Platelet Activation Favours NOX2-Mediated Muscle Damage in Elite Athletes: The Role of Cocoa-Derived Polyphenols
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.1.1. Blood Sampling and Preparations
2.1.2. Evaluation of Platelet Activation Biomarkers
2.1.3. Evaluation of Catecholamine Levels
2.1.4. Evaluation of Oxidative Stress
2.1.5. Evaluation of Muscle Damage
2.2. In Vitro Studies
2.2.1. Platelet Preparation and Aggregation
2.2.2. Cell Culture and Reagents
2.2.3. Protein Detection, Electrophoresis, and Western Blot Analysis
2.2.4. Extraction of Phenolic Fraction from Chocolate and Total Polyphenol Content Evaluation
2.2.5. Extraction and Quantification of Catechin and Epicatechin from Chocolate
2.3. Statistical Methods
2.3.1. Categorical Variables Are Reported as Counts (Percentage) and Continuous Variables as Mean ± SD for Those without Normal Distribution and as Median (Interquartile Range [IQR]) for Continuous Variables without Normal Distribution
2.3.2. Effect Size and Sample Size Determination
3. Results
3.1. In Vivo Study
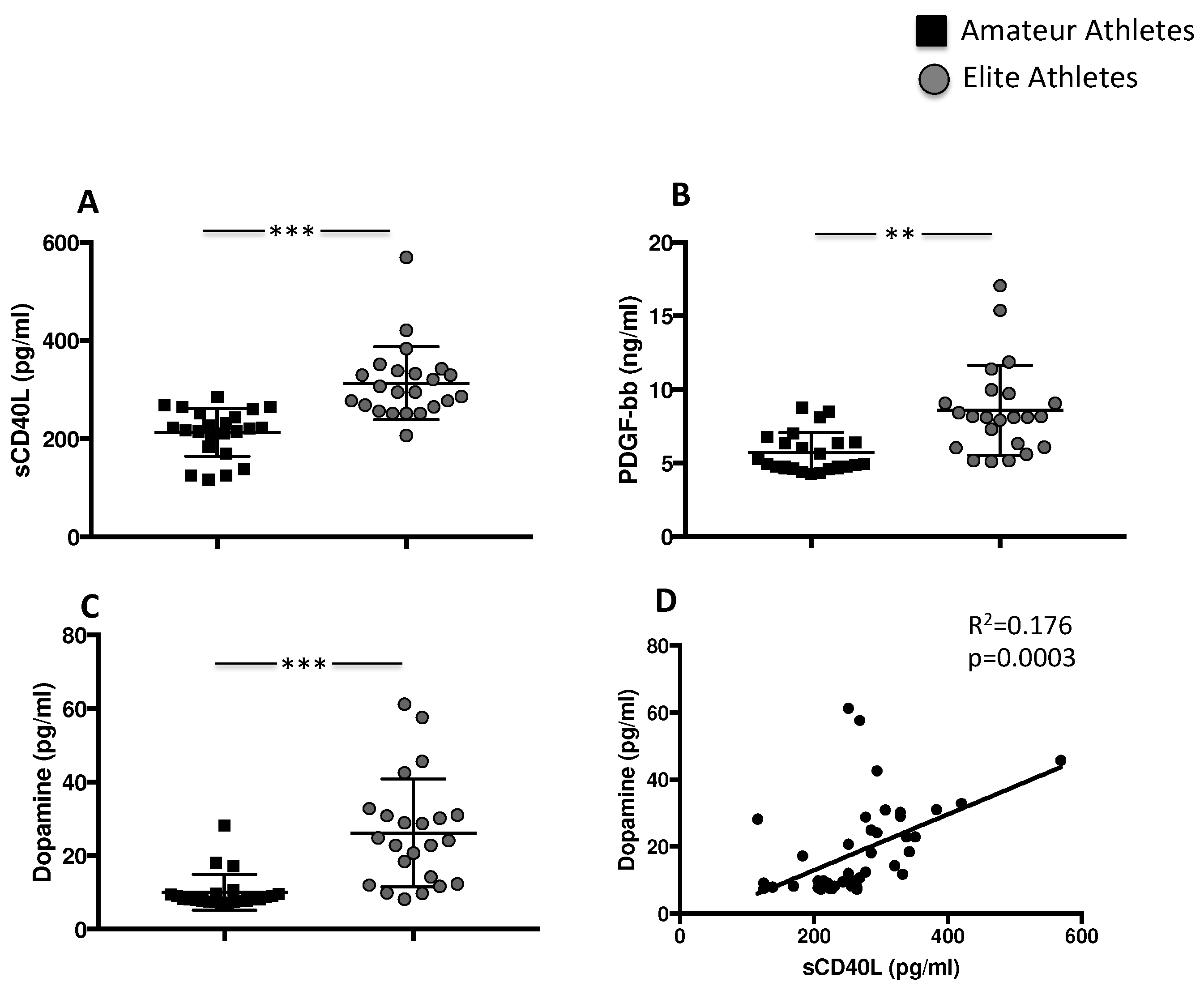
3.1.1. Exercise Induces Platelet Activation and Granule Release
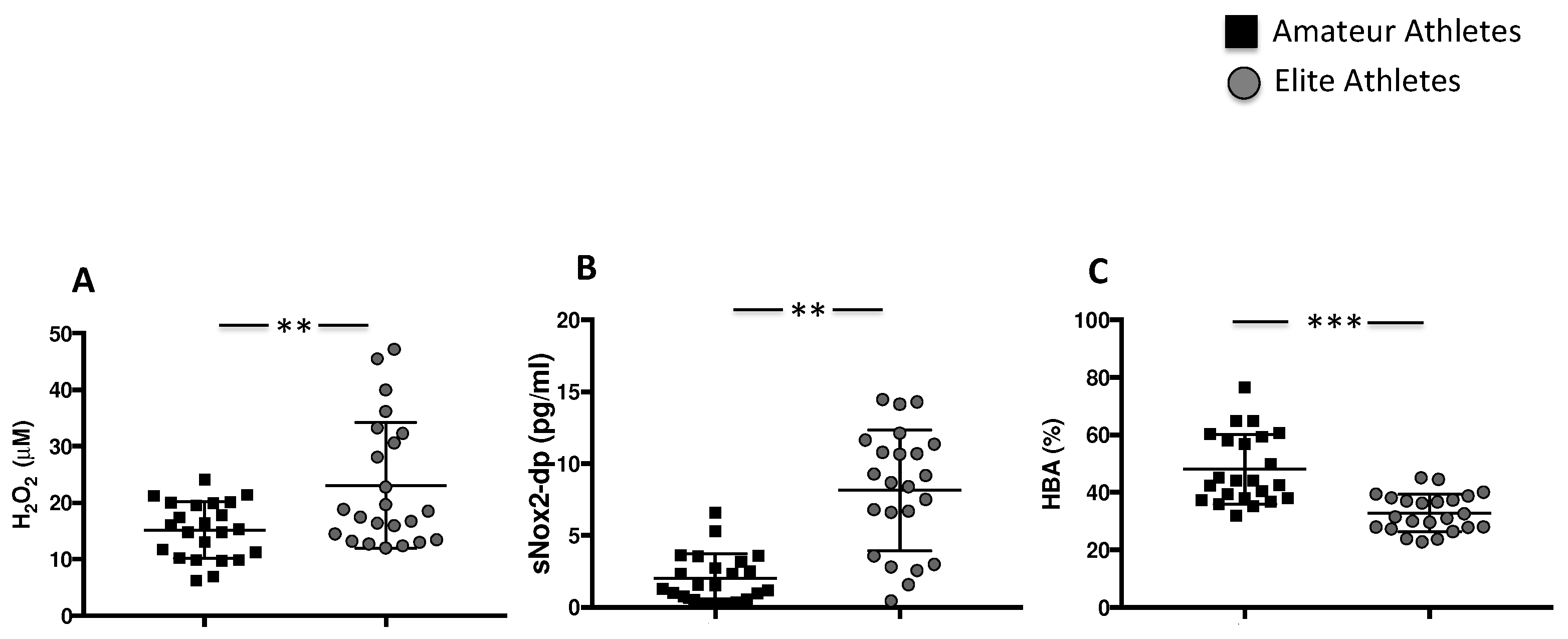
3.1.2. Intensive Exercise Induces Oxidative Stress
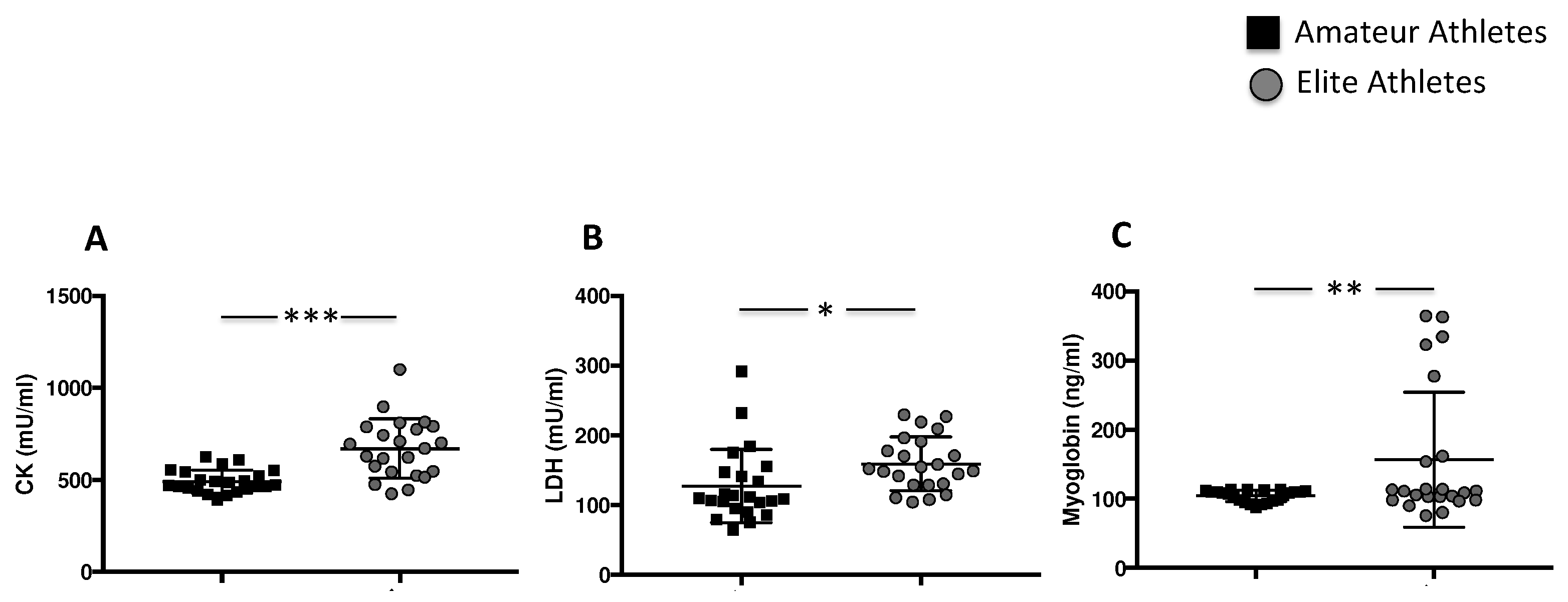
3.1.3. Intensive Exercise Induces Elevation of Specific Muscle Enzymes
3.2. In Vitro Study
3.2.1. Dopamine Induces NOX2-Mediated Oxidative Stress and Platelet Aggregation
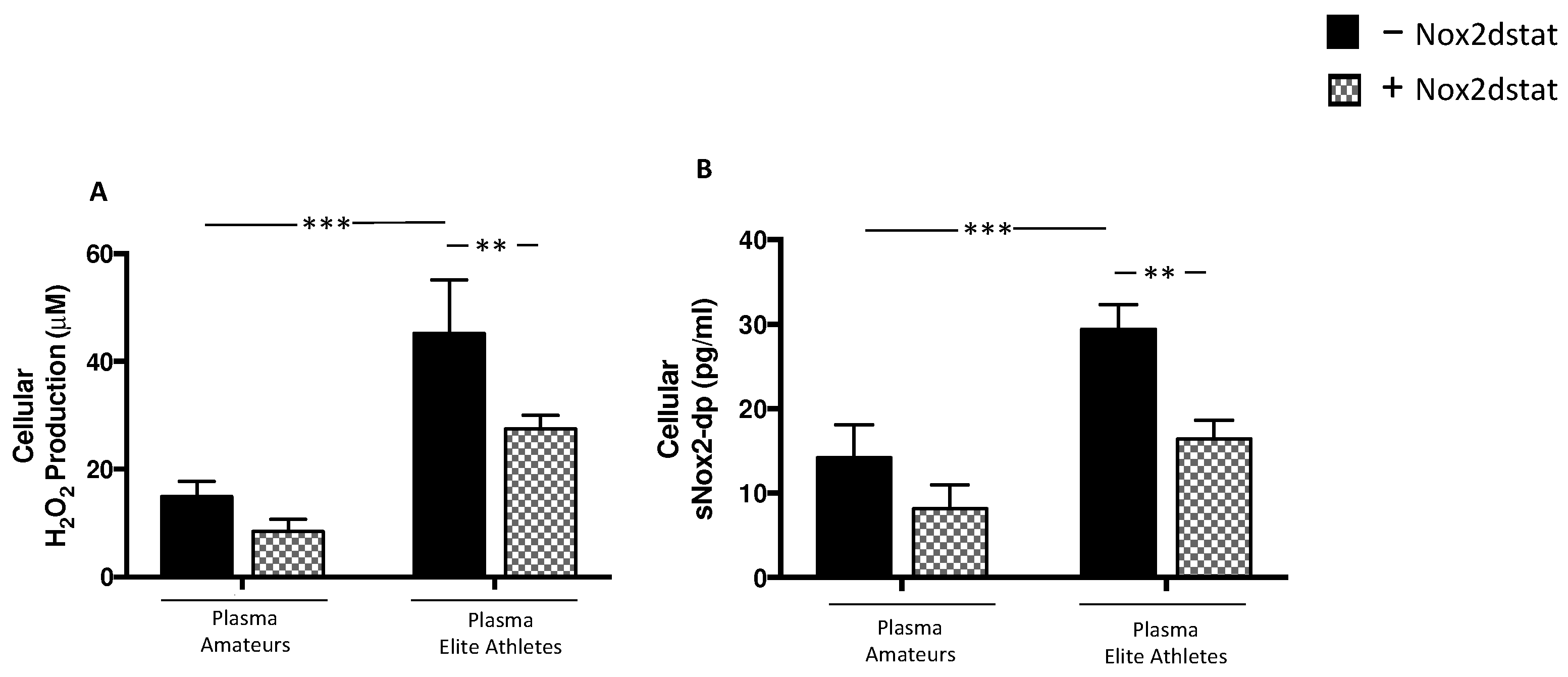
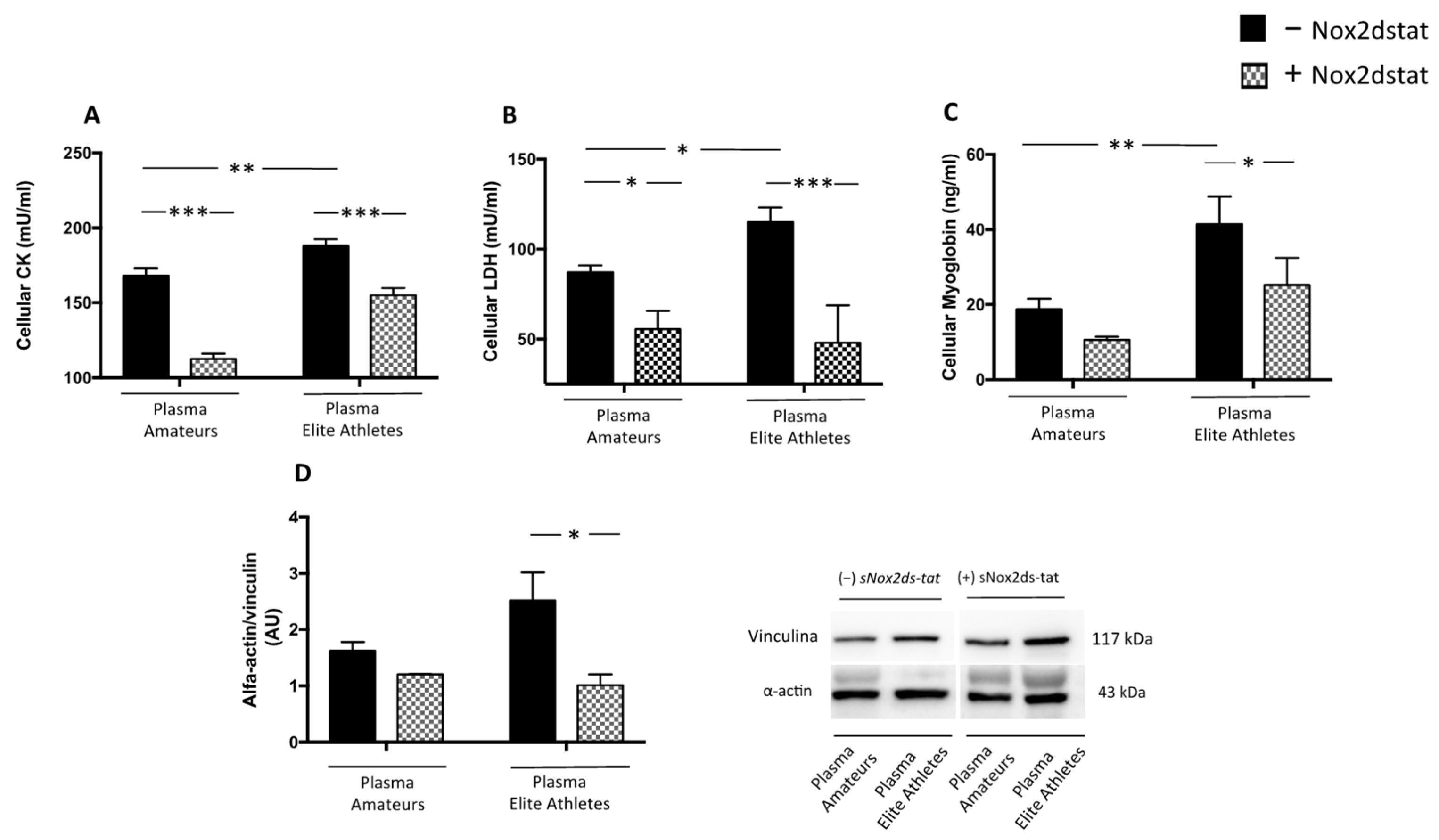
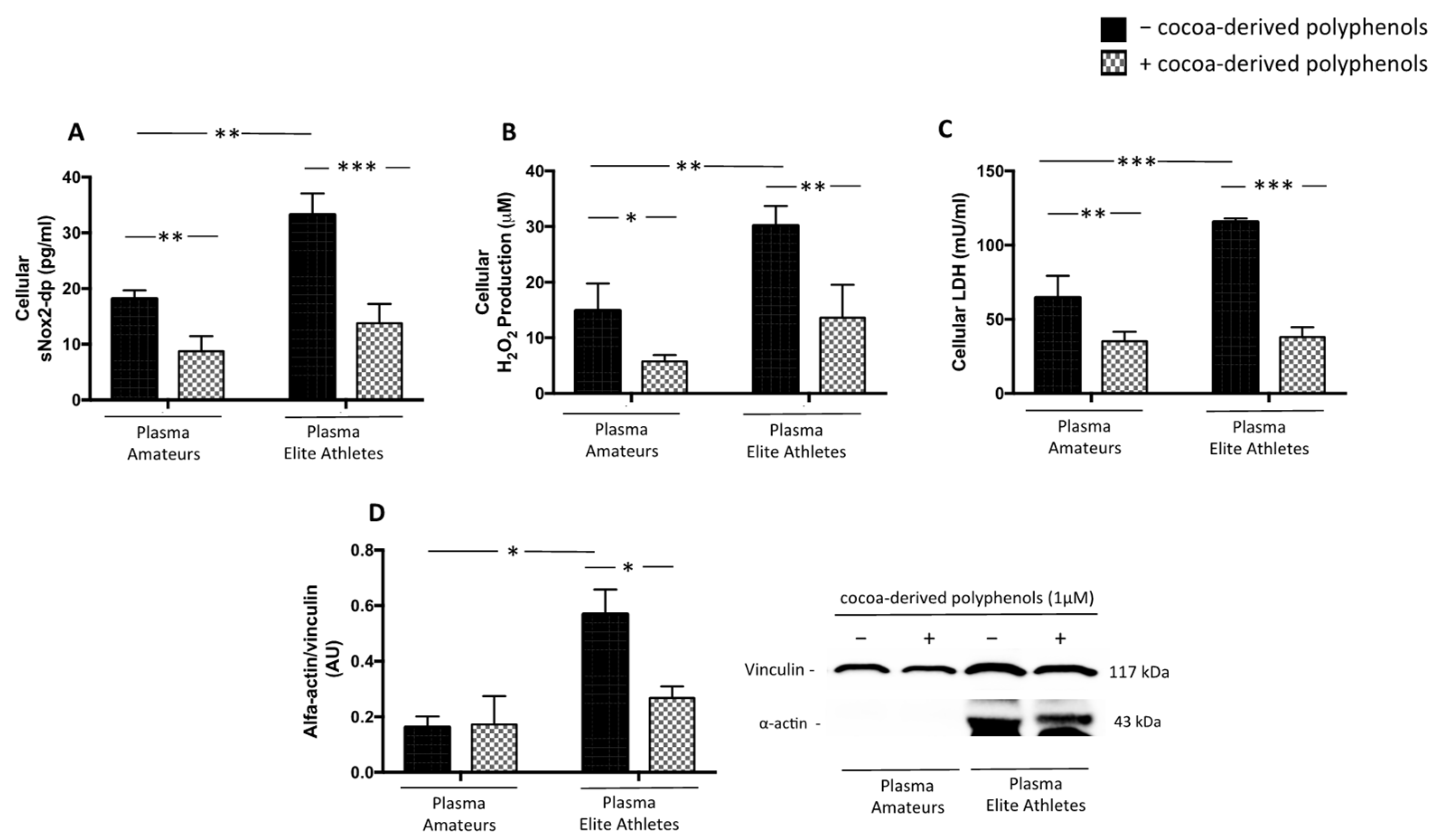
3.2.2. Plasma from Elite Athletes Increases Oxidative Stress and Muscle Injury: The Role of NOX2
3.2.3. Cocoa-Derived Polyphenols Reduces Plasma-Induced Muscle Damage
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Physical Activity. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 1 December 2021).
