Pernicious Anemia: The Hematological Presentation of a Multifaceted Disorder Caused by Cobalamin Deficiency
Abstract
1. Introduction
2. Epidemiology, Pathogenesis, and Clinical Presentations of Pernicious Anemia: Looking beyond Borders of Medical Specialties
2.1. Epidemiology of Pernicious Anemia: A Not Completely Investigated Issue
2.2. Pathogenesis of Pernicious Anemia: More Than One Player on the Field
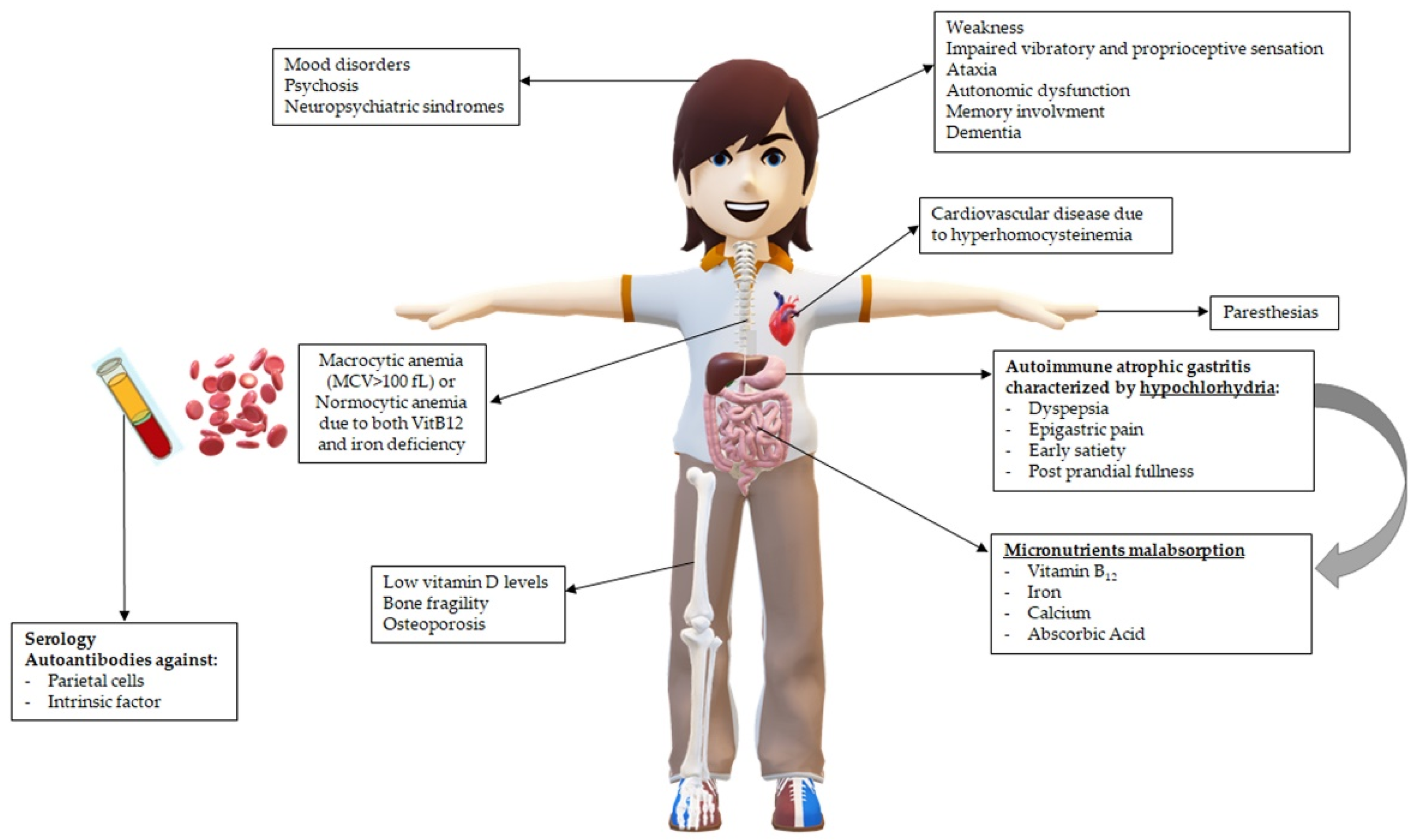
2.3. Clinical Presentation of PA: Many Faces, One Story
3. Micronutrients’ Malabsorption and Deficiencies in Pernicious Anemia: The Known and the Forgotten
3.1. Hypochlorhydria, the Central Player in Pernicious Anemia and Micronutrients’ Malabsorption
3.2. Vitamin B12, the Well-Known Factor Unaccounted for in Pernicious Anemia
3.3. Iron, the often Forgotten Missing Factor in Pernicious Anemia
3.4. Calcium, Vitamin D, and Ascorbic Acid, the Concealed Factors in Pernicious Anemia
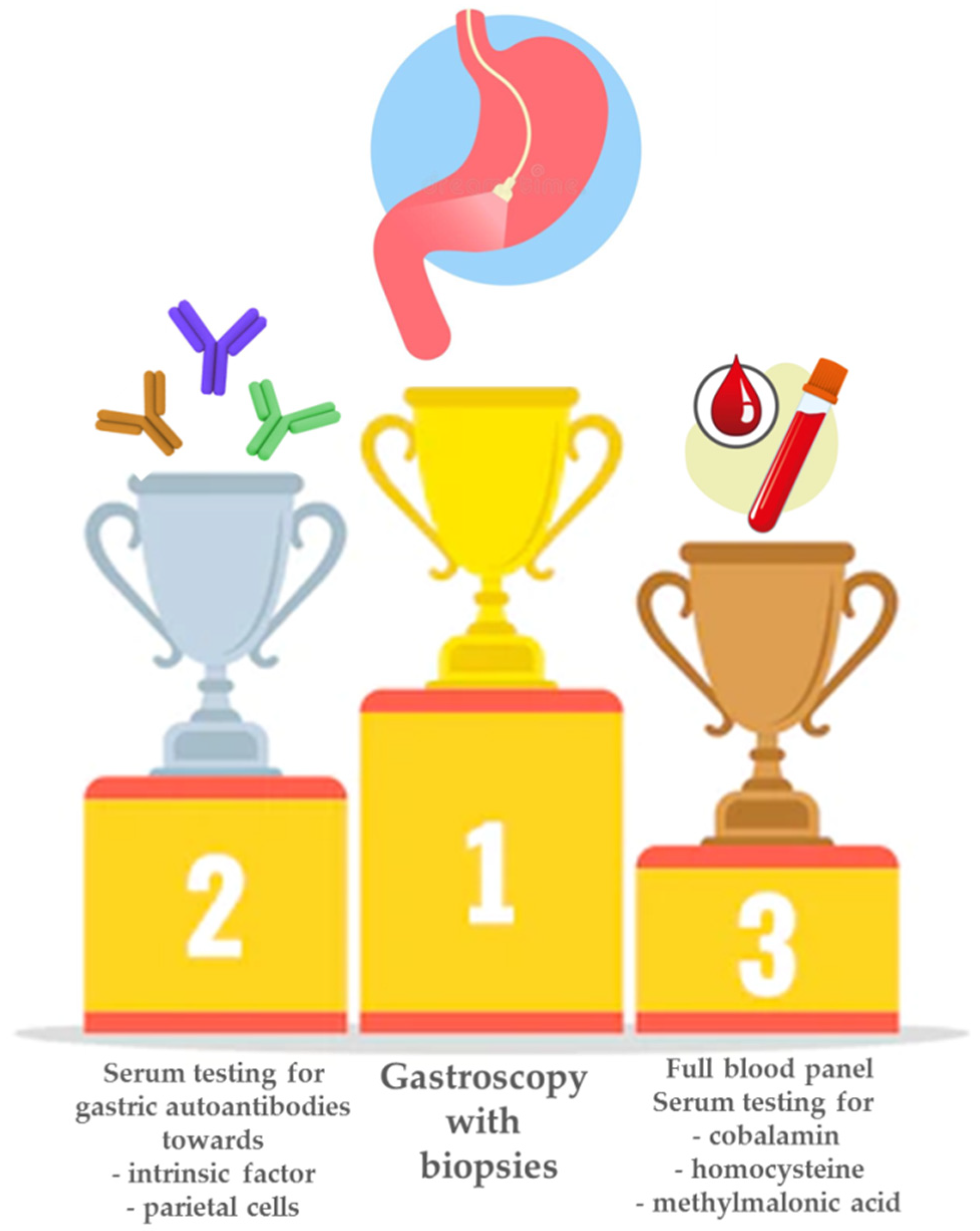
4. Diagnosis of Pernicious Anemia: A Smart Combination of Biochemistry and High-Quality Endoscopy and Histopathology
4.1. Biochemical Tests: The Non-Invasive Approach
4.2. Gastroscopy and Histopathological Assessment: The Required Confirmation of the Diagnosis of Pernicious Anemia
5. Long-Term Complications and Clinical Management of Pernicious Anemia: Good Protocols May Reduce Burden and Long-Term Risks
5.1. Long-Term Risks in Patients with Pernicious Anemia from a Hematological and Gastroenterological Point of View
5.2. Supplementation and Clinical Follow-Up: Always Be Alert
5.3. Endoscopic Follow-Up: New Techniques to Increase the Diagnostic Yield of High-Risk Lesions
6. Conclusive Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Annibale, B.; Lahner, E.; Fave, G.D. Diagnosis and management of pernicious anemia. Curr. Gastroenterol. Rep. 2011, 13, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Annibale, B.; Lahner, E. Common Pitfalls in the Management of Patients with Micronutrient Deficiency: Keep in Mind the Stomach. Nutrients 2021, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Rugge, M.; Lahner, E.; Miceli, E.; Toh, B.H.; Genta, R.M.; De Block, C.; Hershko, C.; Di Sabatino, A. Autoimmune gastritis. Nat. Rev. Dis. Primers 2020, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Lenti, M.V.; Miceli, E.; Cococcia, S.; Klersy, C.; Staiani, M.; Guglielmi, F.; Giuffrida, P.; Vanoli, A.; Luinetti, O.; De Grazia, F.; et al. Determinants of diagnostic delay in autoimmune atrophic gastritis. Aliment. Pharmacol. Ther. 2019, 50, 167–175. [Google Scholar] [CrossRef]
- Kocak, R.; Paydas, S. Pernicious anemia in Turkey. Int. J. Hematol. 1992, 55, 117–119. [Google Scholar] [PubMed]
- Carmel, R. Prevalence of undiagnosed pernicious anemia in the elderly. Arch. Intern. Med. 1996, 156, 1097100. [Google Scholar] [CrossRef]
- Carmel, R.; Johnson, C.S. Racial patterns in pernicious anemia. Early age at onset and increased frequency of intrinsic-factor antibody in black women. N. Engl. J. Med. 1978, 298, 647–650. [Google Scholar] [CrossRef]
- Toh, B.H.; van Driel, I.R.; Gleeson, P.A. Pernicious anemia. N. Engl. J. Med. 1997, 337, 1441–1448. [Google Scholar] [CrossRef]
- Carabotti, M.; Lahner, E.; Esposito, G.; Sacchi, M.C.; Severi, C.; Annibale, B. Upper gastrointestinal symptoms in autoimmune gastritis: A cross-sectional study. Medicine 2017, 96, e5784. [Google Scholar] [CrossRef]
- Vannella, L.; Lahner, E.; Osborn, J.; Annibale, B. Systematic review: Gastric cancer incidence in pernicious anaemia. Aliment. Pharmacol. Ther. 2013, 37, 375–382. [Google Scholar] [CrossRef]
- Green, R.; Allen, L.H.; Bjørke-Monsen, A.L.; Brito, A.; Guéant, J.L.; Miller, J.W.; Molloy, A.M.; Nexo, E.; Stabler, S.; Toh, B.H.; et al. Vitamin B12 deficiency. Nat. Rev. Dis. Primers 2017, 3, 17040. [Google Scholar] [CrossRef] [PubMed]
- Ankar, A.; Kumar, A. Vitamin B12 Deficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lahner, E.; Zagari, R.M.; Zullo, A.; Di Sabatino, A.; Meggio, A.; Cesaro, P.; Lenti, M.V.; Annibale, B.; Corazza, G.R. Chronic atrophic gastritis: Natural history, diagnosis and therapeutic management. A position paper by the Italian Society of Hospital Gastroenterologists and Digestive Endoscopists [AIGO], the Italian Society of Digestive Endoscopy [SIED], the Italian Society of Gastroenterology [SIGE], and the Italian Society of Internal Medicine [SIMI]. Dig. Liver. Dis. 2019, 51, 1621–1632. [Google Scholar]
- Massironi, S.; Zilli, A.; Elvevi, A.; Invernizzi, P. The changing face of chronic autoimmune atrophic gastritis: An updated comprehensive perspective. Autoimmun. Rev. 2019, 18, 215–222. [Google Scholar] [CrossRef]
- Lahner, E.; Annibale, B. Pernicious anemia: New insights from a gastroenterological point of view. World J. Gastroenterol. 2009, 15, 5121–5128. [Google Scholar] [CrossRef] [PubMed]
- Conn, D.A. Detection of type I and type II antibodies to intrinsic factor. Med. Lab. Sci. 1986, 43, 148–151. [Google Scholar] [PubMed]
- Toh, B.H.; Sentry, J.W.; Alderuccio, F. The causative H+/K+ ATPase antigen in the pathogenesis of autoimmune gastritis. Immunol. Today 2000, 21, 348–354. [Google Scholar] [CrossRef]
