4 → 8 or 4 → 6 [24,30].
Tannins are non-hydrolyzable, but when treated with an acid under heat, they degrade into colored pigments formed of anthocyanidins [24,30,33]. Hydrolyzable tannins, unlike condensed tannins, have the capacity to cross the intestinal barrier after hydrolysis [12].
Plums, cocoa beans, carob beans, tea, and wine, as well as pomegranate bark, sorghum and barley seeds contain high levels of tannins [12,29,30,33].
3.1.5. Organosulfur Compounds
Organosulfur compounds include several classes of molecules with a similar basic chemical structure [12]. A carbon atom is surrounded by a glucose molecule via a sulfur bond, a sulfate group via the nitrogen atom of the oxime group and an aglycone, which varies according to the subclass and is derived from an amino acid [34].
The family of organosulfur compounds includes isothiocyanates, indoles, compounds derived from allyl sulfides and sulforaphanes [12,24]. Isothiocyanates are biologically active hydrolysis products of glucosinolates [34]. The two organosulfur compounds most commonly found in plant-based foods are glucosinolates and sulfur derivatives of garlic [12].
Glucosinolates are found in particular in Brassicaceae or cruciferous vegetables (cabbage, cauliflower, turnip, broccoli, black radish, mustard) and are present in varying quantities depending on the species, the part and the plant, as well as the cultivation and climatic conditions [29]. These compounds are responsible for strong odors and tastes.
Sulforaphane is found mainly in cruciferous vegetables (cabbage and broccoli) and isothiocyanate in mustard seeds [10]. Garlic is also a good source of sulfur compounds.
3.1.6. Carotenoids
Carotenoids are a large family of more than 800 different molecules, ranging in color from yellow-orange to red, and of which only approximately 20 are found in food [12,24]. The general structure of a carotenoid is a hydrocarbon chain of polyene composed of 9 to 11 double bonds, possibly terminating in rings [35]. Carotenoids are fat-soluble compounds divided into two classes: xanthophylls and carotenes. In the first class, we find molecules such as lutein, zeaxanthin, β-cryptoxanthin, and astaxanthin. As for carotenes, they are represented by α-carotene, β-carotene and lycopene.
The most extensively studied carotenoids are α-carotene, β-carotene, lycopene, lutein and zeaxanthin. The best known, β-carotene, is a precursor of vitamin A [26,35].
Carotenoids, which are highly sensitive to oxidation, are widely distributed in the natural environment: they accumulate in the chloroplasts of all photosynthetic tissues [36]. β-carotene, lutein, violaxanthin, and neoxanthin are present in the leaves of almost all plants. Carotenoids also accumulate in flower petals (common marigold, pansy, and French marigold), in fruits which may contain chloroplastic carotenoids or accumulate other derivative compounds (capsanthin and lycopene).
Carotenoids are found in carrots, spinach, tomatoes, herbs including parsley and basil, leafy greens such as lettuce and arugula, broccoli, kale, Brussels sprouts, squash and sweet potato, peppers, citrus fruits, seeds, some mushrooms, and in many other plants [29,37,38,39].
3.1.7. Caffeine
Caffeine is a molecule of the alkaloid family, also known as 1,3,7-trimethylxanthine [24,40]. Conversely, caffeine was included as a family in its own right because it accounts for a significant proportion of daily phytochemical intake, has well documented health benefits, and is routinely included in nutritional recommendations such as those issued by the French Agency for Food, Environmental and Occupational Health & Safety (ANSES) [41].
Caffeine is the most widely consumed psychoactive substance in the world and is found in coffee or kola nuts, tea or mate leaves, or guarana seeds [42].
3.2. Therapeutic Applications of the Value of Phytonutrients (Family Health)
3.2.1. Stress and Sleep
Most of the seven families of phytonutrients present a pharmacological effect either in sleep disorders or in the case of problems related to stress. Some have a beneficial effect such as anthocyanins, carotenoids, flavonoids, tannins, and caffeine. Others, on the contrary, are not indicated in the treatment of this health problem. A summary of the effects of phytochemicals in this therapeutic area is presented in Table 3.

Table 3.
Summary of selected studies regarding the therapeutic area of stress and sleep disorders.
Stress
Several studies included in this review assessed the benefit or risk of caffeine consumption in relation to stress [46,47,48]. Bernstein et al. evaluated the acute effects of caffeine consumption on learning mechanisms in children but also on stress [47]. According to the authors, the consumption of 2.5 or 5 mg/kg of caffeine lead to a slight increase in (self-rated) stress in children (p = 0.098). Nevertheless, this non-statistically significant result did not enable a clear claim to be made for a deleterious effect of caffeine on stress in children. In contrast, other studies have reported beneficial effects of caffeine in the management of stress. White et al. evaluated the effects of caffeine on muscular tension and anxiety and their work shows that when heavy coffee drinkers are deprived of caffeine for 3 h, their muscular tension and anxiety significantly increase, compared with low consumers, and that the consumption of caffeine brings these parameters back to the level of subjects treated with placebo [46]. According to the authors, it is therefore the lack of caffeine, and not the caffeine itself, that is responsible for the anxiety. A literature review of 12 observational clinical studies also investigated the effects of coffee, tea, or caffeine consumption on depression [48]. The results of this analysis suggested that a daily consumption of caffeinated coffee could play a protective role with respect to the symptoms of depression in a non-linear dose–effect relationship and with a maximum effect at a consumption of 400 mL of coffee per day. According to the authors, this effect may be due to a stimulation of the central nervous system by caffeine and an improvement of dopaminergic neurotransmission.
Other phytonutrients are of therapeutic interest in the management of stress. The beneficial effects of flavonoids were evaluated by Scholey et al., where the electroencephalogram (EEG) of subjects along with their perceived level of stress before and 120 min after administration of 300 mg of epigallocatechin gallate (EGCG) or placebo were investigated [44]. The results showed an increase in self-rated calmness and a reduction in self-rated stress, as well as EEG modifications, with EGCG use. According to the authors, the mechanism of action could be linked to an effect on nitric oxide (NO) synthesis associated with a modulation of cerebral vascular permeability.
Carotenoids have also shown beneficial effects in the management of stress [43,45]. Kell et al. evaluated the therapeutic benefits of saffron, particularly the crocin (carotenoid) it contains, in the management of mood, stress, and anxiety disorders [45]. After 4 weeks of treatment with 28 mg/day of saffron, subjects saw their stress and anxiety levels decrease and their mood improve significantly compared with placebo. Another study conducted by Stringham et al. sought to demonstrate the value of long-term carotenoid supplementation in the management of stress [43]. Carotenoids or placebo were administered for 12 months and the level of stress associated with cortisol levels was assessed. As early as 6 months, cortisol levels, stress, and anxiety were significantly reduced in subjects who were treated with carotenoids. The authors suggested a mechanism of action based on a direct antioxidant action of carotenoids in neural tissue leading to a decrease in the synthesis of stress-related hormones.
The mechanisms of action described in these studies are most often related to the antioxidant properties of phytonutrients. The antioxidant action of phytonutrients can be either directly linked to their chemical structure and exerted through a direct antioxidant action (hydrogen or electron transfer or chelation of transition metals) or via an indirect action (regulation of enzymatic activity, gene modulation) [54,55,56,57,58,59].
Sleep
The anthocyanin family, and particularly the cyanidin class, has been shown to have therapeutic value in sleep disorders. In a study by Losso et al., subjects who consumed 240 mL of cyanidin-titrated cherry juice for 2 weeks had an average increase in sleep duration of 84 min compared those receiving placebo (p < 0.01) [52]. According to the authors, the beneficial effects on sleep are related to the inhibitory activity of cyanidins on indoleamine 2,3-dioxygenase, an enzyme that degrades tryptophane.
Hachul et al. evaluated the effect of flavonoids, and in particular isoflavones, on the sleep quality of postmenopausal women suffering from insomnia [51]. Subjects received 80 mg of isoflavones or placebo every day for 4 months. A sleep analysis was carried out using polysomnography and questionnaires. The results obtained show a significant decrease in the number of episodes of insomnia at the end of treatment in patients treated with isoflavones versus placebo (p = 0.006) as well as an improvement in sleep efficiency (p < 0.01).
Tannins demonstrated therapeutic potential in the management of sleep disorders in postmenopausal women in a study conducted by Terauchi et al. [49]. In this study, the effect of 100 or 200 mg of proanthocyanidins (derived from grape seeds) on insomnia was compared with placebo when administered over an 8 week period. The results showed a significant decrease (p < 0.01) in the insomnia score after 8 weeks of treatment with 200 mg of proanthocyanidins. According to the authors, the mechanism of action of the active treatment may be linked to an antioxidant effect of the tannins which modulates gamma-aminobutyric acid (GABA)ergic activity, leading to significant hypnosedative and anxiolytic effects (p < 0.01).
