Resolvins’ Obesity-Driven Deficiency: The Implications for Maternal–Fetal Health
Abstract
:1. Introduction
2. Acute and Chronic Inflammation
3. Resolvins
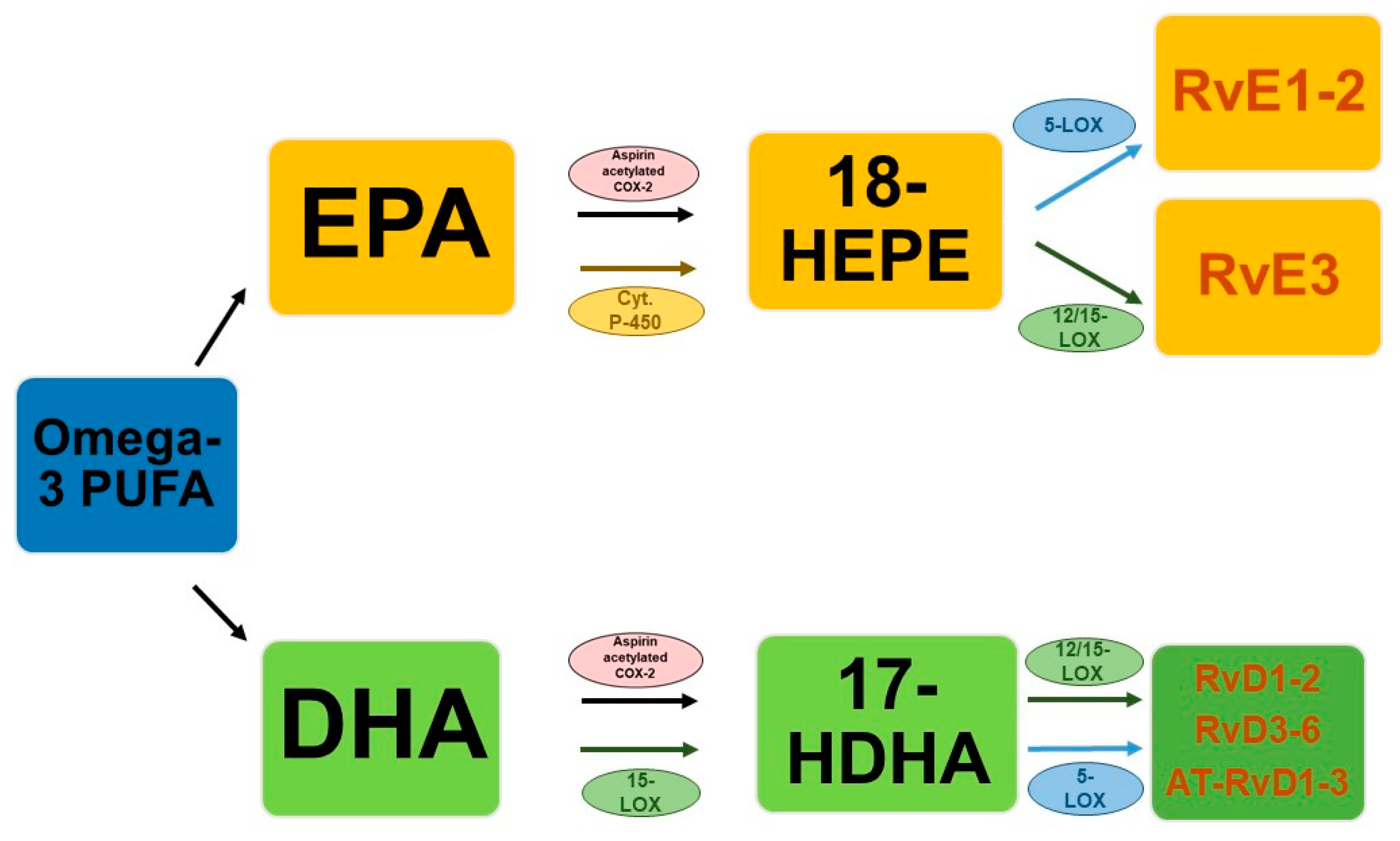
3.1. Types and Biosynthesis
3.2. Maternal–Fetal Concentrations
4. Role in Conditions of Alteration of Maternal–Fetal Health
5. Role in Obesity
6. Correlation with SARS-CoV-2 Infection
7. Utility of Dietary/Supplement Modulation of Resolvin Concentrations?
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poon, L.C.; McIntyre, H.D.; Hyett, J.A.; da Fonseca, E.B.; Hod, M. FIGO Pregnancy and NCD Committee. The first-trimester of pregnancy—A window of opportunity for prediction and prevention of pregnancy complications and future life. Diabetes Res. Clin. Pract. 2018, 145, 20–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and Metabolic Adaptations in Physiological and Complicated Pregnancy: Focus on Obesity and Gestational Diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef] [PubMed]
- Pantham, P.; Aye, I.L.; Powell, T.L. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Xu, X.; Yan, Y. Estimated global overweight and obesity burden in pregnant women based on panel data model. PLoS ONE 2018, 13, e0202183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Sappenfield, W.; Sharma, A.J.; Wilson, H.G.; Bish, C.L.; Salihu, H.M.; England, L.J. Racial/ethnic differences in the prevalence of gestational diabetes mellitus and maternal overweight and obesity, by nativity, Florida, 2004–2007. Obesity 2013, 21, E33–E40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, E.; Boyland, E.; Chisholm, A.; Harrold, J.; Maloney, N.G.; Marty, L.; Mead, B.R.; Noonan, R.; Hardman, C.A. Obesity, eating behavior and physical activity during COVID-19 lockdown: A study of UK adults. Appetite 2021, 156, 104853. [Google Scholar] [CrossRef] [PubMed]
- Ghesquière, L.; Garabedian, C.; Drumez, E.; Lemaître, M.; Cazaubiel, M.; Bengler, C.; Vambergue, A. Effects of COVID-19 pandemic lockdown on gestational diabetes mellitus: A retrospective study. Diabetes Metab. 2021, 47, 101201. [Google Scholar] [CrossRef]
- Cauldwell, M.; van-de-L’Isle, Y.; Watt Coote, I.; Steer, P.J. Seasonal and SARS-CoV-2 pandemic changes in the incidence of gestational diabetes. BJOG 2021, 128, 1881–1887. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Huo, S.; Ma, Y.; Ke, Y.; Wang, P.; Zhao, A. Emotional Eating in Pregnant Women during the COVID-19 Pandemic and Its Association with Dietary Intake and Gestational Weight Gain. Nutrients 2020, 12, 2250. [Google Scholar] [CrossRef]
- Elliott, E.; Hanson, C.K.; Anderson-Berry, A.L.; Nordgren, T.M. The role of specialized pro-resolving mediators in maternal-fetal health. Prostaglandins Leukot. Essent Fat. Acids 2017, 126, 98–104. [Google Scholar] [CrossRef]
- Huizinga, G.P.; Singer, B.H.; Singer, K. The Collision of Meta-Inflammation and SARS-CoV-2 Pandemic Infection. Endocrinology 2020, 161, bqaa154. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Christodoulatos, G.S.; Karampela, I.; Vallianou, N.; Apovian, C.M. Understanding the Co-Epidemic of Obesity and COVID-19: Current Evidence, Comparison with Previous Epidemics, Mechanisms, and Preventive and Therapeutic Perspectives. Curr. Obes. Rep. 2021, 10, 214–243. [Google Scholar] [CrossRef] [PubMed]
