Lower Blood Vitamin D Levels Are Associated with Depressive Symptoms in a Population of Older Adults in Kuwait: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Ethical Approval and Informed Consent
2.3. Primary Outcome
2.4. Data Collection and Covariates
2.5. Biochemical Assessment
2.6. Assessment of Depressive Symptoms
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Study Samples
3.1.1. Association between Symptoms of Depression (GDS-15) and 25-(OH)-D Levels
3.1.2. Association between GDS-15 and 25(OH)D Grouped by Variables
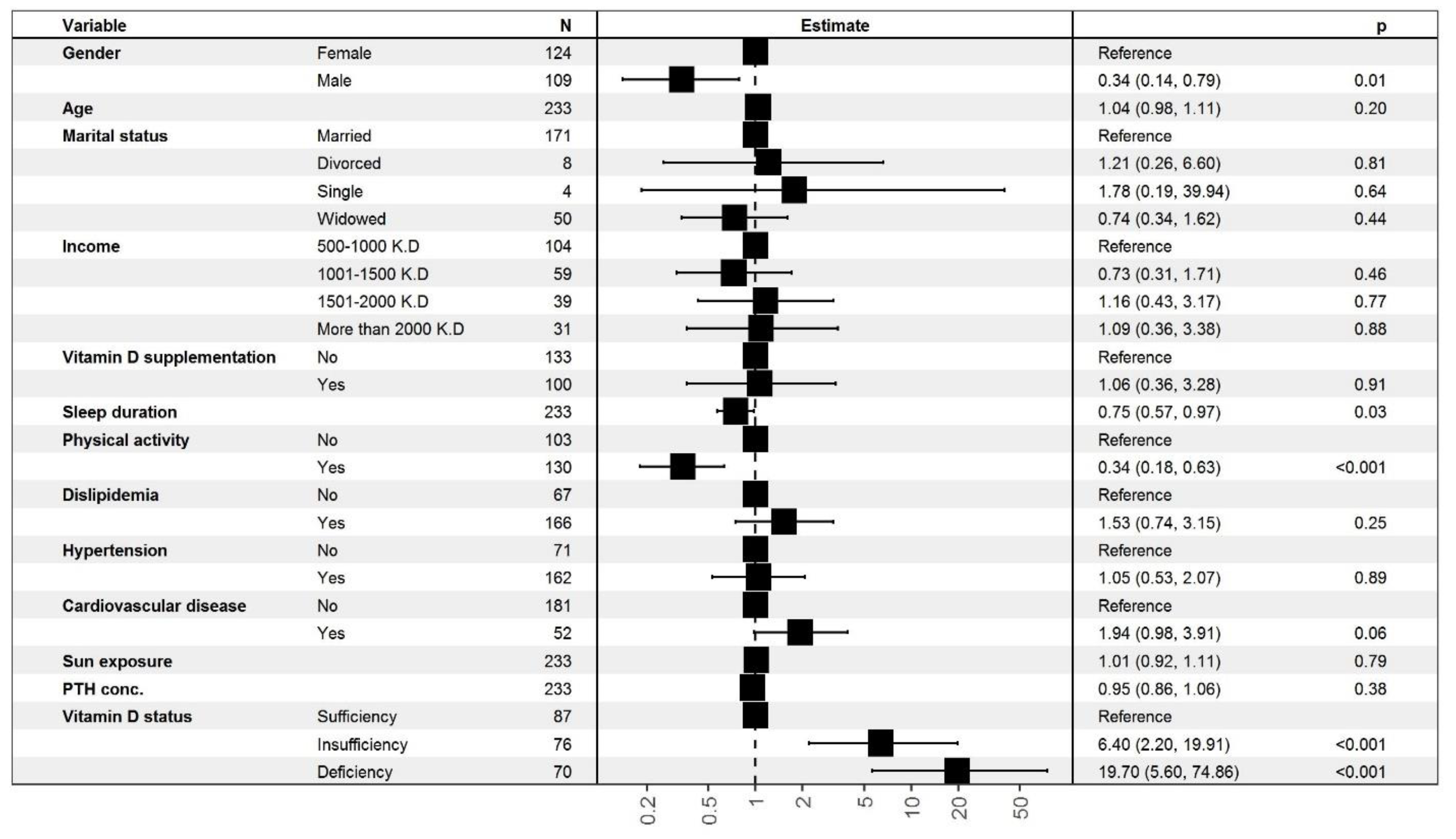
3.1.3. Ordinal Logistic Regression (OLR) Analysis for the Association between Depressive Symptoms and 25-(OH)-D Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-hydroxytryptamine receptors or serotonin receptors |
| 25(OH)D | 25-hydroxy vitamin D |
| BMI | Body mass index |
| CI | Confidence interval |
| GDS-15 | 15-item Geriatric Depression Scale |
| HSE | Health Survey for England |
| KD | Kuwaiti dinars |
| MMSE | Mini-Mental State Exam |
| OR | Odds ratio |
| PTH | Parathyroid hormone |
| WHO | World Health Organization |
References
- Friedrich, M.J. Depression Is the Leading Cause of Disability Around the World. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef]
- Vasiliadis, H.-M.; Dionne, P.-A.; Préville, M.; Gentil, L.; Berbiche, D.; Latimer, E. The Excess Healthcare Costs Associated with Depression and Anxiety in Elderly Living in the Community. Am. J. Geriatr. Psychiatry 2013, 21, 536–548. [Google Scholar] [CrossRef]
- Di Gessa, G.; Biddulph, J.P.; Zaninotto, P.; de Oliveira, C. Changes in vitamin D levels and depressive symptoms in later life in England. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Yao, Y.; Fu, S.; Zhang, H.; Li, N.; Zhu, Q.; Zhang, F.; Luan, F.; Zhao, Y.; He, Y. The prevalence of depressive symptoms in Chinese longevous persons and its correlation with vitamin D status. BMC Geriatr. 2018, 18, 1–7. [Google Scholar] [CrossRef]
- Awad, A.; Hanna, O. Potentially inappropriate medication use among geriatric patients in primary care setting: A cross-sectional study using the Beers, STOPP, FORTA and MAI criteria. PLoS ONE 2019, 14, e0218174. [Google Scholar] [CrossRef]
- Cullum, S.; Metcalfe, C.; Todd, C.; Brayne, C. Does depression predict adverse outcomes for older medical inpatients? A prospective cohort study of individuals screened for a trial. Age Ageing 2008, 37, 690–695. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Naughton, D.P. Vitamin D in health and disease: Current perspectives. Nutr. J. 2010, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Menon, V.; Kar, S.K.; Suthar, N.; Nebhinani, N. Vitamin D and Depression: A Critical Appraisal of the Evidence and Future Directions. Ind. J. Psychol. Med. 2020, 42, 11–21. [Google Scholar] [CrossRef]
- Mithal, A.; Wahl, D.A.; Bonjour, J.-P.; Burckhardt, P.; Dawson-Hughes, B.; Eisman, J.A.; Fuleihan, E.-H.; Josse, R.G.; Lips, P.; Morales-Torres, J. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos. Int. 2009, 20, 1807–1820. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Garcés, L.; Velázquez-Alva, M.C.; Cabrer-Rosales, M.F.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R.; Irigoyen-Camacho, M.E. Vitamin D Deficiency is Associated with Handgrip Strength, Nutritional Status and T2DM in Community-Dwelling Older Mexican Women: A Cross-Sectional Study. Nutrients 2021, 13, 736. [Google Scholar] [CrossRef]
