Genistein Alleviates High-Fat Diet-Induced Obesity by Inhibiting the Process of Gluconeogenesis in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Plasma Biochemical Index Test and Tissue Section
2.3. Glucose Tolerance Test (GTT), Insulin Tolerance Test (ITT) and Pyruvate Tolerance Test (PTT)
2.4. Cell Culture and Transfection
2.5. Real-Time Quantitative PCR
2.6. Dual Luciferase Reporting System
2.7. Analysis of Liver Fatty Acids
2.8. Statistical Analysis
3. Results
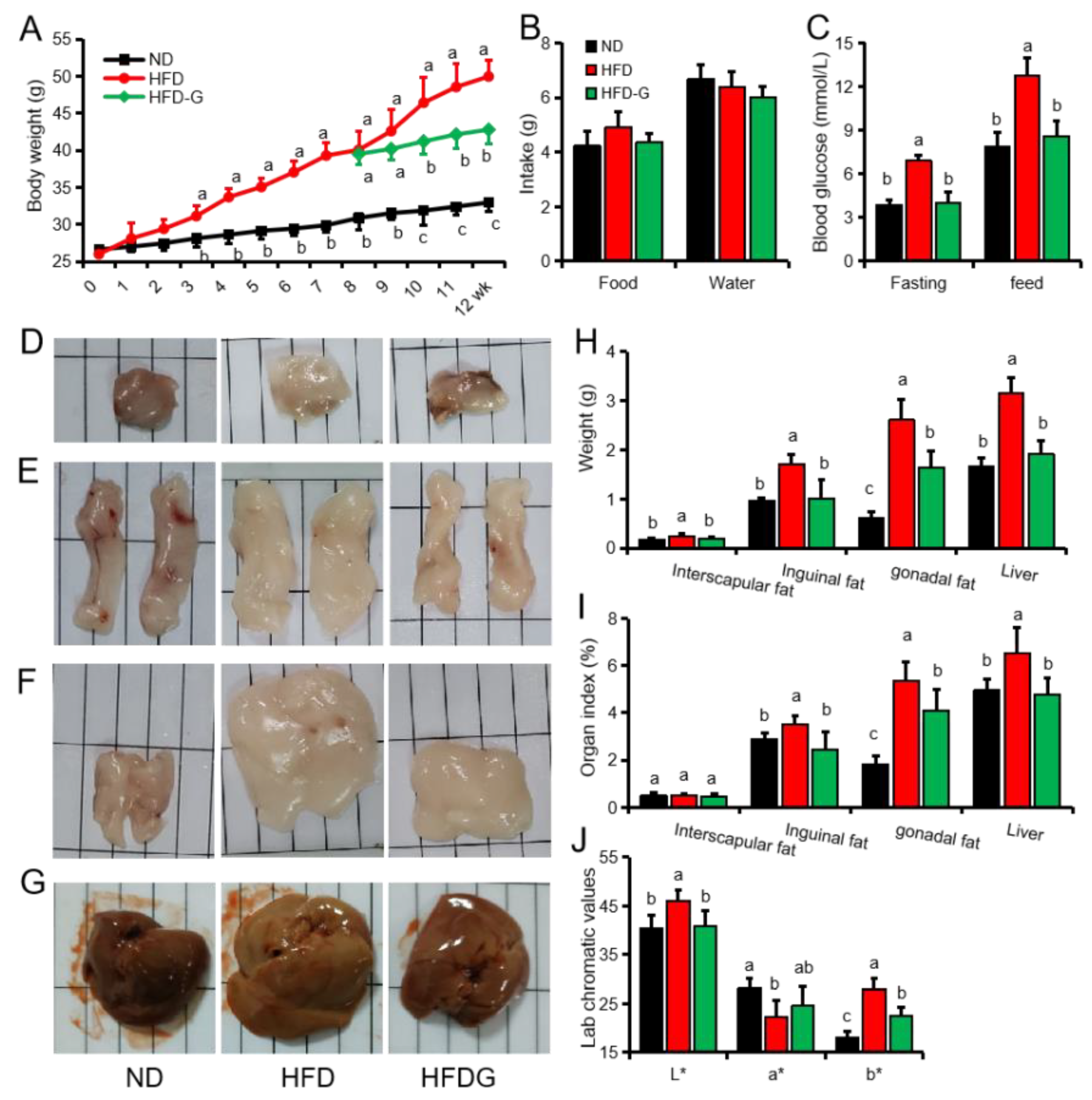
3.1. Genistein Reduces Body Weight and Fat Deposition in Obese Mice
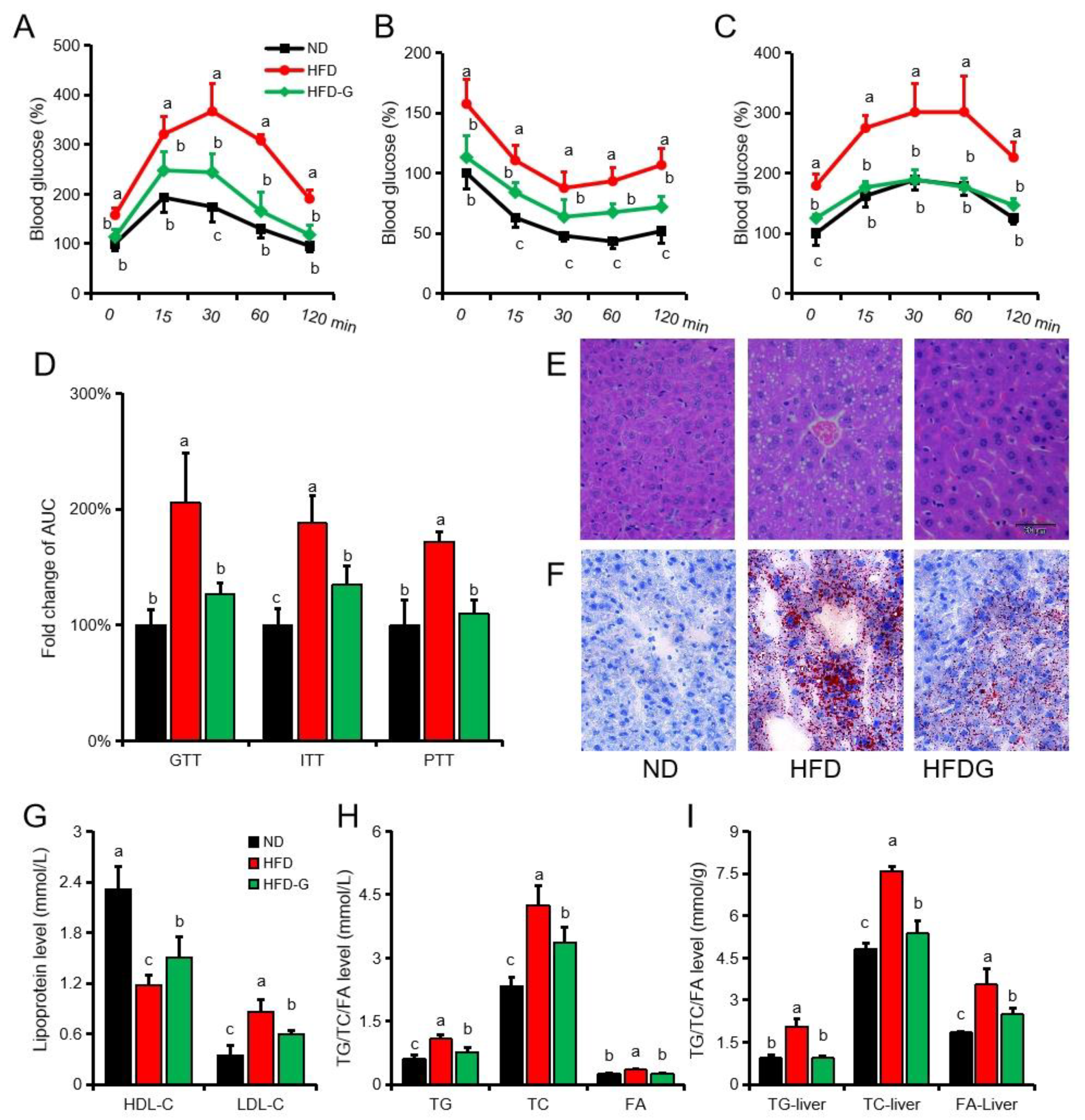
3.2. Genistein Alleviates Insulin Resistance and Inhibits Gluconeogenesis in Obese Mice
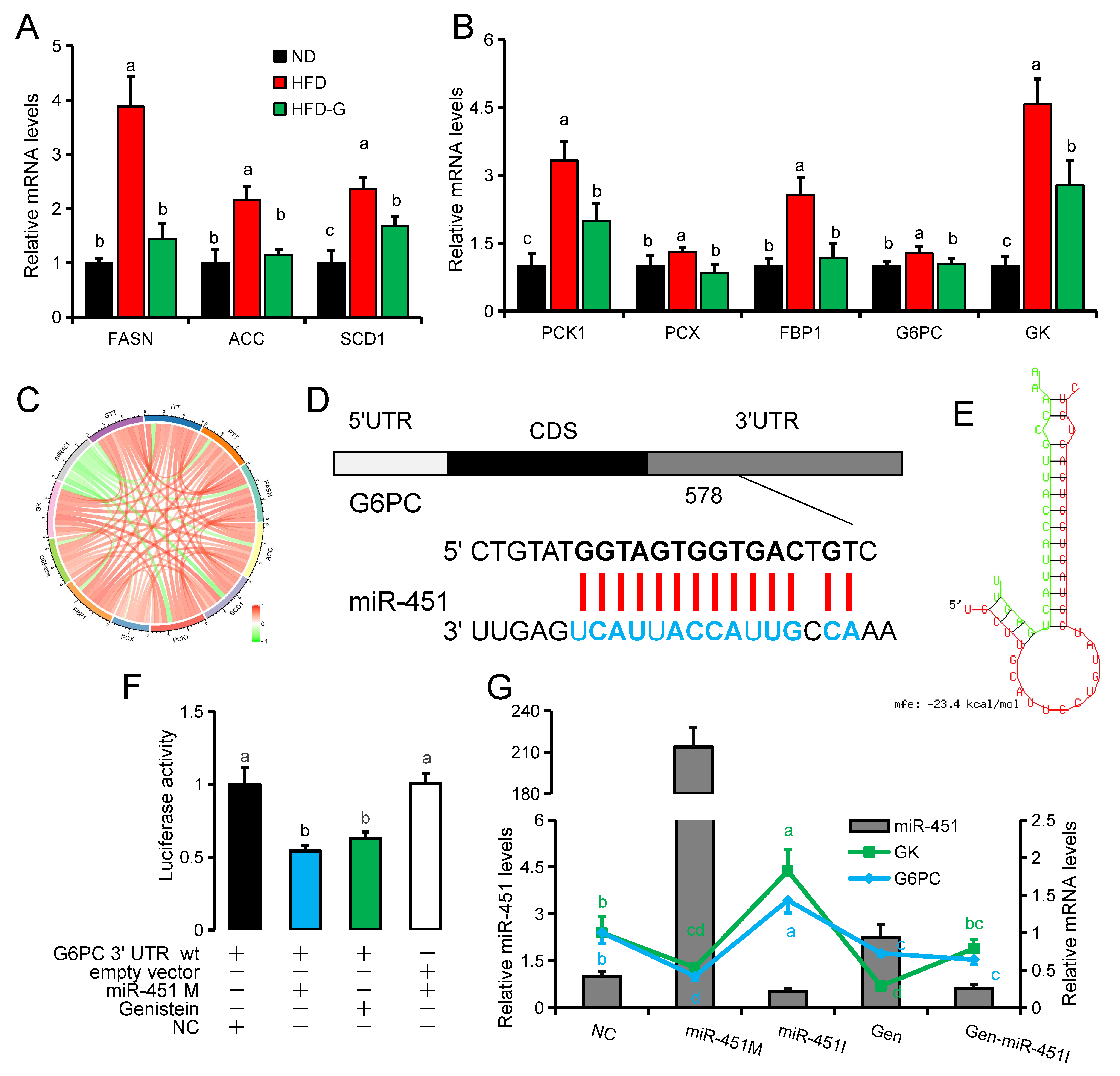
3.3. Genistein Regulates G6pc Expression via miR-451
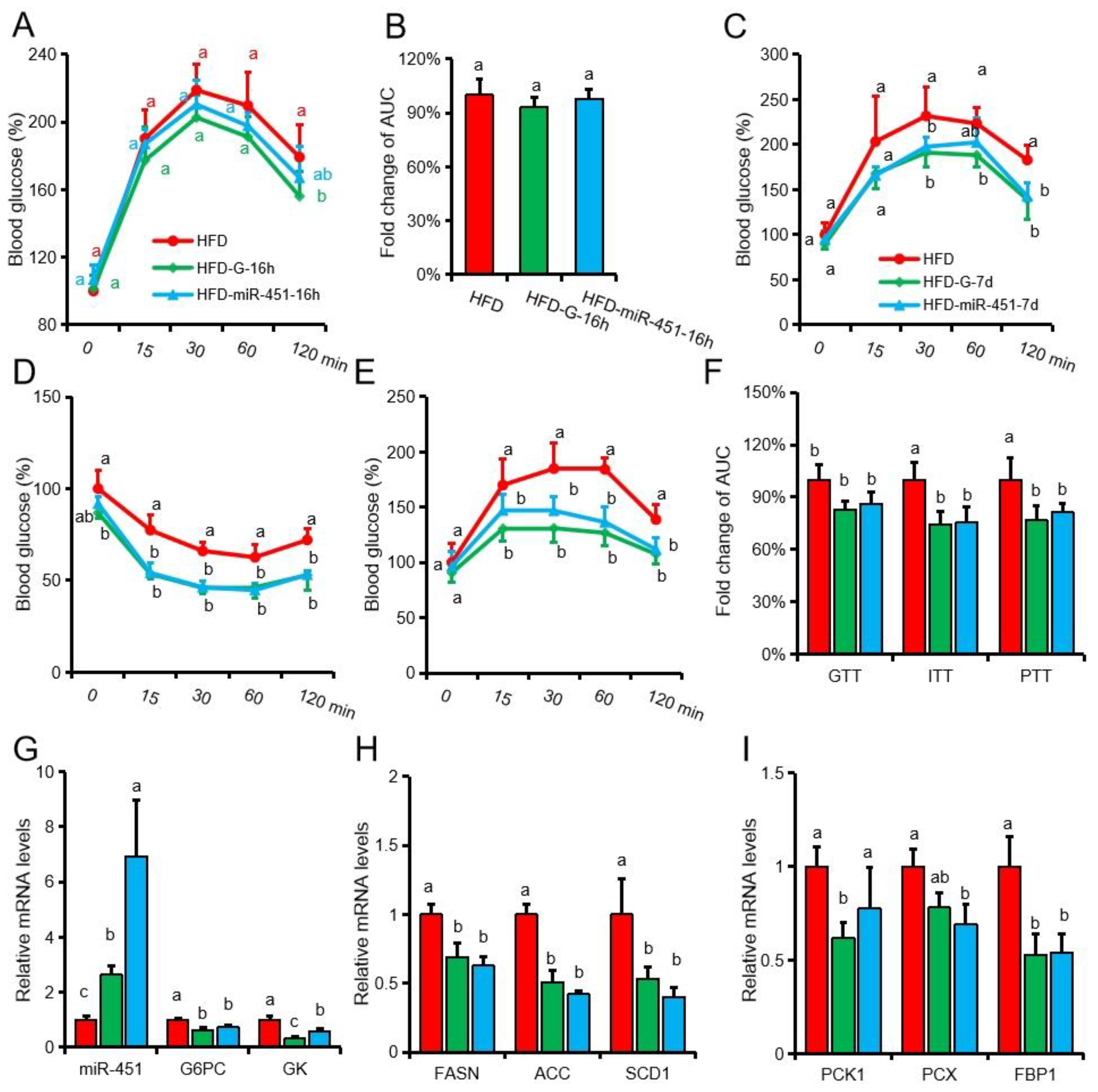
3.4. Both Genistein and miR-451 Agomir Treatment Effectively Inhibit Hepatic Gluconeogenesis in Obese Mice In Vivo
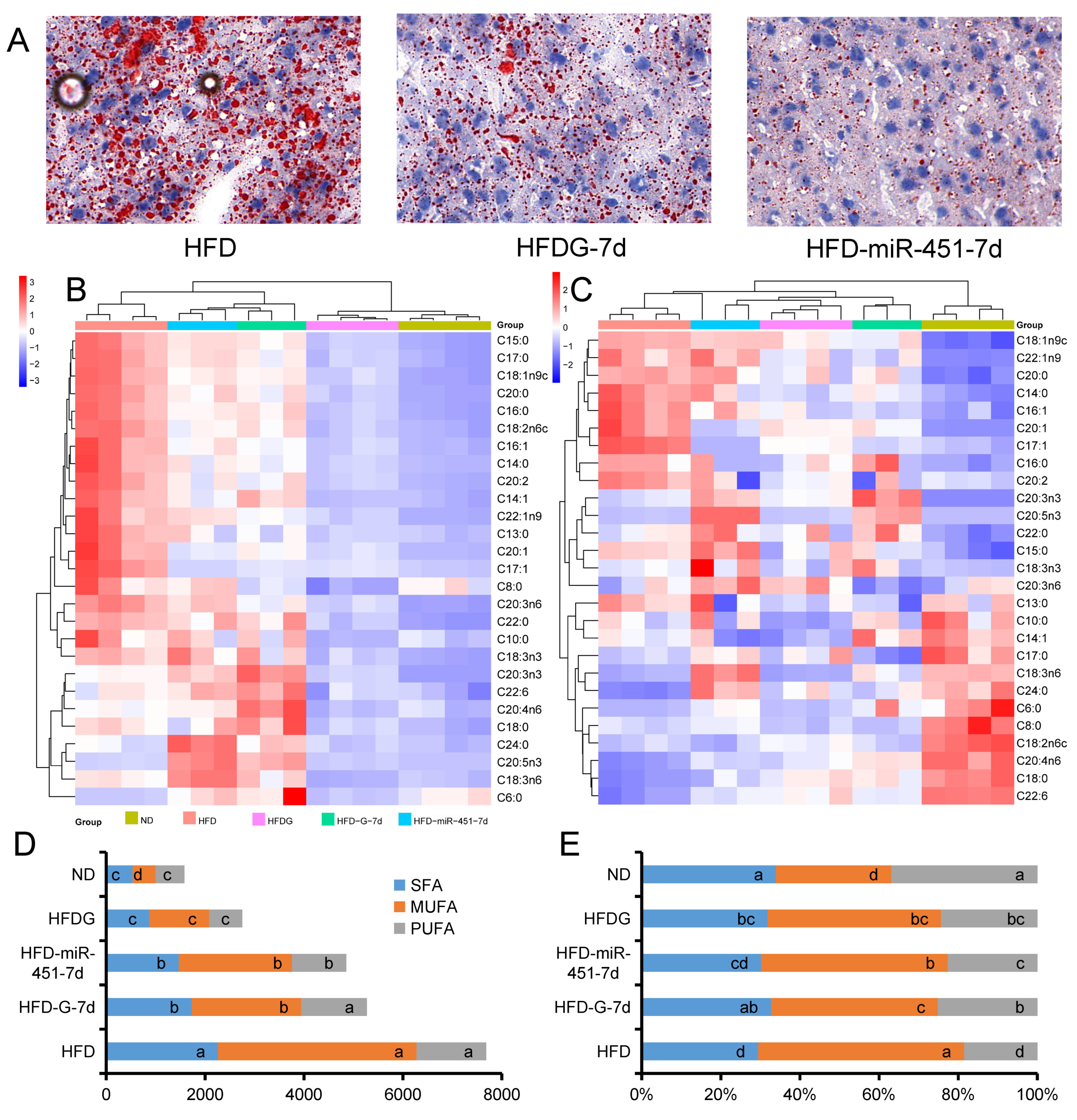
3.5. Genistein and miR-451 Agomir Affect Mouse Liver Fatty Acid Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammed, M.; Sendra, S.; Lloret, J.; Bosch, I. Systems and wbans for controlling obesity. J. Healthc. Eng. 2018, 2018, 1564748. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H. Managing type 2 diabetes mellitus in patients with obesity. Treat. Endocrinol. 2004, 3, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.; Hawley, J. The erosion of physical activity in western societies: An economic death march. Diabetologia 2015, 58, 1730–1734. [Google Scholar] [CrossRef] [PubMed]