- Tognocchi, M.; Conte, M.; Testai, L.; Martucci, M.; Serra, A.; Salvioli, S.; Calderone, V.; Mele, M.; Conte, G. Supplementation of Enriched Polyunsaturated Fatty Acids and CLA Cheese on High Fat Diet: Effects on Lipid Metabolism and Fat Profile. Foods 2022, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Dessardo, N.S.; Dessardo, S.; Mustać, E.; Banac, S.; Petrović, O.; Peter, B. Chronic lung disease of prematurity and early childhood wheezing: Is foetal inflammatory response syndrome to blame? Early Hum. Dev. 2014, 90, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Haglund, B.; Odlind, V.; Altman, M.; Ewald, U.; Kieler, H. Perinatal conditions related to growth restriction and inflammation are associated with an increased risk of bronchopulmonary dysplasia. Acta Paediatr. 2015, 104, 259–263. [Google Scholar] [CrossRef]
- Strunk, T.; Inder, T.; Wang, X.; Burgner, D.; Mallard, C.; Levy, O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect. Dis. 2014, 14, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Koletzko, B.; Boey, C.C.; Campoy, C.; Carlson, S.E.; Chang, N.; Guillermo-Tuazon, M.A.; Joshi, S.; Prell, C.; Quak, S.H.; Sjarif, D.R.; et al. Current information and Asian perspectives on long-chain polyunsaturated fatty acids in pregnancy, lactation, and infancy: Systematic review and practice recommendations from an early nutrition academy workshop. Ann. Nutr. Metab. 2014, 65, 49–80. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Willoughby, D.A.; Moore, A.R.; Colville-Nash, P.R.; Gilroy, D. Resolution of inflammation. Int. J. Immunopharmacol. 2000, 22, 1131–1135. [Google Scholar] [CrossRef]
- Han, J.; Ulevitch, R.J. Limiting inflammatory responses during activation of innate immunity. Nat. Immunol. 2005, 6, 1198–1205. [Google Scholar] [CrossRef]
- Tracey, K.J. Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Investig. 2007, 117, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, N.; Serhan, C.N. Specialized pro-resolving mediator network: An update on production and actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nijhuis, J.; Rensen, S.S.; Slaats, Y.; van Dielen, F.M.; Buurman, W.A.; Greve, J.W. Neutrophil activation in morbid obesity, chronic activation of acute inflammation. Obesity 2009, 17, 2014–2018. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [Green Version]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Spite, M.; Clària, J.; Serhan, C.N. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell Metab. 2014, 19, 21–36. [Google Scholar] [CrossRef] [Green Version]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef] [Green Version]
- Evans, A.C.; Papachristou, G.I.; Whitcomb, D.C. Obesity and the risk of severe acute pancreatitis. Minerva Gastroenterol. Dietol. 2010, 56, 169–179. [Google Scholar]
- Elias, C.F.; Aschkenasi, C.; Lee, C.; Kelly, J.; Ahima, R.S.; Bjorbaek, C.; Flier, J.S.; Saper, C.B.; Elmquist, J.K. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 1999, 23, 775–786. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N. Resolution phase of inflammation: Novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu. Rev. Immunol. 2007, 25, 101–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhan, C.N.; Yacoubian, S.; Yang, R. Anti-inflammatory and proresolving lipid mediators. Annu. Rev. Pathol. 2008, 3, 279–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannenberg, G.; Serhan, C.N. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim. Biophys Acta 2010, 1801, 1260–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozurkewich, E.L.; Greenwood, M.; Clinton, C.; Berman, D.; Romero, V.; Djuric, Z.; Qualls, C.; Gronert, K. Pathway Markers for Pro-resolving Lipid Mediators in Maternal and Umbilical Cord Blood: A Secondary Analysis of the Mothers, Omega-3, and Mental Health Study. Front. Pharmacol. 