Friedelin Alleviates the Pathogenesis of Collagenase-Induced Tendinopathy in Mice by Promoting the Selective Autophagic Degradation of p65
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Animals and Treatment
2.3. Biomechanical Assay
2.4. Histological Assessments
2.5. Quantitative Reverse Transcription PCR (qRT-PCR) Assay
2.6. Cell Culture and Treatment
2.7. Cell Viability Assay
2.8. Plasmids and Transfection
2.9. Protein Degradation Inhibition Assays
2.10. ELISA
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
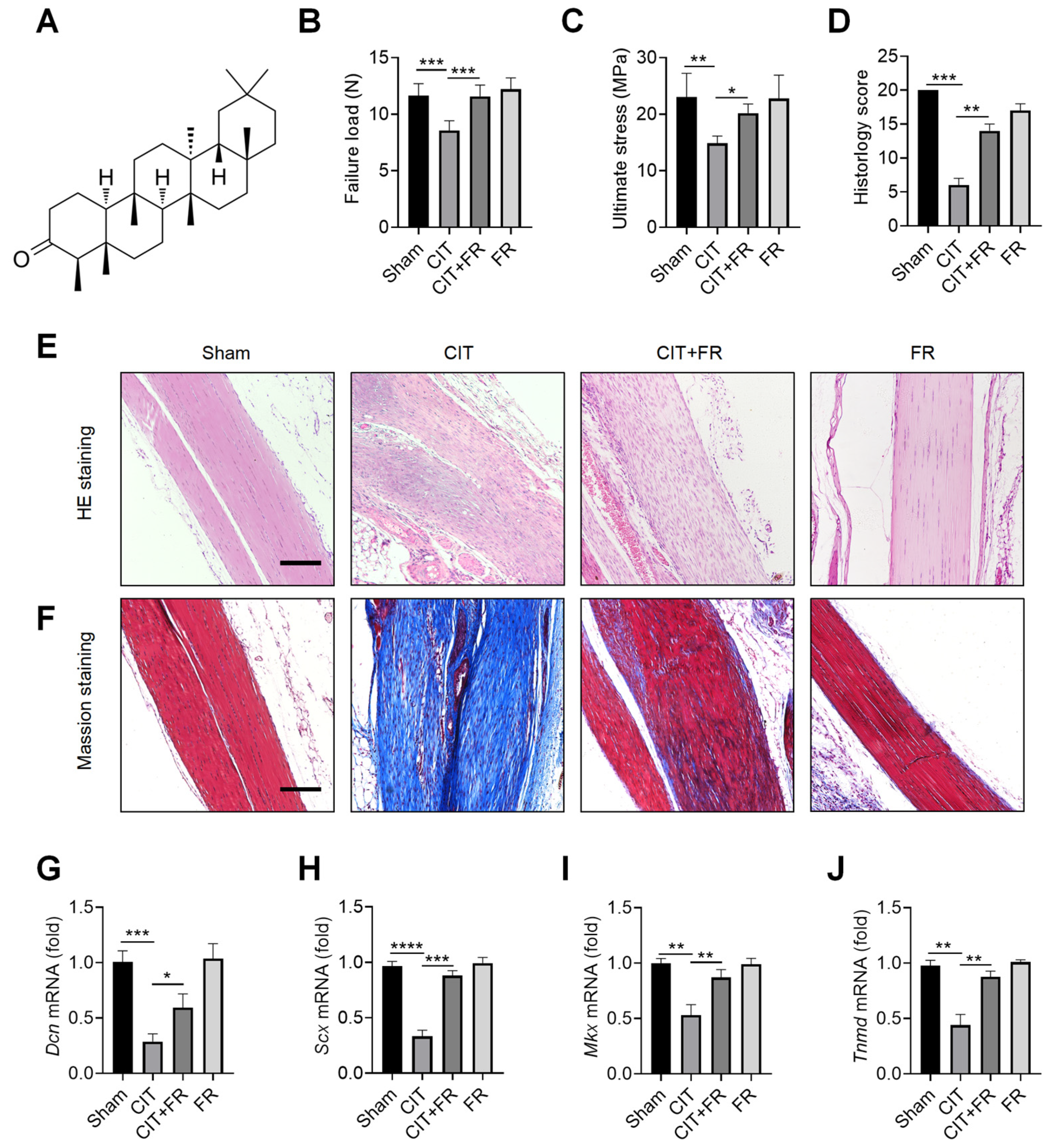
3.1. FR Alleviates the Progression of Tendinopathy in Mice
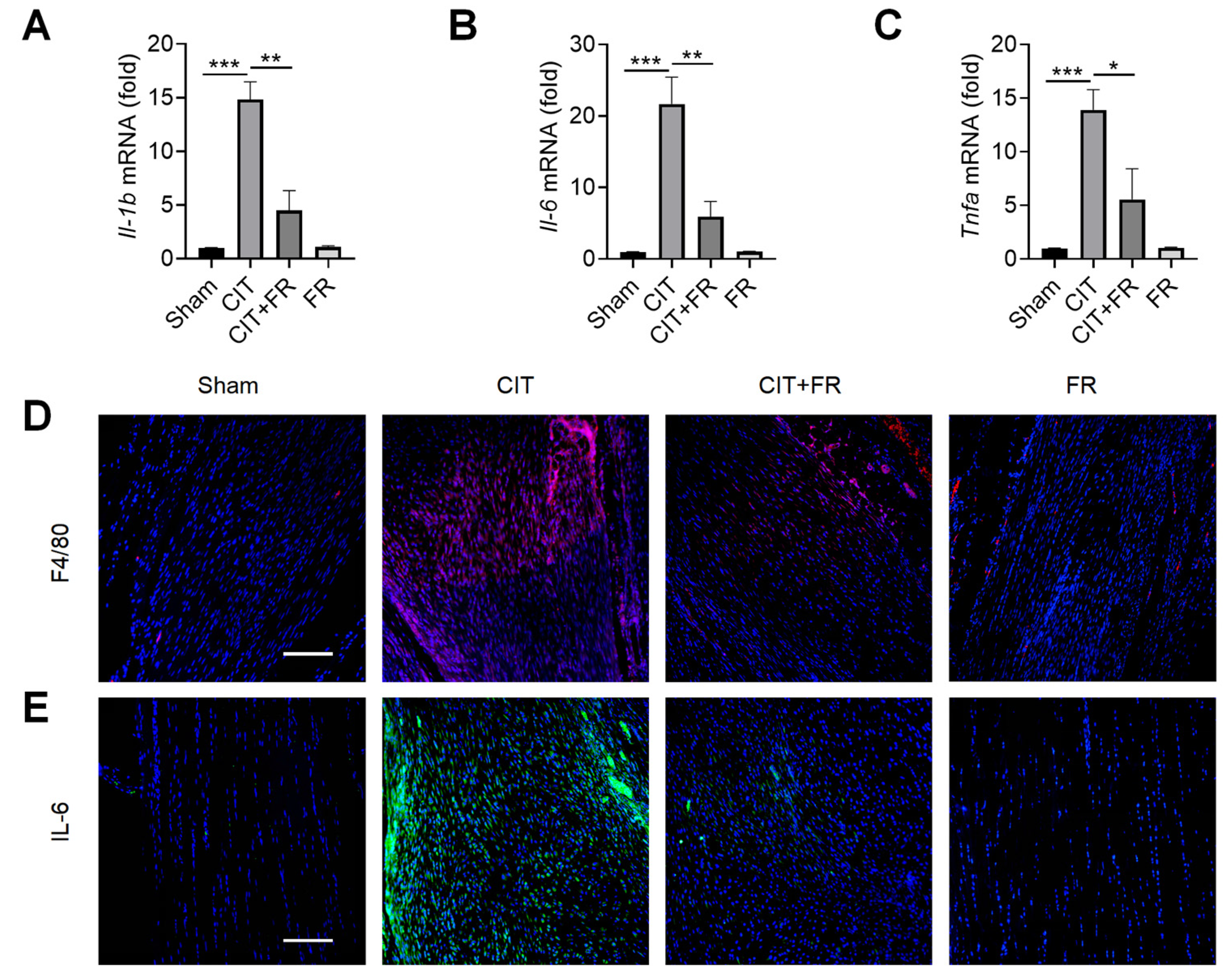
3.2. FR Attenuates the Infiltration of Inflammatory Factors during Tendinopathy
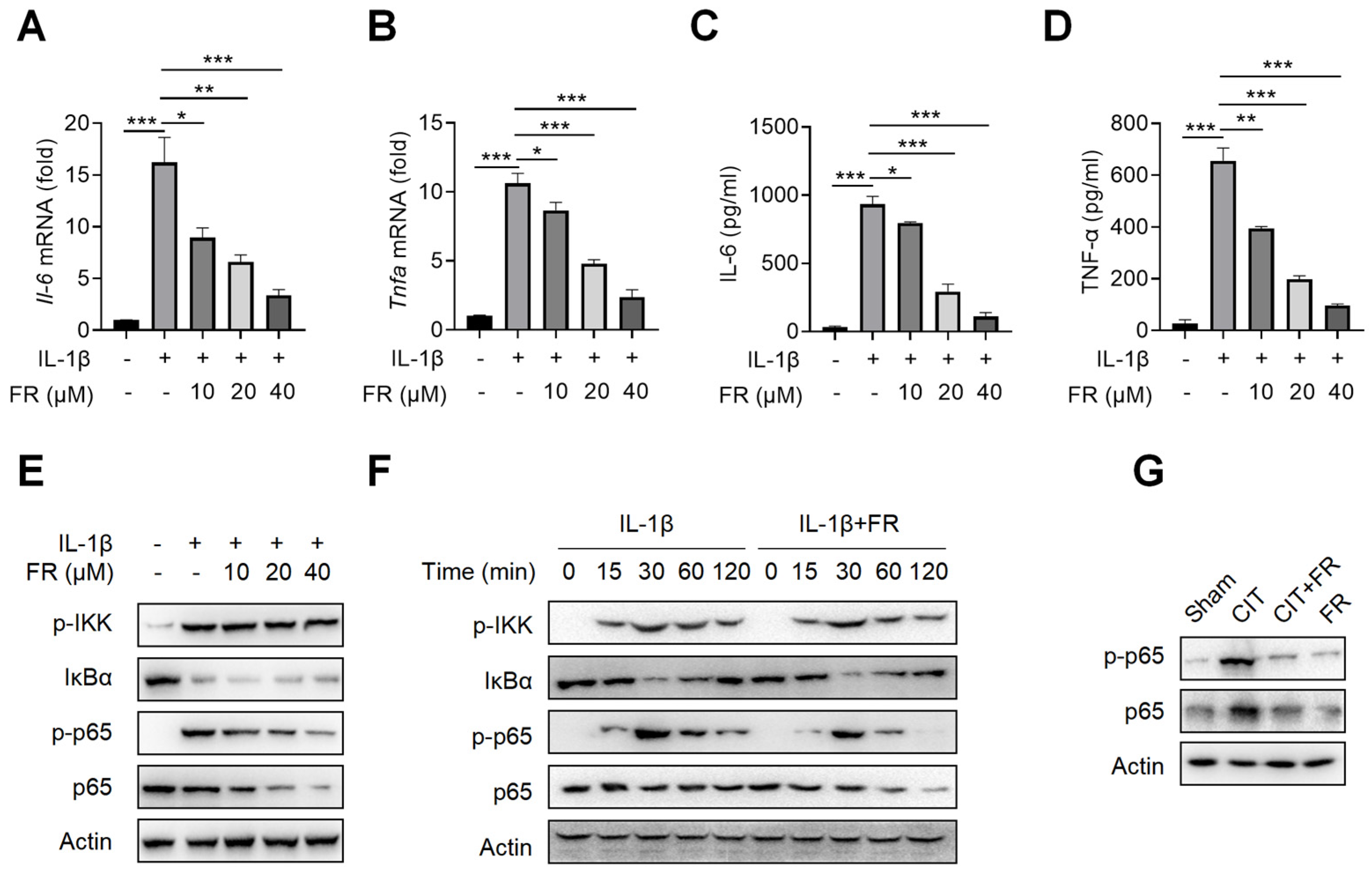
3.3. FR Targets p65 to Regulate NF-κB Signaling to Inhibit the Inflammatory Response in Tenocytes
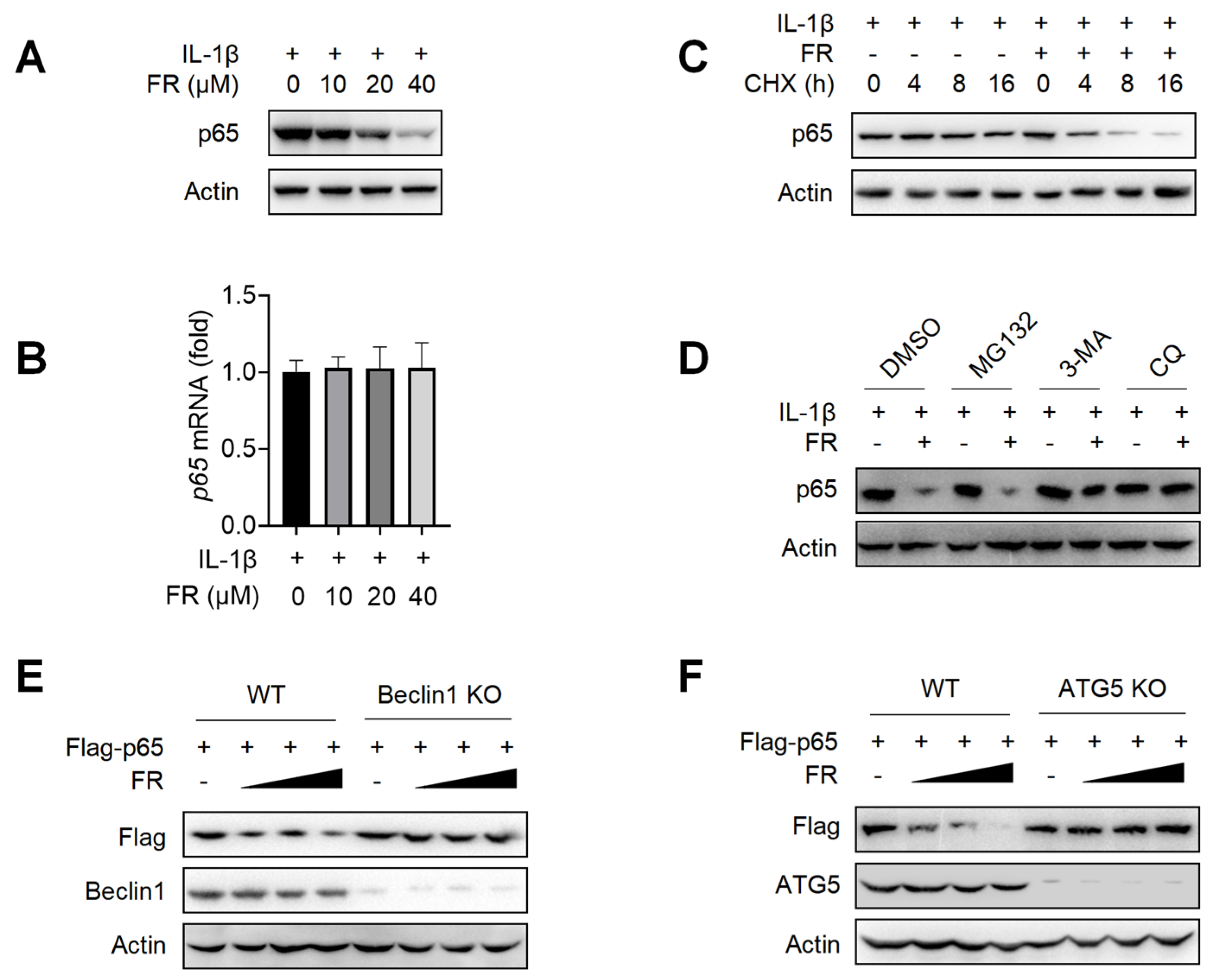
3.4. FR Promotes the Degradation of p65 through the Autophagy-Lysosome Pathway
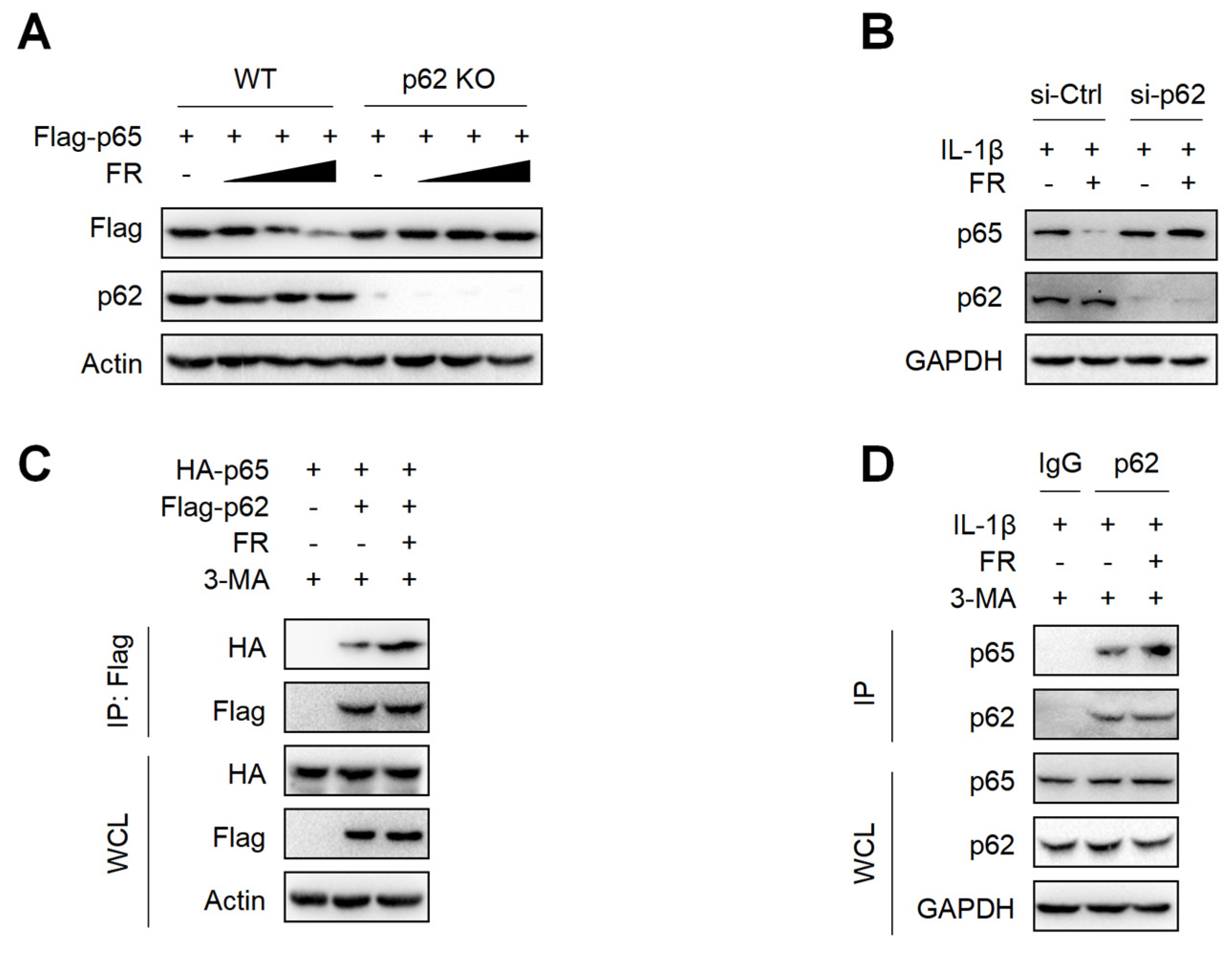
3.5. FR Mediates Selective Autophagic Degradation of p65 via p62-Dependent Pathway
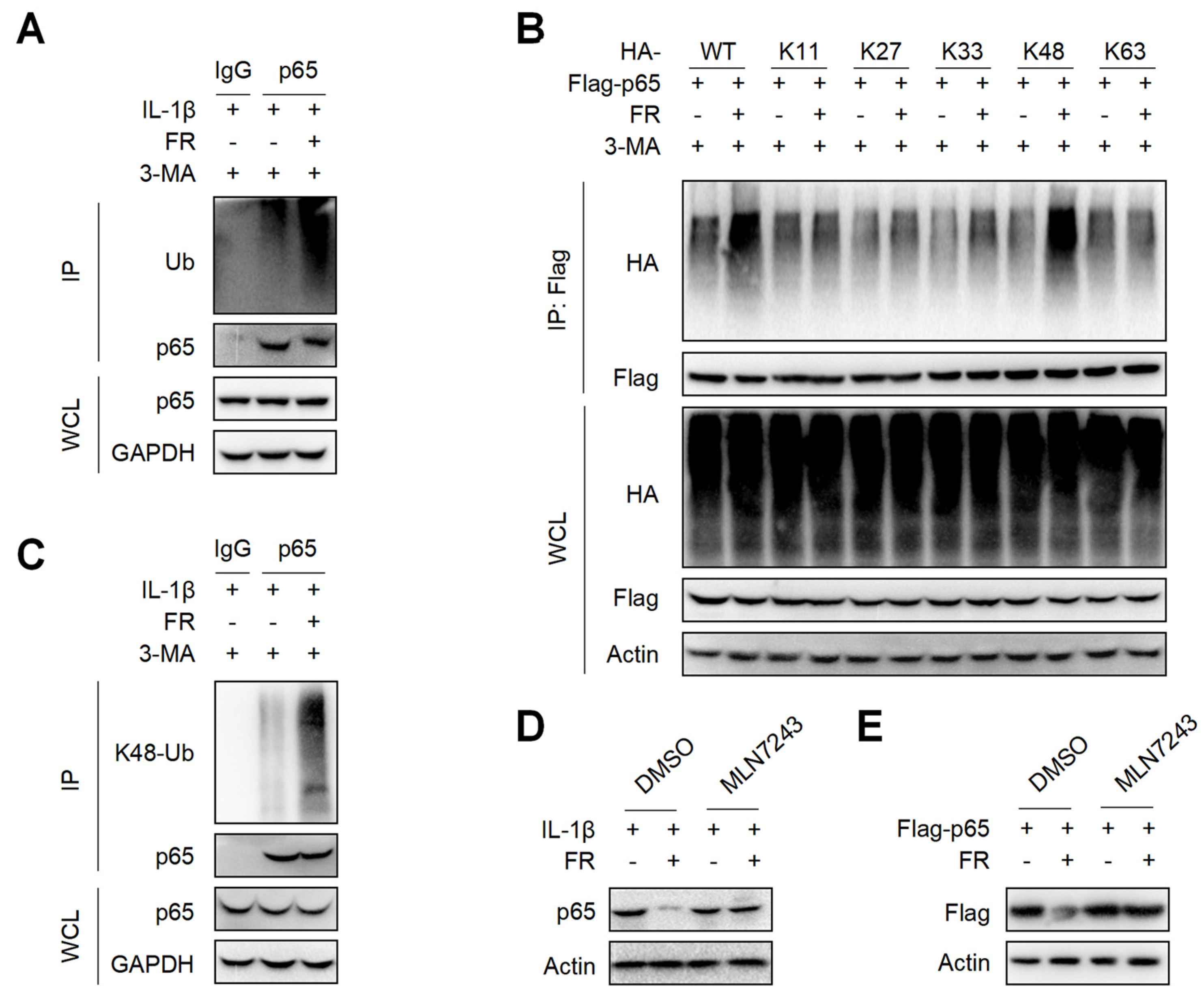
3.6. FR Increases the K48-Linked Ubiquitination of p65 to Promote Its Autophagic Degradation
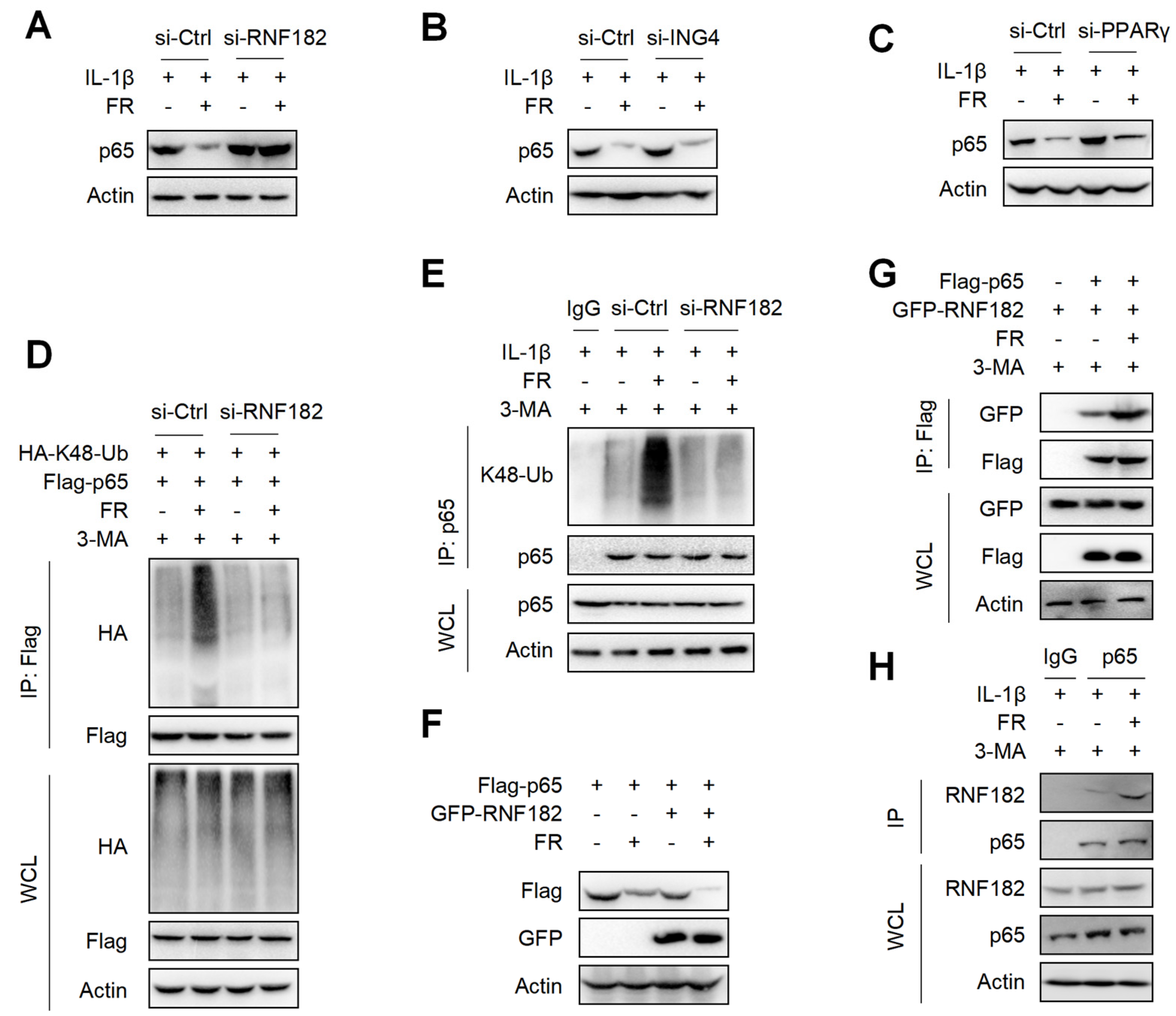
3.7. FR Mediates K48-Linked Ubiquitination of p65 via E3 Ubiquitination Enzyme RNF182
4. Discussion
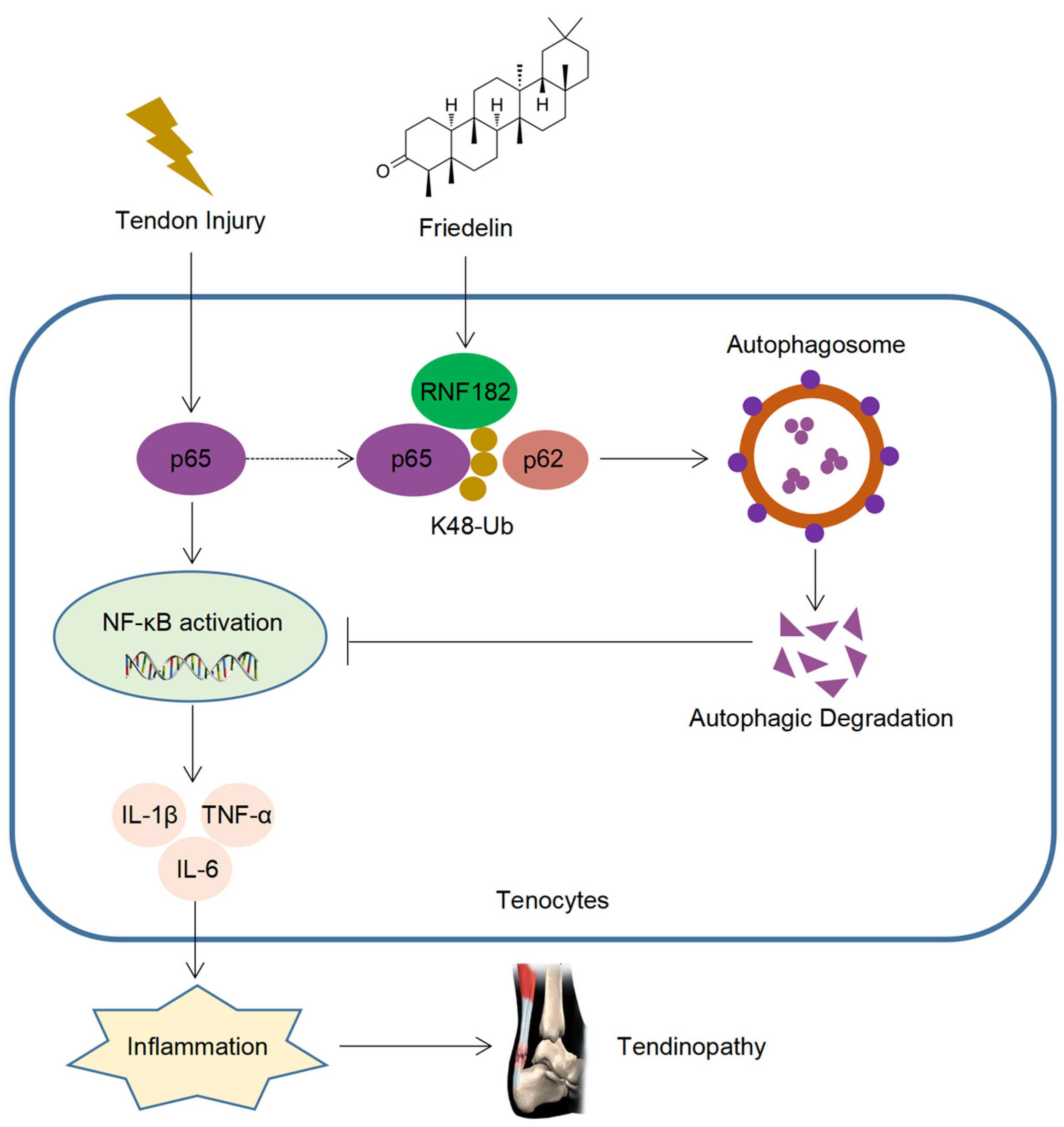
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riley, G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology 2004, 43, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, W.; Snelling, S.J.; Dakin, S.G.; Carr, A.J. Profibrotic mediators in tendon disease: A systematic review. Arthritis Res. Ther. 2016, 18, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, R.; Smith, M.; Clarke, E.; Little, C. Cellular, matrix, and mechano-biological differences in load-bearing versus positional tendons throughout development and aging: A narrative review. Connect. Tissue Res. 2018, 59, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.K.W.; Birch, H.L.; Goodman, S.; Heinegard, D.; Goodship, A.E. The influence of ageing and exercise on tendon growth and degeneration—hypotheses for the initiation and prevention of straininduced tendinopathies. Comp. Biochem. Physiol. 2002, 133, 1039–1350. [Google Scholar] [CrossRef]
