Vitamin D Supplementation in Patients with Juvenile Idiopathic Arthritis
Abstract
1. Introduction
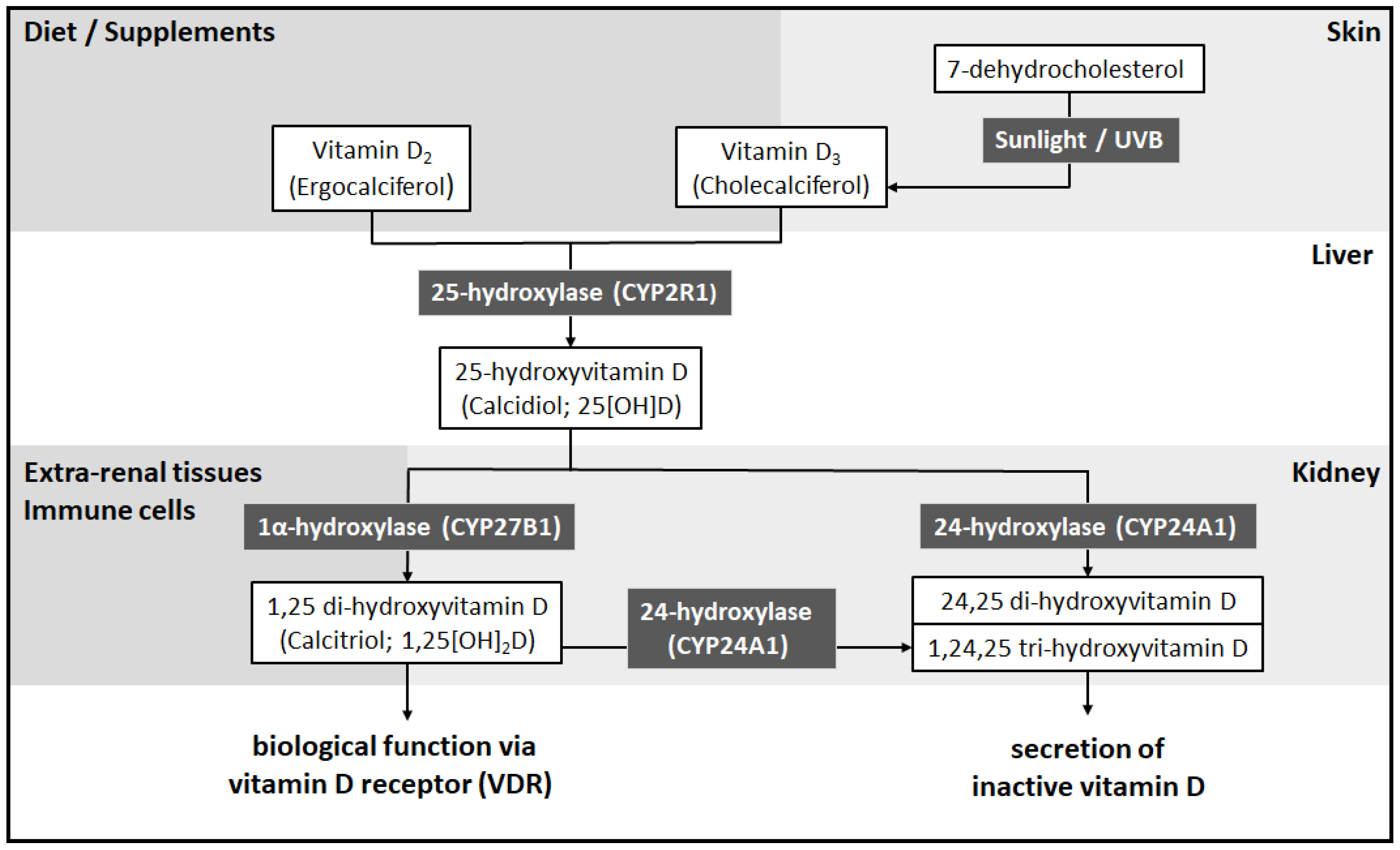
2. Vitamin D
3. Role of Vitamin D in Bone Metabolism
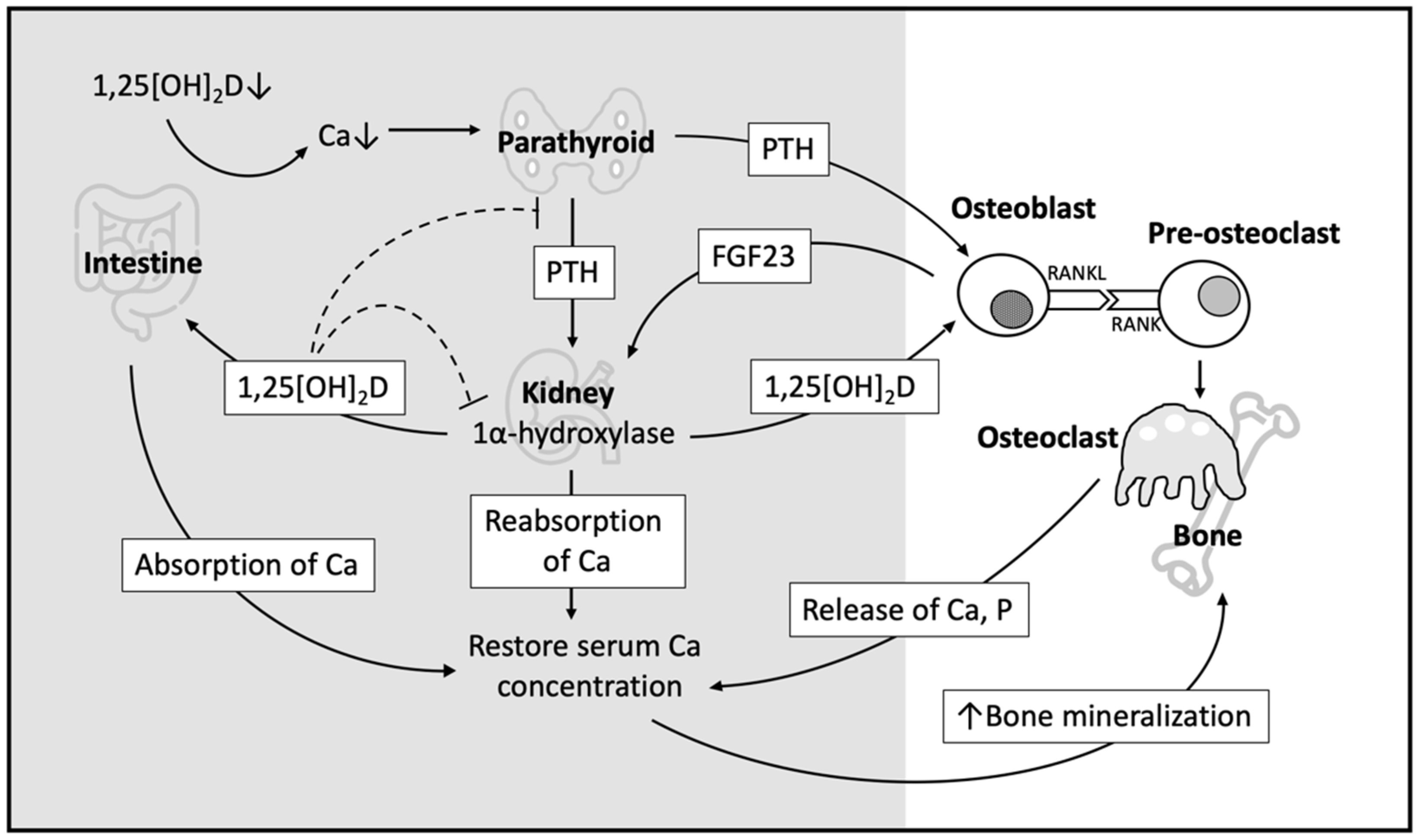
3.1. Indirect Effect of Vitamin D on Bone Homeostasis
3.2. Direct Effect of Vitamin D on Bone Homeostasis
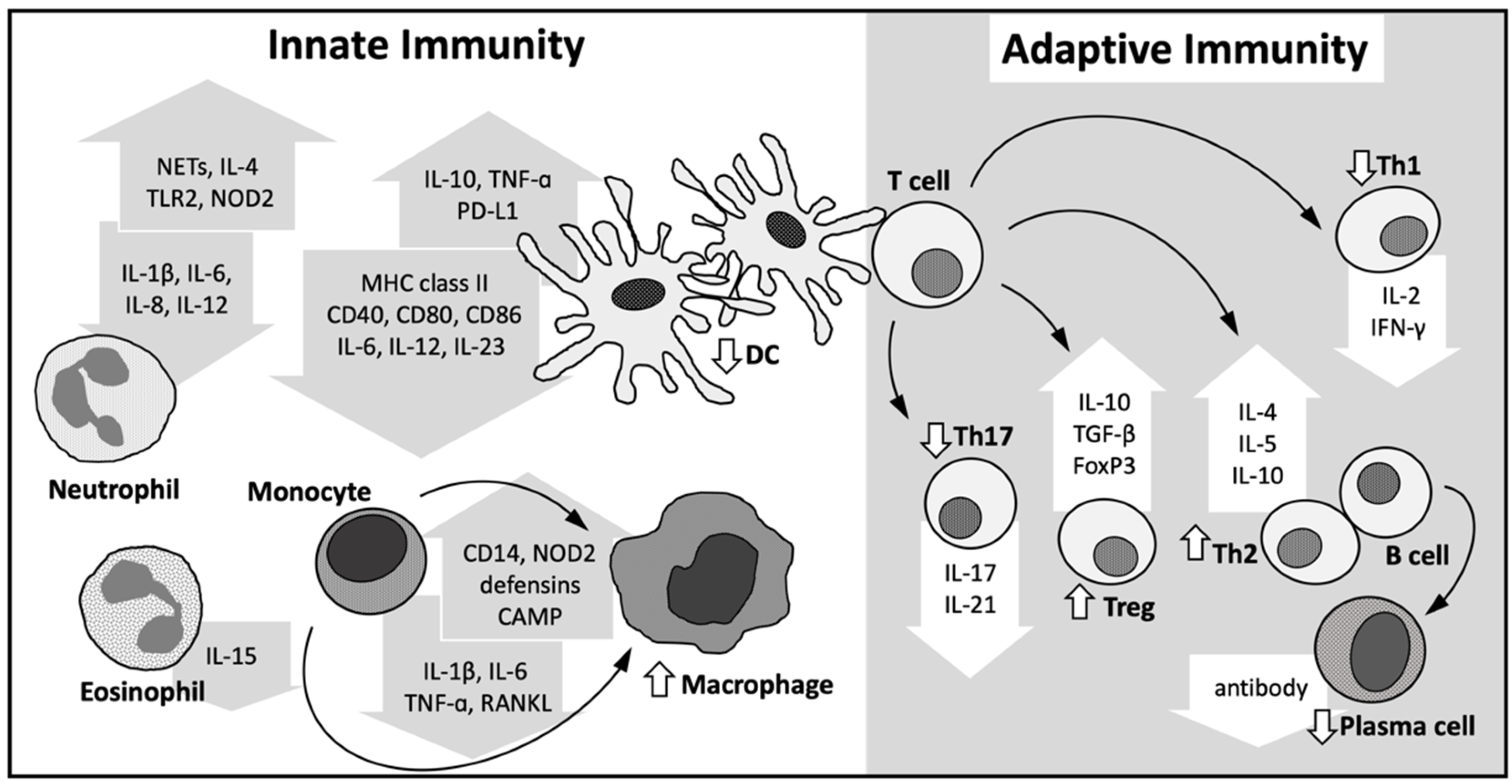
4. Immune Modulatory Role of Vitamin D
4.1. Innate Immune System
4.2. Adaptive Immune System
4.3. Others
5. Clinical Significance of Vitamin D in Patients with JIA
5.1. Vitamin D Level and the Presence of JIA
5.2. Vitamin D Level and BMD in Patients with JIA
5.3. Vitamin D Level and Disease Activity of JIA
5.4. Genomic Association of Vitamin D Signaling and JIA
6. Vitamin D Supplementation in JIA
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMD | bone mineral density |
| DBP | vitamin D-binding protein |
| DMARDs | disease-modifying antirheumatic drugs |
| FGF23 | fibroblast growth factor 23 |
| IFN | interferon |
| IgE | immunoglogulin E |
| IL | interleukin |
| JIA | juvenile idiopathic arthritis |
| NETs | neutrophil extracellular traps |
| NOD2 | oligomerization domain containing 2 |
| OPG | osteoprotegerin |
| PD-L1 | programmed death-ligand 1 |
| PTH | parathyroid hormone |
| RA | rheumatoid arthritis |
| RANK | receptor activator of NFκB |
| RANKL | RANK ligand |
| SOSC | suppressor of cytokine signaling |
| Th1 | type 1 helper T cells |
| Th17 | type 17 helper T cells |
| Th2 | type 2 helper T cells |
| Tregs | regulatory T cells |
| TLR | Toll-like receptor |
| TNF | tumor necrosis factor |
| UVB | ultraviolet B |
| VDREs | vitamin D response elements |
| VDRs | vitamin D receptors |
References
- Dave, M.; Rankin, J.; Pearce, M.; Foster, H.E. Global prevalence estimates of three chronic musculoskeletal conditions: Club foot, juvenile idiopathic arthritis and juvenile systemic lupus erythematosus. Pediatric Rheumatol. 2020, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Prakken, B.; Albani, S.; Martini, A. Juvenile idiopathic arthritis. Lancet 2011, 377, 2138–2149. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wang, C.T.; Gershwin, M.E.; Chiang, B.L. The pathogenesis of oligoarticular/polyarticular vs. systemic juvenile idiopathic arthritis. Autoimmun. Rev. 2011, 10, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Yang, H.Y.; Huang, J.L.; Lai, J.H. Signals and Mechanisms Regulating Monocyte and Macrophage Activation in the Pathogenesis of Juvenile Idiopathic Arthritis. Int. J. Mol. Sci. 2021, 22, 7960. [Google Scholar] [CrossRef] [PubMed]
- Palman, J.; Shoop-Worrall, S.; Hyrich, K.; McDonagh, J.E. Update on the epidemiology, risk factors and disease outcomes of Juvenile idiopathic arthritis. Best Pract. Res. Clin. Rheumatol. 2018, 32, 206–222. [Google Scholar] [CrossRef]
- Sengler, C.; Zink, J.; Klotsche, J.; Niewerth, M.; Liedmann, I.; Horneff, G.; Kessel, C.; Ganser, G.; Thon, A.; Haas, J.P.; et al. Vitamin D deficiency is associated with higher disease activity and the risk for uveitis in juvenile idiopathic arthritis—Data from a German inception cohort. Arthritis Res. Ther. 2018, 20, 276. [Google Scholar] [CrossRef] [PubMed]
- Finch, S.L.; Rosenberg, A.M.; Vatanparast, H. Vitamin D and juvenile idiopathic arthritis. Pediatric Rheumatol. 2018, 16, 34. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and bone. Curr. Osteoporos. Rep. 2012, 10, 151–159. [Google Scholar] [CrossRef]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [PubMed]