2016, 7, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Videla, L.A.; Vargas, R.; Valenzuela, R.; Muñoz, P.; Corbari, A.; Hernandez-Rodas, M.C. Combined administration of docosahexaenoic acid and thyroid hormone synergistically enhances rat liver levels of resolvins RvD1 and RvD2. Prostaglandins Leukot. Essent. Fat. Acids 2019, 140, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Pirault, J.; Bäck, M. Lipoxin and Resolvin Receptors Transducing the Resolution of Inflammation in Cardiovascular Disease. Front. Pharmacol. 2018, 9, 1273. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, X.; Liu, S.; Shen, D.; Zhu, J.; Liu, K. Role of Resolvins in the Inflammatory Resolution of Neurological Diseases. Front. Pharmacol. 2020, 11, 612. [Google Scholar] [CrossRef]
- Ostermann, A.I.; West, A.L.; Schoenfeld, K.; Browning, L.M.; Walker, C.G.; Jebb, S.A.; Calder, P.C.; Schebb, N.H. Plasma oxylipins respond in a linear dose-response manner with increased intake of EPA and DHA: Results from a randomized controlled trial in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1251–1263. [Google Scholar] [CrossRef]
- Isobe, Y.; Arita, M.; Matsueda, S.; Iwamoto, R.; Fujihara, T.; Nakanishi, H.; Taguchi, R.; Masuda, K.; Sasaki, K.; Urabe, D.; et al. Identification and structure determination of novel anti-inflammatory mediator resolvin E3, 17,18-dihydroxyeicosapentaenoic acid. J. Biol. Chem. 2012, 287, 10525–10534. [Google Scholar] [CrossRef] [Green Version]
- Valdes, A.M.; Ravipati, S.; Menni, C.; Abhishek, A.; Metrustry, S.; Harris, J.; Nessa, A.; Williams, F.M.K.; Spector, T.D.; Doherty, M.; et al. Association of the resolvin precursor 17-HDHA, but not D- or E- series resolvins, with heat pain sensitivity and osteoarthritis pain in humans. Sci. Rep. 2017, 7, 10748. [Google Scholar] [CrossRef]
- Mas, E.; Croft, K.D.; Zahra, P.; Barden, A.; Mori, T.A. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 2012, 58, 1476–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keelan, J.A.; Mas, E.; D’Vaz, N.; Dunstan, J.A.; Li, S.; Barden, A.E.; Mark, P.J.; Waddell, B.J.; Prescott, S.L.; Mori, T.A. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction 2015, 149, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.L.; Mark, P.J.; Keelan, J.A.; Barden, A.; Mas, E.; Mori, T.A.; Waddell, B.J. Maternal dietary omega-3 fatty acid intake increases resolvin and protectin levels in the rat placenta. J. Lipid Res. 2013, 54, 2247–2254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, C.; Nadjar, A.; Buaud, B.; Vaysse, C.; Aubert, A.; Pallet, V.; Layé, S.; Joffre, C. Resolvin D1 and E1 promote resolution of inflammation in microglial cells in vitro. Brain Behav. Immun. 2016, 55, 249–259. [Google Scholar] [CrossRef]
- Salas-Hernández, A.; Espinoza-Pérez, C.; Vivar, R.; Espitia-Corredor, J.; Lillo, J.; Parra-Flores, P.; Sánchez-Ferrer, C.F.; Peiró, C.; Díaz-Araya, G. Resolvin D1 and E1 promote resolution of inflammation in rat cardiac fibroblast in vitro. Mol. Biol. Rep. 2021, 48, 57–66. [Google Scholar] [CrossRef]
- Weiss, G.A.; Troxler, H.; Klinke, G.; Rogler, D.; Braegger, C.; Hersberger, M. High level of anti-inflammatory and pro-resolving lipid mediator lipoxins and resolvons and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis. 2013, 12, 89. [Google Scholar] [CrossRef] [Green Version]