- Bunn, H.F. Vitamin B12 and pernicious anemia--the dawn of molecular medicine. N. Engl. J. Med. 2014, 370, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Toh, B.H.; Chan, J.; Kyaw, T.; Alderuccio, F. Cutting edge issues in autoimmune gastritis. Clin. Rev. Allergy Immunol. 2012, 42, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- D’Elios, M.M.; Appelmelk, B.J.; Amedei, A.; Bergman, M.P.; Del Prete, G. Gastric autoimmunity: The role of Helicobacter pylori and molecular mimicry. Trends Mol. Med. 2004, 10, 316–323. [Google Scholar] [CrossRef]
- Appelmelk, B.J.; Faller, G.; Claeys, D.; Kirchner, T.; Vandenbroucke-Grauls, C.M. Bugs on trial: The case of Helicobacter pylori and autoimmunity. Immunol. Today 1998, 19, 296–299. [Google Scholar] [CrossRef]
- Field, J.; Biondo, M.A.; Murphy, K.; Alderuccio, F.; Toh, B.H. Experimental autoimmune gastritis: Mouse models of human organ-specific autoimmune disease. Int. Rev. Immunol. 2005, 24, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Negrini, R.; Lisato, L.; Zanella, I.; Cavazzini, L.; Gullini, S.; Villanacci, V.; Poiesi, C.; Albertini, A.; Ghielmi, S. Helicobacter pylori infection induces antibodies cross-reacting with human gastric mucosa. Gastroenterology 1991, 101, 437–445. [Google Scholar] [CrossRef]
- Negrini, R.; Savio, A.; Poiesi, C.; Appelmelk, B.J.; Buffoli, F.; Paterlini, A.; Cesari, P.; Graffeo, M.; Vaira, D.; Franzin, G. Antigenic mimicry between Helicobacter pylori and gastric mucosa in the pathogenesis of body atrophic gastritis. Gastroenterology 1996, 111, 655–665. [Google Scholar] [CrossRef]
- D’Elios, M.M.; Bergman, M.P.; Azzurri, A.; Amedei, A.; Benagiano, M.; De Pont, J.J.; Cianchi, F.; Vandenbroucke-Grauls, C.M.; Romagnani, S.; Appelmelk, B.J. H(+), K(+)-atpase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology 2001, 120, 377–386. [Google Scholar] [CrossRef]
- Amedei, A.; Bergman, M.P.; Appelmelk, B.J.; Azzurri, A.; Benagiano, M.; Tamburini, C.; van der Zee, R.; Telford, J.L.; Vandenbroucke-Grauls, C.M.; D’Elios, M.M.; et al. Molecular mimicry between Helicobacter pylori antigens and H+, K+–adenosine triphosphatase in human gastric autoimmunity. J. Exp. Med. 2003, 198, 1147–1156. [Google Scholar] [CrossRef]
- D’Elios, M.M.; Amedei, A.; Azzurri, A.; Benagiano, M.; Del Prete, G.; Bergman, M.P.; Vandenbroucke-Grauls, C.M.; Appelmelk, B.J. Molecular specificity and functional properties of autoreactive T-cell response in human gastric autoimmunity. Int. Rev. Immunol. 2005, 24, 111–122. [Google Scholar] [CrossRef]
- Albert, L.J.; Inman, R.D. Molecular mimicry and autoimmunity. N. Engl. J. Med. 1999, 341, 2068–2074. [Google Scholar] [CrossRef]
- Lahner, E.; Spoletini, M.; Buzzetti, R.; Corleto, V.D.; Vannella, L.; Petrone, A.; Annibale, B. HLA-DRB1*03 and DRB1*04 are associated with atrophic gastritis in an Italian population. Dig. Liver. Dis. 2010, 42, 854–859. [Google Scholar] [CrossRef]
- Lahner, E.; Norman, G.L.; Severi, C.; Encabo, S.; Shums, Z.; Vannella, L.; Delle Fave, G.; Annibale, B. Reassessment of intrinsic factor and parietal cell autoantibodies in atrophic gastritis with respect to cobalamin deficiency. Am. J. Gastroenterol. 2009, 104, 2071–2079. [Google Scholar] [CrossRef]
- Fong, T.L.; Dooley, C.P.; Dehesa, M.; Cohen, H.; Carmel, R.; Fitzgibbons, P.L.; Perez-Perez, G.I.; Blaser, M.J. Helicobacter pylori infection in pernicious anemia: A prospective controlled study. Gastroenterology 1991, 100, 328–332. [Google Scholar] [CrossRef]
- Presotto, F.; Sabini, B.; Cecchetto, A.; Plebani, M.; De Lazzari, F.; Pedini, B.; Betterle, C. Helicobacter pylori infection and gastric autoimmune diseases: Is there a link? Helicobacter 2003, 8, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Annibale, B.; Lahner, E.; Santucci, A.; Vaira, D.; Pasquali, A.; Severi, C.; Mini, R.; Figura, N.; Delle Fave, G. CagA and VacA are immunoblot markers of past Helicobacter pylori infection in atrophic body gastritis. Helicobacter 2007, 12, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Annibale, B.; Lahner, E.; Bordi, C.; Martino, G.; Caruana, P.; Grossi, C.; Negrini, R.; Delle Fave, G. Role of Helicobacter pylori infection in pernicious anaemia. Dig. Liver. Dis. 2000, 32, 756–762. [Google Scholar] [CrossRef]
- Rusak, E.; Chobot, A.; Krzywicka, A.; Wenzlau, J. Anti-parietal cell antibodies-diagnostic significance. Adv. Med. Sci. 2016, 61, 175–179. [Google Scholar] [CrossRef]
- Lahner, E.; Brigatti, C.; Marzinotto, I.; Carabotti, M.; Scalese, G.; Davidson, H.W.; Wenzlau, J.M.; Bosi, E.; Piemonti, L.; Annibale, B.L.; et al. Luminescent Immunoprecipitation System (LIPS) for Detection of Autoantibodies Against ATP4A and ATP4B Subunits of Gastric Proton Pump H+, K+-ATPase in Atrophic Body Gastritis Patients. Clin. Transl. Gastroenterol. 2017, 8, e215. [Google Scholar] [CrossRef]
- Rose, N.R.; Bona, C. Defining criteria for autoimmune diseases (Witebsky’s postulates revisited). Immunol. Today 1993, 14, 426–430. [Google Scholar] [CrossRef]
- Ungar, B.; Mathews, J.D.; Tait, B.D.; Cowling, D.C. HLA-DR patterns in pernicious anaemia. Br. Med. J. 1981, 282, 768–770. [Google Scholar] [CrossRef]