- Calton, E.K.; Keane, K.N.; Newsholme, P.; Soares, M.J. The impact of vitamin D levels on inflammatory status: A systematic review of immune cell studies. PLoS ONE 2015, 10, e0141770. [Google Scholar]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Skrobot, A.; Demkow, U.; Wachowska, M. Immunomodulatory role of vitamin D: A review. Adv. Exp. Med. Biol. 2018, 1108, 13–23. [Google Scholar] [PubMed]
- Mailhot, G.; White, J.H. Vitamin D and immunity in infants and children. Nutrients 2020, 12, 1233. [Google Scholar] [CrossRef]
- Zou, J.; Thornton, C.; Chambers, E.S.; Rosser, E.C.; Ciurtin, C. Exploring the evidence for an immunomodulatory role of vitamin D in juvenile and adult rheumatic disease. Front. Immunol. 2020, 11, 616483. [Google Scholar] [CrossRef]
- Bishop, E.; Ismailova, A.; Dimeloe, S.K.; Hewison, M.; White, J.H. Vitamin D and immune regulation: Antibacterial, antiviral, anti-inflammatory. JBMR Plus 2020, 5, e10405. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The effects of vitamin D on immune system and inflammatory diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed]
- Zipitis, C.S.; Akobeng, A.K. Vitamin D supplementation in early childhood and risk of type 1 diabetes: A systematic review and meta-analysis. Arch. Dis. Child. 2008, 93, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Khandker, S.S.; Alam, S.S.; Kotyla, P.; Hassan, R. Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmun. Rev. 2019, 18, 102392. [Google Scholar] [CrossRef] [PubMed]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association between inflammatory bowel disease and vitamin D deficiency: A systematic review and meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Sun, M.H.; Chen, F.; Li, J.R. Vitamin D levels in systemic sclerosis patients: A meta-analysis. Drug Des. Dev. Ther. 2017, 11, 3119–3125. [Google Scholar] [CrossRef]
- Duan, S.; Lv, Z.; Fan, X.; Wang, L.; Han, F.; Wang, H.; Bi, S. Vitamin D status and the risk of multiple sclerosis: A systematic review and meta-analysis. Neurosci. Lett. 2014, 570, 108–113. [Google Scholar] [CrossRef]
- Colotta, F.; Jansson, B.; Bonelli, F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017, 85, 78–97. [Google Scholar] [CrossRef] [PubMed]
- Tavera-Mendoza, L.E.; White, J.H. Cell defenses and the sunshine vitamin. Sci. Am. 2007, 297, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D. Extra Renal Synthesis of 1,25-dihydroxyvitamin D and its Health Implications. Clin. Rev. Bone Miner. Metab. 2009, 7, 114–125. [Google Scholar] [CrossRef]
- Martens, P.J.; Gysemans, C.; Verstuyf, A.; Mathieu, A.C. Vitamin D’s effect on immune function. Nutrients 2020, 12, 1248. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The non-genomic actions of vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Brandi, M.L.; Cusano, N.E.; Mannstadt, M.; Rejnmark, L.; Rizzoli, R.; Rubin, M.R.; Winer, K.K.; Liberman, U.A.; Potts, J.T., Jr. Management of hypoparathyroidism: Present and future. J. Clin. Endocrinol. Metab. 2016, 101, 2313–2324. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grubler, M.R.; Marz, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef]
- Roth, D.E.; Abrams, S.A.; Aloia, J.; Bergeron, G.; Bourassa, M.W.; Brown, K.H.; Calvo, M.S.; Cashman, K.D.; Combs, G.; De-Regil, L.M.; et al. Global prevalence and disease burden of vitamin D deficiency: A roadmap for action in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2018, 1430, 44–79. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. The role of vitamin D in the prevention of osteoporosis. Ann. Med. 2005, 37, 278–285. [Google Scholar] [CrossRef] [PubMed]
