Nutrition in Spondyloarthritis and Related Immune-Mediated Disorders
Abstract
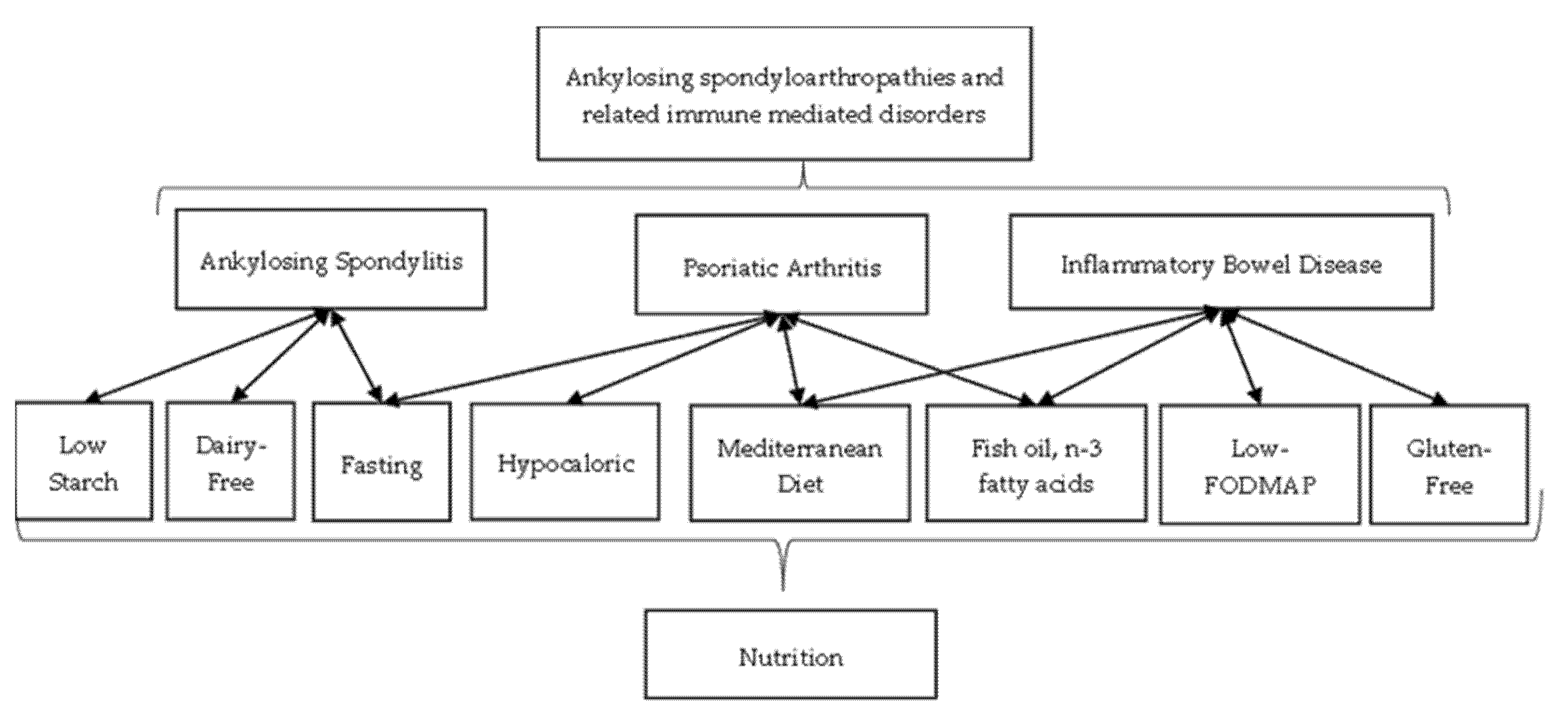
1. Introduction
2. Ankylosing Spondylitis
3. Psoriatic Arthritis
4. Inflammatory Bowel Disease
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; Huang, F.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Akkoc, N.; Brandt, J.; Chou, C.T.; Dougados, M.; Huang, F.; Gu, Y.; Kirazli, Y. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 2011, 70, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Dumas, E.; Venken, K.; Rosenbaum, J.T.; Elewaut, D. Intestinal Microbiota, HLA-B27, and Spondyloarthritis: Dangerous Liaisons. Rheum. Dis. Clin. North Am. 2020, 46, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Sieper, J. Ankylosing spondylitis. Lancet 2007, 369, 1379–1390. [Google Scholar] [CrossRef]
- Weiss, E.H.; Bloemer, K.; Doerner, C.; Kuon, W.; Lang, M.; Pohla, H.; Schattenkirchner, M.; Riethmüller, G. Molecular biology of the HLA-B27 locus. Br. J. Rheumatol. 1988, 27, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Navid, F.; Holt, V.; Colbert, R.A. The enigmatic role of HLA-B*27 in spondyloarthritis pathogenesis. Semin. Immunopathol. 2021, 43, 235–243. [Google Scholar] [CrossRef]
- Rashid, T.; Ebringer, A. Gut-mediated and HLA-B27-associated arthritis: An emphasis on ankylosing spondylitis and Crohn’s disease with a proposal for the use of new treatment. Discov. Med. 2011, 12, 187–194. [Google Scholar]
- Sibley, C.H. Autoinflammation and HLA-B27: Beyond Antigen Presentation. Ocul. Immunol. Inflamm. 2016, 24, 460–469. [Google Scholar] [CrossRef]
- Bowness, P. HLA-B27. Annu. Rev. Immunol. 2015, 33, 29–48. [Google Scholar] [CrossRef]
- Brewerton, D.A.; Cafrey, M.; Nicholls, A.; James, D.C. Proceedings: Histocompatibility antigen (HL-A 27) and its relation to disease. Ann. Rheum. Dis. 1974, 33, 406–407. [Google Scholar] [CrossRef]
- Babaie, F.; Hasankhani, M.; Mohammadi, H.; Safarzadeh, E.; Rezaiemanesch, A.; Salimi, R.; Baradaran, B.; Babaloo, Z. The role of gut microbiota and IL-23/IL-17 pathway in ankylosing spondylitis immunopathogenesis: New insights and updates. Immunol. Lett. 2018, 196, 52–62. [Google Scholar] [CrossRef]
- Rizzo, A.; Ferrante, A.; Guggino, G.; Ciccia, F. Gut inflammation in spondyloarthritis. Best Pract. Res. Clin. Rheumatol. 2017, 31, 863–876. [Google Scholar] [CrossRef]
- Van Praet, L.; Jacques, P.; Van den Bosch, F.; Elewaut, D. The transition of acute to chronic bowel inflammation in spondyloarthritis. Nat. Rev. Rheumatol. 2012, 8, 288–295. [Google Scholar] [CrossRef]
- Gill, T.; Asquith, M.; Brooks, S.R.; Rosenbaum, J.T.; Colbert, R.A. Effects of HLA-B27 on Gut Microbiota in Experimental Spondyloarthritis Implicate an Ecological Model of Dysbiosis. Arthritis Rheumatol. 2018, 70, 555–565. [Google Scholar] [CrossRef]