The effect of carotenoids on the improvement of sleep quality was evaluated by Kuratsune et al. [53]. In this study, patients experiencing moderate sleep disorders received either extract of Gardenia jasminoides titrated to 7.5 mg per day of crocetin or placebo for two periods of 2 weeks separated by a washout period of 2 weeks. The patients’ nocturnal activity measured by an actigraph showed a significant decrease in the number of waking episodes (p < 0.025), while the other parameters showed a trend towards improved sleep without reaching statistical significance. The same protocol was used in 2018 to evaluate the effect of crocetin on new parameters [50]. The results obtained showed no significant effect on electroencephalographic recordings, but an improvement in ‘Sleepiness on rising’ (p = 0.011) and ‘Feeling refreshed’ (p = 0.007) was reported. The mechanism of action supporting this effect is not fully understood but, according to the authors, it may be related to a modulation of the histaminergic system.
3.2.2. Immunity and ENT
Numerous studies have demonstrated the value of phytonutrients in the management of serious chronic diseases, one of the main causes of which is immune deficiency. However, in healthcare, the term immunity refers more to the notion of “maintaining natural defenses” in the context of benign conditions such as colds, allergic rhinitis, and other ENT pathologies. A summary of the effects of phytochemicals in this therapeutic area is presented in Table 4.

Table 4.
Summary of selected studies regarding the therapeutic area of immunity and ENT diseases.
Phytonutrients, and in particular flavonoids, are of therapeutic interest in the field of immunity. In 1995, Crişan et al. evaluated the benefit of a propolis rich in flavonoids in the management and occurrence of colds in children [65]. In this study, children who received 1 mL of product/day by nasal instillation 7 days a month for 5 months had a significantly lower number of colds (p < 0.01) as well as a shorter duration of symptoms (p < 0.05) compared with children in the control group. In 2011, Matsumoto et al. evaluated the effect of taking capsules rich in catechin (378 mg/day) and theanine (210 mg/day) for 5 months on the prevention of influenza viral pathologies [62]. Their results showed that the 98 adults treated with the product developed significantly (p = 0.022) fewer influenza viral pathologies than those in the placebo group. The mechanism of action could be related to an inhibition of the adsorption of the virus to the host cell. The interest of catechins and more particularly of EGCG, the main flavonoid in green tea, has been confirmed by Masuda et al. [60]. In this double-blind clinical study of 51 adults, the effects of EGCG on allergic symptoms were evaluated. In the group that received 700 mL of an EGCG-rich drink daily for 12 weeks, the number of allergic symptoms, such as runny nose (p < 0.05), itchy eyes (p < 0.01), or tearing (p < 0.01) were significantly reduced compared with the control group. The authors suggested that EGCG may limit mast cell activation, thus reducing the synthesis of leukotrienes, histamine, and other inflammatory cytokines.
Organosulfur compounds are also of therapeutic interest in antiviral protection. In 2016, Muller et al. evaluated the effect of sulforaphane-rich broccoli administration on the immune system response to influenza vaccination. Their results show a significant decrease in the number of natural killer T (NKT), T, and N cells as well as a significant increase in the production of granzyme B (an antiviral protein) compared with the placebo group suggesting that sulforaphanes induce an improvement in defenses against viral infections [63].
Tannins also modulate the immune response in ENT pathologies, particularly allergic rhinitis [61]. A randomized, double-blind, placebo-controlled study of 33 patients with allergic rhinitis evaluated the benefit of apple tannins and, in particular, procyanidins on the symptoms of the pathology. The study reported a significant improvement in certain symptoms (sneezing attacks [p < 0.05] and nasal discharge [p < 0.01]) with treatment titrated to 200 mg/day of polyphenols compared with placebo.
In 2013, Nantz et al. evaluated the benefits of consuming cranberry juice rich in pro-anthocyanidins (65–77%) for 10 weeks in the management of ENT Winter pathologies [64]. They demonstrated that the use of active treatment significantly decreased flu symptoms compared with placebo (p = 0.031), along with a proliferation of γδ-T lymphocytes (p < 0.001), immune cells located in the respiratory epithelium, suggesting a strengthening of the first line of defense against viruses.
A Cochrane review has evaluated the effects of caffeine on respiratory parameters in asthmatic patients [66]. This analysis of seven randomized clinical studies and 75 subjects showed a significant improvement in respiratory parameters of up to 4 h, even with doses lower than 5 mg/kg.
Finally, the effect of carotenoids has been evaluated in several clinical studies [67,68]. In 2008, Cingi et al. evaluated the effect of the daily intake of 2 g of spirulina for 6 months versus placebo on symptoms associated with allergic rhinitis [68]. Data from 129 patients showed a significant improvement in nasal discharge, sneezing, nasal congestion, and itching compared with the control group (all p < 0.001). In 2020, Nourollahian et al. also evaluated the effects of a 2 g/day intake of spirulina for 2 months on allergic rhinitis symptoms and associated inflammatory parameters in comparison with cetirizine (control) [67]. The results showed a significant improvement of most of the monitored symptoms as well as an improvement of inflammation markers. The therapeutic benefits observed with carotenoids may be explained by an anti-inflammatory activity that regulates interleukin (IL)-4 and interferon (IFN)-γ expression and restores T helper (Th)1/Th2 balance [67,68].
The principal mechanisms of action involved in this area of health are therefore mainly related to the antimicrobial and anti-inflammatory properties of phytonutrients. Antimicrobial activity is based either on phytonutrients’ direct destabilizing effects on the viral or bacterial membrane, which is well described for EGCG as an example [69,70,71,72], or by their inhibition of microbial enzymes or biofilms [73,74]. Anti-inflammatory activity, often associated with the antioxidant properties of phytonutrients, involves several main mechanisms of action. Families of phytonutrients such as anthocyanins (cyanidin and delphinidin), tannins (proanthocyanidin), flavonoids (quercetin), phenolic acids (ferulic acid), organosulfur compounds (sulforaphane), carotenoids (lycopene), and caffeine have been described as being able to inhibit activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathway, leading to a reduction in inflammation [28,55,75,76,77,78,79]. Flavonoids (luteolin and kaempferol), organosulfur compounds (sulforaphane), anthocyanins (cyanidin and delphinidin), phenolic acids (p-coumaric acid), carotenoids (lycopene), and tannins (proanthocyanidins) can also regulate the mitogen-activated protein kinase pathway, which is also widely implicated in inflammatory processes [28,80,81,82,83,84].
3.2.3. Digestive Health
Digestion is a therapeutic area that is very well represented in family health and includes many benign conditions and symptoms such as constipation, nausea, and diarrhea, but also chronic liver diseases [85,86,87,88,89,90,91,92,93,94,95,96]. Phytonutrients are again of great interest in this field. A summary of the effects of phytochemicals in this therapeutic area is presented in Table 5.

Table 5.
Summary of selected studies regarding the therapeutic area of digestive health.
In 2016, Baek et al. demonstrated the beneficial effect of a flavonoid-rich extract on constipation parameters in a randomized clinical trial [85]. After 8 weeks of treatment, transit time in the colon was significantly reduced versus placebo, stool quality improved, and abdominal discomfort was reduced. The therapeutic effect was thought to be based on the ability of certain flavonoids to stimulate chloride channels and/or serotonin signaling leading to a secretion of water, electrolytes and mucin in the colon. A pilot study has also shown flavonoids present in grape juice to reduce nausea and vomiting during chemotherapy compared with placebo, without reaching the significance threshold [86]. Dryden et al. evaluated the therapeutic benefit of flavonoids in the management of ulcerative colitis and demonstrated that a 56 day administration of an extract rich in EGCG significantly increased (p = 0.003) the rate of remission of the pathology compared with placebo [87]. The observed therapeutic effect was believed to be based on the ability of EGCG to inhibit IkB kinase, thus blocking the activation and nuclear translocation of NF-kB, an important inflammation modulator.
Flavonoids are also of great interest in the management of liver pathologies. In 2013, a team investigated the value of isoflavone supplementation in obese postmenopausal patients as a complement to physical exercise [88]. This randomized, placebo-controlled, double-blind pilot study reported a benefit of isoflavone supplementation for 6 months compared with placebo, notably on the fatty liver index (p < 0.01) and γ-glutamyltransferase levels (p < 0.01). This health benefit was thought to be based on the ability of isoflavones to limit the oxidative stress found in liver pathologies. In 2019, another team evaluated the effects of hesperidin 1 g/day for 12 weeks on the components of non-alcoholic fatty liver disease [89]. Results showed a significant decrease in total cholesterol (p = 0.016), triglyceride (p = 0.049), hepatic steatosis (p = 0.041), and C-reactive protein levels (p = 0.029) versus placebo, probably due to an inhibition of the NF-kB pathway.
Tannins have also demonstrated therapeutic value in the management of digestive pathologies. In 2018, Venancio et al. evaluated the benefit of a daily intake of 300 g of gallotanin-rich mangoes on volunteers with chronic constipation [90]. An improvement in functional (stool frequency and consistency) and inflammatory parameters was demonstrated after 4 weeks of treatment. Tannins, particularly crofelemer, were also evaluated in the management of symptoms related to irritable bowel syndrome in a large randomized, controlled study [91]. After 3 months of treatment, functional parameters had not improved, but abdominal pain and discomfort had significantly improved (p = 0.0076) in those women treated with 500 mg of the product.