- d’Angelo, D.M.; Di Donato, G.; Breda, L.; Chiarelli, F. Growth and puberty in children with juvenile idiopathic arthritis. Pediatric Rheumatol. 2021, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, L.; Carmeliet, G. Integrated view on the role of vitamin D actions on bone and growth plate homeostasis. JBMR Plus 2021, 5, e10577. [Google Scholar] [CrossRef]
- Masuyama, R.; Stockmans, I.; Torrekens, S.; Van Looveren, R.; Maes, C.; Carmeliet, P.; Bouillon, R.; Carmeliet, G. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J. Clin. Investig. 2006, 116, 3150–3159. [Google Scholar] [CrossRef]
- Ma, W.T.; Gao, F.; Gu, K.; Chen, D.K. The role of monocytes and macrophages in autoimmune diseases: A comprehensive review. Front. Immunol. 2019, 10, 1140. [Google Scholar] [CrossRef]
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006, 21, 37–47. [Google Scholar] [CrossRef]
- Stoffels, K.; Overbergh, L.; Bouillon, R.; Mathieu, C. Immune regulation of 1alpha-hydroxylase in murine peritoneal macrophages: Unravelling the IFNgamma pathway. J. Steroid Biochem. Mol. Biol. 2007, 103, 567–571. [Google Scholar] [CrossRef]
- Hewison, M.; Freeman, L.; Hughes, S.V.; Evans, K.N.; Bland, R.; Eliopoulos, A.G.; Kilby, M.D.; Moss, P.A.; Chakraverty, R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J. Immunol. 2003, 170, 5382–5390. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Jain, S.K. 1,25(OH)2D3 inhibits oxidative stress and monocyte adhesion by mediating the upregulation of GCLC and GSH in endothelial cells treated with acetoacetate (ketosis). J. Steroid Biochem. Mol. Biol. 2016, 159, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; He, Y.; Shen, Y.; Zhang, Q.; Chen, D.; Zuo, C.; Qin, J.; Wang, H.; Wang, J.; Yu, Y. Vitamin D inhibits COX-2 expression and inflammatory response by targeting thioesterase superfamily member 4. J. Biol. Chem. 2014, 289, 11681–11694. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Tang, D.H.; Modlin, R.L. Cutting edge: Vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007, 179, 2060–2063. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Jain, S.K.; Micinski, D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Commun. 2013, 437, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Vitamin D signaling in the context of innate immunity: Focus on human monocytes. Front. Immunol. 2019, 10, 2211. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C. Molecular endocrinology of vitamin D on the epigenome level. Mol. Cell. Endocrinol. 2017, 453, 14–21. [Google Scholar] [CrossRef]
- Coutant, F.; Miossec, P. Altered dendritic cell functions in autoimmune diseases: Distinct and overlapping profiles. Nat. Rev. Rheumatol. 2016, 12, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.B.; Gysemans, C.A.; Demengeot, J.; da Cunha, J.P.; Vanherwegen, A.S.; Overbergh, L.; Van Belle, T.L.; Pauwels, F.; Verstuyf, A.; Korf, H.; et al. 1,25-dihydroxyvitamin D3 promotes tolerogenic dendritic cells with functional migratory properties in NOD mice. J. Immunol. 2014, 192, 4210–4220. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.D.; Lutz, W.; Phan, V.A.; Bachman, L.A.; McKean, D.J.; Kumar, R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: A vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 6800–6805. