Abstract
Low tissue creatine characterizes many conditions, including neurodegenerative, cardiopulmonary, and metabolic diseases, with a magnitude of creatine shortfall often corresponds well to a disorder’s severity. A non-invasive monitoring of tissue metabolism with magnetic resonance spectroscopy (MRS) might be a feasible tool to evaluate suboptimal levels of creatine for both predictive, diagnostic, and therapeutic purposes. This mini review paper summarizes disorders with deficient creatine levels and provides arguments for assessing and employing tissue creatine as a relevant target in clinical nutrition.
1. Introduction
Phosphorylated creatine (2-[carbamimidoyl(methyl)amino]acetic acid) is the main high-energy phosphate-storage compound able to promptly regenerate adenosine triphosphate (ATP) by the action of creatine kinase, with ATP being the critical energy-containing molecule found in all living cells. Creatine-phosphocreatine system serves both as a temporal and spatial energy buffer of ATP levels, and intracellular energy transport carrier connecting sites of energy production with sites of energy utilization [1,2]. Humans can obtain creatine via endogenous synthesis (from amino acids glycine and L-arginine) and through different omnivorous foods (especially red meat and fish), accounting for a total daily creatine output of approximately 2 g for a 70 kg young adult man [3,4,5]. Creatine homeostasis appears to be finely tuned to the dynamics of its endogenous–exogenous provision and utilization as an energy facilitator. This is illustrated by the relatively stable intracellular levels of total creatine (creatine plus phosphocreatine) across various energy-demanding tissues in normal conditions, including the brain (4–5 mM) [6], skeletal muscle (25–30 mM) [7], and myocardium (25–30 mM) [8]. On the other hand, a reduction in intracellular creatine concentrations could result in a hypo-energetic state that occurs in a broad spectrum of pathophysiological situations, with a degree of creatine deficit often correlates with a disorder severity. This mini review summarizes disorders with low creatine levels and provides arguments for assessing and employing tissue creatine as a relevant target in clinical nutrition.
2. Creatine Shortfall in Clinical Medicine
The reduced levels of intracellular creatine complement many inherited and acquired disorders, with cerebral creatine deficiency syndromes (CCDS) often considered a clinical paradigm of creatine shortfall. CCDS are rare inborn errors of creatine synthesis machinery and transport, being autosomal recessive or X-linked conditions [9]. CCDS patients typically show clinical features of low bioenergetics (e.g., muscle hypotonia, speech delay, seizures, intellectual impairment, behavioral abnormalities), with low levels of creatine found in the brain, skeletal muscle, and biofluids considered an essential element of preliminary and confirmatory diagnosis of these conditions [10]. However, CCDS often remain underdiagnosed since the assessment of creatine metabolism is not routinely included in standard diagnostic workup [11]. Other neurological pathologies accompanied by insufficient cerebral creatine levels (or impaired creatine-related metabolic ratios) include autism spectrum disorder (ASD) [12], traumatic brain injury [13], multiple sclerosis [14], gyrate atrophy of the choroid and retina [15], post-viral fatigue syndrome [16], and brain malignancies [17,18], while creatine levels were suboptimal in the skeletal muscle of neurological patients suffering from muscular dystrophy and neuromuscular diseases [19,20]. Several cardiovascular conditions exhibit a decrease in myocardial or subendocardial creatine, such as dilated cardiomyopathy [21], aortic valve disease [22], and transplanted heart [23]. Interestingly, it appears that a reduction in myocardial creatine significantly correlates with the clinical severity of heart failure [24], with tissue creatine put forward as an independent multivariate predictor of cardiovascular mortality in patients with failing hearts [25,26]. Tissue creatine levels were also diminished in the skeletal muscle and brain of patients with chronic respiratory failure [27,28], extracts from lung cancer patients [29], pancreatic parenchyma in patients with adenocarcinoma [30], in the brain of patients with hepatitis C and chronic HIV infection [31,32], and in the thalamus of infants with malnutrition [33], while suboptimal creatine levels are suggested in chronic kidney disease [34], and preterm birth [35]. Although the causes of the above conditions are different, they all share a disease-associated creatine deficit. The lack of creatine might not be necessarily the underlying cause for all conditions listed above but perhaps a possible side effect of these diseases. Nevertheless, patients with lower levels appear to be at a higher risk of developing more severe illness [10,24], implying low tissue creatine as a possible prognostic indicator of disease severity. In addition, it might be used as a predictive biomarker to identify individuals with specific conditions who are more likely to experience a favorable effect from the exposure to supplemental creatine. Cut-off points for low tissue creatine are not established so far for specific organs and pathologies, yet various degrees of deficiency of the cerebral creatine pool are seen in neurology, from almost complete depletion of the cerebral creatine pool in CCDS [36,37] to a partial reduction (~10%) seen in other conditions [38,39] (Table 1).

Table 1.
Summary of human studies (excluding CCDS) describing low tissue creatine levels.
Table 1.
