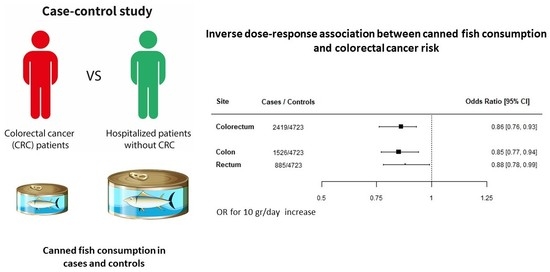
Inverse Association between Canned Fish Consumption and Colorectal Cancer Risk: Analysis of Two Large Case–Control Studies
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Data Collection
2.3. Statistical Analysis
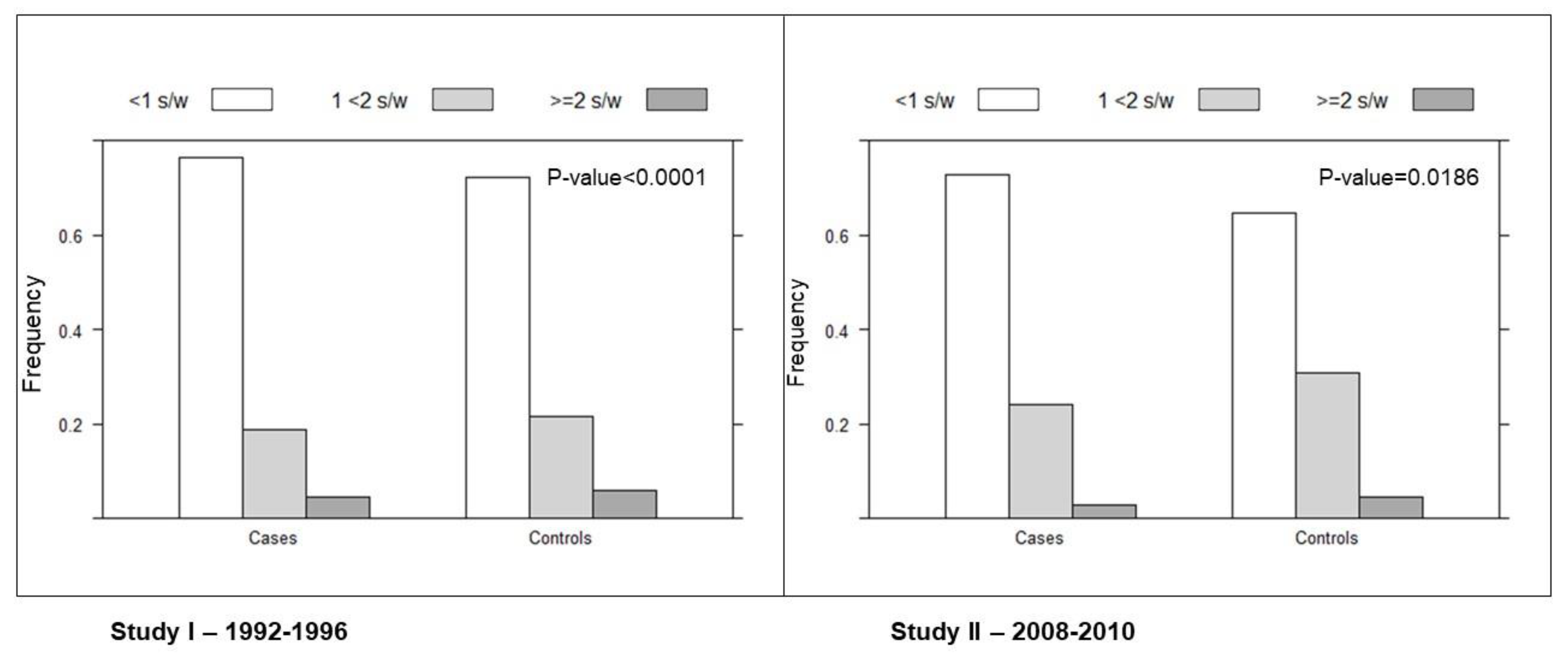
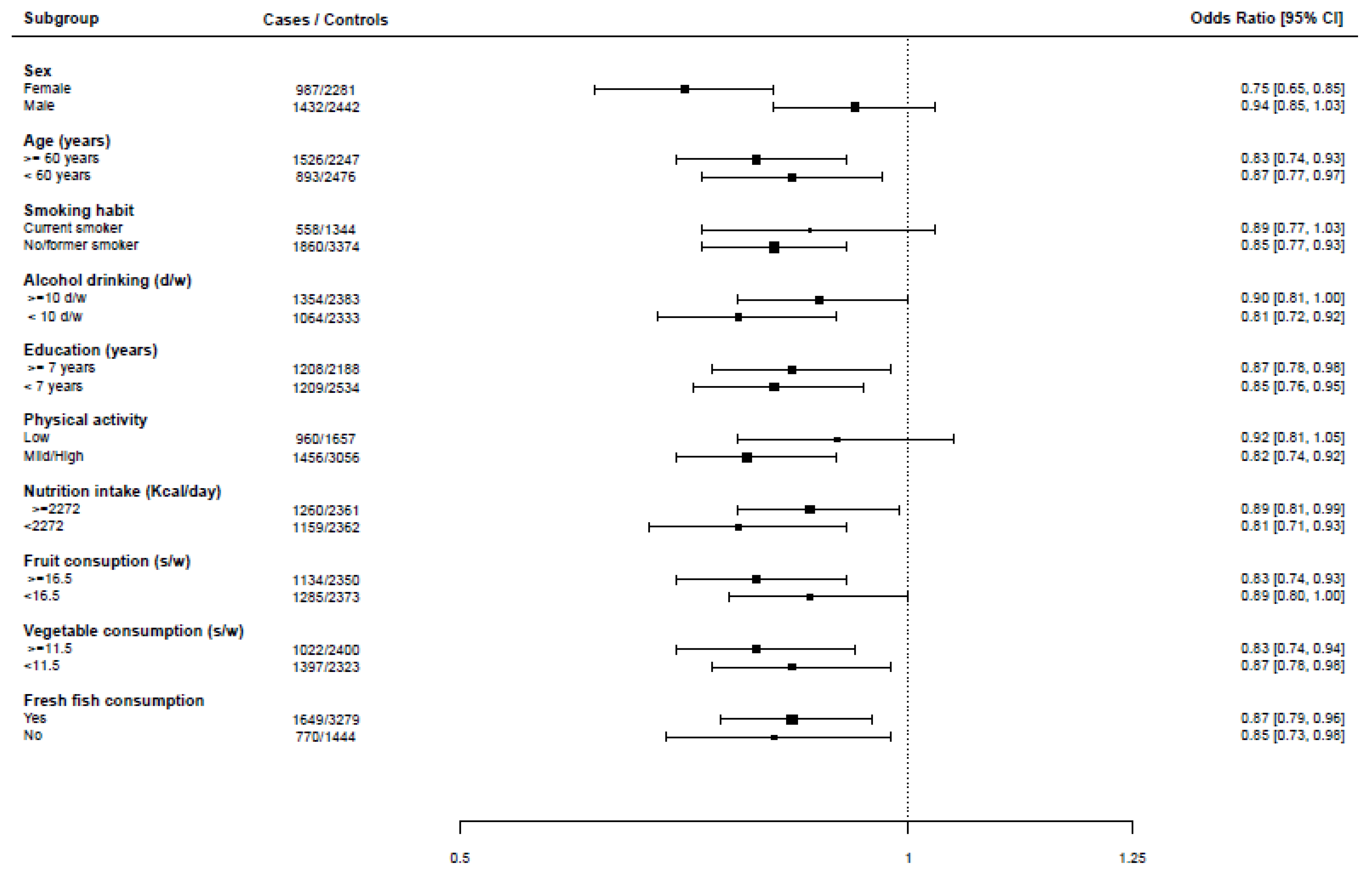
3. Results
4. Discussion
5. Limitations and Strengths
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kohler, L.N.; Garcia, D.O.; Harris, R.B.; Oren, E.; Roe, D.J.; Jacobs, E.T. Adherence to Diet and Physical Activity Cancer Prevention Guidelines and Cancer Outcomes: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1018–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez, E.; Chatenoud, L.; La Vecchia, C.; Negri, E.; Franceschi, S. Fish consumption and cancer risk. Am. J. Clin. Nutr. 1999, 70, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.F.; Zou, J.; Dong, J. Fish consumption and risk of gastrointestinal cancers: A meta-analysis of cohort studies. World J. Gastroenterol. 2014, 20, 15398–15412. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Laure Preterre, A.; Iqbal, K.; Bechthold, A.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; et al. Food groups and risk of colorectal cancer. Int. J. Cancer 2018, 142, 1748–1758. [Google Scholar] [CrossRef] [Green Version]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of Dietary n-3 and n-6 Polyunsaturated Fatty Acids in Inflammation and Cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- Tavani, A.; Franceschi, S.; Levi, F.; La Vecchia, C. Fish, omega-3 polyunsaturated fat intake and cancer at selected sites. World Rev. Nutr. Diet 2005, 94, 166–175. [Google Scholar] [CrossRef]
- Willett, W.C. Specific fatty acids and risks of breast and prostate cancer: Dietary intake. Am. J. Clin. Nutr. 1997, 66 (Suppl. 6), 1557S–1563S. [Google Scholar] [CrossRef] [Green Version]
- de Deckere, E.A. Possible beneficial effect of fish and fish n-3 polyunsaturated fatty acids in breast and colorectal cancer. Eur. J. Cancer Prev. 1999, 8, 213–221. [Google Scholar] [CrossRef]