The antioxidant activity of organosulfur compounds, particularly glucoraphanes, has been shown to improve liver function in patients with fatty liver after 4 months of treatment, possibly due to their antioxidant properties and ability to stimulate detoxifying enzymes by activating the NRF2 transcription factor [92]. In a study by Yanaka et al., the use of glucosinolates, particularly sulforaphanes contained in broccoli sprouts, provided a significant reduction in constipation versus placebo when consumed daily at 20 g/day (i.e., 4.4 mg/day of sulforaphane) for 21 days [93]. According to the authors, this effect was due to the antioxidant action of the sulforaphanes on the digestive tract. Other research teams have attributed the beneficial effects of organosulfur compounds in the digestive tract to a direct effect on the intestinal microbiota [94]. Daily consumption of 200 g of broccoli and 20 g of raw daikon radish for period of 18 days has been shown to result in a significant change (p = 0.03) in the composition of the intestinal microbiota versus a diet without organosulfur compounds; a significant decrease in firmicutes in favor of bacteroides (p = 0.03) compared with the control was noted.
Other phytonutrients may be of interest in the management of digestive pathologies. Biedermann et al. have shown that anthocyanins appear to be active in the management of ulcerative colitis by reducing certain symptoms as well as the Endoscopic Mayo Score [95]. This effect may be related to the anti-inflammatory activity of anthocyanins leading to a reduction in tumor necrosis factor-α (TNF-α) and IFNγ levels in mesenteric lymph nodes. A meta-analysis evaluated the protective potential of caffeine in this type of pathology and the authors suggested a protective effect of this phytonutrient in chronic hepatic pathologies, which may be due to its antioxidant action or modulating effect on insulin resistance [96].
3.2.4. Bones and Joints
Joints are a therapeutic area of interest in family health. Many products are marketed as a first-line treatment for benign tendon and joint disorders, but also as accompanying care for more serious chronic pathologies, such as osteoarthritis or rheumatoid arthritis. Some phytonutrients also demonstrate beneficial properties in this type of pathology while others can be deleterious. A summary of the effects of phytochemicals in this therapeutic area is presented in Table 6.

Table 6.
Summary of selected studies regarding the therapeutic area of bones and joints.
More than 20 years ago, the beneficial effect of flavonoids, particularly ipriflavone, for the prevention of menopausal osteoporosis over the course of a long-term study was reported [108]. The authors concluded that there was an improvement in vertebral bone density with a supplement of ipriflavone 600 mg daily for 2 years, possibly caused by a limited bone resorption effect. The effect of flavonoids has been more recently verified in a study conducted by Law et al. [101]. After 2 months of daily treatment with 100 mL of onion juice rich in flavonoids and phenolic acids, patients with osteoporosis showed significant improvements in oxidation markers and positive modulation in bone loss. The mechanisms of action involved were related to the antioxidant properties of flavonoids as well as their ability to slow the differentiation of progenitors into osteoclasts. A systematic literature review of 26 randomized clinical studies and 2652 patients established a therapeutic benefit of isoflavones in the management of bone loss during menopause, with treatment significantly increasing bone density in the lumbar spine (p < 0.0001) and femoral neck (p < 0.01) [106]. An improvement in the clinical symptoms of rheumatoid arthritis with flavonoids has also been demonstrated in a randomized, controlled trial [103]. The use of quercetin administered at 500 mg/day for 8 weeks significantly improved the clinical (improvement of early morning stiffness, morning pain, and after-activity pain; p < 0.05 for all) and cytokinic profile of patients by inhibiting the NF-kB pathway and the release of associated inflammatory cytokines.
Carotenoids, particularly their anti-inflammatory activity, are of therapeutic interest in the prevention of chronic joint pathologies. A prospective study on more than 25,000 subjects evaluated the effect of carotenoid consumption on the risk of developing rheumatoid arthritis and concluded that an increase in the consumption of β-cryptoxanthin equivalent to a glass of orange juice reduces the risk of developing this pathology due to the antioxidant properties of this phytonutrient [111]. On the contrary, other studies indicate that there is no link between the amount of circulating carotenoids and the occurrence of inflammatory joint disease [102]. Similarly, there is no consensus on the effect of carotenoids on bone preservation. Kim et al. demonstrated that the intake of β-carotene was associated with an improvement in bone mass, particularly in the lumbar spine (p < 0.05), probably due to a stabilization of collagen synthesis and osteoblast differentiation [105]. In contrast, other studies have not indicated any interest in this family of phytonutrients for this indication [97,109,112] or suggested any effect that could be deleterious at high doses via the stimulation of osteoclasts and inhibition of osteoblasts [98].
Caffeine is also a phytonutrient for which there is no consensus on its therapeutic effects in the preservation of bone mass. The results of several meta-analyses, notably those conducted by Li et al. in 2013 and Lee et al. in 2014, indicate that caffeine consumption may induce a slight decrease in the risk of fracture in men and a slight increase in women, with a greater incidence in the elderly [99,107]. The mechanisms of action involved are not clearly defined and are sometimes conflicting. Caffeine has been described both as being able to inhibit osteoclastogenesis and limit osteoclast activity and, on the contrary, to promote it to the detriment of osteoblasts. Conversely, other studies indicate that there is no significant effect of caffeine on bone density or fracture risk [110,114].
A randomized, controlled clinical trial conducted by Wattanathorn et al. reported that the daily consumption of 1.5 g of a phenolic acid-rich extract for 8 weeks significantly increased the amount of markers involved in bone formation (osteocalcin, p < 0.01; alkaline phosphatase, p < 0.05) and decreased those of resorption (β-carboxy-terminal collagen cross-links, p < 0.01) versus baseline [113]. Phenolic acids may therefore be of interest in the management of osteoporosis, thus confirming the results reported by Law et al. (described previously) [101]. Another placebo-controlled study evaluated the therapeutic effects of twice-daily consumption of a spearmint infusion rich in rosmarinic acid (280 mg/day) for 16 weeks in 46 patients suffering from osteoarthritis [100]. Data from this study indicated a significant decrease in pain assessed via the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score in the rosmarinic acid supplemented group versus baseline, whereas there was no improvement in the placebo group. Of note, an improvement in quality of life (QoL) was also reported in the supplemented group at 16 weeks.
The effect of organosulfur compounds was recently evaluated in a randomized, placebo-controlled clinical trial including 50 female patients with osteoarthritis [104]. The results showed that 1 g of garlic taken daily for a period of 12 weeks significantly reduced the WOMAC index (p = 0.013), joint stiffness (p = 0.019), and tended to reduce joint pain (p = 0.073) compared with placebo, due to the anti-inflammatory properties of organosulfur compounds.
3.2.5. Energy and Vitality
The energy and vitality field is a therapeutic area of great interest in family health and includes several indications. It not only covers problems related to fatigue or recovery, but also symptoms associated with cognition. Numerous studies reveal the significant benefit of phytonutrients in this vast area of health. A summary of the effects of phytochemicals in this therapeutic area is presented in Table 7.

Table 7.
Summary of selected studies regarding the therapeutic area of energy and vitality.
Flavonoids have been evaluated with respect to cognitive problems and show improvement in several studies [117,118,124,129,130]. In 2015, Mastroiacovo et al. evaluated the effect of daily flavanol consumption on cognition in a randomized controlled trial of 90 elderly patients [130]. Their results showed that daily consumption of a flavonoid-rich beverage (993 mg/day) for 8 weeks significantly improved results obtained during exercises assessing cognitive function, particularly the trail making test and the verbal fluency test. These effects were, according to the authors, attributable to the antioxidant and neuroprotective properties of flavonoids, associated with their capacity to improve cerebral perfusion by action on NO-dependent endothelial function. Another randomized, controlled trial conducted in 2016 by Alharbi et al. evaluated the immediate cognitive effects of flavonoid consumption [117]. In this study, volunteers who consumed 240 mL of a beverage containing 272 mg of flavonoids showed a significant improvement in their verbal skills and reflexes 6 h after consumption versus placebo (p < 0.05). According to the authors, the cognitive benefits were also supported by the antioxidant properties of flavonoids which improve vascular functions and increase NO bioavailability, leading to an increase in cerebral vascular flow. In 2020, another randomized, controlled clinical trial demonstrated that the consumption of flavonoids, particularly flavanols, lead to increased cerebral oxygenation, resulting in a significant improvement of cognitive performance versus placebo [124]. Strength loss and neuromuscular impairment also significantly improved compared with placebo (p < 0.05), with a daily consumption of 1 g of quercetin for 14 days resulting in the preservation of muscle mass confirming the antireactive oxygen species (ROS) properties of flavonoids [118]. The work of Lamport et al. evaluating the effect of the consumption of 777 mg of flavonoids for 12 weeks on cognitive performance demonstrated a significant improvement in spatial memory (p < 0.05) as well as driving performance (p = 0.05) when associated with the simultaneous intake of 334 mg of proanthocyanins and 167 mg of anthocyanins [129].