- Birch, H.L.; Peffers, M.J.; Clegg, P.D. Influence of Ageing on Tendon Homeostasis. Adv. Exp. Med. Biol. 2016, 920, 247–260. [Google Scholar] [CrossRef]
- Byl, N.; Wilson, F.; Merzenich, M.; Melnick, M.; Scott, P.; Oakes, A.; McKenzie, A. Sensory Dysfunction Associated With Repetitive Strain Injuries of Tendinitis and Focal Hand Dystonia: A Comparative Study. J. Orthop. Sports Phys. Ther. 1996, 23, 234–244. [Google Scholar] [CrossRef]
- Gupta, A.K.; Chalmers, P.N.; Klosterman, E.L.; Harris, J.D.; Bach, B.R., Jr.; Verma, N.N.; Cole, B.J.; Romeo, A.A. Subpectoral biceps tenodesis for bicipital tendonitis with SLAP tear. Orthopedics 2015, 38, e48–e53. [Google Scholar] [CrossRef] [Green Version]
- Dressler, M.R.; Butler, D.L.; Boivin, G.P. Age-related changes in the biomechanics of healing patellar tendon. J. Biomech. 2006, 39, 2205–2212. [Google Scholar] [CrossRef]
- Gumina, S.; Carbone, S.; Campagna, V.; Candela, V.; Sacchetti, F.M.; Giannicola, G. The impact of aging on rotator cuff tear size. Musculoskelet. Surg. 2013, 97 (Suppl. 1), 69–72. [Google Scholar] [CrossRef]
- Riley, G. Tendinopathy--from basic science to treatment. Nat. Clin. Pract. Rheumatol. 2008, 4, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Jarvinen, T.A.; Kannus, P.; Paavola, M.; Jarvinen, T.L.; Jozsa, L.; Jarvinen, M. Achilles tendon injuries. Curr. Opin. Rheumatol. 2001, 13, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.F.; Olewinski, L.H.; Tamminga, K.S. Management of Chronic Tendon Injuries. Am. Fam. Physician. 2019, 100, 147–157. [Google Scholar] [PubMed]
- Jomaa, G.; Kwan, C.K.; Fu, S.C.; Ling, S.K.; Chan, K.M.; Yung, P.S.; Rolf, C. A systematic review of inflammatory cells and markers in human tendinopathy. BMC Musculoskelet. Disord. 2020, 21, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silbernagel, K.G.; Hanlon, S.; Sprague, A. Current Clinical Concepts: Conservative Management of Achilles Tendinopathy. J. Athl. Train. 2020, 55, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.C.M.; Carballar, C.B.; Villafane, J.H.; Perez, S.M.; Perez, J.L.A.; Diaz-Meco, R.; Jimenez, D.G.; Romero, E.A.S. A new ultrasound-guided percutaneous electrolysis and exercise treatment in patellar tendinopathy: Three case reports. Front. Biosci. 2021, 26, 1166–1175. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Sharma, P.; Maffulli, N. Biology of tendon injury: Healing, modeling and remodeling. J. Musculoskelet. Neuronal. Interact. 2006, 6, 181–190. [Google Scholar]