- A Complete Course in Canning and Related Processes, 14th ed.; In Processing Procedures for Canned Food Products, Technology and Nutrition; Woodhead Publishing Series in Food Science: Cambridge, UK, 2016; Volume 3, pp. 423–444.
- CBI.EU—The European Market Potential for Canned Fish—Visited in November 2021. Available online: https://www.cbi.eu/market-information/fish-seafood/canned-fish/market-potential (accessed on 28 February 2022).
- Franceschi, S.; Favero, A.; La Vecchia, C.; Negri, E.; Conti, E.; Montella, M.; Giacosa, A.; Nanni, O.; Decarli, A. Food groups and risk of colorectal cancer in Italy. Int. J. Cancer 1997, 72, 56–61. [Google Scholar] [CrossRef]
- Rosato, V.; Tavani, A.; Gracia-Lavedan, E.; Guinó, E.; Castaño-Vinyals, G.; Villanueva, C.M.; Kogevinas, M.; Polesel, J.; Serraino, D.; Pisa, F.E.; et al. Type 2 Diabetes, Antidiabetic Medications, and Colorectal Cancer Risk: Two Case-Control Studies from Italy and Spain. Front. Oncol. 2016, 6, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decarli, A.; Franceschi, S.; Ferraroni, M.; Gnagnarella, P.; Parpinel, M.T.; La Vecchia, C.; Negri, E.; Salvini, S.; Falcini, F.; Giacosa, A. Validation of a food-frequency questionnaire to assess dietary intakes in cancer studies in Italy. Results for specific nutrients. Ann. Epidemiol. 1996, 6, 110–118. [Google Scholar] [CrossRef]
- Franceschi, S.; Negri, E.; Salvini, S.; Decarli, A.; Ferraroni, M.; Filiberti, R.; Giacosa, A.; Talamini, R.; Nanni, O.; Panarello, G.; et al. Reproducibility of an Italian food frequency questionnaire for cancer studies: Results for specific food items. Eur. J. Cancer 1993, 29A, 2298–2305. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Parpinel, M.; Salvini, S.; Franceschi, S.; Palli, D.; Boyle, P. The update of the Italian food composition database. J. Food Comp. Anal. 2004, 17, 509–522. [Google Scholar] [CrossRef]
- Breslow, N.E.; Day, N.E. Statistical Methods in Cancer Research. The Analysis of Case-Control Studies. In IARC Scientific Publications 32; International Agency for Research on Cancer: Lyon, France, 1980; Volume 1. [Google Scholar]