- Snyder, A.R.; Martinez, J.C.; Bay, R.C.; Parsons, J.T.; Sauers, E.L.; McLeod, T.C.V. Health-Related Quality of Life Differs between Adolescent Athletes and Adolescent Nonathletes. J. Sport Rehabil. 2010, 19, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C.; Wentz, L.M. The Compelling Link between Physical Activity and the Body’s Defense System. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.M.W.; Pedersen, B.K. The Anti-Inflammatory Effect of Exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Angeli, A.; Minetto, M.; Dovio, A.; Paccotti, P. The Overtraining Syndrome in Athletes: A Stress-Related Disorder. J. Endocrinol. Investig. 2004, 27, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, J.M.; Lormes, W.; Reissnecker, S.; Liu, Y. New Aspects of the Hormone and Cytokine Response to Training. Eur. J. Appl. Physiol. 2004, 91, 382–391. [Google Scholar] [CrossRef]
- Delos, D.; Maak, T.G.; Rodeo, S.A. Muscle Injuries in Athletes: Enhancing Recovery Through Scientific Understanding and Novel Therapies. Sports Health 2013, 5, 346–352. [Google Scholar] [CrossRef]
- Gabbett, T.J. The Training-Injury Prevention Paradox: Should Athletes Be Training Smarter and Harder? Br. J. Sports Med. 2016, 50, 273–280. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive Oxygen Species: Impact on Skeletal Muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [CrossRef]
- Steinbacher, P.; Eckl, P. Impact of Oxidative Stress on Exercising Skeletal Muscle. Biomolecules 2015, 5, 356–377. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- El Jamali, A.; Valente, A.J.; Clark, R.A. Regulation of Phagocyte NADPH Oxidase by Hydrogen Peroxide through a Ca(2+)/c-Abl Signaling Pathway. Free Radic. Biol. Med. 2010, 48, 798–810. [Google Scholar] [CrossRef]
- Heber, S.; Volf, I. Effects of Physical (in)Activity on Platelet Function. BioMed Res. Int. 2015, 2015, 165078. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Jen, C.J.; Kung, H.C.; Lin, L.J.; Hsiue, T.R.; Chen, H.I. Different Effects of Strenuous Exercise and Moderate Exercise on Platelet Function in Men. Circulation 1994, 90, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Jen, C.J.; Lee, H.L.; Chen, H.I. Effects of Short-Term Exercise on Female Platelet Function during Different Phases of the Menstrual Cycle. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 1682–1686. [Google Scholar] [CrossRef]