- Li, H.Y.; Hua, Y.H. Achilles Tendinopathy: Current Concepts about the Basic Science and Clinical Treatments. Biomed. Res. Int. 2016, 2016, 6492597. [Google Scholar] [CrossRef] [Green Version]
- Leong, H.T.; Fu, S.C.; He, X.; Oh, J.H.; Yamamoto, N.; Hang, S. Risk factors for rotator cuff tendinopathy: A systematic review and meta-analysis. J. Rehabil. Med. 2019, 51, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Sanchez Romero, E.A.; Melendez Oliva, E.; Alonso Perez, J.L.; Martin Perez, S.; Turroni, S.; Marchese, L.; Villafane, J.H. Relationship between the Gut Microbiome and Osteoarthritis Pain: Review of the Literature. Nutrients 2021, 13, 716. [Google Scholar] [CrossRef]
- Ticinesi, A.; Nouvenne, A.; Cerundolo, N.; Catania, P.; Prati, B.; Tana, C.; Meschi, T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients 2019, 11, 1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerman, J.E.; Best, K.T.; Muscat, S.N.; Loiselle, A.E. Metabolic Regulation of Tendon Inflammation and Healing Following Injury. Curr. Rheumatol. Rep. 2021, 23, 15. [Google Scholar] [CrossRef] [PubMed]
- Arvind, V.; Huang, A.H. Reparative and Maladaptive Inflammation in Tendon Healing. Front. Bioeng. Biotechnol. 2021, 9, 719047. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhou, Z.; Song, W.; Cai, Z.; Ding, Z.; Chen, D.; Xia, F.; He, Y. Inhibition of IKKbeta/NF-kappaB signaling facilitates tendinopathy healing by rejuvenating inflamm-aging induced tendon-derived stem/progenitor cell senescence. Mol. Ther. Nucleic Acids 2022, 27, 562–576. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Tang, H.; Shi, Y.; Kang, X.; Lyu, J.; Zhu, M.; Zhou, M.; Yang, M.; Mu, M.; et al. Aspirin inhibits inflammation and scar formation in the injury tendon healing through regulating JNK/STAT-3 signalling pathway. Cell Prolif. 2019, 52, e12650. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Abraham, A.C.; Shah, S.A.; Golman, M.; Song, L.; Li, X.; Kurtaliaj, I.; Akbar, M.; Millar, N.L.; Abu-Amer, Y.; Galatz, L.M.; et al. Targeting the NF-kB signaling pathway in chronic tendon disease. Sci. Transl. Med. 2019, 11, eaav4319. [Google Scholar] [CrossRef]
- Shi, B.; Liu, S.; Huang, A.; Zhou, M.; Sun, B.; Cao, H.; Shan, J.; Sun, B.; Lin, J. Revealing the Mechanism of Friedelin in the Treatment of Ulcerative Colitis Based on Network Pharmacology and Experimental Verification. Evid. Based Complement. Altern. Med. 2021, 2021, 4451779. [Google Scholar] [CrossRef]