- Xu, M.; Fang, Y.-J.; Chen, Y.-M.; Lu, M.-S.; Pan, Z.-Z.; Yan, B.; Zhong, X.; Zhang, C.-X. Higher freshwater fish and sea fish intake is inversely associated with colorectal cancer risk among Chinese population: A case-control study. Sci. Rep. 2015, 5, 12976. [Google Scholar] [CrossRef] [Green Version]
- Aglago, E.K.; Huybrechts, I.; Murphy, N.; Casagrande, C.; Nicolas, G.; Pischon, T.; Fedirko, V.; Severi, G.; Boutron-Ruault, M.-C.; Fournier, A.; et al. Consumption of Fish and Long-chain n-3 Polyunsaturated Fatty Acids Is Associated with Reduced Risk of Colorectal Cancer in a Large European Cohort. Clin. Gastroenterol. Hepatol. 2020, 18, 654–666.e6. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Fuchs, C.S.; Ogino, S.; Hu, F.B.; Mozaffarian, D.; Ma, J.; Willett, W.C.; Giovannucci, E.L.; Wu, K. Dietary intake of fish, ω-3 and ω-6 fatty acids and risk of colorectal cancer: A prospective study in US men and women. Int. J. Cancer 2014, 135, 2413–2423. [Google Scholar] [CrossRef]
- Wu, S.; Feng, B.; Li, K.; Zhu, X.; Liang, S.; Liu, X.; Han, S.; Wang, B.; Wu, K.; Miao, D.; et al. Fish consumption and colorectal cancer risk in humans: A systematic review and meta-analysis. Am. J. Med. 2012, 125, 551–559.e5. [Google Scholar] [CrossRef]
- Larsson, S.C.; Kumlin, M.; Ingelman-Sundberg, M.; Wolk, A. Dietary long-chain n-3 fatty acids for the prevention of cancer: A review of potential mechanisms. Am. J. Clin. Nutr. 2004, 79, 935–945. [Google Scholar] [CrossRef]
- Narayanan, B.A.; Narayanan, N.K.; Reddy, B.S. Docosahexaenoic acid regulated genes and transcription factors inducing apoptosis in human colon cancer cells. Int. J. Oncol. 2001, 19, 1255–1262. [Google Scholar] [CrossRef]
- Lindner, M.A. A fish oil diet inhibits colon cancer in mice. Nutr. Cancer 1991, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Prabhu, K.S.; Das, A.; Mastro, A.M. Dietary selenium supplementation modifies breast tumor growth and metastasis. Int. J. Cancer 2013, 133, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/1099041/nutrients (accessed on 13 April 2022).