- Omidian, M.; Mahmoudi, M.; Abshirini, M.; Eshraghian, M.R.; Javanbakht, M.H.; Zarei, M.; Hasani, H.; Djalali, M. Effects of vitamin D supplementation on depressive symptoms in type 2 diabetes mellitus patients: Randomized placebo-controlled double-blind clinical trial. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 2375–2380. [Google Scholar] [CrossRef]
- Boucher, B.J. The problems of vitamin d insufficiency in older people. Aging Dis. 2012, 3, 313–329. [Google Scholar]
- Anglin, R.E.S.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef]
- Ju, S.-Y.; Lee, Y.-J.; Jeong, S.-N. Serum 25-hydroxyvitamin d levels and the risk of depression: A systematic review and meta-analysis. J. Nutr. Health Aging 2013, 17, 447–455. [Google Scholar] [CrossRef]
- Han, B.; Lyu, Y.; Sun, H.; Wei, Y.; He, J. Low serum levels of vitamin D are associated with post-stroke depression. Eur. J. Neurol. 2015, 22, 1269–1274. [Google Scholar] [CrossRef]
- Lee, D.M.; Tajar, A.; O’Neill, T.W.; O’Connor, D.B.; Bartfai, G.; Boonen, S.; Bouillon, R.; Casanueva, F.F.; Finn, J.D.; Forti, G.; et al. Lower vitamin D levels are associated with depression among community-dwelling European men. J. Psychopharmacol. 2011, 25, 1320–1328. [Google Scholar] [CrossRef]
- Lapid, M.; Takahashi, P.; Cha, S. Vitamin D and depression in geriatric primary care patients. Clin. Interv. Aging 2013, 8, 509–514. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, C.; Hirani, V.; Biddulph, J.P. Associations between vitamin D levels and depressive symptoms in later life: Evidence from the English Longitudinal Study of Ageing (ELSA). J. Gerontol. Ser. A 2018, 73, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Vidgren, M.; Virtanen, J.; Tolmunen, T.; Nurmi, T.; Tuomainen, T.-P.; Voutilainen, S.; Ruusunen, A. Serum Concentrations of 25-Hydroxyvitamin D and Depression in a General Middle-Aged to Elderly Population in Finland. J. Nutr. Health Aging 2018, 22, 159–164. [Google Scholar] [CrossRef]
- Ceolin, G.; Matsuo, L.H.; Confortin, S.C.; D’Orsi, E.; Rieger, D.K.; Moreira, J.D. Lower serum 25-hydroxycholecalciferol is associated with depressive symptoms in older adults in Southern Brazil. Nutr. J. 2020, 19, 123. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R. Vitamin D and the occurrence of depression: Causal association or circumstantial evidence? Nutr. Rev. 2009, 67, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.; Brotchie, H. ‘D’ for depression: Any role for vitamin D? ‘Food for Thought’II. Acta Psychiatr. Scand. 2011, 124, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Toffanello, E.D.; Sergi, G.; Veronese, N.; Perissinotto, E.; Zambon, S.; Coin, A.; Sartori, L.; Musacchio, E.; Corti, M.-C.; Baggio, G.; et al. Serum 25-Hydroxyvitamin D and the Onset of Late-Life Depressive Mood in Older Men and Women: The Pro.V.A. Study. J. Gerontol. Ser. A 2014, 69, 1554–1561. [Google Scholar] [CrossRef]