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.B.; van Etten, E.; Verstuyf, A.; Waer, M.; Overbergh, L.; Gysemans, C.; Mathieu, C. 1,25-dihydroxyvitamin D3 alters murine dendritic cell behaviour in vitro and in vivo. Diabetes Metab. Res. Rev. 2011, 27, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Vanherwegen, A.S.; Gysemans, C.; Mathieu, C. Vitamin D endocrinology on the cross-road between immunity and metabolism. Mol. Cell. Endocrinol. 2017, 453, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Adorini, L.; Penna, G. Dendritic cell tolerogenicity: A key mechanism in immunomodulation by vitamin D receptor agonists. Hum. Immunol. 2009, 70, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kleijwegt, F.S.; Laban, S.; Duinkerken, G.; Joosten, A.M.; Zaldumbide, A.; Nikolic, T.; Roep, B.O. Critical role for TNF in the induction of human antigen-specific regulatory T cells by tolerogenic dendritic cells. J. Immunol. 2010, 185, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Unger, W.W.; Laban, S.; Kleijwegt, F.S.; van der Slik, A.R.; Roep, B.O. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: Differential role for PD-L1. Eur. J. Immunol. 2009, 39, 3147–3159. [Google Scholar] [CrossRef] [PubMed]
- van Halteren, A.G.; Tysma, O.M.; van Etten, E.; Mathieu, C.; Roep, B.O. 1alpha,25-dihydroxyvitamin D3 or analogue treated dendritic cells modulate human autoreactive T cells via the selective induction of apoptosis. J. Autoimmun. 2004, 23, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Nakayama, Y.; Horiuchi, H.; Ohta, T.; Komoriya, K.; Ohmori, H.; Kamimura, T. Human neutrophils express messenger RNA of vitamin D receptor and respond to 1alpha,25-dihydroxyvitamin D3. Immunopharmacol. Immunotoxicol. 2002, 24, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, K.; Bergman, P.; Henriques-Normark, B. Vitamin D promotes pneumococcal killing and modulates inflammatory responses in primary human neutrophils. J. Innate Immun. 2017, 9, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Long, F.; Zhang, Y.; Yu, R.; Zhang, P.; Li, W.; Li, S.; Jin, X.; Xia, J.; Dong, L.; et al. 1alpha,25-dihydroxyvitamin D3 induces neutrophil apoptosis through the p38 MAPK signaling pathway in chronic obstructive pulmonary disease patients. PLoS ONE 2015, 10, e0120515. [Google Scholar]
- Yip, K.H.; Kolesnikoff, N.; Yu, C.; Hauschild, N.; Taing, H.; Biggs, L.; Goltzman, D.; Gregory, P.A.; Anderson, P.H.; Samuel, M.S.; et al. Mechanisms of vitamin D(3) metabolite repression of IgE-dependent mast cell activation. J. Allergy Clin. Immunol. 2014, 133, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Matheu, V.; Back, O.; Mondoc, E.; Issazadeh-Navikas, S. Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: Enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease. J. Allergy Clin. Immunol. 2003, 112, 585–592. [Google Scholar] [CrossRef]
- Baeke, F.; Korf, H.; Overbergh, L.; van Etten, E.; Verstuyf, A.; Gysemans, C.; Mathieu, C. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J. Steroid Biochem. Mol. Biol. 2010, 121, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, U.; Wakita, D.; Ohkuri, T.; Chamoto, K.; Kitamura, H.; Iwakura, Y.; Nishimura, T. 1alpha,25-Dihydroxyvitamin D3 and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunol. Lett. 2010, 134, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Urry, Z.; Chambers, E.S.; Xystrakis, E.; Dimeloe, S.; Richards, D.F.; Gabrysova, L.; Christensen, J.; Gupta, A.; Saglani, S.; Bush, A.; et al. The role of 1alpha,25-dihydroxyvitamin D3 and cytokines in the promotion of distinct Foxp3+ and IL-10+ CD4+ T cells. Eur. J. Immunol. 