- Arnardottir, H.; Orr, S.K.; Dalli, J.; Serhan, C.N. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 2016, 9, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, A.; Kawana, K.; Tomio, K.; Taguchi, A.; Isobe, Y.; Iwamoto, R.; Masuda, K.; Furuya, H.; Nagamatsu, T.; Nagasaka, K.; et al. Increased tissue levels of omega-3 polyunsaturated fatty acids prevents pathological preterm birth. Sci. Rep. 2013, 3, 3113. [Google Scholar] [CrossRef]
- Maddipati, K.R.; Romero, R.; Chaiworapongsa, T.; Chaemsaithong, P.; Zhou, S.L.; Xu, Z.; Tarca, A.L.; Kusanovic, J.P.; Gomez, R.; Docheva, N.; et al. Clinical chorioamnionitis at term: The amniotic fluid fatty acyl lipidome. J. Lipid Res. 2016, 57, 1906–1916. [Google Scholar] [CrossRef] [Green Version]
- Connor, K.M.; SanGiovanni, J.P.; Lofqvist, C.; Aderman, C.M.; Chen, J.; Higuchi, A.; Hong, S.; Pravda, E.A.; Majchrzak, S.; Carper, D.; et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat. Med. 2007, 13, 868–873. [Google Scholar] [CrossRef] [Green Version]
- Hesselink, J.M.; Chiosi, F.; Costagliola, C. Resolvins and aliamides: Lipid autacoids in ophthalmology—What promise do they hold? Drug Des. Dev. Ther. 2016, 10, 3133–3141. [Google Scholar]
- Nordgren, T.M.; Anderson Berry, A.; Van Ormer, M.; Zoucha, S.; Elliott, E.; Johnson, R.; McGinn, E.; Cave, C.; Rilett, K.; Weishaar, K.; et al. Omega-3 Fatty Acid Supplementation, Pro-Resolving Mediators, and Clinical Outcomes in Maternal-Infant Pairs. Nutrients 2019, 11, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aung, M.T.; Yu, Y.; Ferguson, K.K.; Cantonwine, D.E.; Zeng, L.; McElrath, T.F.; Pennathur, S.; Mukherjee, B.; Meeker, J.D. Prediction and associations of preterm birth and its subtypes with eicosanoid enzymatic pathways and inflammatory markers. Sci. Rep. 2019, 9, 17049. [Google Scholar] [CrossRef] [PubMed]
- Perucci, L.O.; de Castro Pinto, K.M.; da Silva, S.P.G.; Lage, E.M.; Teixeira, P.G.; Barbosa, A.S.; Alpoim, P.N.; de Sousa, L.P.; Talvani, A.; Dusse, L.M.S. Longitudinal assessment of leukotriene B4, lipoxin A4, and resolvin D1 plasma levels in pregnant women with risk factors for preeclampsia. Clin. Biochem. 2021, 98, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Titos, E.; Rius, B.; López-Vicario, C.; Alcaraz-Quiles, J.; García-Alonso, V.; Lopategi, A.; Dalli, J.; Lozano, J.J.; Arroyo, V.; Delgado, S.; et al. Signaling and Immunoresolving Actions of Resolvin D1 in Inflamed Human Visceral Adipose Tissue. J. Immunol. 2016, 197, 3360–3370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, A.; Gowdy, K.M.; Oestreich, K.J.; Beck, M.; Shaikh, S.R. Obesity-Driven Deficiencies of Specialized Pro-resolving Mediators May Drive Adverse Outcomes During SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 1997. [Google Scholar] [CrossRef]
- Alam, I.; Ng, T.P.; Larbi, A. Does inflammation determine whether obesity is metabolically healthy or unhealthy? The aging perspective. Mediat. Inflamm. 2012, 2012, 456456. [Google Scholar] [CrossRef] [Green Version]
- Hellmann, J.; Tang, Y.; Kosuri, M.; Bhatnagar, A.; Spite, M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011, 25, 2399–2407. [Google Scholar] [CrossRef] [Green Version]
- González-Périz, A.; Horrillo, R.; Ferré, N.; Gronert, K.; Dong, B.; Morán-Salvador, E.; Titos, E.; Martínez-Clemente, M.; López-Parra, M.; Arroyo, V.; et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: A role for resolvins and protectins. FASEB J. 2009, 23, 1946–1957. [Google Scholar] [CrossRef] [Green Version]