- Wilkins, C.H.; Sheline, Y.I.; Roe, C.M.; Birge, S.J.; Morris, J.C. Vitamin D Deficiency Is Associated with Low Mood and Worse Cognitive Performance in Older Adults. Am. J. Geriatr. Psychiatry 2006, 14, 1032–1040. [Google Scholar] [CrossRef] [Green Version]
- Hoogendijk, W.J.G.; Lips, P.; Dik, M.G.; Deeg, D.J.H.; Beekman, A.T.F.; Penninx, B.W.J.H. Depression Is Associated With Decreased 25-Hydroxyvitamin D and Increased Parathyroid Hormone Levels in Older Adults. Arch. Gen. Psychiatry 2008, 65, 508–512. [Google Scholar] [CrossRef]
- Song, B.M.; Kim, H.C.; Rhee, Y.; Youm, Y.; Kim, C.O. Association between serum 25-hydroxyvitamin D concentrations and depressive symptoms in an older Korean population: A cross-sectional study. J. Affect. Disord. 2016, 189, 357–364. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1982, 17, 37–49. [Google Scholar] [CrossRef]
- Bigman, G. Vitamin D metabolites, D3 and D2, and their independent associations with depression symptoms among adults in the United States. Nutr. Neurosci. 2020, 1–9. [Google Scholar] [CrossRef]
- Al-Hazzaa, H.M. Health-enhancing physical activity among Saudi adults using the International Physical Activity Questionnaire (IPAQ). Public Health Nutr. 2007, 10, 59–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helou, K.; El Helou, N.; Mahfouz, M.; Mahfouz, Y.; Salameh, P.; Harmouche-Karaki, M. Validity and reliability of an adapted arabic version of the long international physical activity questionnaire. BMC Public Health 2018, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Al Banna, M.; Yehya, A.; Khairi, A.; Dafeeah, E.; Elhadi, A.; Rezgui, L.; Kahlout, S.A.; Yousif, A.; Uthman, B.; Al-Amin, H. Validation and cultural adaptation of the Arabic versions of the Mini–Mental Status Examination—2 and Mini-Cog test. Neuropsychiatr. Dis. Treat. 2017, 13, 793–801. [Google Scholar] [CrossRef] [Green Version]
- Weiderpass, E.; Botteri, E.; Longenecker, J.; Alkandari, A.; Al-Wotayan, R.; Al Duwairi, Q.; Tuomilehto, J. The Prevalence of Overweight and Obesity in an Adult Kuwaiti Population in 2014. Front. Endocrinol. 2019, 10, 449. [Google Scholar] [CrossRef] [Green Version]
- Alyahya, K.O. Vitamin D levels in schoolchildren: A cross-sectional study in Kuwait. BMC Pediatr. 2017, 17, 213. [Google Scholar] [CrossRef] [Green Version]
- Hanwell, H.; Vieth, R.; Cole, D.; Scillitani, A.; Modoni, S.G.; Frusciante, V.; Ritrovato, G.; Chiodini, I.; Minisola, S.; Carnevale, V. Sun exposure questionnaire predicts circulating 25-hydroxyvitamin D concentrations in Caucasian hospital workers in southern Italy. J. Steroid Biochem. Mol. Biol. 2010, 121, 334–337. [Google Scholar] [CrossRef]
- Zareef, T.A.; Jackson, R.T.; Alkahtani, A.A. Vitamin D Intake among Premenopausal Women Living in Jeddah: Food Sources and Relationship to Demographic Factors and Bone Health. J. Nutr. Metab. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Papandreou, D.; Rachaniotis, N.; Lari, M.; Al Mussabi, W. Validation of a Food Frequency Questionnaire for Vitamin D and Calcium Intake in Healthy Female College Students. Food Nutr. Sci. 2014, 5, 2048–2052. [Google Scholar] [CrossRef] [Green Version]