- Fernando, M.M.; Stevens, C.R.; Walsh, E.C.; De Jager, P.L.; Goyette, P.; Plenge, R.M.; Vyse, T.J.; Rioux, J.D. Defining the role of the MHC in autoimmunity: A review and pooled analysis. PLoS Genet. 2008, 4, e1000024. [Google Scholar] [CrossRef]
- Wenzlau, J.M.; Fain, P.R.; Gardner, T.J.; Frisch, L.M.; Annibale, B.; Hutton, J.C. ATPase4A Autoreactivity and Its Association with Autoimmune Phenotypes in the Type 1 Diabetes Genetics Consortium Study. Diabetes Care 2015, 38 (Suppl. 2), S29–S36. [Google Scholar] [CrossRef]
- Laisk, T.; Lepamets, M.; Koel, M.; Abner, E.; Estonian Biobank Research Team; Mägi, R. Genome-wide association study identifies five risk loci for pernicious anemia. Nat. Commun. 2021, 12, 3761. [Google Scholar] [CrossRef] [PubMed]
- Dhir, A.; Dhir, S.; Borowski, L.S.; Jimenez, L.; Teitell, M.; Rötig, A.; Crow, Y.J.; Rice, G.I.; Duffy, D.; Tamby, C.; et al. Mitochondrial double-stranded RNA triggers antiviral signalling in humans. Nature 2018, 560, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Lahner, E.; Gentile, G.; Purchiaroni, F.; Mora, B.; Simmaco, M.; Annibale, B. Single nucleotide polymorphisms related to vitamin B12 serum levels in autoimmune gastritis patients with or without pernicious anaemia. Dig. Liver Dis. 2015, 47, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Healton, E.B.; Savage, D.G.; Brust, J.C.; Garrett, T.J.; Lindenbaum, J. Neurologic aspects of cobalamin deficiency. Medicine 1991, 70, 229–245. [Google Scholar] [CrossRef]
- Htut, T.W.; Thein, K.Z.; Oo, T.H. Pernicious anemia: Pathophysiology and diagnostic difficulties. J. Evid. Based Med. 2021, 14, 161–169. [Google Scholar] [CrossRef]
- Shah, D.R.; Daver, N.; Borthakur, G.; Hirsch-Ginsberg, C.; Oo, T.H. Pernicious anemia with spuriously normal vitamin B12 level might be misdiagnosed as myelodysplastic syndrome. Clin. Lymphoma Myeloma Leuk. 2014, 14, e141–e143. [Google Scholar] [CrossRef]
- Ammouri, W.; Tazi, Z.M.; Harmouche, H.; Maamar, M.; Adnaoui, M. Venous thromboembolism and hyperhomocysteinemia as first manifestation of pernicious anemia: A case series. J. Med. Case Rep. 2017, 11, 250. [Google Scholar] [CrossRef]
- Kharchafi, A.; Oualim, Z.; Amezyane, T.; Mahassin, F.; Ghafir, D.; Ohayon, V.; Archane, M.I. Biermer’s disease and venous thrombosis. Report of two cases. Rev. Med. Interne 2002, 23, 563–566. [Google Scholar] [CrossRef]
- Belen, B.; Hismi, B.O.; Kocak, U. Severe vitamin B12 deficiency with pancytopenia, hepatosplenomegaly and leukoerythroblastosis in two Syrian refugee infants: A challenge to differentiate from acute leukaemia. BMJ Case Rep. 2014, 2014, bcr2014203742. [Google Scholar] [CrossRef]
- Hemmer, B.; Glocker, F.X.; Schumacher, M.; Deuschl, G.; Lücking, C.H. Subacute combined degeneration: Clinical, electrophysiological, and magnetic resonance imaging findings. J. Neurol. Neurosurg. Psychiatry 1998, 65, 822–827. [Google Scholar] [CrossRef]
- Gwathmey, K.G.; Grogan, J. Nutritional neuropathies. Muscle Nerve 2020, 62, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N. Nutritional neuropathies. Neurol. Clin. 2007, 25, 209–255. [Google Scholar] [CrossRef] [PubMed]
- Briani, C.; Dalla Torre, C.; Citton, V.; Manara, R.; Pompanin, S.; Binotto, G.; Adami, F. Cobalamin deficiency: Clinical picture and radiological findings. Nutrients 2013, 5, 4521–4539. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; McCaddon, A.; Herrmann, W. The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin. Chem. Lab. Med. 2007, 45, 1590–1606. [Google Scholar] [CrossRef] [PubMed]
- Dommisse, J. Subtle vitamin-B12 deficiency and psychiatry: A largely unnoticed but devastating relationship? Med. Hypotheses 1991, 34, 131–140. [Google Scholar] [CrossRef]
- Hutto, B.R. Folate and cobalamin in psychiatric illness. Compr. Psychiatry 1997, 38, 305–314. [Google Scholar] [CrossRef]
- van Goor, L.; Woiski, M.D.; Lagaay, A.M.; Meinders, A.E.; Tak, P.P. Review: Cobalamin deficiency and mental impairment in elderly people. Age Ageing 1995, 24, 536–542. [Google Scholar] [CrossRef]
- Stabler, S.P.; Allen, R.H.; Savage, D.G.; Lindenbaum, J. Clinical spectrum and diagnosis of cobalamin deficiency. Blood 1990, 76, 871–881. [Google Scholar] [CrossRef]
- Moore, E.; Mander, A.; Ames, D.; Carne, R.; Sanders, K.; Watters, D. Cognitive impairment and vitamin B12: A review. Int. Psychogeriatr. 2012, 24, 541–556. [Google Scholar] [CrossRef]
- Green, R. Anemias beyond B12 and iron deficiency: The buzz about other B’s, elementary, and nonelementary problems. Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 492–498. [Google Scholar] [CrossRef]
- Tan, I.Y.; de Tilly, L.N.; Gray, T.A. Hypocupremia: An under recognized cause of subacute combined degeneration. Can. J. Neurol. Sci. 2009, 36, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Miceli, E.; Lenti, M.V.; Padula, D.; Luinetti, O.; Vattiato, C.; Monti, C.M.; Di Stefano, M.; Corazza, G.R. Common features of patients with autoimmune atrophic gastritis. Clin. Gastroenterol. Hepatol. 2012, 10, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Tosetti, C.; Stanghellini, V.; Tucci, A.; Poli, L.; Salvioli, B.; Biasco, G.; Paparo, G.F.; Levorato, M.; Corinaldesi, R. Gastric emptying and dyspeptic symptoms in patients with nonautoimmune fundic atrophic gastritis. Dig. Dis. Sci. 2000, 45, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Wauters, L.; Talley, N.J.; Walker, M.M.; Tack, J.; Vanuytsel, T. Novel concepts in the pathophysiology and treatment of functional dyspepsia. Gut 2020, 69, 591–600. [Google Scholar] [CrossRef]