- Wajngot, A.; Chandramouli, V.; Schumann, W.; Ekberg, K.; Jones, P.; Efendic, S.; Landau, B. Quantitative contributions of gluconeogenesis to glucose production during fasting in type 2 diabetes mellitus. Metab. Clin. Exp. 2001, 50, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Accili, D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011, 14, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.; Chacko, S.; Sunehag, A.; Haymond, M. Measurements of gluconeogenesis and glycogenolysis: A methodological review. Diabetes 2015, 64, 3996–4010. [Google Scholar] [CrossRef] [Green Version]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.; Hasan, P.; Shamsi, A. Role of polyphenols in combating type 2 diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Velayudhan, J.; Kelly, D. Analysis of gluconeogenic and anaplerotic enzymes in campylobacter jejuni: An essential role for phosphoenolpyruvate carboxykinase. Microbiology 2002, 148, 685–694. [Google Scholar] [CrossRef] [Green Version]
- Honma, K.; Kamikubo, M.; Mochizuki, K.; Goda, T. Insulin-induced inhibition of gluconeogenesis genes, including glutamic pyruvic transaminase 2, is associated with reduced histone acetylation in a human liver cell line. Metab. Clin. Exp. 2017, 71, 118–124. [Google Scholar] [CrossRef]
- Petersen, M.; Vatner, D.; Shulman, G. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Zhang, J.; Yu, J.; Liu, B.; Guo, Y.; Deng, J.; Chen, S.; Wang, C.; Guo, F. Microrna-214 suppresses gluconeogenesis by targeting activating transcriptional factor 4. J. Biol. Chem. 2015, 290, 8185–8195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, J.; Liu, C.; Qiao, A.; Cui, Y.; Zhang, H.; Cui, A.; Zhang, S.; Yang, Y.; Xiao, X.; Chen, Y.; et al. Microrna-29a-c decrease fasting blood glucose levels by negatively regulating hepatic gluconeogenesis. J. Hepatol. 2013, 58, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B. Humans against obesity: Who will win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Quigley, K.; Wadden, T. Dietary interventions for obesity: Clinical and mechanistic findings. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Ferreira, D. Genistein. Phytochemistry 2002, 60, 205–211. [Google Scholar] [CrossRef]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef]
- Verdrengh, M.; Jonsson, I.M.; Holmdahl, R.; Tarkowski, A. Genistein as an anti-inflammatory agent. Inflamm. Res. 2003, 52, 341–346. [Google Scholar] [CrossRef]
- Xin, X.; Chen, C.; Hu, Y.; Feng, Q. Protective effect of genistein on nonalcoholic fatty liver disease (nafld). Biomed. Pharmacother. 2019, 117, 109047. [Google Scholar] [CrossRef] [PubMed]