- Borzì, A.M.; Biondi, A.; Basile, F.; Luca, S.; Vicari, E.S.D.; Vacante, M. Olive Oil Effects on Colorectal Cancer. Nutrients 2018, 11, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, A.; Cho, S.; Sandin, S.; Lof, M.; Oh, M.Y.; Weiderpass, E. Omega-3 and -6 Fatty Acid Intake and Colorectal Cancer Risk in Swedish Women’s Lifestyle and Health Cohort. Cancer Res. Treat. 2020, 52, 848–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- English, D.R.; MacInnis, R.J.; Hodge, A.M.; Hopper, J.L.; Haydon, A.M.; Giles, G.G. Red meat, chicken, and fish consumption and risk of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1509–1514. [Google Scholar]
- Scuri, S.; Tesauro, M.; Petrelli, F.; Peroni, A.; Kracmarova, L.; Grappasonni, I. Implications of modified food choices and food-related lifestyles following the economic crisis in the Marche Region of Italy. Ann. Ig. 2018, 30, 173–179. [Google Scholar] [CrossRef]
| Cases (N = 2419) | Controls (N = 4723) | Missing | p-Value | ||
|---|---|---|---|---|---|
| Centre | <0.0001 | ||||
| Pordenone | 856 (35.4) | 1606 (34.0) | |||
| Milan | 715 (29.5) | 1403 (29.7) | |||
| Genoa | 225 (9.3) | 498 (10.6) | |||
| Forlì | 94 (3.9) | 247 (5.2) | |||
| Naples | 193 (8.0) | 387 (8.2) | |||
| Rome/Latina | 336 (13.9) | 582 (12.3) | |||
| Sex | <0.0001 | ||||
| Males | 1432 (59.2) | 2442 (51.7) | |||
| Females | 987 (40.8) | 2281 (48.3) | |||
| Age (years) | <0.0001 | ||||
| <40 | 84 (3.5) | 359 (7.6) | |||
| 40–50 | 208 (8.6) | 771 (16.3) | |||
| 50–60 | 601 (24.8) | 1346 (28.5) | |||
| 60–70 | 1018 (42.1) | 1579 (33.5) | |||
| >70 | 508 (21.0) | 668 (14.1) | |||
| BMI (Kg/m2) | 33 | 0.9755 | |||
| <25 | 1076 (44.7) | 2113 (45.0) | |||
| 25–30 | 987 (41.0) | 1915 (40.7) | |||
| >30 | 345 (14.3) | 673 (14.3) | |||
| Education (years) | 3 | <0.0001 | |||
| <7 | 1209 (50.0) | 2534 (53.7) | |||
| 7–11 | 662 (27.4) | 1324 (28.0) | |||
| >12 | 546 (22.6) | 864 (18.3) | |||
| Family history | <0.0001 | ||||
| Yes | 244 (10.1) | 192 (4.1) | |||
| No | 2175 (89.9) | 4531 (95.9) | |||
| Occupational physical activity at age 30–39 | 13 | 0.0009 | |||
| Low | 960 (39.8) | 1657 (35.2) | |||
| Moderate | 806 (33.3) | 1698 (36.0) | |||
| Heavy | 650 (26.9) | 1358 (28.8) |
| Canned Fish Consumption | Model 1 | p-Value for Trend | Model 2 | p-Value for Trend | Model 3 | p-Value for Trend | Model 4 | p-Value for Trend |
|---|---|---|---|---|---|---|---|---|