- Köhnke, C.; Herrmann, M.; Berger, K. Associations of major depressive disorder and related clinical characteristics with 25-hydroxyvitamin D levels in middle-aged adults. Nutr. Neurosci. 2020, 1–10. [Google Scholar] [CrossRef]
- Al-Taiar, A.; Rahman, A.; Al-Sabah, R.; Shaban, L.; Al-Harbi, A. Vitamin D status among adolescents in Kuwait: A cross-sectional study. BMJ Open 2018, 8, e021401. [Google Scholar] [CrossRef] [PubMed]
- Vogeser, M. Quantification of circulating 25-hydroxyvitamin D by liquid chromatography–tandem mass spectrometry. J. Steroid Biochem. Mol. Biol. 2010, 121, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; El Shair, I.H.; Taher, E.; Zyada, F. Prevalence and predictors of depression and anxiety among the elderly population living in geriatric homes in Cairo, Egypt. J. Egypt. Public Health Assoc. 2014, 89, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Chaaya, M.; Sibai, A.-M.; El Roueiheb, Z.; Chemaitelly, H.; Chahine, L.M.; Al-Amin, H.; Mahfoud, Z. Validation of the Arabic version of the short Geriatric Depression Scale (GDS-15). Int. Psychogeriatr. 2008, 20, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Alavi, N.M.; Khademalhoseini, S.; Vakili, Z.; Assarian, F. Effect of vitamin D supplementation on depression in elderly patients: A randomized clinical trial. Clin. Nutr. 2019, 38, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Mahfouz, E.M.; Mahfouz, M.M. Comparing three clinical screening tools in identification of geriatric depression in a rural community setting. Egypt. J. Community Med. 2008, 26, 57–73. [Google Scholar]
- Shipton, E.A.; Shipton, E.E. Vitamin D and Pain: Vitamin D and Its Role in the Aetiology and Maintenance of Chronic Pain States and Associated Comorbidities. Pain Res. Treat. 2015, 2015, 904967. [Google Scholar] [CrossRef] [Green Version]
- Eyles, D.W.; Burne, T.H.; McGrath, J.J. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front. Neuroendocr. 2013, 34, 47–64. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D receptor and 1α-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Patrick, R.P.; Ames, B.N. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: Relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015, 29, 2207–2222. [Google Scholar] [CrossRef] [Green Version]
- Stewart, R.; Hirani, V. Relationship Between Vitamin D Levels and Depressive Symptoms in Older Residents from a National Survey Population. Psychosom. Med. 2010, 72, 608–612. [Google Scholar] [CrossRef]
- de Koning, E.J.; Elstgeest, L.E.M.; Comijs, H.C.; Lips, P.; Rijnhart, J.J.M.; van Marwijk, H.W.J.; Beekman, A.T.F.; Visser, M.; Penninx, B.W.J.H.; van Schoor, N.M. Vitamin D Status and Depressive Symptoms in Older Adults: A Role for Physical Functioning? Am. J. Geriatr. Psychiatry. 2018, 26, 1131–1143. [Google Scholar] [CrossRef] [Green Version]
- Alsharji, K.E. Anxiety and depression during the COVID-19 pandemic in Kuwait: The importance of physical activity. Middle East Curr. Psychiatry 2020, 27, 1–8. [Google Scholar] [CrossRef]
- Elliott, M. Gender differences in causes of depression. Women Health 2001, 33, 183–198. [Google Scholar] [CrossRef]