- Titos, E.; Rius, B.; González-Périz, A.; López-Vicario, C.; Morán-Salvador, E.; Martínez-Clemente, M.; Arroyo, V.; Clària, J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 2011, 187, 5408–5418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clària, J.; Dalli, J.; Yacoubian, S.; Gao, F.; Serhan, C.N. Resolvin D1 and resolvin D2 govern local inflammatory tone in obese fat. J. Immunol. 2012, 189, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Rius, B.; Titos, E.; Morán-Salvador, E.; López-Vicario, C.; García-Alonso, V.; González-Périz, A.; Arroyo, V.; Clària, J. Resolvin D1 primes the resolution process initiated by calorie restriction in obesity-induced steatohepatitis. FASEB J. 2014, 28, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Neuhofer, A.; Zeyda, M.; Mascher, D.; Itariu, B.K.; Murano, I.; Leitner, L.; Hochbrugger, E.E.; Fraisl, P.; Cinti, S.; Serhan, C.N.; et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes 2013, 62, 1945–1956. [Google Scholar] [CrossRef] [Green Version]
- Pascoal, L.B.; Bombassaro, B.; Ramalho, A.F.; Coope, A.; Moura, R.F.; Correa-da-Silva, F.; Ignacio-Souza, L.; Razolli, D.; de Oliveira, D.; Catharino, R.; et al. Resolvin RvD2 reduces hypothalamic inflammation and rescues mice from diet-induced obesity. J. Neuroinflammation 2017, 14, 5. [Google Scholar] [CrossRef] [Green Version]
- Palm, M.; Axelsson, O.; Wernroth, L.; Larsson, A.; Basu, S. Involvement of inflammation in normal pregnancy. Acta Obstet. Gynecol. Scand. 2013, 92, 601–605. [Google Scholar] [CrossRef]
- Menon, R.; Richardson, L.S.; Lappas, M. Fetal membrane architecture, aging and inflammation in pregnancy and parturition. Placenta 2019, 79, 40–45. [Google Scholar] [CrossRef]
- Formoso, G.; Baldassarre, M.P.A.; Ginestra, F.; Carlucci, M.A.; Bucci, I.; Consoli, A. Inositol and antioxidant supplementation: Safety and efficacy in pregnancy. Diabetes Metab. Res. Rev. 2019, 35, e3154. [Google Scholar] [CrossRef]
- Levine, L.D.; Holland, T.L.; Kim, K.; Sjaarda, L.A.; Mumford, S.L.; Schisterman, E.F. The role of aspirin and inflammation on reproduction: The EAGeR trial. Can. J. Physiol. Pharmacol. 2019, 97, 187–192. [Google Scholar] [CrossRef]
- Shafiq, M.; Mathad, J.S.; Naik, S.; Alexander, M.; Yadana, S.; Araújo-Pereira, M.; Kulkarni, V.; Deshpande, P.; Kumar, N.P.; Babu, S.; et al. Association of Maternal Inflammation During Pregnancy With Birth Outcomes and Infant Growth Among Women With or Without HIV in India. JAMA Netw. Open 2021, 4, e2140584. [Google Scholar] [CrossRef]
- Han, V.X.; Patel, S.; Jones, H.F.; Nielsen, T.C.; Mohammad, S.S.; Hofer, M.J.; Gold, W.; Brilot, F.; Lain, S.J.; Nassar, N.; et al. Maternal acute and chronic inflammation in pregnancy is associated with common neurodevelopmental disorders: A systematic review. Transl. Psychiatry 2021, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.G.; Crujeiras, A.B.; Casanueva, F.F.; Carreira, M.C. Leptin, Obesity, and Leptin Resistance: Where Are We 25 Years Later? Nutrients 2019, 11, 2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moynihan, A.T.; Hehir, M.P.; Glavey, S.V.; Smith, T.J.; Morrison, J.J. Inhibitory effect of leptin on human uterine contractility in vitro. Am. J. Obstet. Gynecol. 2006, 195, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Morshedzadeh, N.; Saedisomeolia, A.; Djalali, M.; Eshraghian, M.R.; Hantoushzadeh, S.; Mahmoudi, M. Resolvin D1 impacts on insulin resistance in women with polycystic ovary syndrome and healthy women. Diabetes Metab. Syndr. 2019, 13, 660–664. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Arita, M.; Taguchi, R.; Kang, J.X.; Marette, A. Transgenic restoration of long-chain n-3 fatty acids in insulin target tissues improves resolution capacity and alleviates obesity-linked inflammation and insulin resistance in high-fat-fed mice. Diabetes 2010, 59, 3066–3073. [Google Scholar] [CrossRef] [Green Version]