- Chicharro, J.L.; Sánchez, O.; Bandrés, F.; Guantes, Y.; Yges, A.; Lucía, A.; Legido, J.C. Platelet Aggregability in Relation to the Anaerobic Threshold. Thromb. Res. 1994, 75, 251–257. [Google Scholar] [CrossRef]
- Tozzi-Ciancarelli, M.G.; Penco, M.; Di Massimo, C. Influence of Acute Exercise on Human Platelet Responsiveness: Possible Involvement of Exercise-Induced Oxidative Stress. Eur. J. Appl. Physiol. 2002, 86, 266–272. [Google Scholar] [CrossRef]
- Zouhal, H.; Jacob, C.; Delamarche, P.; Gratas-Delamarche, A. Catecholamines and the Effects of Exercise, Training and Gender. Sports Med. 2008, 38, 401–423. [Google Scholar] [CrossRef]
- Peinado, A.B.; Rojo, J.J.; Calderón, F.J.; Maffulli, N. Responses to Increasing Exercise upon Reaching the Anaerobic Threshold, and Their Control by the Central Nervous System. BMC Sports Sci. Med. Rehabil. 2014, 6, 17. [Google Scholar] [CrossRef]
- Braschi, A. Acute Exercise-Induced Changes in Hemostatic and Fibrinolytic Properties: Analogies, Similarities, and Differences between Normotensive Subjects and Patients with Essential Hypertension. Platelets 2019, 30, 675–689. [Google Scholar] [CrossRef]
- Flaumenhaft, R.; Sharda, A. The Life Cycle of Platelet Granules. F1000Research 2018, 7, 236. [Google Scholar]
- Koupenova, M.; Clancy, L.; Corkrey, H.A.; Freedman, J.E. Circulating Platelets as Mediators of Immunity, Inflammation, and Thrombosis. Circ. Res. 2018, 122, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Perroni, F.; Migliaccio, S.; Borrione, P.; Vetrano, M.; Amatori, S.; Sisti, D.; Rocchi, M.B.L.; Salerno, G.; Del Vescovo, R.; Cavarretta, E.; et al. Can Haematological and Hormonal Biomarkers Predict Fitness Parameters in Youth Soccer Players? A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 6294. [Google Scholar] [CrossRef] [PubMed]