- Wang, S.-J.; Chen, M.-Y. The effects of sunlight exposure therapy on the improvement of depression and quality of life in post-stroke patients: A RCT study. Heliyon 2020, 6, e04379. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.B.; Silva, E.S.; Hallgren, M.; De Leon, A.P.; Dunn, A.L.; Deslandes, A.C.; et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am. J. Psychiatry 2018, 175, 631–648. [Google Scholar] [CrossRef]
- Marques, A.; Bordado, J.; Peralta, M.; Gouveia, E.R.; Tesler, R.; Demetriou, Y.; Baya, D.G. Cross-sectional and prospective relationship between physical activity and depression symptoms. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Babyak, M.A.; Doraiswamy, P.M.; Watkins, L.; Hoffman, B.M.; Barbour, K.A.; Craighead, W.E.; Brosse, A.L.; Waugh, R.; Hinderliter, A.; et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom. Med. 2007, 69, 587. [Google Scholar] [CrossRef] [Green Version]
- Imai, C.M.; Halldorsson, T.I.; Eiriksdottir, G.; Cotch, M.F.; Steingrimsdottir, L.; Thorsdottir, I.; Launer, L.J.; Harris, T.; Gudnason, V.; Gunnarsdottir, I. Depression and serum 25-hydroxyvitamin D in older adults living at northern latitudes–AGES-Reykjavik Study. J. Nutr. Sci. 2015, 4, e37. [Google Scholar] [CrossRef] [Green Version]
- Okasha, T.A.; Sabry, W.M.; Hashim, M.A.; Abdeen, M.S.; Abdelhamid, A.M. Vitamin D serum level in major depressive disorder and schizophrenia. Middle East Curr. Psychiatry 2020, 27, 1–8. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, J.H.; Kim, D.Y.; Kang, J.W. Association between Self-Reported Sleep Duration and Serum Vitamin D Level in Elderly Korean Adults. J. Am. Geriatr. Soc. 2014, 62, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Piovezan, R.D.; Hirotsu, C.; Feres, M.C.; Cintra, F.D.; Andersen, M.L.; Tufik, S.; Poyares, D. Obstructive sleep apnea and objective short sleep duration are independently associated with the risk of serum vitamin D deficiency. PLoS ONE 2017, 12, e0180901. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Moraes, W.; Piovezan, R.; Poyares, D.; Bittencourt, L.; Santos-Silva, R.; Tufik, S. Effects of aging on sleep structure throughout adulthood: A population-based study. Sleep Med. 2014, 15, 401–409. [Google Scholar] [CrossRef]
- Cui, Y.; Gong, Q.; Huang, C.; Guo, F.; Li, W.; Wang, Y.; Cheng, X. The relationship between sunlight exposure duration and depressive symptoms: A cross-sectional study on elderly Chinese women. PLoS ONE 2021, 16, e0254856. [Google Scholar] [CrossRef]
- Epstein, L.J.; Kristo, D.; Strollo, P.J., Jr.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. JCSM 2009, 5, 263–276. [Google Scholar]
- Lambert, G.W.; Reid, C.; Kaye, D.M.; Jennings, G.L.; Esler, M.D. Effect of sunlight and season on serotonin turnover in the brain. Lancet 2002, 360, 1840–1842. [Google Scholar] [CrossRef]
- Svenningsson, P.; Chergui, K.; Rachleff, I.; Flajolet, M.; Zhang, X.; El Yacoubi, M.; Vaugeois, J.-M.; Nomikos, G.G.; Greengard, P. Alterations in 5-HT 1B Receptor Function by p11 in Depression-Like States. Science 2006, 311, 77–80. [Google Scholar] [CrossRef] [Green Version]