- de Bortoli, N.; Martinucci, I.; Savarino, E.; Franchi, R.; Bertani, L.; Russo, S.; Ceccarelli, L.; Costa, F.; Bellini, M.; Blandizzi, C.; et al. Lower pH values of weakly acidic refluxes as determinants of heartburn perception in gastroesophageal reflux disease patients with normal esophageal acid exposure. Dis. Esophagus 2016, 29, 3–9. [Google Scholar] [CrossRef]
- Lahner, E.; Centanni, M.; Agnello, G.; Gargano, L.; Vannella, L.; Iannoni, C.; Delle Fave, G.; Annibale, B. Occurrence and risk factors for autoimmune thyroid disease in patients with atrophic body gastritis. Am. J. Med. 2008, 121, 136–141. [Google Scholar] [CrossRef]
- Centanni, M.; Gargano, L.; Canettieri, G.; Viceconti, N.; Franchi, A.; Delle Fave, G.; Annibale, B. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N. Engl. J. Med. 2006, 354, 1787–1795. [Google Scholar] [CrossRef]
- Nenna, R.; Magliocca, F.M.; Tiberti, C.; Mastrogiorgio, G.; Petrarca, L.; Mennini, M.; Lucantoni, F.; Luparia, R. Endoscopic and histological gastric lesions in children with celiac disease: Mucosal involvement is not only confined to the duodenum. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 728–732. [Google Scholar] [CrossRef]
- Massironi, S.; Cavalcoli, F.; Rossi, R.E.; Conte, D.; Spampatti, M.P.; Ciafardini, C.; Verga, U.; Beck-Peccoz, P.; Peracchi, M. Chronic autoimmune atrophic gastritis associated with primary hyperparathyroidism: A transversal prospective study. Eur. J. Endocrinol. 2013, 168, 755–761. [Google Scholar] [CrossRef][Green Version]
- Chang, K.H.; Lyu, R.K.; Ro, L.S.; Wu, Y.R.; Chen, C.M. Coexistence of pernicious anemia and myasthenia gravis—A rare combination of autoimmune diseases in Taiwan. J. Formos. Med. Assoc. 2006, 105, 946–949. [Google Scholar] [CrossRef]
- Jevremovic, D.; Torbenson, M.; Murray, J.A.; Burgart, L.J.; Abraham, S.C. Atrophic autoimmune pangastritis: A distinctive form of antral and fundic gastritis associated with systemic autoimmune disease. Am. J. Surg. Pathol. 2006, 30, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Vanderlocht, J.; van der Cruys, M.; Stals, F.; Bakker-Jonges, L.; Damoiseaux, J. Multiplex autoantibody detection for autoimmune liver diseases and autoimmune gastritis. J. Immunol. Methods 2017, 448, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Warren, M.J.; Refsum, H. Vitamin B12. Adv. Food Nutr. Res. 2018, 83, 215–279. [Google Scholar] [PubMed]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- Zaric, B.L.; Obradovic, M.; Bajic, V.; Haidara, M.A.; Jovanovic, M.; Isenovic, E.R. Homocysteine and Hyperhomocysteinaemia. Curr. Med. Chem. 2019, 26, 2948–2961. [Google Scholar] [CrossRef]
- Lahner, E.; Persechino, S.; Annibale, B. Micronutrients (Other than iron) and Helicobacter pylori infection: A systematic review. Helicobacter 2012, 17, 1–15. [Google Scholar] [CrossRef]
- Annibale, B.; Capurso, G.; Fave, G.D. Consequences of Helicobacter pylori infection on the absorption of micronutrients. Dig. Liver Dis. 2002, 34 (Suppl. 2), S72–S77. [Google Scholar] [CrossRef]
- Zilli, A.; Cavalcoli, F.; Ciafardini, C.; Massironi, S. Deficiency of micronutrients in patients affected by chronic atrophic autoimmune gastritis: A single-institution observational study. Dig. Liver Dis. 2019, 51, 505–509. [Google Scholar] [CrossRef]
- Saenz, J.B.; Mills, J.C. Acid and the basis for cellular plasticity and reprogramming in gastric repair and cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 257–273. [Google Scholar] [CrossRef]
- Kopic, S.; Geibel, J.P. Gastric acid, calcium absorption, and their impact on bone health. Physiol. Rev. 2013, 93, 189–268. [Google Scholar] [CrossRef]
- Engevik, A.C.; Kaji, I.; Goldenring, J.R. The Physiology of the Gastric Parietal Cell. Physiol. Rev. 2020, 100, 573–602. [Google Scholar] [CrossRef] [PubMed]
- Festen, H.P. Intrinsic factor secretion and cobalamin absorption. Physiology and pathophysiology in the gastrointestinal tract. Scand. J. Gastroenterol. Suppl. 1991, 188, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P.; Allen, R.H. Vitamin B12 deficiency as a worldwide problem. Annu. Rev. Nutr. 2004, 24, 299–326. [Google Scholar] [CrossRef]
- Fedosov, S.N.; Fedosova, N.U.; Krautler, B.; Nexo, E.; Petersen, T.E. Mechanisms of discrimination between cobalamins and their natural analogues during their binding to the specific B12-transporting proteins. Biochemistry 2007, 46, 6446–6458. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.J.; Rasmussen, M.R.; Andersen, C.B.; Nexo, E.; Moestrup, S.K. Vitamin B12 transport from food to the body’s cells–A sophisticated, multistep pathway. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P. Clinical practice. Vitamin B12 deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Annibale, B.; Capurso, G.; Fave, G.D. The stomach and iron deficiency anaemia: A forgotten link. Dig. Liver Dis. 2003, 35, 288–295. [Google Scholar] [CrossRef]