- Lubich, T.; Cesaretti, D. Review Frame Proposals and Classification of Sport Activities. Med. Sport 1990, 43, 223–229. [Google Scholar]
- Pelliccia, A.; Caselli, S.; Sharma, S.; Basso, C.; Bax, J.J.; Corrado, D.; D’Andrea, A.; D’Ascenzi, F.; Di Paolo, F.M.; Edvardsen, T.; et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) Joint Position Statement: Recommendations for the Indication and Interpretation of Cardiovascular Imaging in the Evaluation of the Athlete’s Heart. Eur. Heart J. 2018, 39, 1949–1969. [Google Scholar] [PubMed]
- Becatti, M.; Mannucci, A.; Barygina, V.; Mascherini, G.; Emmi, G.; Silvestri, E.; Wright, D.; Taddei, N.; Galanti, G.; Fiorillo, C. Redox Status Alterations during the Competitive Season in Élite Soccer Players: Focus on Peripheral Leukocyte-Derived ROS. Intern. Emerg. Med. 2017, 12, 777–788. [Google Scholar] [CrossRef]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a Component of Extra Virgin Olive Oil, Lowers Postprandial Glycaemia in Healthy Subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef]
- Cifuentes-Pagano, E.; Csanyi, G.; Pagano, P.J. NADPH Oxidase Inhibitors: A Decade of Discovery from Nox2ds to HTS. Cell. Mol. Life Sci. 2012, 69, 2315–2325. [Google Scholar] [CrossRef]
- Gottumukkala, R.V.S.S.; Nadimpalli, N.; Sukala, K.; Subbaraju, G.V. Determination of Catechin and Epicatechin Content in Chocolates by High-Performance Liquid Chromatography. Int. Sch. Res. Not. 2014, 2014, 628196. [Google Scholar] [CrossRef]
- Pingitore, A.; Lima, G.P.P.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and Oxidative Stress: Potential Effects of Antioxidant Dietary Strategies in Sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef]
- Cavarretta, E.; Peruzzi, M.; Del Vescovo, R.; Di Pilla, F.; Gobbi, G.; Serdoz, A.; Ferrara, R.; Schirone, L.; Sciarretta, S.; Nocella, C.; et al. Dark Chocolate Intake Positively Modulates Redox Status and Markers of Muscular Damage in Elite Football Athletes: A Randomized Controlled Study. Oxid. Med. Cell. Longev. 