- Pan, A.; Lu, L.; Franco, O.; Yu, Z.; Li, H.; Lin, X. Association between depressive symptoms and 25-hydroxyvitamin D in middle-aged and elderly Chinese. J. Affect. Disord. 2009, 118, 240–243. [Google Scholar] [CrossRef]
- Zhao, G.; Ford, E.S.; Li, C.; Balluz, L.S. No associations between serum concentrations of 25-hydroxyvitamin D and parathyroid hormone and depression among US adults. Br. J. Nutr. 2010, 104, 1696–1702. [Google Scholar] [CrossRef] [Green Version]
- Øgard, C.G.; Engelmann, M.D.; Kistorp, C.; Nielsen, S.L.; Vestergaard, H. Increased plasma N-terminal pro-B-type natriuretic peptide and markers of inflammation related to atherosclerosis in patients with primary hyperparathyroidism. Clin. Endocrinol. 2005, 63, 493–498. [Google Scholar] [CrossRef]
- Young, S.N. Has the time come for clinical trials on the antidepressant effect of vitamin D? J. Psychiatry Neurosci. 2009, 34, 3. [Google Scholar] [PubMed]
- Johnson, M.A.; Kimlin, M.G. Vitamin D, aging, and the 2005 Dietary Guidelines for Americans. Nutr. Rev. 2006, 64, 410–421. [Google Scholar] [CrossRef]
- Milaneschi, Y.; Shardell, M.; Corsi, A.M.; Vazzana, R.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Serum 25-Hydroxyvitamin D and Depressive Symptoms in Older Women and Men. J. Clin. Endocrinol. Metab. 2010, 95, 3225–3233. [Google Scholar] [CrossRef] [Green Version]
- Gugger, A.; Marzel, A.; Orav, E.J.; Willett, W.C.; Dawson-Hughes, B.; Theiler, R.; Freystätter, G.; Egli, A.; Bischoff-Ferrari, H.A. Effect of Monthly High-Dose Vitamin D on Mental Health in Older Adults: Secondary Analysis of a RCT. J. Am. Geriatr. Soc. 2019, 67, 1211–1217. [Google Scholar] [CrossRef]
- Kjærgaard, M.; Waterloo, K.; Wang, C.E.A.; Almås, B.; Figenschau, Y.; Hutchinson, M.S.; Svartberg, J.; Jorde, R. Effect of vitamin D supplement on depression scores in people with low levels of serum 25-hydroxyvitamin D: Nested case—Control study and randomised clinical trial. Br. J. Psychiatry 2012, 201, 360–368. [Google Scholar] [CrossRef] [Green Version]
| [ALL] | Normal | Mild | Moderate | Severe | p-Value | |
|---|---|---|---|---|---|---|
| Variables | n = 237 | n = 34 | n = 100 | n = 100 | n = 3 | |
| Age, mean (SD), year | 71.4 (4.94) | 70.3 (4.12) | 71.3 (4.58) | 71.9 (5.46) | 69.5 (6.36) | 0.158 |
| Gender n % | 0.003 | |||||
| Female | 128 (54.0%) | 16 (47.1%) | 43 (43.0%) | 66 (66.0%) | 3 (100%) | 0.001 |
| Male | 109 (46.0%) | 18 (52.9%) | 57 (57.0%) | 34 (34.0%) | 0 (0.00%) | 0.001 |
| Marital status, n % | 0.042 | |||||
| Divorced | 8 (3.38%) | 2 (5.88%) | 1 (1.00%) | 5 (5.00%) | 0 (0.00%) | 0.072 |
| Married | 173 (73.0%) | 26 (76.5%) | 81 (81.0%) | 65 (65.0%) | 1 (33.3%) | <0.001 |
| Single | 4 (1.69%) | 0 (0.00%) | 1 (1.00%) | 3 (3.00%) | 0 (0.00%) | 0.112 |
| Widowed | 52 (21.9%) | 6 (17.6%) | 17 (17.0%) | 27 (27.0%) | 2 (66.7%) | <0.001 |
| Income per month, n (%) | 0.012 | |||||
| 500–1000 K.D | 104 (44.6%) | 9 (26.5%) | 39 (40.2%) | 53 (53.5%) | 3 (100%) | <0.000 |
| 1001–1500 K.D | 59 (25.3%) | 14 (41.2%) | 24 (24.7%) | 21 (21.2%) | 0 (0.00%) | <0.000 |
| 1501–2000 K.D | 39 (16.7%) | 5 (14.7%) | 20 (20.6%) | 14 (14.1%) | 0 (0.00%) | <0.000 |
| More than 2000 K.D | 31 (13.3%) | 6 (17.6%) | 14 (14.4%) | 11 (11.1%) | 0 (0.00%) | <0.002 |