Summary of human studies (excluding CCDS) describing low tissue creatine levels.
| Pathology | Refs. |
|---|---|
| Autism spectrum disorder | [40,41,42,43,44,45] |
| Concussion and mild traumatic brain injury | [13,46] |
| Multiple sclerosis | [47,48,49,50,51] |
| Gyrate atrophy of the choroid and retina | [15,52] |
| Post-viral fatigue syndrome | [53,54] |
| Primary and secondary brain tumors | [17,18] |
| Neuromuscular disease | [19] |
| Facioscapulohumeral muscular dystrophy | [20] |
| Dilated cardiomyopathy | [21,24,25] |
| Aortic valve disease | [22] |
| Heart transplantation | [23] |
| Coronary disease | [26] |
| Chronic obstructive pulmonary disease | [27,28] |
| Lung cancer | [29] |
| Pancreatic cancer | [30] |
| Hepatitis C | [31] |
| Chronic HIV infection | [32] |
| Infant malnutrition | [33] |
3. Methods for Tissue Creatine Evaluation
A breakthrough in monitoring creatine levels has occurred with magnetic resonance spectroscopy (MRS), a state-of-the-art analytical technique that can be used to non-invasively measure biochemical changes across different tissues and organs. By observing local magnetic fields around atomic nuclei, MRS allows the measurement of in vivo chemical information in a specific volume of interest, with creatine included in routine MRS profiling [55]. Each metabolite has a different peak in the spectrum, which appears at a known frequency (e.g., creatine at 3.03 ppm). Both proton and non-proton MRS techniques are available, with 1H MRS appearing to be more convenient than other methods to evaluate creatine concentrations. More accurate MRS methods of creatine assessment involve absolute quantification of creatine levels instead of resonance amplitudes presented as ratios [56]. The experts’ working group on reporting standards for MRS recently provided a set of rules required for the acquisition, post-processing, and analysis required for appropriate interpretation of results [57], facilitating MRS use in everyday practice. MRS is a relatively expensive technique and requires an educated technician, yet it has gained popularity in recent years, as illustrated by more studies employing MRS in nutritional research [58]. The fact that MRS systems are now available in most medical centers allows the routine evaluation of creatine metabolism in many pathologies, at least those that affect energy-demanding organs. Alternatively, tissue biopsy might provide a means to assess creatine levels in specific tissues (predominantly the skeletal muscle), yet this procedure’s invasiveness and possible complications prevent its ample applicability in regular practice. Possible surrogate indices of tissue creatine, including serum or salivary creatine, liquid biopsy, and creatine in cerebrospinal fluid, require more evidence to support or prove its use as MRS substitutes.
4. Transition of Tissue Creatine Evaluation from Research to Routine Practice
A meaningful application of any biomarker holds a considerable value in nutrition and medicine, yet a quest from its recognition to clinical use is often slow and arduous [59]. In the case of tissue creatine quantified by MRS, a number of requirements for a successful transition from the research environment to routine clinical practice appear to be nearing completion, with its possible uses potentially encompassing both predictive and diagnostic purposes. The analytical validity of MRS suggests relatively high reproducibility for tissue metabolites measurements (including total creatine) in various tissues [60,61]. MRS employing creatine-related metabolic ratios has high diagnostic accuracy and clinical validity in differentiating neoplasms from non-neoplastic brain tumors [62], brain metastases and primary high-grade gliomas [63], tremor-dominant Parkinson’s disease (PD) and non-PD tremor [64], various parkinsonian syndromes [65], high-functioning adults with ASD and typically developing peers [66], and bone and soft tissue tumors versus normal muscle [67]. In addition, MRS sensitivity (92%) and specificity (76%) were equivalent to other superior methods in aiding the localization of prostate metabolic abnormalities that might include creatine [68]. Evaluating the clinical utility of tissue creatine is still under critical observation. Early studies suggest that MRS-driven creatine indices can help distinguish responders and non-responders to drug treatment in insomnia patients [69], with the test likely improving healthcare and changing outcomes. Although no extensive cost-effectiveness trials are available at this moment, a study suggests that abnormal creatine levels consistent with myocardial ischemia predicted cardiovascular outcomes, higher rates of anginal hospitalization, repeat catheterization, and more significant treatment costs [26]. Another trial suggests that the use of MRS may be cost-effective in specific contexts, for example, in settings where the cost of transrectal ultrasound-guided prostate biopsy exceeds the cost of obtaining an MRS sequence by ~£115 [68]. The above studies suggest added value and costs saved by knowing a patient’s tissue creatine levels.