- Han, J.Y.; Ahn, C.H.; Adhikari, P.B.; Kondeti, S.; Choi, Y.E. Functional characterization of an oxidosqualene cyclase (PdFRS) encoding a monofunctional friedelin synthase in Populus davidiana. Planta 2019, 249, 95–111. [Google Scholar] [CrossRef]
- Antonisamy, P.; Duraipandiyan, V.; Ignacimuthu, S. Anti-inflammatory, analgesic and antipyretic effects of friedelin isolated from Azima tetracantha Lam. in mouse and rat models. J. Pharm. Pharmacol. 2011, 63, 1070–1077. [Google Scholar] [CrossRef]
- Sunil, C.; Duraipandiyan, V.; Ignacimuthu, S.; Al-Dhabi, N.A. Antioxidant, free radical scavenging and liver protective effects of friedelin isolated from Azima tetracantha Lam. leaves. Food Chem. 2013, 139, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Subash-Babu, P.; Li, D.K.; Alshatwi, A.A. In vitro cytotoxic potential of friedelin in human MCF-7 breast cancer cell: Regulate early expression of Cdkn2a and pRb1, neutralize mdm2-p53 amalgamation and functional stabilization of p53. Exp. Toxicol. Pathol. 2017, 69, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Mokoka, T.A.; McGaw, L.J.; Mdee, L.K.; Bagla, V.P.; Iwalewa, E.O.; Eloff, J.N. Antimicrobial activity and cytotoxicity of triterpenes isolated from leaves of Maytenus undata (Celastraceae). BMC Complement. Altern. Med. 2013, 13, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonisamy, P.; Duraipandiyan, V.; Aravinthan, A.; Al-Dhabi, N.A.; Ignacimuthu, S.; Choi, K.C.; Kim, J.H. Protective effects of friedelin isolated from Azima tetracantha Lam. against ethanol-induced gastric ulcer in rats and possible underlying mechanisms. Eur. J. Pharmacol. 2015, 750, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kokubu, S.; Inaki, R.; Hoshi, K.; Hikita, A. Adipose-derived stem cells improve tendon repair and prevent ectopic ossification in tendinopathy by inhibiting inflammation and inducing neovascularization in the early stage of tendon healing. Regen. Ther. 2020, 14, 103–110. [Google Scholar] [CrossRef]
- Lin, X.; Huang, M.; Yin, G.; Zhang, J.; Zhang, Z.; Lai, P.; Yan, B.; Chen, Y.; Jin, D.; Wang, L. Characterization of a Novel Calcific Achilles Tendinopathy Model in Mice: Contralateral Tendinopathy Induced by Unilateral Tenotomy. Calcif. Tissue Int. 2018, 103, 698–707. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Tang, H.; Shi, Y.; Zhu, M.; Kang, X.; Bian, X.; Lyu, J.; Zhou, M.; Yang, M.; et al. Aspirin promotes tenogenic differentiation of tendon stem cells and facilitates tendinopathy healing through regulating the GDF7/Smad1/5 signaling pathway. J. Cell Physiol. 2020, 235, 4778–4789. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Wu, Y.T.; Huang, T.C.; Su, F.C.; Jou, I.M.; Wu, C.C. Sequential inflammation model for Achilles tendinopathy by elastin degradation with treadmill exercise. J. Orthop. Translat. 2020, 23, 113–121. [Google Scholar] [CrossRef]
- Dakin, S.G.; Newton, J.; Martinez, F.O.; Hedley, R.; Gwilym, S.; Jones, N.; Reid, H.A.B.; Wood, S.; Wells, G.; Appleton, L.; et al. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br. J. Sports Med. 2018, 52, 359–367. [Google Scholar] [CrossRef]
- Sunwoo, J.Y.; Eliasberg, C.D.; Carballo, C.B.; Rodeo, S.A. The role of the macrophage in tendinopathy and tendon healing. J. Orthop. Res. 2020, 38, 1666–1675. [Google Scholar] [CrossRef]
- September, A.V.; Nell, E.M.; O’Connell, K.; Cook, J.; Handley, C.J.; van der Merwe, L.; Schwellnus, M.; Collins, M. A pathway-based approach investigating the genes encoding interleukin-1beta, interleukin-6 and the interleukin-1 receptor antagonist provides new insight into the genetic susceptibility of Achilles tendinopathy. Br. J. Sports Med. 2011, 45, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Tang, Q.; Liu, K.; Xie, W.; Liu, X.; Wang, H.; Wang, R.F.; Cui, J. TRIM11 Suppresses AIM2 Inflammasome by Degrading AIM2 via p62-Dependent Selective Autophagy. Cell Rep. 2016, 16, 1988–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikic, I. Proteasomal and Autophagic Degradation Systems. Annu. Rev. Biochem. 2017, 86, 193–224. [Google Scholar] [CrossRef] [PubMed]
- Buetow, L.; Huang, D.T. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2016, 17, 626–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Sun, Y.; Chang, H.; Sun, X.; Yang, S. The E3 ubiquitin ligase RNF182 inhibits TLR-triggered cytokine production through promoting p65 ubiquitination and degradation. FEBS Lett. 2019, 593, 3210–3219. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, Z.; Xu, Q.; Wang, H.; Xu, Y.; Chen, K. Inhibitor of growth 4 induces NFkappaB/p65 ubiquitin-dependent degradation. Oncogene 2014, 33, 1997–2003. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Moreau, F.; Chadee, K. PPARgamma is an E3 ligase that induces the degradation of NFkappaB/p65. Nat. Commun. 2012, 3, 1300. [Google Scholar] [CrossRef] [Green Version]