| Education level, n (%) | 0.071 | |||||
| No formal education | 51 (21.5%) | 9 (26.5%) | 15 (15.0%) | 26 (26.0%) | 1 (33.3%) | 0.001 |
| Completed primary/intermediate school | 36 (15.2%) | 1 (2.94%) | 23 (23.0%) | 11 (11.0%) | 1 (33.3%) | 0.001 |
| Completed secondary school | 55 (23.2%) | 7 (20.6%) | 22 (22.0%) | 25 (25.0%) | 1 (33.3%) | 0.004 |
| Completed Diploma | 43 (18.1%) | 7 (20.6%) | 16 (16.0%) | 20 (20.0%) | 0 (0.00%) | 0.050 |
| University degree or above | 52 (21.9%) | 10 (29.4%) | 24 (24.0%) | 18 (18.0%) | 0 (0.00%) | 0.058 |
| Type of housing, n (%) | 0.170 | |||||
| Rental flat | 3 (1.27%) | 0 (0.00%) | 1 (1.00%) | 2 (2.00%) | 0 (0.00%) | NA |
| Rental house | 2 (0.84%) | 0 (0.00%) | 0 (0.00%) | 2 (2.00%) | 0 (0.00%) | NA |
| Owned flat | 1 (0.42%) | 0 (0.00%) | 0 (0.00%) | 1 (1.00%) | 0 (0.00%) | NA |
| Owned house | 231 (97.5%) | 34 (100%) | 99 (99.0%) | 95 (95.0%) | 3 (100%) | <0.001 |
| Having children | 6.59 (3.07) | 6.50 (3.10) | 6.81 (2.98) | 6.33 (3.14) | 8.67 (3.51) | 0.757 |
| Sleep duration (day/hours; mean (SD) | 6.41 (1.10) | 6.82 (1.10) | 6.52 (1.00) | 6.16 (1.15) | 6.50 (2.12) | 0.002 |
| Smoking cigarettes (yes/no), n (%) | 17 (7.17%) | 2 (5.88%) | 7 (7.00%) | 8 (8.00%) | 0 (0.00%) | 0.772 |
| Drinking alcohol (yes/no), n (%) | 4 (1.69%) | 0 (0.00%) | 2 (2.00%) | 2 (2.00%) | 0 (0.00%) | 0.585 |
| Walking per minutes, mean (SD) | 3.16 (2.99) | 4.1 (2.90) | 3.6 (3.00) | 2.5 (3.10) | 0.00 (0.00) | 0.004 |
| Physical activity n (%) | 108 (46.2%) | 9 (28.1%) | 37 (37.4%) | 59 (59.0%) | 3 (100%) | <0.001 |
| Vitamin D supplement (yes/no), n (%) | 100 (42.2%) | 28 (82.4%) | 43 (43.0%) | 29 (29.0%) | 0 (0.00%) | <0.001 |
| Calcium supplement (yes/no), n (%) | 7 (2.95%) | 2 (5.88%) | 3 (3.00%) | 2 (2.00%) | 0 (0.00%) | 0.261 |
| Dietary intake of vitamin D (IU) * | 247 (261) | 307 (277) | 245 (242) | 233 (277) | 114 (76.0) | 0.157 |
| Dietary intake of calcium (mg) * | 871 (565) | 1072 (564) | 919 (552) | 772 (564) | 404 (177) | 0.002 |
| Sun Exposure | 1.27 (3.03) | 2.21 (2.85) | 1.42 (3.44) | 0.83 (2.62) | 0.00 (0.00) | 0.014 |
| [ALL] | Normal | Mild | Moderate | Severe | p-Value | |
|---|---|---|---|---|---|---|
| Variables | n = 237 | n = 34 | n = 100 | n = 100 | n = 3 | |
| Systolic mm Hg; mean (SD) | 133 (12.2) | 131 (11.1) | 132 (11.7) | 135 (13.0) | 133 (15.3) | 0.361 |
| Diastolic mm Hg; mean (SD) | 78.7 (11.6) | 80.2 (20.8) | 77.7 (6.62) | 79.2 (11.6) | 77.7(4.04) | 0.681 |
| Serum 25-OH-D level (nmol/L), n (%) | <0.001 | |||||
| Deficiency < 50 nmol/L | 72 (30.4%) | 1 (2.94%) | 27 (27.0%) | 42 (42.0%) | 2 (66.7%) | <0.001 |
| Insufficiency: 50–75 nmol/L | 78 (32.9%) | 3 (8.82%) | 37 (37.0%) | 37 (37.0%) | 1 (33.3%) | <0.001 |
| Sufficiency ≥ 75 nmol/L | 87 (36.7%) | 30 (88.2%) | 36 (36.0%) | 21 (21.0%) | 0 (0.00%) | 0.140 |
| Dyslipidemia | 170 (71.7%) | 18 (52.9%) | 72 (72.0%) | 77 (77.0%) | 3 (100%) | 0.008 |
| Hypertension | 165 (69.6%) | 21 (61.8%) | 66 (66.0%) | 76 (76.0%) | 2 (66.7%) | 0.084 |
| Type 2 diabetes | 152 (64.1%) | 19 (55.9%) | 62 (62.0%) | 68 (68.0%) | 3 (100%) | 0.099 |
| Cardiovascular disease | 54 (22.9%) | 4 (11.8%) | 22 (22.0%) | 26 (26.3%) | 2 (66.7%) | 0.037 |