Anthocyanins alone have been shown to be effective in numerous clinical studies in this area of health, with a marked effect on improving physical performance [116,122,128]. A randomized, controlled trial involving 19 healthy subjects studied the effect of daily supplementation with 35 mg of anthocyanins for one month on recovery from physical performance [128]. At the end of the supplementation period, blood levels of cellular oxidation markers such as glutathione peroxidase, superoxide dismutase, and thiobarbituric acid reactive substances were significantly decreased versus placebo (p < 0.05 for all). The authors concluded that anthocyanins have a protective effect on oxidative stress in red blood cells, possibly by increasing the endogenous antioxidant defense system. In 2015, another team evaluated the effect of a daily intake of 27.6 mg of anthocyanins from an açai extract on the physical performance and blood biomarkers of 14 athletes [116]. The study demonstrated that anthocyanins increased the time to exhaustion during high intensity exercise (p = 0.045) and decreased the metabolic stress caused by exercise (p < 0.05), along with decreasing the intensity of perceived exertion and improving cardiorespiratory responses (p < 0.05). According to the authors, these effects were due to the antioxidant properties of anthocyanins, which reduce oxidative stress during exercise. The efficacy of anthocyanins on physical performance was also demonstrated in another randomized, controlled clinical trial conducted by Cook et al. in 2017 which evaluated the effects of a 7 day supplementation of a blackcurrant extract containing 210 mg of anthocyanins [122]. The authors reported that the supplement significantly improved cardiovascular capacity by causing vasodilation and a decrease in muscle oxygen saturation, along with an increase in hemoglobin levels.
Anthocyanins have shown therapeutic benefits in the field of cognition [120,133,137,138]. A 2015 pilot study by Whyte et al. involving 14 children aged 8–10 years of age described how daily supplementation for 7 days with a blueberry drink containing 143 mg of anthocyanins improved response time assessed by the Rey Auditory Verbal Learning Test compared with placebo (p < 0.001), but did not improve visuospatial memory or attention [133]. Another study evaluating the effect of a 12 week supplementation with 387 mg of anthocyanidins on the cognitive performance of elderly subjects also concluded that this family of phytonutrients had beneficial effects [120]. The results indicated a significant increase in brain activity (p < 0.001) and a significant increase in gray matter perfusion in the parietal (p = 0.013) and occipital (p = 0.031) lobes following supplementation. A significant improvement in working memory vs. placebo (p = 0.05) was also reported. The authors associated these clinical effects with an improvement in cerebral vascular function induced by anthocyanins. Beneficial effects on the maintenance of cognitive functions in elderly patients with mild cognitive impairment were also reported in a double-blind, randomized, placebo-controlled trial conducted by Do Rosario in 2021 [137]. The effects of a drink (250 mL/day) containing 201 or 47 mg of anthocyanins were evaluated on vascular functions and circulating inflammatory markers. The results suggested a significant decrease in blood TNF-α levels compared with placebo for both doses tested with no change in other parameters; these results were consistent with a decrease in the decline of cognitive function. The effect of anthocyanins on cognitive parameters in an elderly population was evaluated by Calapai et al. [138]. In this randomized, double-blind, placebo-controlled study, the effect of supplementation with 250 mg/d of anthocyanins for 12 weeks was evaluated with a battery of cognitive tests. The results showed a significant improvement in attention (p < 0.001), language (p < 0.05) and memory (p < 0.0001) with anthocyanins compared with placebo, as well as a decrease in anxiety (p < 0.05) and depression (p < 0.0001) scores, which could be explained by their antioxidant, anti-inflammatory, and antiapoptotic properties.
The action of phenolic acids has been studied in this area of health and this family of phytonutrients presents beneficial effects on agility [119,123,134]. A randomized, controlled study by Falcone et al. evaluated the effects of supplementation with a spearmint extract rich in phenolic acids (900 mg/d) versus placebo in 142 adults for 7–90 days [123]. The study demonstrated an overall significant effect on reactive agility (p = 0.049) and, more specifically, a beneficial effect on the stationary test (p = 0.04 at Day 30 and p = 0.002 at Day 90), reaction time (p = 0.049 at Day 30), and accuracy (p = 0.007 at Day 30 and p = 0.026 at Day 90), which was attributed to the cerebral anti-inflammatory properties of phenolic acids. Another spearmint extract has also shown beneficial effects on cognition in a study published in 2019 by the same team [134]. Young adults received 900 mg of a spearmint extract or placebo daily for 90 days and a battery of cognitive tests involving sleep, mood, and QoL were performed on Day 0 and after 7, 30, and 90 days of treatment. The results indicated a significant improvement in attention with the spearmint extract compared with placebo after 30 days (p = 0.001) and 90 days (p = 0.007) without significant improvement in the other parameters measured. Another randomized, controlled study evaluated the effect of supplementation with 300 mg of chlorogenic acid for 16 weeks on the cognitive functions of 38 subjects with memory problems [119]. The authors reported that chlorogenic acid significantly improved certain cognitive functions, particularly attention, motor speed, psychomotor speed, and executive function compared with placebo. This effect could be linked to an effect of the phytonutrient on the synthesis of apolipoprotein A1 and transthyretin, two markers associated with early cognitive decline.
Tannins have shown therapeutic interest in the area of energy and vitality as they have been shown to reduce fatigue and improve physical performance [132,136]. In a randomized, controlled study conducted in 2007, the daily intake of a tannin-rich extract (1200 mg) for 8 days resulted in a better resistance to fatigue induced by physical exercise compared with placebo (p < 0.05) without modification of cardiovascular parameters [136]. Other work conducted in 2010 by Trombold et al. also demonstrated that consumption of a pomegranate extract rich in ellagitannins (650 mg) for 9 days significantly increased muscle strength recovery 2 to 3 days after an eccentric elbow flexion exercise compared with placebo [132]. The authors suggested that the antioxidant properties of tannins may limit the production of free radicals and ROS during exercise and act as the source of the therapeutic benefits of these phytonutrients.
Carotenoids are also of interest in the area of health, particularly for problems related to fatigue and recovery [121,127,135]. A randomized, controlled clinical trial conducted by Imai et al. in 2018 evaluated the benefit of the antioxidant properties of carotenoids in the management of fatigue [127]. Their work consisted of evaluating the effects of supplementation with 3 mg astaxanthin and 5 mg sesamin for 4 weeks on mental fatigue. At the end of the treatment, they observed a significant decrease in mental fatigue versus placebo (p < 0.05) and a lower increase in circulating levels of phosphatidylcholine hydroperoxide (p < 0.05), a marker of oxidative stress. The authors attribute these results to the significant antioxidant properties of the carotenoids evaluated. A double-blind, randomized, controlled trial involving 2983 subjects evaluated the long-term cognitive effects of a diet rich in carotenoids over a period of 8 years [121]. The results indicated that this type of diet was associated with an improvement in the composite cognitive score (p = 0.002) as well as in six neuropsychological tests: cued recall task, backward digit span task, trail making test, and semantic fluency task compared with a diet less rich in carotenoids. Once again, the antioxidant and anti-inflammatory properties of carotenoids were thought to explain these therapeutic benefits. Another study conducted by Johnson et al. in 2008 evaluated the effect of lutein supplementation on cognition and more particularly on memory and speech [135]. This study demonstrated an improvement in verbal fluency with a daily treatment of 12 mg of lutein compared with placebo (p < 0.03), but no positive effect on memory. Nevertheless, the authors reported a significant improvement in memory and learning level when lutein was associated with docosahexaenoic acid (p < 0.03), validating the therapeutic interest of combining phytonutrients.
The most well-known phytonutrient in the field of cognition and vitality is caffeine. Numerous scientific studies have reported that this phytonutrient acts on the problems of fatigue, improves concentration and physical performance and facilitates recovery [115,125,126,131].
A recent randomized, controlled trial evaluated the short-term effects on cognition and mood of caffeinated and non-caffeinated black coffee compared with placebo in 59 volunteers [126]. The results suggested that while decaffeinated coffee increased alertness versus placebo, only coffee containing 100 mg of caffeine significantly improved accuracy and reduced fatigue and headaches 30 min after ingestion; significant differences were reported versus both placebo and decaffeinated coffee. These effects could be attributed to the ability of caffeine to antagonize A1 and A2A adenosine receptors, thus increasing oxygen metabolism and increasing the synthesis of neurotransmitters such as noradrenaline, dopamine, serotonin, and GABA. Another study conducted in 2014 by Borota et al. reported beneficial effects of caffeine on memory function [115]. This randomized, controlled study in 160 subjects demonstrated an improvement in memory performance up to 24 h after coffee consumption with an inverted U-shaped dose response effect. The authors concluded that this effect was compatible with a consolidation of long-term memory and could be due to an inhibition of norepinephrine via direct blocking of adenosine by caffeine or an effect of caffeine in the CA2 area of the hippocampus.
In terms of improving physical performance, caffeine is often used by athletes as an ergogenic aid. A 2018 review evaluated 20 clinical studies involving 294 subjects to investigate the effects of caffeine on muscle strength and power [125]. The results of this analysis indicated that caffeine significantly improved strength (p = 0.023) and muscle power (p = 0.047), particularly in the upper extremities. A randomized, controlled trial by Duvnjak-Zaknich et al. evaluated the effect of caffeine 6 mg/kg or placebo administered 60 min before a team sport session (80 min duration) on performance [131]. Intermediate exercises of reactivity, agility, and decision making were conducted. The study demonstrated that caffeine significantly improved most of the parameters measured versus placebo (total time, p = 0.001; reactive agility time, p = 0.001; decision time, p = 0.045; movement time, p = 0.043). The mechanism of action involved was thought to be related to a blocking of adenosine receptors by caffeine, leading to stimulation of the CNS [131].