5. Creatine in Clinical Nutrition
A lack of cellular energy as a cause of disease has recently regained attention [70,71], and tissue creatine shortage might be a suitable proxy that characterizes poor bioenergetics seen in conditions that impact top energy-consuming organs. Low creatine levels perhaps indicate a high-scale utilization of this energy-facilitating compound for replenishing in-demand ATP for dysfunctional cells and/or an impaired internal production and intra- and extra-cellular transport of creatine (at least in CCDS). Whether low creatine in many acquired conditions is a consequence of a disease or a causative factor (or both) remains poorly addressed. However, supplemental creatine or creatine analogs appear to partially or totally restore tissue creatine levels and attenuate clinical features of many maladies, including CCDS [36,72,73,74], mitochondrial encephalomyopathy, lactic acidosis, stroke-like episodes (MELAS) syndrome [75], acute oxygen deprivation [76], chronic fatigue syndrome [77], Huntington disease [78], treatment-resistant major depressive disorder [79,80], methamphetamine-complemented depression [81], chronic heart failure [82], and rheumatoid arthritis [83], to name just a few. How much creatine needs to be compensated likely depends on the degree and type of creatine shortage; some conditions may entail covering amounts one magnitude over average dietary requirements (for a detailed review, see Ref. [84]). A vast majority of pharmacovigilance studies demonstrated favorable safety of supplemental creatine, with creatine posing no adverse health risks in humans across various life stages and conditions, at dosages ranging from 0.03 to 0.8 g per kilogram of body weight per day for up to 5 years, and dosages commonly prescribed for CCDS and other diseases/syndromes 2 to 20 g/day (for a detailed review, see Refs. [39,84]). In line with affirmative evidence from safety trials, the U.S. Food and Drug Administration (FDA) recently recognized creatine monohydrate as a safe ingredient (Generally Recognized as Safe, GRAS) [85]. This labels creatine as a non-toxic food substance under the conditions of its intended use, and perhaps expands its use across various nutritional domains. Still, determining the most bioactive chemical formulation of creatine remains a challenge [86].
6. Conclusions
A drop in creatine levels accompanies various hereditary and non-hereditary diseases, and the human brain, skeletal muscle, and heart appear to be affected rather badly by creatine shortage. Being either an etiological factor or a secondary finding, lower tissue creatine concentrations almost always refer to a more severe phenotype, implying a key role of creatine in normal homeostasis and health protection. Evaluating tissue creatine (predominantly by non-invasive techniques) might thus assist in an appropriate diagnosis, prediction, and even a nutritional treatment of a specific disease characterized by creatine shortfall, with the assay thus providing an additional value in terms of health care (Figure 1). Still, to recognize tissue creatine as a potential novel biomarker candidate, additional studies are highly warranted to substantiate its biological plausibility, practicability in real-life situations, sensitivity and specificity, and cost-effectiveness, along with its responsiveness to treatment (such as exogenous creatine) in various clinical populations.
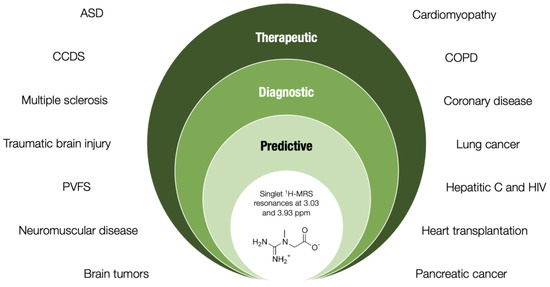
Figure 1.
Theoretical framework of tissue creatine assessment via proton magnetic resonance spectroscopy (MRS) in clinical science. Abbreviations: ASD, autism spectrum disorder; CCDS, cerebral creatine deficiency syndromes; PFVS, post-viral fatigue syndrome; COPD, chronic obstructive pulmonary disease.
Funding
This work was not supported by any public or private entity.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
S.M.O. serves as a member of the Scientific Advisory Board on creatine in health and medicine (AlzChem LLC). S.M.O. owns patent “Sports Supplements Based on Liquid Creatine” at European Patent Office (WO2019150323 A1), and active patent application “Synergistic Creatine” at UK Intellectual Property Office (GB2012773.4). S.M.O. has served as a speaker at Abbott Nutrition, a consultant of Allied Beverages Adriatic and IMLEK, and has received research funding related to creatine from the Serbian Ministry of Education, Science, and Technological Development, Provincial Secretariat for Higher Education and Scientific Research, AlzChem GmbH, KW Pfannenschmidt GmbH, ThermoLife International LLC, and Hueston Hennigan LLP. S.M.O. does not own stocks and shares in any organization.