- Behloul, N.; Wu, G. Genistein: A promising therapeutic agent for obesity and diabetes treatment. Eur. J. Pharmacol. 2013, 698, 31–38. [Google Scholar] [CrossRef]
- Cotter, A.; Cashman, K. Genistein appears to prevent early postmenopausal bone loss as effectively as hormone replacement therapy. Nutr. Rev. 2003, 61, 346–351. [Google Scholar] [CrossRef]
- Hsieh, P.; Liao, Y.; Hsieh, C.; Chen, P.; Yu, C. Soy isoflavone genistein impedes cancer stemness and mesenchymal transition in head and neck cancer through activating mir-34a/rtcb axis. Nutrients 2020, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Huang, W.-L.; Xing, B.-P.; Fang, X.; Feng, M.; Jiang, C.-M. Genistein improves the major depression through suppressing the expression of mir-221/222 by targeting connexin 43. Psychiatry Investig. 2018, 15, 919–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, M.; Shen, L.; Wang, S.; Guo, Z.; Zheng, T.; Tan, Y.; Fan, Y.; Liu, L.; Chen, L.; Jiang, A.; et al. Genistein inhibits high fat diet-induced obesity through mir-222 by targeting btg2 and adipor1. Food Funct. 2020, 11, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Guo, X. The clinical potential of circulating micrornas in obesity. Nat. Rev. Endocrinol. 2019, 15, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Agbu, P.; Carthew, R. Microrna-mediated regulation of glucose and lipid metabolism. Nat. Rev. Mol. Cell Biol. 2021, 22, 425–438. [Google Scholar] [CrossRef]