All the therapeutic benefits of phytonutrients are based on the same mechanisms of action described in the previous paragraphs and are mainly based on their antioxidant and anti-inflammatory activities.
4. Conclusion and Perspectives
This analysis of the literature allows highlighting of the significant benefits of phytonutrients in the prevention and management of pathologies and symptoms in the field of family health. To date, natural healthcare has primarily been based on phytotherapy, which consists of using the therapeutic properties of so-called medicinal plants to prevent or treat certain pathologies [139]. Phytonutrition is concerned with the action of molecules derived from plants that can be integrated into a balanced diet with beneficial effects on health. The phytonutritional approach is positioned at the interface between phytoaromatherapy and nutrition, constitutes an original and innovative breakthrough, and provides a source of reflection for the field of phytotherapy. Phytonutrition adds to existing data on medicinal plants and may open up new areas of therapeutic activity for some of them. In addition, phytonutrition sheds additional light on the classical mode of action of plants and their active ingredients, and could become a discipline in its own right in the near future.
This review assessed the seven largest families of phytonutrients found in food and the diet [9] and demonstrated that each of them had significant therapeutic potential in the healthcare field. Moreover, this evaluation also enabled complementary effects of the different families of phytonutrients in the same area of health to be recognized. Nevertheless, there are many other phytonutrients that were not included in this review of the literature. Similarly, our analysis focused on healthcare, but it is clear that phytonutrients also play an important role in the prevention of serious chronic diseases such as diabetes, obesity, and hypertension, along with different types of cancer or degenerative diseases [21,78,83,140]. Thus, it would be worthwhile to further investigate the mechanisms of action of phytonutrients associated with these effects in chronic diseases.
To the best of our knowledge, this review provides a deeper analysis of the potential benefits of phytonutrients in human healthcare. A phytonutrient-based approach appears to provide an innovative way to address natural health and could be a useful additional therapeutic option for physicians.
Author Contributions
Conceptualization, N.M., M.J.A., J.F., J.M.M. and S.R.; methodology, N.M. and M.J.A.; formal analysis, N.M., M.J.A., J.F., J.M.M. and S.R.; validation, N.M., M.J.A., J.F., J.M.M. and S.R.; writing—original draft preparation, N.M., M.J.A., J.F., J.M.M. and S.R.; writing—review and editing, N.M., M.J.A., J.F., J.M.M. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
Editorial assistance for this manuscript and open access was funded by Pierre Fabre Laboratories, France.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Emilie Ondet, publication manager at Pierre Fabre, who ensured the follow-up of the redaction of this paper and communication between the authors and the medical writer. They also thank Christophe Long, Clement Laur, Ines Fechtner, and Sandrine Le Hello for their scientific help in this topic and Matthew Joynson of Springer Healthcare Communications who edited and formatted the manuscript for publication. This editorial assistance was funded by Pierre Fabre Laboratories, France.
Conflicts of Interest
M.J. Amiot, J. Fleurentin and J.M. Morel have acted as consultants for, and as expert witnesses on behalf of, Pierre Fabre Laboratories, France. N. Monjotin and S. Raynal are employees of Pierre Fabre Laboratories, France.
References
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune function and micronutrient requirements change over the life course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Poiroux-Gonord, F.; Bidel, L.P.; Fanciullino, A.L.; Gautier, H.; Lauri-Lopez, F.; Urban, L. Health benefits of vitamins and secondary metabolites of fruits and vegetables and prospects to increase their concentrations by agronomic approaches. J. Agric. Food Chem. 2010, 58, 12065–12082. [Google Scholar] [CrossRef]
- Aune, D. Plant foods, antioxidant biomarkers, and the risk of cardiovascular disease, cancer, and mortality: A review of the evidence. Adv. Nutr. 2019, 10 (Suppl. 4), S404–S421. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health-promoting components of fruits and vegetables in the diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113 (Suppl. 9B), 71S–88S. [Google Scholar] [CrossRef]
- Tennant, D.R.; Davidson, J.; Day, A.J. Phytonutrient intakes in relation to European fruit and vegetable consumption patterns observed in different food surveys. Br. J. Nutr. 2014, 112, 1214–1225. [Google Scholar] [CrossRef]
- Amiot, M.J.; Latgé, C.; Plumey, L.; Raynal, S. Intake estimation of phytochemicals in a French well-balanced diet. Nutrients 2021, 13, 3628. [Google Scholar] [CrossRef]
- Jeffery, E.H.; Brown, A.F.; Kurilich, A.C.; Keck, A.S.; Matusheski, N.; Klein, B.P.; Juvik, J.A. Variation in content of bioactive components in broccoli. J. Food Compos. Anal. 2003, 16, 323–330. [Google Scholar] [CrossRef]
- King, A.; Young, G. Characteristics and occurrence of phenolic phytochemicals. J. Am. Diet Assoc. 1999, 99, 213–218. [Google Scholar] [CrossRef]
- Amiot, M.J.; Coxam, V.; Strigler, F. Les Phytomicronutriments, 1st ed.; Lavoisier: Paris, France, 2012; p. 386. [Google Scholar]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on plant antimicrobials: A mechanistic viewpoint. AntiMicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, P.D.; Pollier, J.; Panda, S.; Szymanski, J.; Massalha, H.; Yona, M.; Unger, T.; Malitsky, S.; Arendt, P.; Pauwels, L.; et al. Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nat. Plants 2016, 3, 16205. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef] [PubMed]
- Kaulmann, A.; Bohn, T. Carotenoids, inflammation, and oxidative stress—Implications of cellular signaling pathways and relation to chronic disease prevention. Nutr. Res. 2014, 34, 907–929. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef]
- Chandrasekara, A. Phenolic Acids. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 535–545. [Google Scholar]
- Liu, R.H. Potential synergy of phytochemicals in cancer prevention: Mechanism of action. J. Nutr. 2004, 134 (Suppl. 12), 3479S–3485S. [Google Scholar] [CrossRef]
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78 (Suppl. 1), A18–A25. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Bruneton, J. Pharmacognosie Phytochimie Plantes Médicinales, 5th ed.; Lavoisier Tec&Doc: Paris, France, 2016; pp. 287–1485. [Google Scholar]
- Banjarnahor, S.D.S.; Artanti, N. Antioxidant properties of flavonoids. Med. J. Indones. 2015, 23, 239–244. [Google Scholar] [CrossRef]
- Gupta, C.; Prakash, D. Phytonutrients as therapeutic agents. J. Complement. Integr. Med. 2014, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.L.; Wang, X.H.; Pazmiño, J.; Engeseth, N.J. Antioxidant capacity and phenolic content of grapes, sun-dried raisins, and golden raisins and their effect on ex vivo serum antioxidant capacity. J. Agric. Food Chem. 2007, 55, 8472–8477. [Google Scholar] [CrossRef]
- Lin, B.W.; Gong, C.C.; Song, H.F.; Cui, Y.Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef]
- Derbel, S.; Ghedira, K. Les phytonutriments et leur impact sur la santé. Phytothérapie 2005, 3, 28–34. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130 (Suppl. S8), 2073S–2085S. [Google Scholar] [CrossRef]
- Vlachojannis, C.; Zimmermann, B.F.; Chrubasik-Hausmann, S. Quantification of anthocyanins in elderberry and chokeberry dietary supplements. Phytother. Res. 2015, 29, 561–565. [Google Scholar] [CrossRef]
- Ghosh, D. Tannins from foods to combat diseases. Int. J. Pharma. Res. Rev. 2015, 4, 40–44. [Google Scholar]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef]
- Sugiyama, R.; Hirai, M.Y. Atypical myrosinase as a mediator of glucosinolate functions in plants. Front. Plant Sci. 2019, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613. [Google Scholar] [CrossRef] [PubMed]
- Bohn, T. Carotenoids and markers of oxidative stress in human observational studies and intervention trials: Implications for chronic diseases. Antioxidants 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Niroula, A.; Khatri, S.; Timilsina, R.; Khadka, D.; Khadka, A.; Ojha, P. Profile of chlorophylls and carotenoids of wheat (Triticum aestivum L.) and barley (Hordeum vulgare L.) microgreens. J. Food Sci. Technol. 2019, 56, 2758–2763. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Carotenoids: How effective are they to prevent age-related diseases? Molecules 2019, 24, 1801. [Google Scholar] [CrossRef]
- Ashihara, H.; Sano, H.; Crozier, A. Caffeine and related purine alkaloids: Biosynthesis, catabolism, function and genetic engineering. Phytochemistry 2008, 69, 841–856. [Google Scholar] [CrossRef]
- Actualisation des Repères du PNNS: ÉTude des Relations Entre Consommation de Groupes d’aliments et Risque de Maladies Chroniques Non Transmissibles. November 2016. Available online: https://www.researchgate.net/publication/312665902_Actualisation_des_reperes_du_PNNS_etude_des_relations_entre_consommation_de_groupes_d’aliments_et_risque_de_maladies_chroniques_non_transmissibles (accessed on 1 March 2022).