- Echeverría, F.; Valenzuela, R.; Espinosa, A.; Bustamante, A.; Álvarez, D.; Gonzalez-Mañan, D.; Ortiz, M.; Soto-Alarcon, S.A.; Videla, L.A. Reduction of high-fat diet-induced liver proinflammatory state by eicosapentaenoic acid plus hydroxytyrosol supplementation: Involvement of resolvins RvE1/2 and RvD1/2. J. Nutr. Biochem. 2019, 63, 35–43. [Google Scholar] [CrossRef]
- López-Vicario, C.; Titos, E.; Walker, M.E.; Alcaraz-Quiles, J.; Casulleras, M.; Durán-Güell, M.; Flores-Costa, R.; Pérez-Romero, N.; Forné, M.; Dalli, J.; et al. Leukocytes from obese individuals exhibit an impaired SPM signature. FASEB J. 2019, 33, 7072–7083. [Google Scholar] [CrossRef] [Green Version]
- Bashir, S.; Sharma, Y.; Jairajpuri, D.; Rashid, F.; Nematullah, M.; Khan, F. Alteration of adipose tissue immune cell milieu towards the suppression of inflammation in high fat diet fed mice by flaxseed oil supplementation. PLoS ONE 2019, 14, e0223070. [Google Scholar] [CrossRef]
- Kosaraju, R.; Guesdon, W.; Crouch, M.J.; Teague, H.L.; Sullivan, E.M.; Karlsson, E.A.; Schultz-Cherry, S.; Gowdy, K.; Bridges, L.C.; Reese, L.R.; et al. B Cell Activity Is Impaired in Human and Mouse Obesity and Is Responsive to an Essential Fatty Acid upon Murine Influenza Infection. J. Immunol. 2017, 198, 4738–4752. [Google Scholar] [CrossRef] [Green Version]
- Misumi, I.; Starmer, J.; Uchimura, T.; Beck, M.A.; Magnuson, T.; Whitmire, J.K. Obesity Expands a Distinct Population of T Cells in Adipose Tissue and Increases Vulnerability to Infection. Cell Rep. 2019, 27, 514–524.e5. [Google Scholar] [CrossRef] [Green Version]
- Petrakis, D.; Margină, D.; Tsarouhas, K.; Tekos, F.; Stan, M.; Nikitovic, D.; Kouretas, D.; Spandidos, D.A.; Tsatsakis, A. Obesity—A risk factor for increased COVID-19 prevalence, severity and lethality (Review). Mol. Med. Rep. 2020, 22, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Simonnet, A.; Chetboun, M.; Poissy, J.; Raverdy, V.; Noulette, J.; Duhamel, A.; Labreuche, J.; Mathieu, D.; Pattou, F.; Jourdain, M. LICORN and the Lille COVID-19 and Obesity study group. High Prevalence of Obesity in Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) Requiring Invasive Mechanical Ventilation. Obesity 2020, 28, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; He, P.; Liu, H.G.; Wang, X.J.; Li, F.J.; Chen, S.; Lin, J.; Chen, P.; Liu, J.H.; Li, C.H. Clinical characteristics of 30 medical workers infected with new coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, E016. [Google Scholar] [PubMed]
- Peng, Y.D.; Meng, K.; Guan, H.Q.; Leng, L.; Zhu, R.R.; Wang, B.Y.; He, M.A.; Cheng, L.X.; Huang, K.; Zeng, Q.T. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi 2020, 48, 450–455. [Google Scholar] [PubMed]
- Lighter, J.; Phillips, M.; Hochman, S.; Sterling, S.; Johnson, D.; Francois, F.; Stachel, A. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin. Infect. Dis. 2020, 71, 896–897. [Google Scholar] [CrossRef] [Green Version]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Neuzil, K.M.; Reed, G.W.; Mitchel, E.F.; Simonsen, L.; Griffin, M.R. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am. J. Epidemiol. 1998, 148, 1094–1102. [Google Scholar] [CrossRef] [Green Version]