- Toledo, C.R.; Pereira, V.V.; Duarte, L.P.; Sousa, G.F.; Silva-Cunha, A. Anti-angiogenic activity and safety of intraocular application of triterpenes. Doc. Ophthalmol. 2021, 143, 259–270. [Google Scholar] [CrossRef]
- Sunil, C.; Irudayaraj, S.S.; Duraipandiyan, V.; Alrashood, S.T.; Alharbi, S.A.; Ignacimuthu, S. Friedelin exhibits antidiabetic effect in diabetic rats via modulation of glucose metabolism in liver and muscle. J. Ethnopharmacol. 2021, 268, 113659. [Google Scholar] [CrossRef]
- Millar, N.L.; Akbar, M.; Campbell, A.L.; Reilly, J.H.; Kerr, S.C.; McLean, M.; Frleta-Gilchrist, M.; Fazzi, U.G.; Leach, W.J.; Rooney, B.P.; et al. IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci. Rep. 2016, 6, 27149. [Google Scholar] [CrossRef]
- Chisari, E.; Rehak, L.; Khan, W.S.; Maffulli, N. Tendon healing in presence of chronic low-level inflammation: A systematic review. Br. Med. Bull. 2019, 132, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Gravanis, M.B.; Gaffney, E.F. Idiopathic calcifying tenosynovitis. Histopathologic features and possible pathogenesis. Am. J. Surg. Pathol. 1983, 7, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Hori, N.; Nakamoto, N.; Akita, M.; Yoda, T. Masticatory muscle tendon-aponeurosis hyperplasia exhibits heterotopic calcification in tendons. Oral. Dis. 2014, 20, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Dakin, S.G.; Martinez, F.O.; Yapp, C.; Wells, G.; Oppermann, U.; Dean, B.J.F.; Smith, R.D.J.; Wheway, K.; Watkins, B.; Roche, L.; et al. Inflammation activation and resolution in human tendon disease. Sci. Transl. Med. 2015, 7, 311ra173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moqbel, S.A.A.; Xu, K.; Chen, Z.; Xu, L.; He, Y.; Wu, Z.; Ma, C.; Ran, J.; Wu, L.; Xiong, Y. Tectorigenin Alleviates Inflammation, Apoptosis, and Ossification in Rat Tendon-Derived Stem Cells via Modulating NF-Kappa B and MAPK Pathways. Front. Cell Dev. Biol. 2020, 8, 568894. [Google Scholar] [CrossRef] [PubMed]
- Shaid, S.; Brandts, C.H.; Serve, H.; Dikic, I. Ubiquitination and selective autophagy. Cell Death Differ. 2013, 20, 21–30. [Google Scholar] [CrossRef]
- Behl, T.; Chadha, S.; Sachdeva, M.; Kumar, A.; Hafeez, A.; Mehta, V.; Bungau, S. Ubiquitination in rheumatoid arthritis. Life Sci. 2020, 261, 118459. [Google Scholar] [CrossRef]