- Heckman, M.A.; Weil, J.; Gonzalez de Mejia, E. Caffeine (1,3,7-trimethylxanthine) in foods: A comprehensive review on consumption, functionality, safety, and regulatory matters. J. Food Sci. 2010, 75, R77–R87. [Google Scholar] [CrossRef]
- Stringham, N.T.; Holmes, P.V.; Stringham, J.M. Supplementation with macular carotenoids reduces psychological stress, serum cortisol, and sub-optimal symptoms of physical and emotional health in young adults. Nutr. Neurosci. 2018, 21, 286–296. [Google Scholar] [CrossRef]
- Scholey, A.; Downey, L.A.; Ciorciari, J.; Pipingas, A.; Nolidin, K.; Finn, M.; Wines, M.; Catchlove, S.; Terrens, A.; Barlow, E.; et al. Acute neurocognitive effects of epigallocatechin gallate (EGCG). Appetite 2012, 58, 767–770. [Google Scholar] [CrossRef]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-García, A.M.; Prodanov, M. Affron® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef] [PubMed]
- White, B.C.; Lincoln, C.A.; Pearce, N.W.; Reeb, R.; Vaida, C. Anxiety and muscle tension as consequences of caffeine withdrawal. Science 1980, 209, 1547–1548. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, G.A.; Carroll, M.E.; Crosby, R.D.; Perwien, A.R.; Go, F.S.; Benowitz, N.L. Caffeine effects on learning, performance, and anxiety in normal school-age children. J. Am. Acad. Child Adolesc. Psychiatry 1994, 33, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Micek, A.; Castellano, S.; Pajak, A.; Galvano, F. Coffee, tea, caffeine and risk of depression: A systematic review and dose-response meta-analysis of observational studies. Mol. Nutr. Food Res. 2016, 60, 223–234. [Google Scholar] [CrossRef]
- Terauchi, M.; Horiguchi, N.; Kajiyama, A.; Akiyoshi, M.; Owa, Y.; Kato, K.; Kubota, T. Effects of grape seed proanthocyanidin extract on menopausal symptoms, body composition, and cardiovascular parameters in middle-aged women: A randomized, double-blind, placebo-controlled pilot study. Menopause 2014, 21, 990–996. [Google Scholar] [CrossRef]
- Umigai, N.; Takeda, R.; Mori, A. Effect of crocetin on quality of sleep: A randomized, double-blind, placebo-controlled, crossover study. Complement. Ther. Med. 2018, 41, 47–51. [Google Scholar] [CrossRef]
- Hachul, H.; Brandão, L.C.; D’Almeida, V.; Bittencourt, L.R.; Baracat, E.C.; Tufik, S. Isoflavones decrease insomnia in postmenopause. Menopause 2011, 18, 178–184. [Google Scholar] [CrossRef]
- Losso, J.N.; Finley, J.W.; Karki, N.; Liu, A.G.; Prudente, A.; Tipton, R.; Yu, Y.; Greenway, F.L. Pilot study of the tart cherry juice for the treatment of insomnia and investigation of mechanisms. Am. J. Ther. 2018, 25, e194–e201. [Google Scholar] [CrossRef]
- Kuratsune, H.; Umigai, N.; Takeno, R.; Kajimoto, Y.; Nakano, T. Effect of crocetin from Gardenia jasminoides Ellis on sleep: A pilot study. Phytomedicine 2010, 17, 840–843. [Google Scholar] [CrossRef]
- Valko, M.; Izakovic, M.; Mazur, M.; Rhodes, C.J.; Telser, J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. [Google Scholar] [CrossRef]
- Stefanello, N.; Spanevello, R.M.; Passamonti, S.; Porciúncula, L.; Bonan, C.D.; Olabiyi, A.A.; Teixeira da Rocha, J.B.; Assmann, C.E.; Morsch, V.M.; Schetinger, M.R.C. Coffee, caffeine, chlorogenic acid, and the purinergic system. Food Chem. Toxicol. 2019, 123, 298–313. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Vrolijk, M.F.; Opperhuizen, A.; Jansen, E.H.; Godschalk, R.W.; Van Schooten, F.J.; Bast, A.; Haenen, G.R. The shifting perception on antioxidants: The case of vitamin E and β-carotene. Redox Biol. 2015, 4, 272–278. [Google Scholar] [CrossRef]
- Cos, P.; De Bruyne, T.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2004, 11, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, S.A.; van den Berg, D.J.; Tromp, M.N.; Griffioen, D.H.; van Bennekom, W.P.; van der Vijgh, W.J.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Masuda, S.; Maeda-Yamamoto, M.; Usui, S.; Fujisawa, T. ‘Benifuuki’ green tea containing O-methylated catechin reduces symptoms of Japanese Cedar Pollinosis: A randomized, double-blind, placebo-controlled trial. Allergol. Int. 2014, 63, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, T.; Nagasako-Akazome, Y.; Kanda, T.; Ikeda, M.; Dake, Y. Clinical effects of apple polyphenols on persistent allergic rhinitis: A randomized double-blind placebo-controlled parallel arm study. J. Investig. Allergol. Clin. Immunol. 2006, 16, 283–289. [Google Scholar] [PubMed]
- Matsumoto, K.; Yamada, H.; Takuma, N.; Niino, H.; Sagesaka, Y.M. Effects of green tea catechins and theanine on preventing influenza infection among healthcare workers: A randomized controlled trial. BMC Complement. Altern. Med. 2011, 11, 15. [Google Scholar] [CrossRef]
- Muller, L.; Meyer, M.; Bauer, R.N.; Zhou, H.; Zhang, H.; Jones, S.; Robinette, C.; Noah, T.L.; Jaspers, I. Effect of broccoli sprouts and live attenuated influenza virus on peripheral blood natural killer cells: A randomized, double-blind study. PLoS ONE 2016, 11, e0147742. [Google Scholar] [CrossRef]
- Nantz, M.P.; Rowe, C.A.; Muller, C.; Creasy, R.; Colee, J.; Khoo, C.; Percival, S.S. Consumption of cranberry polyphenols enhances human γδ-T cell proliferation and reduces the number of symptoms associated with colds and influenza: A randomized, placebo-controlled intervention study. Nutr. J. 2013, 12, 161. [Google Scholar] [CrossRef]
- Crişan, I.; Zaharia, C.N.; Popovici, F.; Jucu, V.; Belu, O.; Dascălu, C.; Mutiu, A.; Petrescu, A. Natural propolis extract NIVCRISOL in the treatment of acute and chronic rhinopharyngitis in children. Rom. J. Virol. 1995, 46, 115–133. [Google Scholar] [PubMed]
- Welsh, E.J.; Bara, A.; Barley, E.; Cates, C.J. Caffeine for asthma. Cochrane Database Syst. Rev. 2010, 2010, CD001112. [Google Scholar] [CrossRef] [PubMed]
- Nourollahian, M.; Rasoulian, B.; Gafari, A.; Anoushiravani, M.; Jabari, F.; Bakhshaee, M. Clinical comparison of the efficacy of spirulina platensis and cetirizine for treatment of allergic rhinitis. Acta Otorhinolaryngol. Ital. 2020, 40, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Cingi, C.; Conk-Dalay, M.; Cakli, H.; Bal, C. The effects of spirulina on allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2008, 265, 1219–1223. [Google Scholar] [CrossRef]
- Cui, Y.; Oh, Y.J.; Lim, J.; Youn, M.; Lee, I.; Pak, H.K.; Park, W.; Jo, W.; Park, S. AFM study of the differential inhibitory effects of the green tea polyphenol (-)-epigallocatechin-3-gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiol. 2012, 29, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Yoda, Y.; Hu, Z.Q.; Zhao, W.H.; Shimamura, T. Different susceptibilities of Staphylococcus and Gram-negative rods to epigallocatechin gallate. J. Infect. Chemother. 2004, 10, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Oneda, H.; Shiihara, M.; Inouye, K. Inhibitory effects of green tea catechins on the activity of human matrix metalloproteinase 7 (matrilysin). J. Biochem. 2003, 133, 571–576. [Google Scholar] [CrossRef]
- Yi, S.M.; Zhu, J.L.; Fu, L.L.; Li, J.R. Tea polyphenols inhibit Pseudomonas aeruginosa through damage to the cell membrane. Int. J. Food Microbiol. 2010, 144, 111–117. [Google Scholar] [CrossRef]
- Chen, C.C.; Huang, C.Y. Inhibition of Klebsiella pneumoniae DnaB helicase by the flavonol galangin. Protein J. 2011, 30, 59–65. [Google Scholar] [CrossRef]
- Huang, Y.H.; Huang, C.C.; Chen, C.C.; Yang, K.J.; Huang, C.Y. Inhibition of Staphylococcus aureus PriA helicase by flavonol kaempferol. Protein J. 2015, 34, 169–172. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An overview on the role of dietary phenolics for the treatment of cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- De Stefano, D.; Maiuri, M.C.; Simeon, V.; Grassia, G.; Soscia, A.; Cinelli, M.P.; Carnuccio, R. Lycopene, quercetin and tyrosol prevent macrophage activation induced by gliadin and IFN-gamma. Eur. J. Pharmacol. 2007, 566, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Mann, G.E.; Chapple, S.J. Concerted redox modulation by sulforaphane alleviates diabetes and cardiometabolic syndrome. Free Radic. Biol. Med. 2018, 122, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.S.; Fan, Y.E.; Lin, C.Y.; Hu, M.L. Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. J. Nutr. Biochem. 2007, 18, 449–456. [Google Scholar] [CrossRef]