- Conrad, M.E.; Umbreit, J.N.; Moore, E.G. Iron absorption and transport. Am. J. Med. Sci. 1999, 318, 213–229. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1559S–1566S. [Google Scholar] [CrossRef]
- Conrad, M.E.; Schade, S.G. Ascorbic acid chelates in iron absorption: A role for hydrochloric acid and bile. Gastroenterology 1968, 55, 35–45. [Google Scholar] [CrossRef]
- Cook, J.D.; Brown, G.M.; Valberg, L.S. The Effect of Achylia Gastrica on Iron Absorption. J. Clin. Investig. 1964, 43, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Dickey, W. Iron deficiency, gastric atrophy and Helicobacter pylori. Dig. Liver Dis. 2002, 34, 313–315. [Google Scholar] [CrossRef]
- Dickey, W.; Kenny, B.D.; McMillan, S.A.; Porter, K.G.; McConnell, J.B. Gastric as well as duodenal biopsies may be useful in the investigation of iron deficiency anaemia. Scand. J. Gastroenterol. 1997, 32, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Marignani, M.; Delle Fave, G.; Mecarocci, S.; Bordi, C.; Angeletti, S.; D’Ambra, G.; Aprile, M.R.; Corleto, V.D.; Monarca, B.; Annibale, B. High prevalence of atrophic body gastritis in patients with unexplained microcytic and macrocytic anemia: A prospective screening study. Am. J. Gastroenterol. 1999, 94, 766–772. [Google Scholar] [CrossRef]
- Annibale, B.; Capurso, G.; Chistolini, A.; D’Ambra, G.; DiGiulio, E.; Monarca, B.; DelleFave, G. Gastrointestinal causes of refractory iron deficiency anemia in patients without gastrointestinal symptoms. Am. J. Med. 2001, 111, 439–445. [Google Scholar] [CrossRef]
- Hershko, C.; Ronson, A.; Souroujon, M.; Maschler, I.; Heyd, J.; Patz, J. Variable hematologic presentation of autoimmune gastritis: Age-related progression from iron deficiency to cobalamin depletion. Blood 2006, 107, 1673–1679. [Google Scholar] [CrossRef]
- Zagari, R.M.; Romano, M.; Ojetti, V.; Stockbrugger, R.; Gullini, S.; Annibale, B.; Farinati, F.; Ierardi, E.; Maconi, G.; Rugge, M. Guidelines for the management of Helicobacter pylori infection in Italy: The III Working Group Consensus Report 2015. Dig. Liver Dis. 2015, 47, 903–912. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Boyce, B.F. Stomaching calcium for bone health. Nat. Med. 2009, 15, 610–612. [Google Scholar] [CrossRef]
- Sipponen, P.; Harkonen, M. Hypochlorhydric stomach: A risk condition for calcium malabsorption and osteoporosis? Scand. J. Gastroenterol. 2010, 45, 133–138. [Google Scholar] [CrossRef]
- Kitay, A.M.; Geibel, J.P. Stomach and Bone. Adv. Exp. Med. Biol. 2017, 1033, 97–131. [Google Scholar] [PubMed]
- Antico, A.; Tozzoli, R.; Giavarina, D.; Tonutti, E.; Bizzaro, N. Hypovitaminosis D as predisposing factor for atrophic type A gastritis: A case-control study and review of the literature on the interaction of Vitamin D with the immune system. Clin. Rev. Allergy Immunol. 2012, 42, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Massironi, S.; Cavalcoli, F.; Zilli, A.; Del Gobbo, A.; Ciafardini, C.; Bernasconi, S.; Felicetta, I.; Conte, D.; Peracchi, M. Relevance of vitamin D deficiency in patients with chronic autoimmune atrophic gastritis: A prospective study. BMC Gastroenterol. 2018, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, B.J.; Johnson, A.W.; Wyatt, J.I.; Kelleher, J.; Heatley, R.V.; Losowsky, M.S. Ascorbic acid: A factor concentrated in human gastric juice. Clin. Sci. 1989, 76, 237–241. [Google Scholar] [CrossRef]
- Lombard, M.; Chua, E.; O’Toole, P. Regulation of intestinal non-haem iron absorption. Gut 1997, 40, 435–439. [Google Scholar] [CrossRef]
- Bothwell, T.H.; Baynes, R.D.; MacFarlane, B.J.; MacPhail, A.P. Nutritional iron requirements and food iron absorption. J. Intern. Med. 1989, 226, 357–365. [Google Scholar] [CrossRef]
- Annibale, B.; Capurso, G.; Lahner, E.; Passi, S.; Ricci, R.; Maggio, F.; Delle Fave, G. Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut 2003, 52, 496–501. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Abdullahi, M.; Farthing, M.J. Effect of physiological concentrations of vitamin C on gastric cancer cells and Helicobacter pylori. Gut 2002, 50, 165–169. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Patchett, S.E.; Perrett, D.; Katelaris, P.H.; Domizio, P.; Farthing, M.J. The relation between gastric vitamin C concentrations, mucosal histology, and CagA seropositivity in the human stomach. Gut 1998, 43, 322–326. [Google Scholar] [CrossRef]
- Ruiz, B.; Rood, J.C.; Fontham, E.T.; Malcom, G.T.; Hunter, F.M.; Sobhan, M.; Johnson, W.D.; Correa, P. Vitamin C concentration in gastric juice before and after anti-Helicobacter pylori treatment. Am. J. Gastroenterol. 1994, 89, 533–539. [Google Scholar]