2012, 42, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Mijnheer, G.; Lutter, L.; Mokry, M.; van der Wal, M.; Scholman, R.; Fleskens, V.; Pandit, A.; Tao, W.; Wekking, M.; Vervoort, S.; et al. Conserved human effector Treg cell transcriptomic and epigenetic signature in arthritic joint inflammation. Nat. Commun. 2021, 12, 2710. [Google Scholar] [CrossRef]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Drozdenko, G.; Scheel, T.; Heine, G.; Baumgrass, R.; Worm, M. Impaired T cell activation and cytokine production by calcitriol-primed human B cells. Clin. Exp. Immunol. 2014, 178, 364–372. [Google Scholar] [CrossRef]
- Heine, G.; Niesner, U.; Chang, H.D.; Steinmeyer, A.; Zugel, U.; Zuberbier, T.; Radbruch, A.; Worm, M. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur. J. Immunol. 2008, 38, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Na, S.; Rathnachalam, R. Noncalcemic actions of vitamin D receptor ligands. Endocr. Rev. 2005, 26, 662–687. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Lv, C.; Wang, F.; Gan, K.; Zhang, M.; Tan, W. Modulatory effect of 1,25-dihydroxyvitamin D 3 on IL1 beta -induced RANKL, OPG, TNF alpha, and IL-6 expression in human rheumatoid synoviocyte MH7A. Clin. Dev. Immunol. 2013, 2013, 160123. [Google Scholar] [CrossRef]
- Laragione, T.; Shah, A.; Gulko, P.S. The vitamin D receptor regulates rheumatoid arthritis synovial fibroblast invasion and morphology. Mol. Med. 2012, 18, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Gu, B.; Lv, X.; Yu, Z.; Wang, R.; Zhou, X.; Qiao, W.; Mao, Z.; Zuo, G.; Li, Q.; et al. 1, 25-dihydroxy-vitamin D3 with tumor necrosis factor-alpha protects against rheumatoid arthritis by promoting p53 acetylation-mediated apoptosis via Sirt1 in synoviocytes. Cell Death Dis. 2016, 7, e2423. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.R.; Li, D.; Jeffery, L.E.; Raza, K.; Hewison, M. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif. Tissue Int. 2020, 106, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Bouaddi, I.; Rostom, S.; El Badri, D.; Hassani, A.; Chkirate, B.; Abouqal, R.; Amine, B.; Hajjaj-Hassouni, N. Vitamin D concentrations and disease activity in Moroccan children with juvenile idiopathic arthritis. BMC Musculoskelet. Disord. 2014, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Stagi, S.; Bertini, F.; Cavalli, L.; Matucci-Cerinic, M.; Brandi, M.L.; Falcini, F. Determinants of vitamin D levels in children, adolescents, and young adults with juvenile idiopathic arthritis. J. Rheumatol. 2014, 41, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Comak, E.; Dogan, C.S.; Uslu-Gokceoglu, A.; Akbas, H.; Ozdem, S.; Koyun, M.; Akman, S. Association between vitamin D deficiency and disease activity in juvenile idiopathic arthritis. Turk. J. Pediatrics 2014, 56, 626–631. [Google Scholar] [CrossRef]
- Dagdeviren-Cakir, A.; Arvas, A.; Barut, K.; Gur, E.; Kasapcopur, O. Serum vitamin D levels during activation and remission periods of patients with juvenile idiopathic arthritis and familial Mediterranean fever. Turk. J. Pediatrics 2016, 58, 125–131. [Google Scholar] [CrossRef]
- Sumi, S.K.; Rahman, S.A.; Islam, M.I.; Islam, M.M.; Talukder, M.K. Vitamin D profile in juvenile idiopathic arthritis patients in a tertiary care hospital in Bangladesh. Mymensingh Med. J. 2020, 29, 311–316. [Google Scholar] [PubMed]