- Cockram, P.E.; Kist, M.; Prakash, S.; Chen, S.H.; Wertz, I.E.; Vucic, D. Ubiquitination in the regulation of inflammatory cell death and cancer. Cell Death Differ. 2021, 28, 591–605. [Google Scholar] [CrossRef]
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-F4/80 | ABclonal | A18637 |
| Anti-IL-6 | Cell Signaling Technology | #12912 |
| Anti-p-IKK | Cell Signaling Technology | #2697 |
| Anti-IκBa | Cell Signaling Technology | #4814 |
| Anti-p-p65 | Cell Signaling Technology | #3039 |
| Anti-p65 | Santa Cruz Biotechnology | sc-8008 |
| Anti-Actin | ABclonal | AC026 |
| Anti-Flag agarose gels | Sigma-Aldrich | A2220 |
| Horseradish peroxidase (HRP)-anti-Flag (M2) | Sigma-Aldrich | A8592 |
| Anti-HA | Roche Applied Science | 12013819001 |
| Anti-Beclin1 | Cell Signaling Technology | #3738 |
| Anti-ATG5 | Cell Signaling Technology | #12994 |
| Anti-p62 | Santa Cruz Biotechnology | sc-48402 |
| Anti-Ub | Santa Cruz Biotechnology | sc-8017 |
| Anti-K48 Ub | ABclonal | A3606 |
| Anti-RNF182 | Novus Biologicals | NBP1-82707 |
| Anti-ING4 | ABclonal | A5833 |
| Anti-PPARγ | ABclonal | A11183 |
| Goat anti-rabbit IgG (H&L) | Beijing Ray Antibody Biotech | RM3002 |
| Goat anti-mouse IgG (H&L) | Beijing Ray Antibody Biotech | RM3001 |
| Goat anti-rabbit IgG (H&L) Alexa Fluor 488 | Immunoway | RS23220 |
| Goat anti-rabbit IgG (H&L) Alexa Fluor 594 | Immunoway | RS23420 |
| Anti-IL-1β (ELISA) | Elabscience Biotechnology | #E-EL-M0037c |
| Anti-IL-6 (ELISA) | Elabscience Biotechnology | #E-EL-M0044c |
| Anti-TNF-α (ELISA) | Elabscience Biotechnology | #E-EL-M1084c |
| Reagents | ||
| Friedelin | MedChemExpress | HY-N4110 |
| LPS | Sigma-Aldrich | L2630 |
| Recombinant IL-1β | Abcam | ab9723 |
| Recombinant TNFα | Biovision | 1051-1000 |
| MG-132 | Sigma-Aldrich | C-2211 |
| Chloroquine | Glpbio | 1954/5/7 |
| Cycloheximide | Sigma-Aldrich | C7698 |
| 3-methyladenine | Sigma-Aldrich | 5142-23-4 |
| MLN7243 | Sigma-Aldrich | HY-100487 |
| DMSO | Sigma-Aldrich | D2650 |
| Paraformaldehyde | Sigma-Aldrich | P6148 |
| Lipofectamine 2000 | ThermoFisher | 11668019 |
| Protein G beads | GenScript | L00209 |
| Type I collagenase | Wako | 031-17601 |
| Hematoxylin and eosin solution | Beyotime | C0105S |
| Masson's trichrome kit | Solarbio | G1340 |
| TRIZOL | Beyotime | R0016 |
| Fetal bovine serum | Sigma-Aldrich | 12103C |
| Penicillin/streptomycin | Gibco | 15140122 |
| Amphotericin B | Gibco | 15290026 |
| Trypsinization | Gibco | R001100 |
| Cell Counting Kit-8 | Beyotime | C0037 |