References
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [PubMed]
- Wallimann, T.; Dolder, M.; Schlattner, U.; Eder, M.; Hornemann, T.; Kraft, T.; Stolz, M. Creatine kinase: An enzyme with a central role in cellular energy metabolism. Magn. Reson. Mater. Phys. Biol. Med. 1998, 6, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, J.T.; Brosnan, M.E. Creatine: Endogenous Metabolite, Dietary, and Therapeutic Supplement. Annu. Rev. Nutr. 2007, 27, 241–261. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, M.E.; Brosnan, J.T. The role of dietary creatine. Amino Acids 2016, 48, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Forbes, S.C. Perspective: Creatine, a Conditionally Essential Nutrient: Building the Case. Adv. Nutr. Int. Rev. J. 2021, 13, 34–37. [Google Scholar] [CrossRef] [PubMed]
- De Graf, R.A. In Vivo NMR Spectroscopy: Priniciples and Techniques, 3rd ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Wiedermann, D.; Schneider, J.; Fromme, A.; Thorwesten, L.; Möller, H.E. Creatine loading and resting skeletal muscle phosphocreatine flux: A saturation-transfer NMR study. Magn. Reson. Mater. Phys. Biol. Med. 2001, 13, 118–126. [Google Scholar] [CrossRef]
- Nakae, I.; Mitsunami, K.; Matsuo, S.; Matsumoto, T.; Morikawa, S.; Inubushi, T.; Koh, T.; Horie, M. Assessment of Myocardial Creatine Concentration in Dysfunctional Human Heart by Proton Magnetic Resonance Spectroscopy. Magn. Reson. Med. Sci. 2004, 3, 19–25. [Google Scholar] [CrossRef]
- Mercimek-Andrews, S.; Salomons, G.S. Creatine Deficiency Syndromes. In GeneReviews [Internet]; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Mirzaa, G., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2009. [Google Scholar]
- Sharer, J.D.; Bodamer, O.; Longo, N.; Tortorelli, S.; Wamelink, M.M.; Young, S. Laboratory diagnosis of creatine deficiency syndromes: A technical standard and guideline of the American College of Medical Genetics and Genomics. Genet. Med. 2017, 19, 256–263. [Google Scholar] [CrossRef]
- Schulze, A. Creatine deficiency syndromes. Handb. Clin. Neurol. 2013, 113, 1837–1843. [Google Scholar] [CrossRef]
- Ford, T.C.; Crewther, D.P. A Comprehensive Review of the 1H-MRS Metabolite Spectrum in Autism Spectrum Disorder. Front. Mol. Neurosci. 2016, 9, 14. [Google Scholar] [CrossRef]
- Vagnozzi, R.; Signoretti, S.; Floris, R.; Marziali, S.; Manara, M.; Amorini, A.M.; Belli, A.; Di Pietro, V.; D’Urso, S.; Pastore, F.S.; et al. Decrease in N-Acetylaspartate Following Concussion May Be Coupled to Decrease in Creatine. J. Head Trauma Rehabil. 2013, 28, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Creatine and multiple sclerosis. Nutr. Neurosci. 2020. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Nanto-Salonen, K.; Komu, M.; Lundbom, N.; Heinanen, K.; Alanen, A.; Sipila, I.; Simell, O. Reduced brain creatine in gyrate atrophy of the choroid and retina with hyperornithinemia. Neurology 1999, 53, 303. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S. Diagnostic and Pharmacological Potency of Creatine in Post-Viral Fatigue Syndrome. Nutrients 2021, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Chernov, M.F.; Hayashi, M.; Izawa, M.; Ono, Y.; Hori, T. Proton magnetic resonance spectroscopy (MRS) of metastatic brain tumors: Variations of metabolic profile. Int. J. Clin. Oncol. 2006, 11, 375–384. [Google Scholar] [CrossRef]
- Jia, C.; Li, Z.; Guo, D.; Zhang, Z.; Yu, J.; Jiang, G.; Xing, X.; Ji, S.; Jin, F. Brain Metastases of Non-Small Cell Lung Cancer: Magnetic Resonance Spectroscopy for Clinical Outcome Assessment in Patients with Stereotactic Radiotherapy. OncoTargets Ther. 2020, 13, 13087–13096. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Parise, G. Direct measurement of high-energy phosphate compounds in patients with neuromuscular disease. Muscle Nerve 1999, 22, 1228–1233. [Google Scholar] [CrossRef]
- Leung, D.G.; Wang, X.; Barker, P.B.; Carrino, J.A.; Wagner, K.R. Multivoxel proton magnetic resonance spectroscopy in facioscapulohumeral muscular dystrophy. Muscle Nerve 2018, 57, 958–963. [Google Scholar] [CrossRef]
- Hardy, C.J.; Weiss, R.G.; Bottomley, P.A.; Gerstenblith, G. Altered myocardial high-energy phosphate metabolites in patients with dilated cardiomyopathy. Am. Heart J. 1991, 122 Pt 1, 795–801. [Google Scholar] [CrossRef]