2018, 2018, 4061901. [Google Scholar] [CrossRef]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative Biology of Exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Boccatonda, A.; Tripaldi, R.; Davì, G.; Santilli, F. Oxidative Stress Modulation Through Habitual Physical Activity. Curr. Pharm. Des. 2016, 22, 3648–3680. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, A.; Gaeini, A.; Shekarfroush, S.; Khoshbaten, A. Cardioprotective Effect of High Intensity Interval Training and Nitric Oxide Metabolites (NO2−, NO3−). Iran. J. Public Health 2015, 44, 1270–1276. [Google Scholar] [PubMed]
- Kumral, Z.N.O.; Şener, G.; Ozgur, S.; Koç, M.; Suleymanoglu, S.; Hurdag, C.; Yeğen, B. Regular Exercise Alleviates Renovascular Hypertension-Induced Cardiac/Endothelial Dysfunction and Oxidative Injury in Rats. J. Physiol. Pharmacol. 2016, 67, 45–55. [Google Scholar]
- Banerjee, A.K.; Mandal, A.; Chanda, D.; Chakraborti, S. Oxidant, Antioxidant and Physical Exercise. Mol. Cell. Biochem. 2003, 253, 307–312. [Google Scholar] [CrossRef]
- Packer, L.; Cadenas, E.; Davies, K.J.A. Free Radicals and Exercise: An Introduction. Free Radic. Biol. Med. 2008, 44, 123–125. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.; Bloomer, R.J. Acute Exercise and Oxidative Stress: A 30 Year History. Dyn. Med. 2009, 8, 1. [Google Scholar] [CrossRef]
- Metin, G.; Gümüştaş, M.K.; Uslu, E.; Belce, A.; Kayserilioglu, A. Effect of regular training on plasma thiols, malondialdehyde and carnitine concentrations in young soccer players. Chin. J. Physiol. 2003, 46, 35–39. [Google Scholar]
- Hoppel, F.; Calabria, E.; Pesta, D.H.; Kantner-Rumplmair, W.; Gnaiger, E.; Burtscher, M. Effects of Ultramarathon Running on Mitochondrial Function of Platelets and Oxidative Stress Parameters: A Pilot Study. Front. Physiol. 2021, 12, 12. [Google Scholar] [CrossRef]
- Lippi, G.; Montagnana, M.; Salvagno, G.L.; Franchini, M.; Guidi, G.C. Comparison of Platelet Function between Sedentary Individuals and Competitive Athletes at Rest. Thromb. J. 2006, 4, 10. [Google Scholar] [CrossRef][Green Version]