- Zamani-Garmsiri, F.; Hashemnia, S.; Shabani, M.; Bagherieh, M.; Emamgholipour, S.; Meshkani, R. Combination of metformin and genistein alleviates non-alcoholic fatty liver disease in high-fat diet-fed mice. J. Nutr. Biochem. 2021, 87, 108505. [Google Scholar] [CrossRef]
- Gan, M.; Shen, L.; Fan, Y.; Tan, Y.; Zheng, T.; Tang, G.; Niu, L.; Zhao, Y.; Chen, L.; Jiang, D.; et al. Microrna-451 and genistein ameliorate nonalcoholic steatohepatitis in mice. Int. J. Mol. Sci. 2019, 20, 6084. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Lu, Y.; Jiao, Y.; Liu, B.; Li, S.; Li, Y.; Xing, F.; Chen, D.; Liu, X.; Zhao, J.; et al. Paternal psychological stress reprograms hepatic gluconeogenesis in offspring. Cell Metab. 2016, 23, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rehmsmeier, M.; Steffen, P.; Hochsmann, M.; Giegerich, R. Fast and effective prediction of microrna/target duplexes. RNA 2004, 10, 1507–1517. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, E.; Liu, D. Anti-diabetic functions of soy isoflavone genistein: Mechanisms underlying its effects on pancreatic β-cell function. Food Funct. 2013, 4, 200–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, L.; Zhang, F.; Wang, R.; Ma, W.; Song, Y. A review on protective role of genistein against oxidative stress in diabetes and related complications. Chem. Biol. Interact. 2019, 310, 108665. [Google Scholar] [CrossRef] [PubMed]