- Chen, C.C.; Chow, M.P.; Huang, W.C.; Lin, Y.C.; Chang, Y.J. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: Structure-activity relationships. Mol. Pharmacol. 2004, 66, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Matsui, T. Protective role of sulphoraphane against vascular complications in diabetes. Pharm. Biol. 2016, 54, 2329–2339. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Kwon, D.; Um, E.; Kim, B. Could polyphenols help in the control of rheumatoid arthritis? Molecules 2019, 24, 1589. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.D.; et al. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; González-Abuín, N.; Ardèvol, A.; Pinent, M.; Blay, M.T. Procyanidins and inflammation: Molecular targets and health implications. Biofactors 2012, 38, 257–265. [Google Scholar] [CrossRef]
- Baek, H.I.; Ha, K.C.; Kim, H.M.; Choi, E.K.; Park, E.O.; Park, B.H.; Yang, H.J.; Kim, M.J.; Kang, H.J.; Chae, S.W. Randomized, double-blind, placebo-controlled trial of Ficus carica paste for the management of functional constipation. Asia Pac. J. Clin. Nutr. 2016, 25, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, G.L.; Wasilewski, A.; Haller, M.; Pandya, K.; Bennett, J.; He, H.; Hoffmire, C.; Berry, C. Effect of concord grape juice on chemotherapy-induced nausea and vomiting: Results of a pilot study. Oncol. Nurs. Forum. 2010, 37, 213–221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dryden, G.W.; Lam, A.; Beatty, K.; Qazzaz, H.H.; McClain, C.J. A pilot study to evaluate the safety and efficacy of an oral dose of (-)-epigallocatechin-3-gallate-rich polyphenon E in patients with mild to moderate ulcerative colitis. Inflamm. Bowel Dis. 2013, 19, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- Barsalani, R.; Riesco, E.; Lavoie, J.M.; Dionne, I.J. Effect of exercise training and isoflavones on hepatic steatosis in overweight postmenopausal women. Climacteric 2013, 16, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Cheraghpour, M.; Imani, H.; Ommi, S.; Alavian, S.M.; Karimi-Shahrbabak, E.; Hedayati, M.; Yari, Z.; Hekmatdoost, A. Hesperidin improves hepatic steatosis, hepatic enzymes, and metabolic and inflammatory parameters in patients with nonalcoholic fatty liver disease: A randomized, placebo-controlled, double-blind clinical trial. Phytother. Res. 2019, 33, 2118–2125. [Google Scholar] [CrossRef]
- Venancio, V.P.; Kim, H.; Sirven, M.A.; Tekwe, C.D.; Honvoh, G.; Talcott, S.T.; Mertens-Talcott, S.U. Polyphenol-rich mango (Mangifera indica L.) ameliorate functional constipation symptoms in humans beyond equivalent amount of fiber. Mol. Nutr. Food Res. 2018, 62, e1701034. [Google Scholar] [CrossRef]
- Mangel, A.W.; Chaturvedi, P. Evaluation of crofelemer in the treatment of diarrhea-predominant irritable bowel syndrome patients. Digestion 2008, 78, 180–186. [Google Scholar] [CrossRef]
- Kikuchi, M.; Ushida, Y.; Shiozawa, H.; Umeda, R.; Tsuruya, K.; Aoki, Y.; Suganuma, H.; Nishizaki, Y. Sulforaphane-rich broccoli sprout extract improves hepatic abnormalities in male subjects. World J. Gastroenterol. 2015, 21, 12457–12467. [Google Scholar] [CrossRef]
- Yanaka, A. Daily intake of broccoli sprouts normalizes bowel habits in human healthy subjects. J. Clin. Biochem. Nutr. 2018, 62, 75–82. [Google Scholar] [CrossRef]
- Kaczmarek, J.L.; Liu, X.; Charron, C.S.; Novotny, J.A.; Jeffery, E.H.; Seifried, H.E.; Ross, S.A.; Miller, M.J.; Swanson, K.S.; Holscher, H.D. Broccoli consumption affects the human gastrointestinal microbiota. J. Nutr. Biochem. 2019, 63, 27–34. [Google Scholar] [CrossRef]
- Biedermann, L.; Mwinyi, J.; Scharl, M.; Frei, P.; Zeitz, J.; Kullak-Ublick, G.A.; Vavricka, S.R.; Fried, M.; Weber, A.; Humpf, H.U.; et al. Bilberry ingestion improves disease activity in mild to moderate ulcerative colitis—An open pilot study. J. Crohns. Colitis 2013, 7, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, C.E.; Everhart, J.E. Coffee and tea consumption are associated with a lower incidence of chronic liver disease in the United States. Gastroenterology 2005, 129, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.L.; Bremner, A.P.; Reid, A.; Mackerras, D.; Alfonso, H.; Olsen, N.J.; Musk, A.W.; de Klerk, N.H. No dose-dependent increase in fracture risk after long-term exposure to high doses of retinol or beta-carotene. Osteoporos Int. 2013, 24, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Huang, C.Q.; Lin, Z.K.; Tian, N.F.; Ni, W.F.; Wang, X.Y.; Xu, H.Z.; Chi, Y.L. The relationship between vitamin A and risk of fracture: Meta-analysis of prospective studies. J. Bone Miner. Res. 2014, 29, 2032–2039. [Google Scholar] [CrossRef]
- Lee, D.R.; Lee, J.; Rota, M.; Lee, J.; Ahn, H.S.; Park, S.M.; Shin, D. Coffee consumption and risk of fractures: A systematic review and dose-response meta-analysis. Bone 2014, 63, 20–28. [Google Scholar] [CrossRef]
- Connelly, A.E.; Tucker, A.J.; Tulk, H.; Catapang, M.; Chapman, L.; Sheikh, N.; Yurchenko, S.; Fletcher, R.; Kott, L.S.; Duncan, A.M.; et al. High-rosmarinic acid spearmint tea in the management of knee osteoarthritis symptoms. J. Med. Food 2014, 17, 1361–1367. [Google Scholar] [CrossRef]
- Law, Y.Y.; Chiu, H.F.; Lee, H.H.; Shen, Y.C.; Venkatakrishnan, K.; Wang, C.K. Consumption of onion juice modulates oxidative stress and attenuates the risk of bone disorders in middle-aged and post-menopausal healthy subjects. Food Funct. 2016, 7, 902–912. [Google Scholar] [CrossRef]
- Hu, Y.; Cui, J.; Sparks, J.A.; Malspeis, S.; Costenbader, K.H.; Karlson, E.W.; Lu, B. Circulating carotenoids and subsequent risk of rheumatoid arthritis in women. Clin. Exp. Rheumatol. 2017, 35, 309–312. [Google Scholar]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Rahimi Foroushani, A.; Jazayeri, S. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: A double-blind, randomized controlled trial. J. Am. Coll. Nutr. 2017, 36, 9–15. [Google Scholar] [CrossRef]
- Hosseinzadeh-Attar, M.J.; Alipoor, E.; Dehghani, S.; Salimzadeh, A. Increased efficacy of a garlic supplement on knee osteoarthritis symptoms in patients with obesity. J. Herb. Med. 2020, 24, 100392. [Google Scholar] [CrossRef]
- Kim, D.E.; Cho, S.H.; Park, H.M.; Chang, Y.K. Relationship between bone mineral density and dietary intake of β-carotene, vitamin C, zinc and vegetables in postmenopausal Korean women: A cross-sectional study. J. Int. Med. Res. 2016, 44, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.N.T.; Hu, L.M.; Jeppesen, P.B. A systematic review and meta-analysis of the effects of isoflavone formulations against estrogen-deficient bone resorption in peri- and postmenopausal women. Am. J. Clin. Nutr. 2017, 106, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Xu, J.H. Coffee consumption and hip fracture risk: A meta-analysis. J. Nutr. Sci. 2013, 2, e23. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Ciaponi, M.; Cappagli, B.; Piaggesi, L.; Genazzani, A.R. Effects of combined low dose of the isoflavone derivative ipriflavone and estrogen replacement on bone mineral density and metabolism in postmenopausal women. Maturitas 1997, 28, 75–81. [Google Scholar] [CrossRef]
- Ambrosini, G.L.; Alfonso, H.; Reid, A.; Mackerras, D.; Bremner, A.P.; Beilby, J.; Olsen, N.J.; Musk, A.W.; de Klerk, N.H. Plasma retinol and total carotenes and fracture risk after long-term supplementation with high doses of retinol. Nutrition 2014, 30, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, C.M.; Ichikawa, L.; LaCroix, A.Z.; Ott, S.M.; Scholes, D. Association between caffeine intake and bone mass among young women: Potential effect modification by depot medroxyprogesterone acetate use. Osteoporos. Int. 2008, 19, 519–527. [Google Scholar] [CrossRef]