- Nisar, M.K.; Masood, F.; Cookson, P.; Sansome, A.; Ostor, A.J. What do we know about juvenile idiopathic arthritis and vitamin D? A systematic literature review and meta-analysis of current evidence. Clin. Rheumatol. 2013, 32, 729–734. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Studart, S.A.; Leite, A.C.; Marinho, A.L.; Pinto, A.C.; Rabelo Junior, C.N.; de Melo Nunes, R.; Rocha, H.A.; Rocha, F.A. Vitamin D levels in juvenile idiopathic arthritis from an equatorial region. Rheumatol. Int. 2015, 35, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Alhomaidah, D.; Alsagheir, A.; Al-Mayouf, S.M. Coexistence of endocrinopathies in children with rheumatic diseases. Int. J. Pediatrics Adolesc. Med. 2016, 3, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Finch, S.L.; Rosenberg, A.M.; Kusalik, A.J.; Maleki, F.; Rezaei, E.; Baxter-Jones, A.; Benseler, S.; Boire, G.; Cabral, D.; Campillo, S.; et al. Higher concentrations of vitamin D in Canadian children with juvenile idiopathic arthritis compared to healthy controls are associated with more frequent use of vitamin D supplements and season of birth. Nutr. Res. 2021, 92, 139–149. [Google Scholar] [CrossRef]
- Thorsen, S.U.; Pipper, C.B.; Alberdi-Saugstrup, M.; Nielsen, S.; Cohen, A.; Lundqvist, M.; Thygesen, L.C.; Ascherio, A.; Svensson, J. No association between vitamin D levels around time of birth and later risk of developing oligo- and polyarticular juvenile idiopathic arthritis: A Danish case-cohort study. Scand. J. Rheumatol. 2017, 46, 104–111. [Google Scholar] [CrossRef]
- Clarke, S.L.; Mitchell, R.E.; Sharp, G.C.; Ramanan, A.V.; Relton, C.L. Vitamin D levels and risk of juvenile idiopathic arthritis: A Mendelian randomization study. Arthritis Care Res. 2021. [Google Scholar] [CrossRef]
- Henderson, C.J.; Cawkwell, G.D.; Specker, B.L.; Sierra, R.I.; Wilmott, R.W.; Campaigne, B.N.; Lovell, D.J. Predictors of total body bone mineral density in non-corticosteroid-treated prepubertal children with juvenile rheumatoid arthritis. Arthritis Rheum. 1997, 40, 1967–1975. [Google Scholar] [CrossRef]
- Bianchi, M.L.; Bardare, M.; Caraceni, M.P.; Cohen, E.; Falvella, S.; Borzani, M.; DeGaspari, M.G. Bone metabolism in juvenile rheumatoid arthritis. Bone Miner. 1990, 9, 153–162. [Google Scholar] [CrossRef]
- Hillman, L.S.; Cassidy, J.T.; Chanetsa, F.; Hewett, J.E.; Higgins, B.J.; Robertson, J.D. Percent true calcium absorption, mineral metabolism, and bone mass in children with arthritis: Effect of supplementation with vitamin D3 and calcium. Arthritis Rheum. 2008, 58, 3255–3263. [Google Scholar] [CrossRef]
- Elsasser, U.; Wilkins, B.; Hesp, R.; Thurnham, D.I.; Reeve, J.; Ansell, B.M. Bone rarefaction and crush fractures in juvenile chronic arthritis. Arch. Dis. Child. 1982, 57, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Sung, Y.K. Update on glucocorticoid induced osteoporosis. Endocrinol. Metab. 2021, 36, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Epsley, S.; Tadros, S.; Farid, A.; Kargilis, D.; Mehta, S.; Rajapakse, C.S. The effect of inflammation on bone. Front. Physiol. 2020, 11, 511799. [Google Scholar] [CrossRef] [PubMed]
- Nandi, M.; Mullick, M.A.S.; Nandy, A.; Samanta, M.; Sarkar, S.; Sabui, T.K. Evaluation of vitamin D profile in juvenile idiopathic arthritis. Mod. Rheumatol. 2021, roab053. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, N.; Khadzhynova, Y. Juvenile idiopathic arthritis and vitamin D status in Ukrainian patients. Georgian Med. News 2019, 294, 88–91. [Google Scholar]