- Mei, H.; Tu, H. Vitamin C and Helicobacter pylori Infection: Current Knowledge and Future Prospects. Front. Physiol. 2018, 9, 1103. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.B.; Brodsky, I. Metabolic interrelationship between vitamin B12 and ascorbic acid in pernicious anemia. Blood 1968, 31, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.A.; Adil, A. Macrocytic Anemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Green, R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood 2017, 129, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Cavalcoli, F.; Zilli, A.; Conte, D.; Massironi, S. Micronutrient deficiencies in patients with chronic atrophic autoimmune gastritis: A review. World J. Gastroenterol. 2017, 23, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.; Moller, J.; Lyngbak, M.; Pedersen, A.M.; Dybkjaer, L. Age-and gender-specific reference intervals for total homocysteine and methylmalonic acid in plasma before and after vitamin supplementation. Clin. Chem. 1996, 42, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Vogiatzoglou, A.; Oulhaj, A.; Smith, A.D.; Nurk, E.; Drevon, C.A.; Ueland, P.M.; Vollset, S.E.; Tell, G.S.; Refsum, H. Determinants of plasma methylmalonic acid in a large population: Implications for assessment of vitamin B12 status. Clin. Chem. 2009, 55, 2198–2206. [Google Scholar] [CrossRef]
- Chuang, J.S.; Callaghan, J.M.; Gleeson, P.A.; Toh, B.H. Diagnostic ELISA for parietal cell autoantibody using tomato lectin-purified gastric H+/K(+)-ATPase (proton pump). Autoimmunity 1992, 12, 1–7. [Google Scholar] [CrossRef]
- Conti, L.; Lenti, M.V.; Di Sabatino, A.; Miceli, E.; Galli, G.; Cazzato, M.; Falangone, F.; Annibale, B.; Lahner, E. Seronegative autoimmune atrophic gastritis is more common in elderly patients. Dig. Liver. Dis. 2020, 52, 1310–1314. [Google Scholar] [CrossRef]
- Korstanje, A.; den Hartog, G.; Biemond, I.; Lamers, C.B. The serological gastric biopsy: A non-endoscopical diagnostic approach in management of the dyspeptic patient: Significance for primary care based on a survey of the literature. Scand. J. Gastroenterol. Suppl. 2002, 2002, 22–26. [Google Scholar] [CrossRef]
- Yoshida, T.; Kato, J.; Inoue, I.; Yoshimura, N.; Deguchi, H.; Mukoubayashi, C.; Oka, M.; Watanabe, M.; Enomoto, S.; Niwa, T.; et al. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int. J. Cancer 2014, 134, 1445–1457. [Google Scholar] [CrossRef]
- Lahner, E.; Marzinotto, I.; Lampasona, V.; Dottori, L.; Bazzigaluppi, E.; Brigatti, C.; Secchi, M.; Piemonti, L.; Conti, L.; Pilozzi, E.; et al. Autoantibodies Toward ATP4A and ATP4B Subunits of Gastric Proton Pump H+,K+-ATPase Are Reliable Serological Pre-endoscopic Markers of Corpus Atrophic Gastritis. Clin. Transl. Gastroenterol. 2020, 11, e00240. [Google Scholar] [CrossRef] [PubMed]
- Marzinotto, I.; Dottori, L.; Baldaro, F.; Dilaghi, E.; Brigatti, C.; Bazzigaluppi, E.; Esposito, G.; Davidson, H.W.; Piemonti, L.; Lampasona, V.; et al. Intrinsic factor autoantibodies by luminescent immuno-precipitation system in patients with corpus atrophic gastritis. J. Transl. Autoimmun. 2021, 4, 100131. [Google Scholar] [CrossRef] [PubMed]
- Kotelevets, S.M.; Chekh, S.A.; Chukov, S.Z. Updated Kimura-Takemoto classification of atrophic gastritis. World J. Clin. Cases 2021, 9, 3014–3023. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Meggio, A.; Pennelli, G.; Piscioli, F.; Giacomelli, L.; De Pretis, G.; Graham, D.Y. Gastritis staging in clinical practice: The OLGA staging system. Gut 2007, 56, 631–636. [Google Scholar] [CrossRef]
- Capelle, L.G.; de Vries, A.C.; Haringsma, J.; Ter Borg, F.; de Vries, R.A.; Bruno, M.J.; van Dekken, H.; Meijer, J.; van Grieken, N.C.; Kuipers, E.J. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest. Endosc. 2010, 71, 1150–1158. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Dinis-Ribeiro, M.; Soares, J.B.; Marcos-Pinto, R.; Santos, C.; Rolanda, C.; Bastos, R.P.; Areia, M.; Afonso, L.; Bergman, J.; et al. A multicenter validation of an endoscopic classification with narrow band imaging for gastric precancerous and cancerous lesions. Endoscopy 2012, 44, 236–246. [Google Scholar] [CrossRef]
- Kikuste, I.; Marques-Pereira, R.; Monteiro-Soares, M.; Pimentel-Nunes, P.; Areia, M.; Leja, M.; Dinis-Ribeiro, M. Systematic review of the diagnosis of gastric premalignant conditions and neoplasia with high-resolution endoscopic technologies. Scand. J. Gastroenterol. 2013, 48, 1108–1117. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Lage, J.; Abrantes, D.; Coimbra, M.; Esposito, G.; Hormozdi, D.; Pepper, M.; Drasovean, S.; White, J.R.; et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy 2016, 48, 723–730. [Google Scholar] [CrossRef]
- Buxbaum, J.L.; Hormozdi, D.; Dinis-Ribeiro, M.; Lane, C.; Dias-Silva, D.; Sahakian, A.; Jayaram, P.; Pimentel-Nunes, P.; Shue, D.; Pepper, M.; et al. Narrow-band imaging versus white light versus mapping biopsy for gastric intestinal metaplasia: A prospective blinded trial. Gastrointest. Endosc. 2017, 86, 857–865. [Google Scholar] [CrossRef]
- Lage, J.; Pimentel-Nunes, P.; Figueiredo, P.C.; Libanio, D.; Ribeiro, I.; Jacome, M.; Afonso, L.; Dinis-Ribeiro, M. Light-NBI to identify high-risk phenotypes for gastric adenocarcinoma: Do we still need biopsies? Scand. J. Gastroenterol. 2016, 51, 501–506. [Google Scholar] [CrossRef]