| Lipofectamine 2000 | Invitrogen | 11668030 |
| Lipofectamine RNAiMAX | Invitrogen | 13778150 |
| Enhanced chemiluminescence kit | Cell Signaling Technology | #12630 |
| Gene | Sequences | Species |
|---|---|---|
| Dcn | Forward: AGACTCACAGCCGAGTAGGA Reverse: ACATTCGCATCTCAGACACC | Mouse |
| Scx | Forward: CCTTCTGCCTCAGCAACCAG Reverse: GGTCCAAAGTGGGGCTCTCCGTGACT | Mouse |
| Mkx | Forward: CCCCGGACATCGGATCTACTA Reverse: CTCTTAGGATGAGGATTTAGGTA | Mouse |
| Tnmd | Forward: GGGTGGTCCCGCAAGTGAAGGTG Reverse: GCCTCGACGACAGTAAATACAACAGT | Mouse |
| Il-1b | Forward: GCAACTGTTCCTGAACTCAACT Reverse: GTGCTCATGTCCTCATCCTG | Mouse |
| Il-6 | Forward: CTCTGGGAAATCGTGGAAAT Reverse: CCAGTTTGGTAGCATCCATC | Mouse |
| Tnfa | Forward: GACGTGGAACTGGCAGAAGAG Reverse: TTGGTGGTTTGTGAGTGTGAG | Mouse |
| Gapdh | Forward: AGGTCGGTGTGAACGGATTTG Reverse: TGTAGACCATGTAGTTGAGGTCA | Mouse |
| Gene | Sequences | Species |
|---|---|---|
| p62 siRNA | 5’-GCUGAAACAUGGACACUUUTT-3’ 3’-AAAGUGUCCAUGUUUCAGCTT-5’ | Mouse |
| RNF182 siRNA | 5’-GCGCCAAAUGCCUCUACAATT-3’ 3’-UUGUAGAGGCAUUUGGCGCTT-5’ | Mouse |
| RNF182 siRNA | 5’-GACAACAACAUCCUUGUAATT-3’ 3’-UUACAAGGAUGUUGUUGUCTT-5’ | Human |
| ING4 siRNA | 5’-GAUCCCAACGAACCCACAUTT-3’ 3’-AUGUGGGUUCGUUGGGAUCTT-5’ | Mouse |
| PPARγ siRNA | 5’-GCAAGAGAUCACAGAGUAUTT-3’ 3’-AUACUCUGUGAUCUCUUGCTT-5’ | Mouse |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Lin, X.; Liang, W.; Li, Y.; Yu, X. Friedelin Alleviates the Pathogenesis of Collagenase-Induced Tendinopathy in Mice by Promoting the Selective Autophagic Degradation of p65. Nutrients 2022, 14, 1673. https://doi.org/10.3390/nu14081673
Jiang H, Lin X, Liang W, Li Y, Yu X. Friedelin Alleviates the Pathogenesis of Collagenase-Induced Tendinopathy in Mice by Promoting the Selective Autophagic Degradation of p65. Nutrients. 2022; 14(8):1673. https://doi.org/10.3390/nu14081673
Chicago/Turabian StyleJiang, Huaji, Xuemei Lin, Wei Liang, Yiqiang Li, and Xiao Yu. 2022. "Friedelin Alleviates the Pathogenesis of Collagenase-Induced Tendinopathy in Mice by Promoting the Selective Autophagic Degradation of p65" Nutrients 14, no. 8: 1673. https://doi.org/10.3390/nu14081673
APA StyleJiang, H., Lin, X., Liang, W., Li, Y., & Yu, X. (2022). Friedelin Alleviates the Pathogenesis of Collagenase-Induced Tendinopathy in Mice by Promoting the Selective Autophagic Degradation of p65. Nutrients, 14(8), 1673. https://doi.org/10.3390/nu14081673