| Osteoporosis (OA) | 59 (24.9%) | 6 (17.6%) | 24 (24.0%) | 28 (28.0%) | 1 (33.3%) | 0.208 |
| Pigmentary phototype: | 0.107 | |||||
| II | 3 (1.27%) | 0 (0.00%) | 3 (3.00%) | 0 (0.00%) | 0 (0.00%) | NA |
| III | 58 (24.5%) | 12 (35.3%) | 22 (22.0%) | 22 (22.0%) | 2 (66.7%) | 0.118 |
| IV | 154 (65.0%) | 22 (64.7%) | 65 (65.0%) | 66 (66.0%) | 1 (33.3%) | <0.001 |
| V | 22 (9.28%) | 0 (0.00%) | 10 (10.0%) | 12 (12.0%) | 0 (0.00%) | 0.670 |
| Seasonality, n (%) | 0.338 | |||||
| Summer/Fall | 82 (34.6%) | 11 (32.4%) | 30 (30.0%) | 41 (41.0%) | 0 (0.00%) | <0.001 |
| Winter/Spring | 155 (65.4%) | 23 (67.6%) | 70 (70.0%) | 59 (59.0%) | 3 (100%) | <0.001 |
| Variables | Normal | Mild | Moderate | Severe | p-Value |
|---|---|---|---|---|---|
| n = 34 | n = 100 | n = 100 | n = 3 | ||
| BMI (kg/m2), n (%) | <0.001 | ||||
| Normal weight Overweight Obese | 8 (23.5%) 16 (47.1%) 10 (29.4%) | 12 (12.2%) 43 (43.9%) 43 (43.9%) | 42 (42.0%) 37 (37.0%) 21 (21.0%) | 2 (66.7%) 1 (33.3%) 0 (0.00%) | <0.001 <0.01 <0.001 |
| Waist–hip ratio WHR, n (%) | <0.001 | ||||
| Low Moderate High | 10 (29.4%) 9 (26.5%) 15 (44.1%) | 33 (33.0%) 16 (16.0%) 51 (51.0%) | 26 (26.3%) 14 (14.1%) 59 (59.6%) | 0 (0.00%) 1 (33.3%) 2 (66.7%) | <0.01 0.342 <0.001 |
| Waist (cm) mean (SD) | 98.3 (26.0) | 103 (17.8) | 102 (16.8) | 101 (15.3) | 0.610 |
| Hip (cm) mean (SD) | 102 (30.5) | 108 (15.2) | 109 (12.7) | 111 (9.83) | 0.064 |
| 25-OH-D nmol/L | 100 (31.7) | 71.2 (38.6) | 58.6 (30.1) | 49.0 (6.93) | <0.001 |
| ALP IU/L | 72.6 (22.8) | 71.0 (19.1) | 76.2 (29.0) | 102 (40.5) | 0.104 |
| PO4 mmol/L | 1.13 (0.13) | 1.12 (0.17) | 1.12 (0.16) | 1.43 (0.29) | 0.013 |
| Ca mmol/L | 2.30 (0.10) | 2.30 (0.11) | 2.31 (0.17) | 2.21 (0.07) | 0.600 |
| PTH pmol/L | 5.55 (2.17) | 5.89 (2.45) | 6.19 (3.23) | 15.4 (18.6) | <0.001 |
| Cortisol (AM) nmol/L | 379 (159) | 384 (135) | 375 (143) | 252 (64.3) | 0.464 |
| Glucose (F) mmol/L | 6.15 (1.55) | 6.74 (2.12) | 7.77 (8.66) | 12.6 (11.1) | 0.169 |
| Insulin ulU/mL | 13.4 (6.62) | 16.2 (11.6) | 15.3 (10.7) | 10.2 (7.40) | 0.466 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albolushi, T.; Bouhaimed, M.; Spencer, J. Lower Blood Vitamin D Levels Are Associated with Depressive Symptoms in a Population of Older Adults in Kuwait: A Cross-Sectional Study. Nutrients 2022, 14, 1548. https://doi.org/10.3390/nu14081548
Albolushi T, Bouhaimed M, Spencer J. Lower Blood Vitamin D Levels Are Associated with Depressive Symptoms in a Population of Older Adults in Kuwait: A Cross-Sectional Study. Nutrients. 2022; 14(8):1548. https://doi.org/10.3390/nu14081548
Chicago/Turabian StyleAlbolushi, Thurayya, Manal Bouhaimed, and Jeremey Spencer. 2022. "Lower Blood Vitamin D Levels Are Associated with Depressive Symptoms in a Population of Older Adults in Kuwait: A Cross-Sectional Study" Nutrients 14, no. 8: 1548. https://doi.org/10.3390/nu14081548
APA StyleAlbolushi, T., Bouhaimed, M., & Spencer, J. (2022). Lower Blood Vitamin D Levels Are Associated with Depressive Symptoms in a Population of Older Adults in Kuwait: A Cross-Sectional Study. Nutrients, 14(8), 1548. https://doi.org/10.3390/nu14081548