- Kraus, T.A.; Engel, S.M.; Sperling, R.S.; Kellerman, L.; Lo, Y.; Wallenstein, S.; Escribese, M.M.; Garrido, J.L.; Singh, T.; Loubeau, M.; et al. Characterizing the pregnancy immune phenotype: Results of the viral immunity and pregnancy (VIP) study. J. Clin. Immunol. 2012, 32, 300–311. [Google Scholar] [CrossRef]
- Wegmann, T.G.; Lin, H.; Guilbert, L.; Mosmann, T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunol. Today 1993, 14, 353–356. [Google Scholar] [CrossRef]
- Klein, S.L.; Passaretti, C.; Anker, M.; Olukoya, P.; Pekosz, A. The impact of sex, gender and pregnancy on 2009 H1N1 disease. Biol. Sex Differ. 2010, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- GREENBERG, M.; JACOBZINER, H.; PAKTER, J.; WEISL, B.A. Maternal mortality in the epidemic of Asian influenza, New York City, 1957. Am. J. Obstet. Gynecol. 1958, 76, 897–902. [Google Scholar] [CrossRef]
- Harris, J.W. Influenza occurring in pregnant women: A statistical study of thirteen hundred and fifty cases. JAMA 1919, 72, 978–980. [Google Scholar] [CrossRef]
- Stockman, L.J.; Lowther, S.A.; Coy, K.; Saw, J.; Parashar, U.D. SARS during pregnancy, United States. Emerg. Infect. Dis. 2004, 10, 1689–1690. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Kalafat, E.; Benlioglu, C.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; Le Doare, K.; Heath, P.; Ladhani, S.; et al. SARS-CoV-2 infection in pregnancy: A systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine 2020, 25, 100446. [Google Scholar] [CrossRef]
- Panagiotakopoulos, L.; Myers, T.R.; Gee, J.; Lipkind, H.S.; Kharbanda, E.O.; Ryan, D.S.; Williams, J.T.B.; Naleway, A.L.; Klein, N.P.; Hambidge, S.J.; et al. SARS-CoV-2 Infection Among Hospitalized Pregnant Women: Reasons for Admission and Pregnancy Characteristics—Eight U.S. Health Care Centers, 1 March–May 30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1355–1359. [Google Scholar] [CrossRef]
- Lapolla, A.; Dalfrà, M.G.; Burlina, S. Vaccination against COVID-19 infection: The need of evidence for diabetic and obese pregnant women. Acta Diabetol. 2021, 58, 1581–1585. [Google Scholar] [CrossRef]
- Nordgren, T.M.; Lyden, E.; Anderson-Berry, A.; Hanson, C. Omega-3 Fatty Acid Intake of Pregnant Women and Women of Childbearing Age in the United States: Potential for Deficiency? Nutrients 2017, 9, 197. [Google Scholar] [CrossRef] [Green Version]
- Jandacek, R.J. Linoleic Acid: A Nutritional Quandary. Healthcare 2017, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Marchix, J.; Catheline, D.; Duby, C.; Monthéan-Boulier, N.; Boissel, F.; Pédrono, F.; Boudry, G.; Legrand, P. Interactive effects of maternal and weaning high linoleic acid intake on hepatic lipid metabolism, oxylipins profile and hepatic steatosis in offspring. J. Nutr. Biochem. 2020, 75, 108241. [Google Scholar] [CrossRef]
- Suratt, B.T.; Ubags, N.D.J.; Rastogi, D.; Tantisira, K.G.; Marsland, B.J.; Petrache, I.; Allen, J.B.; Bates, J.H.T.; Holguin, F.; McCormack, M.C.; et al. Allergy, Immunology, and Inflammation Assembly. An Official American Thoracic Society Workshop Report: Obesity and Metabolism. An Emerging Frontier in Lung Health and Disease. Ann. Am. Thorac. Soc. 2017, 14, 1050–1059. [Google Scholar] [CrossRef]