- Conway, M.; Allis, J.; Ouwerkerk, R.; Niioka, T.; Rajagopalan, B.; Radda, G. Detection of low phosphocreatine to ATP ratio in failing hypertrophied human myocardium by 31P magnetic resonance spectroscopy. Lancet 1991, 338, 973–976. [Google Scholar] [CrossRef]
- Evanochko, W.; Buchthal, S.D.; den Hollander, J.A.; Katholi, C.R.; Bourge, R.C.; Benza, R.L.; Kirklin, J.K.; Pohost, G.M. Cardiac transplant patients response to the 31P MRS stress test. J. Heart Lung Transplant. 2002, 21, 522–529. [Google Scholar] [CrossRef]
- Neubauer, S.; Krahe, T.; Schindler, R.; Horn, M.; Hillenbrand, H.; Entzeroth, C.; Mader, H.; Kromer, E.P.; Riegger, G.A.; Lackner, K. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation 1992, 86, 1810–1818. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, S.; Horn, M.; Cramer, M.; Harre, K.; Newell, J.B.; Peters, W.; Pabst, T.; Ertl, G.; Hahn, D.; Ingwall, J.S.; et al. Myocardial Phosphocreatine-to-ATP Ratio Is a Predictor of Mortality in Patients with Dilated Cardiomyopathy. Circulation 1997, 96, 2190–2196. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.D.; Shaw, L.J.; Buchthal, S.D.; Merz, C.N.B.; Kim, H.-W.; Scott, K.N.; Doyle, M.; Olson, M.B.; Pepine, C.J.; Hollander, J.D.; et al. Prognosis in Women With Myocardial Ischemia in the Absence of Obstructive Coronary Disease. Circulation 2004, 109, 2993–2999. [Google Scholar] [CrossRef] [PubMed]
- Payen, J.-F.; Wuyam, B.; Reutenauer, H.; Laurent, D.; Levy, P.; Le Bas, J.-F.; Benabid, A.L. Impairment of muscular metabolism in chronic respiratory failure. A human31P MRS study. NMR Biomed. 1991, 4, 41–45. [Google Scholar] [CrossRef]
- Mathur, R.; Cox, I.J.; Oatridge, A.; Shephard, D.T.; Shaw, R.J.; Taylor-Robinson, S.D. Cerebral Bioenergetics in Stable Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1999, 160, 1994–1999. [Google Scholar] [CrossRef]
- Hanaoka, H.; Yoshioka, Y.; Ito, I.; Niitu, K.; Yasuda, N. In vitro characterization of lung cancers by the use of1H nuclear magnetic resonance spectroscopy of tissue extracts and discriminant factor analysis. Magn. Reson. Med. 1993, 29, 436–440. [Google Scholar] [CrossRef]
- Chang, C.-K.; Shih, T.T.-F.; Tien, Y.-W.; Chang, M.-C.; Chang, Y.-T.; Yang, S.-H.; Cheng, M.-F.; Chen, B.-B. Metabolic Alterations in Pancreatic Cancer Detected by In Vivo 1H-MR Spectroscopy: Correlation with Normal Pancreas, PET Metabolic Activity, Clinical Stages, and Survival Outcome. Diagnostics 2021, 11, 1541. [Google Scholar] [CrossRef]
- Bladowska, J.; Zimny, A.; Knysz, B.; Małyszczak, K.; Kołtowska, A.; Szewczyk, P.; Gąsiorowski, J.; Furdal, M.; Sąsiadek, M.J. Evaluation of early cerebral metabolic, perfusion and microstructural changes in HCV-positive patients: A pilot study. J. Hepatol. 2013, 59, 651–657. [Google Scholar] [CrossRef]
- Boban, J.; Thurnher, M.M.; Brkic, S.; Lendak, D.; Ignjatovic, V.B.; Todorovic, A.; Kozic, D. Neurometabolic Remodeling in Chronic Hiv Infection: A Five-Year Follow-up Multi-Voxel Mrs Study. Sci. Rep. 2019, 9, 19799. [Google Scholar] [CrossRef]
- Cakir, M.; Senyuva, S.; Kul, S.; Sag, E.; Cansu, A.; Yucesan, F.B.; Yaman, S.O.; Orem, A. Neurocognitive Functions in Infants with Malnutrition; Relation with Long-chain Polyunsaturated Fatty Acids, Micronutrients Levels and Magnetic Resonance Spectroscopy. Pediatr. Gastroenterol. Hepatol. Nutr. 2019, 22, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Post, A.; Tsikas, D.; Bakker, S.J. Creatine is a Conditionally Essential Nutrient in Chronic Kidney Disease: A Hypothesis and Narrative Literature Review. Nutrients 2019, 11, 1044. [Google Scholar] [CrossRef] [PubMed]
- Muccini, A.M.; Tran, N.T.; de Guingand, D.L.; Philip, M.; Della Gatta, P.A.; Galinsky, R.; Sherman, L.S.; Kelleher, M.A.; Palmer, K.R.; Berry, M.J.; et al. Creatine Metabolism in Female Reproduction, Pregnancy and Newborn Health. Nutrients 2021, 13, 490. [Google Scholar] [CrossRef] [PubMed]
- Mercimek-Mahmutoglu, S.; Stoeckler-Ipsiroglu, S.; Adami, A.; Appleton, R.; Araújo, H.C.; Duran, M.; Ensenauer, R.; Fernandez-Alvarez, E.; Garcia, P.; Grolik, C.; et al. GAMT deficiency: Features, treatment, and outcome in an inborn error of creatine synthesis. Neurology 2006, 67, 480–484. [Google Scholar] [CrossRef]