- Pattison, D.J.; Symmons, D.P.; Lunt, M.; Welch, A.; Bingham, S.A.; Day, N.E.; Silman, A.J. Dietary beta-cryptoxanthin and inflammatory polyarthritis: Results from a population-based prospective study. Am. J. Clin. Nutr. 2005, 82, 451–455. [Google Scholar] [CrossRef]
- Rejnmark, L.; Vestergaard, P.; Charles, P.; Hermann, A.P.; Brot, C.; Eiken, P.; Mosekilde, L. No effect of vitamin A intake on bone mineral density and fracture risk in perimenopausal women. Osteoporos. Int. 2004, 15, 872–880. [Google Scholar] [CrossRef]
- Wattanathorn, J.; Somboonporn, W.; Sungkamanee, S.; Thukummee, W.; Muchimapura, S. A double-blind placebo-controlled randomized trial evaluating the effect of polyphenol-rich herbal congee on bone turnover markers of the perimenopausal and menopausal women. Oxid. Med. Cell Longev. 2018, 2018, 2091872. [Google Scholar] [CrossRef]
- Li, S.; Dai, Z.; Wu, Q. Effect of coffee intake on hip fracture: A meta-analysis of prospective cohort studies. Nutr. J. 2015, 14, 38. [Google Scholar] [CrossRef]
- Borota, D.; Murray, E.; Keceli, G.; Chang, A.; Watabe, J.M.; Ly, M.; Toscano, J.P.; Yassa, M.A. Post-study caffeine administration enhances memory consolidation in humans. Nat. Neurosci. 2014, 17, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Peixoto, J.; Moura, M.R.; Cunha, F.A.; Lollo, P.C.; Monteiro, W.D.; Carvalho, L.M.; Farinatti Pde, T. Consumption of açai (Euterpe oleracea Mart.) functional beverage reduces muscle stress and improves effort tolerance in elite athletes: A randomized controlled intervention study. Appl. Physiol. Nutzr. Metab. 2015, 40, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.H.; Lamport, D.J.; Dodd, G.F.; Saunders, C.; Harkness, L.; Butler, L.T.; Spencer, J.P. Flavonoid-rich orange juice is associated with acute improvements in cognitive function in healthy middle-aged males. Eur. J. Nutr. 2016, 55, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Bazzucchi, I.; Patrizio, F.; Ceci, R.; Duranti, G.; Sgrò, P.; Sabatini, S.; Di Luigi, L.; Sacchetti, M.; Felici, F. The effects of quercetin supplementation on eccentric exercise-induced muscle damage. Nutrients 2019, 11, 205. [Google Scholar] [CrossRef]
- Saitou, K.; Ochiai, R.; Kozuma, K.; Sato, H.; Koikeda, T.; Osaki, N.; Katsuragi, Y. Effect of chlorogenic acids on cognitive function: A randomized, double-blind, placebo-controlled trial. Nutrients 2018, 10, 1337. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Aboo-Bakkar, Z.; Conway, M.E.; Adlam, A.R.; Fulford, J. Enhanced task-related brain activation and resting perfusion in healthy older adults after chronic blueberry supplementation. Appl. Physiol. Nutr. Metab. 2017, 42, 773–779. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Andreeva, V.A.; Ducros, V.; Jeandel, C.; Julia, C.; Hercberg, S.; Galan, P. Carotenoid-rich dietary patterns during midlife and subsequent cognitive function. Br. J. Nutr. 2014, 111, 915–923. [Google Scholar] [CrossRef]
- Cook, M.D.; Myers, S.D.; Gault, M.L.; Willems, M.E.T. Blackcurrant alters physiological responses and femoral artery diameter during sustained isometric contraction. Nutrients 2017, 9, 556. [Google Scholar] [CrossRef]
- Falcone, P.H.; Tribby, A.C.; Vogel, R.M.; Joy, J.M.; Moon, J.R.; Slayton, C.A.; Henigman, M.M.; Lasrado, J.A.; Lewis, B.J.; Fonseca, B.A.; et al. Efficacy of a nootropic spearmint extract on reactive agility: A randomized, double-blind, placebo-controlled, parallel trial. J. Int. Soc. Sports Nutr. 2018, 15, 58. [Google Scholar] [CrossRef]
- Gratton, G.; Weaver, S.R.; Burley, C.V.; Low, K.A.; Maclin, E.L.; Johns, P.W.; Pham, Q.S.; Lucas, S.J.E.; Fabiani, M.; Rendeiro, C. Dietary flavanols improve cerebral cortical oxygenation and cognition in healthy adults. Sci. Rep. 2020, 10, 19409. [Google Scholar] [CrossRef]
- Grgic, J.; Trexler, E.T.; Lazinica, B.; Pedisic, Z. Effects of caffeine intake on muscle strength and power: A systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2018, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Haskell-Ramsay, C.F.; Jackson, P.A.; Forster, J.S.; Dodd, F.L.; Bowerbank, S.L.; Kennedy, D.O. The acute effects of caffeinated black coffee on cognition and mood in healthy young and older adults. Nutrients 2018, 10, 1386. [Google Scholar] [CrossRef] [PubMed]
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Effects of dietary supplementation of Astaxanthin and Sesamin on daily fatigue: A randomized, double-blind, placebo-controlled, two-way crossover study. Nutrients 2018, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Pilaczynska-Szczesniak, L.; Skarpanska-Steinborn, A.; Deskur, E.; Basta, P.; Horoszkiewicz-Hassan, M. The influence of chokeberry juice supplementation on the reduction of oxidative stress resulting from an incremental rowing ergometer exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.J.; Lawton, C.L.; Merat, N.; Jamson, H.; Myrissa, K.; Hofman, D.; Chadwick, H.K.; Quadt, F.; Wightman, J.D.; Dye, L. Concord grape juice, cognitive function, and driving performance: A 12-wk, placebo-controlled, randomized crossover trial in mothers of preteen children. Am. J. Clin. Nutr. 2016, 103, 775–783. [Google Scholar] [CrossRef]
- Mastroiacovo, D.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Raffaele, A.; Pistacchio, L.; Righetti, R.; Bocale, R.; Lechiara, M.C.; Marini, C.; et al. Cocoa flavanol consumption improves cognitive function, blood pressure control, and metabolic profile in elderly subjects: The Cocoa, Cognition, and Aging (CoCoA) Study—A randomized controlled trial. Am. J. Clin Nutr. 2015, 101, 538–548. [Google Scholar] [CrossRef]
- Duvnjak-Zaknich, D.M.; Dawson, B.T.; Wallman, K.E.; Henry, G. Effect of caffeine on reactive agility time when fresh and fatigued. Med. Sci. Sports Exerc. 2011, 43, 1523–1530. [Google Scholar] [CrossRef]
- Trombold, J.R.; Barnes, J.N.; Critchley, L.; Coyle, E.F. Ellagitannin consumption improves strength recovery 2-3 d after eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 493–498. [Google Scholar] [CrossRef]
- Whyte, A.R.; Williams, C.M. Effects of a single dose of a flavonoid-rich blueberry drink on memory in 8 to 10 y old children. Nutrition 2015, 31, 531–534. [Google Scholar] [CrossRef]
- Falcone, P.H.; Nieman, K.M.; Tribby, A.C.; Vogel, R.M.; Joy, J.M.; Moon, J.R.; Slayton, C.A.; Henigman, M.M.; Lasrado, J.A.; Lewis, B.J.; et al. The attention-enhancing effects of spearmint extract supplementation in healthy men and women: A randomized, double-blind, placebo-controlled, parallel trial. Nutr. Res. 2019, 64, 24–38. [Google Scholar] [CrossRef]
- Johnson, E.J.; McDonald, K.; Caldarella, S.M.; Chung, H.Y.; Troen, A.M.; Snodderly, D.M. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr. Neurosci. 2008, 11, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Ataka, S.; Tanaka, M.; Nozaki, S.; Mizuma, H.; Mizuno, K.; Tahara, T.; Sugino, T.; Shirai, T.; Kajimoto, Y.; Kuratsune, H.; et al. Effects of Applephenon and ascorbic acid on physical fatigue. Nutrition 2007, 23, 419–423. [Google Scholar] [CrossRef]
- Do Rosario, V.A.; Fitzgerald, Z.; Broyd, S.; Paterson, A.; Roodenrys, S.; Thomas, S.; Bliokas, V.; Potter, J.; Walton, K.; Weston-Green, K.; et al. Food anthocyanins decrease concentrations of TNF-α in older adults with mild cognitive impairment: A randomized, controlled, double blind clinical trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Calapai, G.; Bonina, F.; Bonina, A.; Rizza, L.; Mannucci, C.; Arcoraci, V.; Laganà, G.; Alibrandi, A.; Pollicino, C.; Inferrera, S.; et al. A randomized, double-blinded, clinical trial on effects of a Vitis vinifera extract on cognitive function in healthy older adults. Front Pharmacol. 2017, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.I.; Apostolidis, E.; Kim, Y.C.; Shetty, K. Health benefits of traditional corn, beans, and pumpkin: In vitro studies for hyperglycemia and hypertension management. J. Med. Food. 2007, 10, 266–275. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).