- Cutolo, M.; Pizzorni, C.; Sulli, A. Vitamin D endocrine system involvement in autoimmune rheumatic diseases. Autoimmun. Rev. 2011, 11, 84–87. [Google Scholar] [CrossRef]
- Vojinovic, J.; Cimaz, R. Vitamin D-update for the pediatric rheumatologists. Pediatrics Rheumatol. 2015, 13, 18. [Google Scholar] [CrossRef]
- Marini, F.; Falcini, F.; Stagi, S.; Fabbri, S.; Ciuffi, S.; Rigante, D.; Cerinic, M.M.; Brandi, M.L. Study of vitamin D status and vitamin D receptor polymorphisms in a cohort of Italian patients with juvenile idiopathic arthritis. Sci. Rep. 2020, 10, 17550. [Google Scholar] [CrossRef]
- Kostik, M.M.; Smirnov, A.M.; Demin, G.S.; Scheplyagina, L.A.; Larionova, V.I. Juvenile idiopathic arthritis patients and their skeletal status: Possible role of vitamin D receptor gene polymorphism. Mol. Biol. Rep. 2014, 41, 1937–1943. [Google Scholar] [CrossRef]
- Basic, J.; Vojinovic, J.; Jevtovic-Stoimenov, T.; Despotovic, M.; Susic, G.; Lazarevic, D.; Milosevic, V.; Cvetkovic, M.; Pavlovic, D. Vitamin D receptor gene polymorphism influences lipid profile in patients with juvenile idiopathic arthritis. Clin. Rheumatol. 2019, 38, 117–124. [Google Scholar] [CrossRef]
- Ellis, J.A.; Scurrah, K.J.; Li, Y.R.; Ponsonby, A.L.; Chavez, R.A.; Pezic, A.; Dwyer, T.; Akikusa, J.D.; Allen, R.C.; Becker, M.L.; et al. Epistasis amongst PTPN2 and genes of the vitamin D pathway contributes to risk of juvenile idiopathic arthritis. J. Steroid Biochem. Mol. Biol. 2015, 145, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Makitie, O.; et al. Global consensus recommendations on prevention and management of nutritional rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Carucci, J.A.; Spencer, J.M.; Rigel, D.S. Commentary: A responsible approach to maintaining adequate serum vitamin D levels. J. Am. Acad. Dermatol. 2007, 57, 594–595. [Google Scholar] [CrossRef]
- von Scheven, E.; Burnham, J.M. Vitamin D supplementation in the pediatric rheumatology clinic. Curr. Rheumatol. Rep. 2011, 13, 110–116. [Google Scholar] [CrossRef]
- Grober, U.; Spitz, J.; Reichrath, J.; Kisters, K.; Holick, M.F. Vitamin D: Update 2013: From rickets prophylaxis to general preventive healthcare. Dermatoendocrinology 2013, 5, 331–347. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Shao, A.; Vieth, R.; Heaney, R. Risk assessment for vitamin D. Am. J. Clin. Nutr. 2007, 85, 6–18. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, Y.; Luo, C.; Liu, M.; Xu, L.; Tang, X. Adjunctive vitamin D for the treatment of active juvenile idiopathic arthritis: An open-label, prospective, randomized controlled trial. Exp. Ther. Med. 2019, 18, 4921–4926. [Google Scholar] [CrossRef]
- Warady, B.D.; Lindsley, C.B.; Robinson, F.G.; Lukert, B.P. Effects of nutritional supplementation on bone mineral status of children with rheumatic diseases receiving corticosteroid therapy. J. Rheumatol. 1994, 21, 530–535. [Google Scholar] [CrossRef]
- Reed, A.; Haugen, M.; Pachman, L.M.; Langman, C.B. 25-Hydroxyvitamin D therapy in children with active juvenile rheumatoid arthritis: Short-term effects on serum osteocalcin levels and bone mineral density. J. Pediatrics 1991, 119, 657–660. [Google Scholar] [CrossRef]
- Carrasco, R.; Lovell, D.J.; Giannini, E.H.; Henderson, C.J.; Huang, B.; Kramer, S.; Ranz, J.; Heubi, J.; Glass, D. Biochemical markers of bone turnover associated with calcium supplementation in children with juvenile rheumatoid arthritis: Results of a double-blind, placebo-controlled intervention trial. Arthritis Rheum. 2008, 58, 3932–3940. [Google Scholar] [CrossRef]