- Dkhar, B.; Khongsti, K.; Thabah, D.; Syiem, D.; Satyamoorthy, K.; Das, B. Genistein represses pepck-c expression in an insulin-independent manner in hepg2 cells and in alloxan-induced diabetic mice. J. Cell. Biochem. 2018, 119, 1953–1970. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, C.; Li, H.; Huang, L.; Sun, Q.; Dong, Y.; Tian, C.; Gao, S.; Dong, H.; Guan, D.; et al. Identification and characterization of micrornas in raw milk during different periods of lactation, commercial fluid, and powdered milk products. Cell Res. 2010, 20, 1128–1137. [Google Scholar] [CrossRef]
- Xu, P.; Palmer, L.; Lechauve, C.; Zhao, G.; Yao, Y.; Luan, J.; Vourekas, A.; Tan, H.; Peng, J.; Schuetz, J.; et al. Regulation of gene expression by mir-144/451 during mouse erythropoiesis. Blood 2019, 133, 2518–2528. [Google Scholar] [CrossRef]
- Zhuo, S.; Yang, M.; Zhao, Y.; Chen, X.; Zhang, F.; Li, N.; Yao, P.; Zhu, T.; Mei, H.; Wang, S.; et al. Microrna-451 negatively regulates hepatic glucose production and glucose homeostasis by targeting glycerol kinase-mediated gluconeogenesis. Diabetes 2016, 65, 3276–3288. [Google Scholar] [CrossRef] [Green Version]