| <1 serving/week | 1 | <0.0001 | 1 | <0.0001 | 1 | <0.0001 | 1 | <0.0001 |
| 1 < 2 serving/week | 0.80 (0.71–0.91) | 0.80 (0.71–0.91) | 0.80 (0.71–0.91) | 0.81 (0.71–0.92) | ||||
| ≥2 servings/week | 0.66 (0.51–0.84) | 0.67 (0.52–0.87) | 0.66 (0.51–0.85) | 0.66 (0.51–0.85) | ||||
| 10 gr/die | 0.87 (0.80–0.94) | 0.0003 | 0.87 (0.80–0.94) | 0.0005 | 0.86 (0.79–0.93) | 0.0002 | 0.86 (0.79–0.93) | 0.0002 |
| Type of Fish Consumption | Cases N (%) | Controls N (%) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| No fish | 617 (25.5) | 1092 (23.1) | 1 | |
| Only canned fish | 153 (6.3) | 352 (7.5) | 0.77 (0.62–0.97) | |
| Only non-canned fish | 1226 (50.7) | 2282 (48.3) | 0.88 (0.77–1.00) | |
| Both | 423 (17.5) | 997 (21.1) | 0.69 (0.58–0.81) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchi, C.; Ardoino, I.; Bosetti, C.; Negri, E.; Serraino, D.; Crispo, A.; Giacosa, A.; Fattore, E.; Dolci, A.; Bravi, F.; et al. Inverse Association between Canned Fish Consumption and Colorectal Cancer Risk: Analysis of Two Large Case–Control Studies. Nutrients 2022, 14, 1663. https://doi.org/10.3390/nu14081663
Franchi C, Ardoino I, Bosetti C, Negri E, Serraino D, Crispo A, Giacosa A, Fattore E, Dolci A, Bravi F, et al. Inverse Association between Canned Fish Consumption and Colorectal Cancer Risk: Analysis of Two Large Case–Control Studies. Nutrients. 2022; 14(8):1663. https://doi.org/10.3390/nu14081663
Chicago/Turabian StyleFranchi, Carlotta, Ilaria Ardoino, Cristina Bosetti, Eva Negri, Diego Serraino, Anna Crispo, Attilio Giacosa, Elena Fattore, Alberto Dolci, Francesca Bravi, and et al. 2022. "Inverse Association between Canned Fish Consumption and Colorectal Cancer Risk: Analysis of Two Large Case–Control Studies" Nutrients 14, no. 8: 1663. https://doi.org/10.3390/nu14081663
APA StyleFranchi, C., Ardoino, I., Bosetti, C., Negri, E., Serraino, D., Crispo, A., Giacosa, A., Fattore, E., Dolci, A., Bravi, F., Turati, F., La Vecchia, C., & D’Avanzo, B. (2022). Inverse Association between Canned Fish Consumption and Colorectal Cancer Risk: Analysis of Two Large Case–Control Studies. Nutrients, 14(8), 1663. https://doi.org/10.3390/nu14081663