- Wang, J.S.; Liao, C.H. Moderate-Intensity Exercise Suppresses Platelet Activation and Polymorphonuclear Leukocyte Interaction with Surface-Adherent Platelets under Shear Flow in Men. Thromb. Haemost. 2004, 91, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Nailin, L.; Wallén, N.H.; Hjemdahl, P. Evidence for Prothrombotic Effects of Exercise and Limited Protection by Aspirin. Circulation 1999, 100, 1374–1379. [Google Scholar] [CrossRef]
- Wang, J.S.; Chow, S.E.; Chen, J.K. Strenuous, Acute Exercise Affects Reciprocal Modulation of Platelet and Polymorphonuclear Leukocyte Activities under Shear Flow in Men. J. Thromb. Haemost. 2003, 1, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Kratz, A.; Wood, M.J.; Siegel, A.J.; Hiers, J.R.; Cott, E.M. Van Effects of Marathon Running on Platelet Activation Markers: Direct Evidence for In Vivo Platelet Activation. Am. J. Clin. Pathol. 2006, 125, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Bronzetti, E.; Mannino, F.; Mignini, F.; Morosetti, C.; Tayebati, S.K.; Amenta, F. Dopamine Receptors in Human Platelets. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 363, 376–382. [Google Scholar] [CrossRef]
- Amat, A.M.; Corrales, J.A.M.; Serrano, F.R.; Boulaiz, H.; Salazar, J.C.P.; Contreras, F.H.; Perez, O.C.; Delgado, E.C.; Martín, I.; Jimenez, A.A. Role of α-Actin in Muscle Damage of Injured Athletes in Comparison with Traditional Markers. Br. J. Sports Med. 2007, 41, 442–446. [Google Scholar] [CrossRef]
- Williams, M.H. Dietary Supplements and Sports Performance: Introduction and Vitamins. J. Int. Soc. Sports Nutr. 2004, 1, 1–6. [Google Scholar] [CrossRef]
- Amiri, M.; Ghiasvand, R.; Kaviani, M.; Forbes, S.C.; Salehi-Abargouei, A. Chocolate Milk for Recovery from Exercise: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. Eur. J. Clin. Nutr. 2019, 73, 835–849. [Google Scholar] [CrossRef]