- Gan, M.; Zheng, T.; Shen, L.; Tan, Y.; Fan, Y.; Shuai, S.; Bai, L.; Li, X.; Wang, J.; Zhang, S.; et al. Genistein reverses isoproterenol-induced cardiac hypertrophy by regulating mir-451/timp2. Biomed. Pharmacother. 2019, 112, 108618. [Google Scholar] [CrossRef]
- Wang, Y.; Kwon, H.; Su, X.; Wondisford, F. Glycerol not lactate is the major net carbon source for gluconeogenesis in mice during both short and prolonged fasting. Mol. Metab. 2020, 31, 36–44. [Google Scholar] [CrossRef]
- Chou, J.; Mansfield, B. Mutations in the glucose-6-phosphatase-alpha (g6pc) gene that cause type ia glycogen storage disease. Hum. Mutat. 2008, 29, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Hodson, L.; Rosqvist, F.; Parry, S. The influence of dietary fatty acids on liver fat content and metabolism. Proc. Nutr. Soc. 2020, 79, 30–41. [Google Scholar] [CrossRef]
- Paiva, P.; Medina, F.; Viegas, M.; Ferreira, P.; Neves, R.; Sousa, J.; Ramos, M.; Fernandes, P. Animal fatty acid synthase: A chemical nanofactory. Chem. Rev. 2021, 121, 9502–9553. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free fatty acid receptors in health and disease. Physiol. Rev. 2020, 100, 171–210. [Google Scholar] [CrossRef] [PubMed]
- Sheashea, M.; Xiao, J.; Farag, M. Mufa in metabolic syndrome and associated risk factors: Is mufa the opposite side of the pufa coin? Food Funct. 2021, 12, 12221–12234. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.; Varbo, A. Triglycerides and cardiovascular disease. Lancet 2014, 384, 626–635. [Google Scholar] [CrossRef]