- Dey, S.; Jahan, A.; Yadav, T.P.; Bhagwani, D.K.; Sachdev, N. Measurement of bone mineral density by dual energy X-ray absorptiometry in juvenile idiopathic arthritis. Indian J. Pediatrics 2014, 81, 126–132. [Google Scholar] [CrossRef]
- Lien, G.; Selvaag, A.M.; Flato, B.; Haugen, M.; Vinje, O.; Sorskaar, D.; Dale, K.; Egeland, T.; Forre, O. A two-year prospective controlled study of bone mass and bone turnover in children with early juvenile idiopathic arthritis. Arthritis Rheum. 2005, 52, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Pepmueller, P.H.; Cassidy, J.T.; Allen, S.H.; Hillman, L.S. Bone mineralization and bone mineral metabolism in children with juvenile rheumatoid arthritis. Arthritis Rheum. 1996, 39, 746–757. [Google Scholar] [CrossRef]
- Valta, H.; Lahdenne, P.; Jalanko, H.; Aalto, K.; Makitie, O. Bone health and growth in glucocorticoid-treated patients with juvenile idiopathic arthritis. J. Rheumatol. 2007, 34, 831–836. [Google Scholar] [PubMed]
- Markula-Patjas, K.P.; Valta, H.L.; Kerttula, L.I.; Soini, I.H.; Honkanen, V.E.; Toiviainen-Salo, S.M.; Makitie, O.M. Prevalence of vertebral compression fractures and associated factors in children and adolescents with severe juvenile idiopathic arthritis. J. Rheumatol. 2012, 39, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R.; Chan, P.C.; MacFarlane, G.D. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am. J. Clin. Nutr. 2001, 73, 288–294. [Google Scholar] [CrossRef] [PubMed]
| Year | Subjects | Vitamin D Supplementation | Sample Size (F/M) | Age (Range) | Baseline 25[OH]D (ng/mL) | Major Findings | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Regimen | Duration | |||||||
| 1991 | Children with sustained active polyarticular JIA | 25[OH]D (1–2 μg/kg/day) | 12 months | 13 (12/1) | 5–18 | 28 ± 16 |
| [110] |
| 1994 | Children with rheumatic disease and osteoporosis, treated with corticosteroid | Ca ± Vit D | 6 months | 10 (six with JIA) | 13 (10.9–18.0) | 28.04 ± 8.48 |
| [109] |
| 2008 | Children with oligo-arthritis or polyarthritis | Vit D3 400 IU/day + Ca (1000 mg/day) or Vit D3 (1600 IU/day), or both | 6 months | 18 (12/6) | 10 (5–15) | 32.84 ± 15.48 |
| [90] |
| 2008 | Children with JIA free of systemic corticosteroid 3 months prior to enrollment | Vit D3 (400 IU/day) ± Ca (1000 mg/day) | 24 months | 198 (141/57) | 11.7 (6–18) | NA |
| [111] |
| 2019 | Children with JIA | ±Vit D3 (2000 IU/day) | 6 months | 36 (13/23) | 6.9 ± 3.1 | 13.316 ± 5.148 |
| [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-Y.; Yang, H.-Y.; Luo, S.-F.; Huang, J.-L.; Lai, J.-H. Vitamin D Supplementation in Patients with Juvenile Idiopathic Arthritis. Nutrients 2022, 14, 1538. https://doi.org/10.3390/nu14081538
Wu C-Y, Yang H-Y, Luo S-F, Huang J-L, Lai J-H. Vitamin D Supplementation in Patients with Juvenile Idiopathic Arthritis. Nutrients. 2022; 14(8):1538. https://doi.org/10.3390/nu14081538
Chicago/Turabian StyleWu, Chao-Yi, Huang-Yu Yang, Shue-Fen Luo, Jing-Long Huang, and Jenn-Haung Lai. 2022. "Vitamin D Supplementation in Patients with Juvenile Idiopathic Arthritis" Nutrients 14, no. 8: 1538. https://doi.org/10.3390/nu14081538
APA StyleWu, C.-Y., Yang, H.-Y., Luo, S.-F., Huang, J.-L., & Lai, J.-H. (2022). Vitamin D Supplementation in Patients with Juvenile Idiopathic Arthritis. Nutrients, 14(8), 1538. https://doi.org/10.3390/nu14081538