- Van De Kamp, J.; Mancini, G.; Pouwels, P.; Betsalel, O.; Van Dooren, S.; De Koning, I.; Steenweg, M.; Jakobs, C.; Van Der Knaap, M.; Salomons, G. Clinical features and X-inactivation in females heterozygous for creatine transporter defect. Clin. Genet. 2011, 79, 264–272. [Google Scholar] [CrossRef]
- Balestrino, M.; Adriano, E. Beyond sports: Efficacy and safety of creatine supplementation in pathological or paraphysiological conditions of brain and muscle. Med. Res. Rev. 2019, 39, 2427–2459. [Google Scholar] [CrossRef]
- Ostojic, S.M. Creatine as a food supplement for the general population. J. Funct. Foods 2021, 83, 104568. [Google Scholar] [CrossRef]
- Friedman, S.; Shaw, D.; Artru, A.; Richards, T.; Gardner, J.; Dawson, G.; Posse, S.; Dager, S. Regional brain chemical alterations in young children with autism spectrum disorder. Neurology 2003, 60, 100–107. [Google Scholar] [CrossRef]
- Levitt, J.G.; O’Neill, J.; Blanton, R.E.; Smalley, S.; Fadale, D.; McCracken, J.T.; Guthrie, D.; Toga, A.W.; Alger, J.R. Proton magnetic resonance spectroscopic imaging of the brain in childhood autism. Biol. Psychiatry 2003, 54, 1355–1366. [Google Scholar] [CrossRef]
- Friedman, S.D.; Shaw, D.W.W.; Artru, A.A.; Dawson, G.; Petropoulos, H.; Dager, S.R. Gray and White Matter Brain Chemistry in Young Children With Autism. Arch. Gen. Psychiatry 2006, 63, 786–794. [Google Scholar] [CrossRef]
- Hardan, A.Y.; Minshew, N.J.; Melhem, N.M.; Srihari, S.; Jo, B.; Bansal, R.; Keshavan, M.S.; Stanley, J.A. An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Res. Neuroimaging 2008, 163, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kleinhans, N.M.; Richards, T.; Weaver, K.E.; Liang, O.; Dawson, G.; Aylward, E. Brief report: Biochemical correlates of clinical impairment in high functioning autism and Asperger’s disorder. J. Autism. Dev. Disord. 2009, 39, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Golomb, B.A.; Erickson, L.C.; Zeeland, A.A.S.-V.; Koperski, S.; Haas, R.H.; Wallace, D.C.; Naviaux, R.K.; Lincoln, A.J.; Reiner, G.E.; Hamilton, G. Assessing bioenergetic compromise in autism spectrum disorder with 31P magnetic resonance spectroscopy: Preliminary report. J. Child Neurol. 2014, 29, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kirov, I.; Fleysher, L.; Babb, J.; Silver, J.M.; Grossman, R.I.; Gonen, O. Characterizing ‘mild’ in traumatic brain injury with proton MR spectroscopy in the thalamus: Initial findings. Brain Inj. 2007, 21, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Cadoux-Hudson, T.A.; Kermode, A.; Rajagopalan, B.; Taylor, D.; Thompson, A.; Ormerod, I.E.; McDonald, W.I.; Radda, G.K. Biochemical changes within a multiple sclerosis plaque in vivo. J. Neurol. Neurosurg. Psychiatry 1991, 54, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Hanefeld, F.; Bauer, H.J.; Christen, H.-J.; Kruse, B.; Bruhn, H.; Frahm, J. Multiple sclerosis in childhood: Report of 15 cases. Brain Dev. 1991, 13, 410–416. [Google Scholar] [CrossRef]
- Bruhn, H.; Frahm, J.; Merboldt, K.D.; Hanefeld, F.; Christen, H.J.; Kruse, B.; Bauer, H.J. Multiple sclerosis in children: Cerebral metabolic alterations monitored by localized proton magnetic resonance spectroscopy in vivo. Ann. Neurol. 1992, 32, 140–150. [Google Scholar] [CrossRef]
- Koschorek, F.; O’ermann, W.; Stelten, J.; Braunsdorf, W.E.; Steller, U.; Gremmel, H.; Leibfritz, D. High-resolution 1H NMR spectroscopy of cerebrospinal fluid in spinal diseases. Neurosurg. Rev. 1993, 16, 307–315. [Google Scholar] [CrossRef]
- Joanna, L.; James, P.; Anthony, A.; Garnette, R.S. Nuclear mag- netic resonance study of cerebrospinal fluid from patients with multiple sclerosis. Can. J. Neurol. Sci. 1993, 20, 194–198. [Google Scholar] [CrossRef]
- Valayannopoulos, V.; Boddaert, N.; Mention, K.; Touati, G.; Barbier, V.; Chabli, A.; Sedel, F.; Kaplan, J.; Dufier, J.; Seidenwurm, D.; et al. Secondary creatine deficiency in ornithine delta-aminotransferase deficiency. Mol. Genet. Metab. 2009, 97, 109–113. [Google Scholar] [CrossRef]