- Ranucci, M.; Aloisio, T.; Di Dedda, U.; Menicanti, L.; de Vincentiis, C.; Baryshnikova, E. Gender-Based Differences in Platelet Function and Platelet Reactivity to P2Y12 Inhibitors. PLoS ONE 2019, 14, e0225771. [Google Scholar] [CrossRef]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender Difference in Oxidative Stress: A New Look at the Mechanisms for Cardiovascular Diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Raparelli, V.; Nocella, C.; Proietti, M.; Romiti, G.F.; Corica, B.; Bartimoccia, S.; Stefanini, L.; Lenzi, A.; Viceconte, N.; Tanzilli, G.; et al. Testosterone-to-Estradiol Ratio and Platelet Thromboxane Release in Ischemic Heart Disease: The EVA Project. J. Endocrinol. Investig. 2022. [Google Scholar] [CrossRef] [PubMed]


| Amateurs Athletes (n = 23) | Elite Football Players (n = 23) | p | |
|---|---|---|---|
| Age (years) | 30.2 ± 4.7 | 30.1 ± 4.8 | 0.943 |
| Gender (M/F) | 23/0 | 23/0 | - |
| WBC (×103 μL) | 7.2 ± 2.2 | 5.9 ± 1.4 | 0.02 |
| PLT (×103 μL) | 215.3 ± 40.3 | 210.6 ± 49.5 | 0.725 |
| RBC (×106 μL) | 5.1 ± 0.4 | 5.1 ± 0.39 | 0.99 |
| Colesterol (mg/dL) | 185.1 ± 30.8 | 172.3 ± 29.4 | 0.163 |
| Glycaemia (mg/dL) | 89.0 ± 28.8 | 83.5 ± 15.2 | 0.422 |
| Height (cm) | 179.8 ± 4.4 | 185.5 ± 5.6 | <0.001 |
| Weight (kg) | 78.8 ± 6.8 | 81 ± 6.7 | 0.549 |
| BMI | 24.3 ± 1.9 | 24.3 ± 1.3 | 0.99 |
| Heart rate at rest (bpm) | 62.1 ± 10.4 | 56.3 ± 11.6 | 0.08 |
| Systolic blood pressure (mmHg) | 114.8 ± 11.2 | 111.7 ± 7.9 | 0.284 |
| Diastolic blood pressure (mmHg) | 74.1 ± 7.2 | 70.9 ± 6.8 | 0.128 |
| Training per week (h) | 5.1 ± 2.0 | 14.4 ± 1.1 | <0.001 |
| Sport practice (years) | 12.7 ± 4.6 | 16 ± 1.2 | 0.002 |
| Maximum workload (METs) | 12.2 ± 1.8 | 15.4 ± 1.9 | <0.001 |
| Peak heart rate (bpm) | 164.7 ± 6.9 | 169 ± 11.5 | 0.131 |
| sCD40L (pg/mL) | 220 [183–251] | 294 [264–338] | <0.001 |
| PDGF-bb (ng/mL) | 4.9 [4.7–6.4] | 8.1 [6.0–9.7] | <0.001 |
| Dopamin (pg/mL) | 8.1 [7.6–9.6] | 24 [12–31] | <0.001 |
| H2O2 (μM) | 15 [10–19] | 18 [13–32] | 0.02 |
| sNOX2dp (pg/mL) | 1.5 [0.6–3.1] | 8.6 [3.5–11] | <0.001 |
| HBA (%) | 44 ± 12 | 32 ± 6 | <0.001 |
| CK (mU/mL) | 491 ± 62 | 669 ± 160 | 0.04 |