- Castro, R.; Rodriguez, M.; Libânio, D.; Esposito, G.; Pita, I.; Patita, M.; Santos, C.; Pimentel-Nunes, P.; Dinis-Ribeiro, M. Reliability and accuracy of blue light imaging for staging of intestinal metaplasia in the stomach. Scand. J. Gastroenterol. 2019, 54, 1301–1305. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Pimentel-Nunes, P.; Angeletti, S.; Castro, R.; Libânio, D.; Galli, G.; Lahner, E.; Di Giulio, E.; Annibale, B.; Dinis-Ribeiro, M. Endoscopic grading of gastric intestinal metaplasia (EGGIM): A multicenter validation study. Endoscopy 2019, 51, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Dilaghi, E.; Ida, K.; Kato, T.; Uedo, N.; Ando, T.; Watanabe, H.; Shimbo, T.; Study Group for Investigating Endoscopic Diagnosis of Gastric Intestinal Metaplasia. Endoscopic diagnosis of gastric intestinal metaplasia in patients with autoimmune gastritis using narrow-band imaging: Does pseudopyloric metaplasia muddy the waters? Endosc. Int. Open 2022, 25, 526–534. [Google Scholar] [CrossRef]
- Correa, P. Human gastric carcinogenesis: A multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992, 52, 6735–6740. [Google Scholar] [PubMed]
- Xia, H.H.; Kalantar, J.S.; Talley, N.J.; Wyatt, J.M.; Adams, S.; Chueng, K.; Mitchell, H.M. Antral-type mucosa in the gastric incisura, body, and fundus (antralization): A link between Helicobacter pylori infection and intestinal metaplasia? Am. J. Gastroenterol. 2000, 95, 114–121. [Google Scholar] [CrossRef]
- Dilaghi, E.; Baldaro, F.; Pilozzi, E.; Conti, L.; Palumbo, A.; Esposito, G.; Annibale, B.; Lahner, E. Pseudopyloric Metaplasia Is Not Associated with the Development of Gastric Cancer. Am. J. Gastroenterol. 2021, 116, 1859–1867. [Google Scholar] [CrossRef]
- Dinis-Ribeiro, M.; Areia, M.; de Vries, A.C.; Marcos-Pinto, R.; Monteiro-Soares, M.; O’Connor, A.; Pereira, C.; Pimentel-Nunes, P.; Correia, R.; Ensari, A.; et al. Management of precancerous conditions and lesions in the stomach (MAPS): Guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED). Endoscopy 2012, 44, 74–94. [Google Scholar]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar]
- Banks, M.; Graham, D.; Jansen, M.; Gotoda, T.; Coda, S.; di Pietro, M.; Uedo, N.; Bhandari, P.; Pritchard, D.M.; Kuipers, E.J.; et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut 2019, 68, 1545–1575. [Google Scholar] [CrossRef]
- Shah, S.C.; Piazuelo, M.B.; Kuipers, E.J.; Li, D. AGA Clinical Practice Update on the Diagnosis and Management of Atrophic Gastritis: Expert Review. Gastroenterology 2021, 161, 1325–1332.e7. [Google Scholar] [CrossRef]
- Vannella, L.; Lahner, E.; Osborn, J.; Bordi, C.; Miglione, M.; Delle Fave, G.; Annibale, B. Risk factors for progression to gastric neoplastic lesions in patients with atrophic gastritis. Aliment. Pharmacol. Ther. 2010, 31, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Ribeiro, M.; Lopes, C.; da Costa-Pereira, A.; Guilherme, M.; Barbosa, J.; Lomba-Viana, H.; Silva, R.; Moreira-Dias, L. A follow up model for patients with atrophic chronic gastritis and intestinal metaplasia. J. Clin. Pathol. 2004, 57, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenburg, S.A.V.; Mommersteeg, M.C.; Eikenboom, E.L.; Yu, B.; den Hollander, W.J.; Holster, I.L.; den Hoed, C.M.; Capelle, L.G.; Tang, T.J.; Anten, M.P.; et al. Factors associated with the progression of gastric intestinal metaplasia: A multicenter, prospective cohort study. Endosc. Int. Open. 2021, 9, E297–E305. [Google Scholar] [PubMed]
- Vannella, L.; Sbrozzi-Vanni, A.; Lahner, E.; Bordi, C.; Pilozzi, E.; Corleto, V.D.; Osborn, J.F.; Delle Fave, G.; Annibale, B. Development of type I gastric carcinoid in patients with chronic atrophic gastritis. Aliment. Pharmacol. Ther. 2011, 33, 1361–1369. [Google Scholar] [CrossRef]
- Lahner, E.; Esposito, G.; Pilozzi, E.; Purchiaroni, F.; Corleto, V.D.; Di Giulio, E.; Annibale, B. Occurrence of gastric cancer and carcinoids in atrophic gastritis during prospective long-term follow up. Scand. J. Gastroenterol. 2015, 50, 856–865. [Google Scholar] [CrossRef]
- Song, H.; Zhu, J.; Lu, D. Long-term proton pump inhibitor (PPI) use and the development of gastric pre-malignant lesions. Cochrane Database Syst. Rev. 2014, 40, CD010623. [Google Scholar] [CrossRef]
- Annibale, B.; Esposito, G.; Lahner, E. A current clinical overview of atrophic gastritis. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 93–102. [Google Scholar] [CrossRef]
- Lahner, E.; Capasso, M.; Carabotti, M.; Annibale, B. Incidence of cancer (other than gastric cancer) in pernicious anaemia: A systematic review with meta-analysis. Dig. Liver. Dis. 2018, 50, 780–786. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Qin, L.L.; Song, Y.; Vidal-Alaball, J.; Liu, T.H. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst. Rev. 2018, 3, CD004655. [Google Scholar] [CrossRef]
- Evstatiev, R.; Marteau, P.; Iqbal, T.; Khalif, I.L.; Stein, J.; Bokemeyer, B.; Chopey, I.V.; Gutzwiller, F.S.; Riopel, L.; Gasche, C.; et al. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology 2011, 141, 846–853.e1-2. [Google Scholar] [CrossRef]