- Mueller, C.; Lin, J.; Sheriff, S.; Maudsley, A.A.; Younger, J.W. Evidence of widespread metabolite abnormalities in myalgic encephalomyelitis/chronic fatigue syndrome: Assessment with whole-brain magnetic resonance spectroscopy. Brain Imaging Behav. 2020, 14, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Van Der Schaaf, M.E.; de Lange, F.; Schmits, I.C.; Geurts, D.E.; Roelofs, K.; Van Der Meer, J.W.; Toni, I.; Knoop, H. Prefrontal structure varies as a function of pain symptoms in chronic fatigue syndrome. Biol. Psychiatry 2017, 81, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Holloway, C.; Clarke, K. Is MR Spectroscopy of the Heart Ready for Humans? Heart Lung Circ. 2010, 19, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E.; Gant, N. The biochemistry of creatine. In Magnetic Resonance Spectroscopy; Stagg, C.J., Rothman, D.L., Eds.; Academic Press: London, UK, 2014; pp. 91–110. [Google Scholar]
- Lin, A.; Andronesi, O.; Bogner, W.; Choi, I.; Coello, E.; Cudalbu, C.; Juchem, C.; Kemp, G.J.; Kreis, R.; Krššák, M.; et al. Minimum reporting standards for in vivo magnetic resonance spectroscopy (MRSinMRS): Experts’ consensus recommendations. NMR Biomed. 2021, 34, e4484. [Google Scholar] [CrossRef]
- Inoue, T.; Kozawa, E.; Ishikawa, M.; Okada, H. Application of Magnetic Resonance Imaging in the Evaluation of Nutritional Status: A Literature Review with Focus on Dialysis Patients. Nutrients 2021, 13, 2037. [Google Scholar] [CrossRef]
- Selleck, M.J.; Senthil, M.; Wall, N.R. Making Meaningful Clinical Use of Biomarkers. Biomark. Insights 2017, 12, 1177271917715236. [Google Scholar] [CrossRef]
- Bakermans, A.J.; Boekholdt, S.M.; de Vries, D.K.; Reckman, Y.J.; Farag, E.S.; de Heer, P.; Uthman, L.; Denis, S.W.; Zuurbier, C.J.; Houtkooper, R.H.; et al. Quantification of Myocardial Creatine and Triglyceride Content in the Human Heart: Precision and Accuracy of in vivo Proton Magnetic Resonance Spectroscopy. J. Magn. Reson. Imaging 2021, 54, 411–420. [Google Scholar] [CrossRef]
- Gupta, A.; Houston, B. Cardiac 1H MR spectroscopy: Development of the past five decades and future perspectives. Heart Fail. Rev. 2021, 26, 839–859. [Google Scholar] [CrossRef]
- Alshammari, Q.T.; Salih, M.; Gameraddin, M.; Yousef, M.; Abdelmalik, B.; Loaz, O. Accuracy of Magnetic Resonance Spectroscopy in Discrimination of Neoplastic and Non-Neoplastic Brain Lesions. Curr. Med. Imaging 2021, 17, 904–910. [Google Scholar] [CrossRef]
- Server, A.; Josefsen, R.; Kulle, B.; Maehlen, J.; Schellhorn, T.; Gadmar, Ø.; Kumar, T.; Haakonsen, M.; Langberg, C.W.; Nakstad, P.H. Proton magnetic resonance spectroscopy in the distinction of high-grade cerebral gliomas from single metastatic brain tumors. Acta Radiol. 2010, 51, 316–325. [Google Scholar] [CrossRef]
- Barbagallo, G.; Arabia, G.; Morelli, M.; Nisticò, R.; Novellino, F.; Salsone, M.; Rocca, F.; Quattrone, A.; Caracciolo, M.; Sabatini, U.; et al. Thalamic neurometabolic alterations in tremulous Parkinson’s disease: A preliminary proton MR spectroscopy study. Park. Relat. Disord. 2017, 43, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zanigni, S.; Testa, C.; Calandra-Buonaura, G.; Sambati, L.; Guarino, M.; Gabellini, A.; Evangelisti, S.; Cortelli, P.; Lodi, R.; Tonon, C. The contribution of cerebellar proton magnetic resonance spectroscopy in the differential diagnosis among parkinsonian syndromes. Park. Relat. Disord. 2015, 21, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Libero, L.E.; DeRamus, T.; Lahti, A.C.; Deshpande, G.; Kana, R.K. Multimodal neuroimaging based classification of autism spectrum disorder using anatomical, neurochemical, and white matter correlates. Cortex 2015, 66, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.-H.; Li, C.-F.; Li, Z.-F.; Zhang, K.; Wang, Q.; Yu, D.-X. Preliminary study of 3T 1H MR spectroscopy in bone and soft tissue tumors. Chin. Med. J. 2009, 122, 39–43. [Google Scholar] [PubMed]
- Mowatt, G.; Scotland, G.; Boachie, C.; Cruickshank, M.; Ford, J.A.; Fraser, C.; Kurban, L.; Lam, T.B.; Padhani, A.; Royle, J.; et al. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: A systematic review and economic evaluation. Health Technol. Assess. 2013, 17, vii–xix, 1–281. [Google Scholar] [CrossRef]
- Izuhara, M.; Miura, S.; Otsuki, K.; Nagahama, M.; Hayashida, M.; Hashioka, S.; Asou, H.; Kitagaki, H.; Inagaki, M. Magnetic Resonance Spectroscopy in the Ventral Tegmental Area Distinguishes Responders to Suvorexant Prior to Treatment: A 4-Week Prospective Cohort Study. Front. Psychiatry 2021, 12, 714376. [Google Scholar] [CrossRef]