| LDH (mU/mL) | 109 [94–147] | 152 [128–191] | <0.001 |
| Myoglobin (ng/mL) | 108 [96–111] | 111 [98–161] | 0.135 |
| CK | ||
|---|---|---|
| Rs | p Value | |
| sNOX2dp | 0.112 | p = 0.02 |
| H2O2 | 0.016 | p = 0.124 |
| sCD40L | 0.08 | p < 0.05 |
| PDGF-bb | 0.268 | p < 0.001 |
| LDH | ||
| Rs | pValue | |
| sNOX2dp | 0.121 | p = 0.017 |
| Myoglobin | ||
| Rs | pValue | |
| sCD40L | 0.09 | p < 0.05 |
| Compounds | Dark Chocolate |
|---|---|
| Total polyphenols, μg GAE/mL | 799 |
| Epicatechin, mg/g | 0.65 |
| Catechin, mg/g | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amico, A.; Cavarretta, E.; Fossati, C.; Borrione, P.; Pigozzi, F.; Frati, G.; Sciarretta, S.; Costa, V.; De Grandis, F.; Nigro, A.; et al. Platelet Activation Favours NOX2-Mediated Muscle Damage in Elite Athletes: The Role of Cocoa-Derived Polyphenols. Nutrients 2022, 14, 1558. https://doi.org/10.3390/nu14081558
D’Amico A, Cavarretta E, Fossati C, Borrione P, Pigozzi F, Frati G, Sciarretta S, Costa V, De Grandis F, Nigro A, et al. Platelet Activation Favours NOX2-Mediated Muscle Damage in Elite Athletes: The Role of Cocoa-Derived Polyphenols. Nutrients. 2022; 14(8):1558. https://doi.org/10.3390/nu14081558
Chicago/Turabian StyleD’Amico, Alessandra, Elena Cavarretta, Chiara Fossati, Paolo Borrione, Fabio Pigozzi, Giacomo Frati, Sebastiano Sciarretta, Vincenzo Costa, Fabrizio De Grandis, Antonia Nigro, and et al. 2022. "Platelet Activation Favours NOX2-Mediated Muscle Damage in Elite Athletes: The Role of Cocoa-Derived Polyphenols" Nutrients 14, no. 8: 1558. https://doi.org/10.3390/nu14081558
APA StyleD’Amico, A., Cavarretta, E., Fossati, C., Borrione, P., Pigozzi, F., Frati, G., Sciarretta, S., Costa, V., De Grandis, F., Nigro, A., Peruzzi, M., Miraldi, F., Saade, W., Calogero, A., Rosa, P., Galardo, G., Loffredo, L., Pignatelli, P., Nocella, C., & Carnevale, R. (2022). Platelet Activation Favours NOX2-Mediated Muscle Damage in Elite Athletes: The Role of Cocoa-Derived Polyphenols. Nutrients, 14(8), 1558. https://doi.org/10.3390/nu14081558