- Avni, T.; Bieber, A.; Grossman, A.; Green, H.; Leibovici, L.; Gafter-Gvili, A. The safety of intravenous iron preparations: Systematic review and meta-analysis. Mayo Clin. Proc. 2015, 90, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Areia, M.; Dinis-Ribeiro, M.; Goncalves, F.R. Cost-utility analysis of endoscopic surveillance of patients with gastric premalignant conditions. Helicobacter 2014, 19, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Lahner, E.; Hassan, C.; Esposito, G.; Carabotti, M.; Zullo, A.; Dinis-Ribeiro, M.; Annibale, B. Cost of detecting gastric neoplasia by surveillance endoscopy in atrophic gastritis in Italy: A low risk country. Dig. Liver. Dis. 2017, 49, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Rugge, M.; Meggio, A.; Pravadelli, C.; Barbareschi, M.; Fassan, M.; Gentilini, M.; Zorzi, M.; Pretis, G.; Graham, D.Y.; Genta, R.M. Gastritis staging in the endoscopic follow-up for the secondary prevention of gastric cancer: A 5-year prospective study of 1755 patients. Gut 2019, 68, 11–17. [Google Scholar] [CrossRef]
- Zullo, A.; Hassan, C.; Repici, A.; Annibale, B. Intestinal metaplasia surveillance: Searching for the road-map. World J. Gastroenterol. 2013, 19, 1523–1526. [Google Scholar] [CrossRef]
- Rugge, M.; Genta, R.M.; Fassan, M.; Valentini, E.; Coati, I.; Guzzinati, S.; Savarino, E.; Zorzi, M.; Farinati, F.; Malfertheiner, P. OLGA Gastritis Staging for the Prediction of Gastric Cancer Risk: A Long-term Follow-up Study of 7436 Patients. Am. J. Gastroenterol. 2018, 113, 1621–1628. [Google Scholar] [CrossRef]
- de Vries, A.C.; van Grieken, N.C.; Looman, C.W.; Casparie, M.K.; de Vries, E.; Meijer, G.A.; Kuipers, E.J. Gastric cancer risk in patients with premalignant gastric lesions: A nationwide cohort study in the Netherlands. Gastroenterology 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Cho, S.J.; Choi, I.J.; Kook, M.C.; Nam, B.H.; Kim, C.G.; Lee, J.Y.; Ryu, K.W.; Kim, Y.W. Staging of intestinal- and diffuse-type gastric cancers with the OLGA and OLGIM staging systems. Aliment. Pharmacol. Ther. 2013, 38, 1292–1302. [Google Scholar] [CrossRef]
- Esposito, G.; Dilaghi, E.; Cazzato, M.; Pilozzi, E.; Conti, L.; Carabotti, M.; Di Giulio, E.; Annibale, B.; Lahner, E. Endoscopic surveillance at 3 years after diagnosis, according to European guidelines, seems safe in patients with atrophic gastritis in a low-risk region. Dig. Liver Dis. 2021, 53, 467–473. [Google Scholar] [CrossRef]
- Panzuto, F.; Magi, L.; Esposito, G.; Rinzivillo, M.; Annibale, B. Comparison of Endoscopic Techniques in the Management of Type I Gastric Neuroendocrine Neoplasia: A Systematic Review. Gastroenterol. Res. Pract. 2021, 2021, 6679397. [Google Scholar] [CrossRef]
- Fave, G.D.; O’Toole, D.; Sundin, A.; Taal, B.; Ferolla, P.; Ramage, J.K.; Ferone, D.; Ito, T.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Cazzato, M.; Rinzivillo, M.; Pilozzi, E.; Lahner, E.; Annibale, B.; Panzuto, F. Management of type-I gastric neuroendocrine neoplasms: A 10-years prospective single centre study. Dig. Liver Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yin, Z.; Wang, S.; Wang, J.; Bai, B.; Qiu, Z.; Zhao, Q. Meta-analysis: The diagnostic efficacy of chromoendoscopy for early gastric cancer and premalignant gastric lesions. J. Gastroenterol. Hepatol. 2016, 31, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carrasco, M.; Esposito, G.; Libânio, D.; Pimentel-Nunes, P.; Dinis-Ribeiro, M. Image-enhanced endoscopy for gastric preneoplastic conditions and neoplastic lesions: A systematic review and meta-analysis. Endoscopy 2020, 52, 1048–1065. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Angeletti, S.; Cazzato, M.; Galli, G.; Conti, L.; Di Giulio, E.; Annibale, B.; Lahner, E. Narrow band imaging characteristics of gastric polypoid lesions: A single-center prospective pilot study. Eur. J. Gastroenterol. Hepatol. 2020, 32, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Pecere, S.; Milluzzo, S.M.; Esposito, G.; Dilaghi, E.; Telese, A.; Eusebi, L.H. Applications of Artificial Intelligence for the Diagnosis of Gastrointestinal Diseases. Diagnostics 2021, 11, 1575. [Google Scholar] [CrossRef]
- Dilaghi, E.; Lahner, E.; Annibale, B.; Esposito, G. Systematic review and meta-analysis: Artificial intelligence for the diagnosis of gastric precancerous lesions and Helicobacter pylori infection. Dig. Liver Dis. 2022. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, G.; Dottori, L.; Pivetta, G.; Ligato, I.; Dilaghi, E.; Lahner, E. Pernicious Anemia: The Hematological Presentation of a Multifaceted Disorder Caused by Cobalamin Deficiency. Nutrients 2022, 14, 1672. https://doi.org/10.3390/nu14081672
Esposito G, Dottori L, Pivetta G, Ligato I, Dilaghi E, Lahner E. Pernicious Anemia: The Hematological Presentation of a Multifaceted Disorder Caused by Cobalamin Deficiency. Nutrients. 2022; 14(8):1672. https://doi.org/10.3390/nu14081672
Chicago/Turabian StyleEsposito, Gianluca, Ludovica Dottori, Giulia Pivetta, Irene Ligato, Emanuele Dilaghi, and Edith Lahner. 2022. "Pernicious Anemia: The Hematological Presentation of a Multifaceted Disorder Caused by Cobalamin Deficiency" Nutrients 14, no. 8: 1672. https://doi.org/10.3390/nu14081672
APA StyleEsposito, G., Dottori, L., Pivetta, G., Ligato, I., Dilaghi, E., & Lahner, E. (2022). Pernicious Anemia: The Hematological Presentation of a Multifaceted Disorder Caused by Cobalamin Deficiency. Nutrients, 14(8), 1672. https://doi.org/10.3390/nu14081672