- Johnson, T.A.; Jinnah, H.A.; Kamatani, N. Shortage of cellular ATP as a cause of diseases and strategies to enhance ATP. Front. Pharmacol. 2019, 10, 98. [Google Scholar] [CrossRef]
- Chu, X.-Y.; Wang, G.; Zhang, H.-Y. ATP as an anti-aging agent: Beyond the energy reservoir. Drug Discov. Today 2021, 26, 2783–2785. [Google Scholar] [CrossRef]
- Leuzzi, V.; Bianchi, M.C.; Tosetti, M.; Carducci, C.; Cerquiglini, C.A.; Cioni, G.; Antonozzi, I. Brain creatine depletion: Guanidinoacetate methyltransferase deficiency (improving with creatine supplementation). Neurology 2000, 55, 1407–1410. [Google Scholar] [CrossRef]
- Bianchi, M.; Tosetti, M.; Battini, R.; Leuzzi, V.; Alessandri’, M.; Carducci, C.; Antonozzi, I.; Cioni, G. Treatment Monitoring of Brain Creatine Deficiency Syndromes: A 1H- and 31P-MR Spectroscopy Study. Am. J. Neuroradiol. 2007, 28, 548–554. [Google Scholar]
- Valayannopoulos, V.; Boddaert, N.; Chabli, A.; Barbier, V.; Desguerre, I.; Philippe, A.; Afenjar, A.; Mazzuca, M.; Cheillan, D.; Munnich, A.; et al. Treatment by oral creatine, L-arginine and L-glycine in six severely affected patients with creatine transporter defect. J. Inherit. Metab. Dis. 2012, 35, 151–157. [Google Scholar] [CrossRef]
- Barisic, N.; Bernert, G.; Ipsiroglu, O.; Stromberger, C.; Müller, T.; Gruber, S.; Prayer, D.; Moser, E.; Bittner, R.E.; Stöckler-Ipsiroglu, S. Effects of Oral Creatine Supplementation in a Patient with MELAS Phenotype and Associated Nephropathy. Neuropediatrics 2002, 33, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.; Byblow, W.; Gant, N. Creatine Supplementation Enhances Corticomotor Excitability and Cognitive Performance during Oxygen Deprivation. J. Neurosci. 2015, 35, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Stojanovic, M.; Drid, P.; Hoffman, J.R.; Sekulic, D.; Zenic, N. Supplementation with Guanidinoacetic Acid in Women with Chronic Fatigue Syndrome. Nutrients 2016, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Hersch, S.M.; Gevorkian, S.; Marder, K.; Moskowitz, C.; Feigin, A.; Cox, M.; Como, P.; Zimmerman, C.; Lin, M.; Zhang, L.; et al. Creatine in Huntington disease is safe, tolerable, bioavailable in brain and reduces serum 8OH2’dG. Neurology 2006, 66, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Kondo, D.G.; Sung, Y.-H.; Hellem, T.L.; Fiedler, K.K.; Shi, X.; Jeong, E.-K.; Renshaw, P.F. Open-label adjunctive creatine for female adolescents with SSRI-resistant major depressive disorder: A 31-phosphorus magnetic resonance spectroscopy study. J. Affect. Disord. 2011, 135, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Kondo, D.G.; Forrest, L.N.; Shi, X.; Sung, Y.-H.; Hellem, T.L.; Huber, R.S.; Renshaw, P.F. Creatine target engagement with brain bioenergetics: A dose-ranging phosphorus-31 magnetic resonance spectroscopy study of adolescent females with SSRI-resistant depression. Amino Acids 2016, 48, 1941–1954. [Google Scholar] [CrossRef] [PubMed]
- Hellem, T.L.; Sung, Y.-H.; Shi, X.-F.; Pett, M.A.; Latendresse, G.; Morgan, J.; Huber, R.S.; Kuykendall, D.; Lundberg, K.J.; Renshaw, P.F. Creatine as a Novel Treatment for Depression in Females Using Methamphetamine: A Pilot Study. J. Dual Diagn. 2015, 11, 189–202. [Google Scholar] [CrossRef]
- Gordon, A.; Hultman, E.; Kaijser, L.; Kristjansson, S.; Rolf, C.J.; Nyquist, O.; Sylvén, C. Creatine supplementation in chronic heart failure increases skeletal muscle creatine phosphate and muscle performance. Cardiovasc. Res. 1995, 30, 413–418. [Google Scholar] [CrossRef]
- Willer, B.; Stucki, G.; Hoppeler, H.; Brühlmann, P.; Krähenbühl, S. Effects of creatine supplementation on muscle weakness in patients with rheumatoid arthritis. Rheumatology 2000, 39, 293–298. [Google Scholar] [CrossRef][Green Version]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. GRAS Notice for Creatine Monohydrate. 2020. Available online: https://www.fda.gov/media/143525/download (accessed on 17 November 2021).
- Ostojic, S.M. Overcoming restraints of dietary creatine. Trends Food Sci. Technol. 2020, 100, 246–247. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).