- Chapman, M.; Ginsberg, H.; Amarenco, P.; Andreotti, F.; Borén, J.; Catapano, A.; Descamps, O.; Fisher, E.; Kovanen, P.; Kuivenhoven, J.; et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur. Heart J. 2011, 32, 1345–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, M.; Chen, X.; Chen, Z.; Chen, L.; Zhang, S.; Zhao, Y.; Niu, L.; Li, X.; Shen, L.; Zhu, L. Genistein Alleviates High-Fat Diet-Induced Obesity by Inhibiting the Process of Gluconeogenesis in Mice. Nutrients 2022, 14, 1551. https://doi.org/10.3390/nu14081551
Gan M, Chen X, Chen Z, Chen L, Zhang S, Zhao Y, Niu L, Li X, Shen L, Zhu L. Genistein Alleviates High-Fat Diet-Induced Obesity by Inhibiting the Process of Gluconeogenesis in Mice. Nutrients. 2022; 14(8):1551. https://doi.org/10.3390/nu14081551
Chicago/Turabian StyleGan, Mailin, Xinquan Chen, Zongjian Chen, Lei Chen, Shunhua Zhang, Ye Zhao, Lili Niu, Xuewei Li, Linyuan Shen, and Li Zhu. 2022. "Genistein Alleviates High-Fat Diet-Induced Obesity by Inhibiting the Process of Gluconeogenesis in Mice" Nutrients 14, no. 8: 1551. https://doi.org/10.3390/nu14081551
APA StyleGan, M., Chen, X., Chen, Z., Chen, L., Zhang, S., Zhao, Y., Niu, L., Li, X., Shen, L., & Zhu, L. (2022). Genistein Alleviates High-Fat Diet-Induced Obesity by Inhibiting the Process of Gluconeogenesis in Mice. Nutrients, 14(8), 1551. https://doi.org/10.3390/nu14081551