- Souza, P.R.; Marques, R.M.; Gomez, E.A.; Colas, R.A.; De Matteis, R.; Zak, A.; Patel, M.; Collier, D.J.; Dalli, J. Enriched Marine Oil Supplements Increase Peripheral Blood Specialized Pro-Resolving Mediators Concentrations and Reprogram Host Immune Responses: A Randomized Double-Blind Placebo-Controlled Study. Circ. Res. 2020, 126, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Quiros, M.; Feier, D.; Birkl, D.; Agarwal, R.; Zhou, D.W.; García, A.J.; Parkos, C.A.; Nusrat, A. Resolvin E1 is a pro-repair molecule that promotes intestinal epithelial wound healing. Proc. Natl. Acad. Sci. USA 2020, 117, 9477–9482. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Al-Shaer, A.E.; Guesdon, W.; Torres, M.J.; Armstrong, M.; Quinn, K.; Davis, T.; Reisdorph, N.; Neufer, P.D.; Spangenburg, E.E.; et al. Resolvin E1 derived from eicosapentaenoic acid prevents hyperinsulinemia and hyperglycemia in a host genetic manner. FASEB J. 2020, 34, 10640–10656. [Google Scholar] [CrossRef] [PubMed]
- Fanos, V.; Atzori, L.; Makarenko, K.; Melis, G.B.; Ferrazzi, E. Metabolomics application in maternal-fetal medicine. Biomed. Res. Int. 2013, 2013, 720514. [Google Scholar] [CrossRef]
- Fanos, V.; Antonucci, R.; Barberini, L.; Noto, A.; Atzori, L. Clinical application of metabolomics in neonatology. J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. 1), 104–109. [Google Scholar] [CrossRef]

| Authors, Year | Patients | Sample | Technique | Main Results | Clinical Significance |
|---|---|---|---|---|---|
| Nordgren et al. [52], 2019 | 136 mothers and 138 infants at the time of delivery. | Maternal and cord plasma. | Commercially available enzyme immunoassays | ↑ SPM in maternal versus cord plasma ↑ SPM levels associated with at-risk outcomes ↑ maternal DHA intake associated with ↑ maternal plasma RvD1 e RvD2 | Increased n-3 fatty acid intake may provide increased substrate for the production of SPM during high-risk pregnancy/delivery conditions Increased maternal plasma SPM could serve as a biomarker for negative neonatal outcomes |
| Aung et al. [53], 2019 | 58 cases of preterm birth and 115 controls | Blood samples | 6490 Triple Quadrupole mass spectrometer | RvD1 were among the most predictive markers Lipid biomarkers were the best in separating cases from controls Eicosanoids resulting from the lipoxygenase pathway showed the strongest association with preterm childbirth | The combination of lipid biomarkers may have good utility in clinical settings to predict preterm birth Most of the observed associations relate to spontaneous preterm birth |
| Perucci et al. [54], 2021 | 28 pregnant Brazilian women, 11 women developed PE and 17 remained normotensive | Blood samples | Competitive enzyme-linked immunosorbent assays (ELISA) | ↓ RvD1 levels and ↓ RvD1/LTB4 ratio at 30–34 weeks in pregnant women with PE In pregnant women who developed PE ↑RvD1 levels at weeks 12–19 and ↑ LXA4 and RvD1 levels at 30–34 weeks than those at 20–29 weeks | Potential use of lipid mediators (RvD1) as clinical markers for PE development |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosco, A.; Dessì, A.; Zanza, C.; Pintus, R.; Fanos, V. Resolvins’ Obesity-Driven Deficiency: The Implications for Maternal–Fetal Health. Nutrients 2022, 14, 1662. https://doi.org/10.3390/nu14081662
Bosco A, Dessì A, Zanza C, Pintus R, Fanos V. Resolvins’ Obesity-Driven Deficiency: The Implications for Maternal–Fetal Health. Nutrients. 2022; 14(8):1662. https://doi.org/10.3390/nu14081662
Chicago/Turabian StyleBosco, Alice, Angelica Dessì, Caterina Zanza, Roberta Pintus, and Vassilios Fanos. 2022. "Resolvins’ Obesity-Driven Deficiency: The Implications for Maternal–Fetal Health" Nutrients 14, no. 8: 1662. https://doi.org/10.3390/nu14081662
APA StyleBosco, A., Dessì, A., Zanza, C., Pintus, R., & Fanos, V. (2022). Resolvins’ Obesity-Driven Deficiency: The Implications for Maternal–Fetal Health. Nutrients, 14(8), 1662. https://doi.org/10.3390/nu14081662