- Ebringer, A.; Wilson, C. The use of a low starch diet in the treatment of patients suffering from ankylosing spondylitis. Clin. Rheumatol. 1996, 15, 62–66. [Google Scholar] [CrossRef]
- Schreiner, P.; Yilmaz, B.; Rossel, J.B.; Franc, Y.; Misselwitz, B.; Scharl, M.; Zeitz, J.; Frei, P.; Greuter, T.; Vavricka, S.R.; et al. Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United Eur. Gastroenterol. J. 2019, 7, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Ben Nessib, D.; Maatallah, K.; Ferjani, H.; Kaffel, D.; Hamdi, W. Impact of Ramadan diurnal intermittent fasting on rheumatic diseases. Clin. Rheumatol. 2020, 39, 2433–2440. [Google Scholar] [CrossRef]
- Herfarth, H.H.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D.; Long, M.D. Prevalence of a gluten-free diet and improvement of clinical symptoms in patients with inflammatory bowel diseases. Inflamm. Bowel Dis. 2014, 20, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Caso, F.; Navarini, L.; Carubbi, F.; Picchianti-Diamanti, A.; Chimenti, M.S.; Tasso, M.; Currado, D.; Ruscitti, P.; Ciccozzi, M.; Annarumma, A.; et al. Mediterranean diet and Psoriatic Arthritis activity: A multicenter cross-sectional study. Rheumatol. Int. 2020, 40, 951–958. [Google Scholar] [CrossRef]
- Klingberg, E.; Bilberg, A.; Björkman, S.; Hedberg, M.; Jacobsson, L.; Forsblad-d’Elia, H.; Carlsten, H.; Eliasson, B.; Larsson, I. Weight loss improves disease activity in patients with psoriatic arthritis and obesity: An interventional study. Arthritis Res. Ther. 2019, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Gearry, R.B.; Irving, P.M.; Barrett, J.S.; Nathan, D.M.; Shepherd, S.J.; Gibson, P.R. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease-a pilot study. J. Crohns Colitis 2009, 3, 8–14. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.G.; Goulis, D.G.; Gkiouras, K.; Nigdelis, M.P.; Papageorgiou, S.T.; Papamitsou, T.; Forbes, A.; Bogdanos, D.P. Low FODMAP Diet for Functional Gastrointestinal Symptoms in Quiescent Inflammatory Bowel Disease: A Systematic Review of Randomized Controlled Trials. Nutrients 2020, 12, 3648. [Google Scholar] [CrossRef]
- Gudu, T.; Jadon, D.R. Multidisciplinary working in the management of axial and peripheral spondyloarthritis. Ther. Adv. Musculoskelet Dis. 2020, 12, 1–14. [Google Scholar] [CrossRef]
- Asquith, M.; Sternes, P.R.; Costello, M.E.; Karstens, L.; Diamond, S.; Martin, T.M.; Li, Z.; Marshall, M.S.; Spector, T.D.; le Cao, K.A.; et al. HLA Alleles Associated with Risk of Ankylosing Spondylitis and Rheumatoid Arthritis Influence the Gut Microbiome. Arthritis Rheumatol. 2019, 71, 1642–1650. [Google Scholar] [CrossRef]
- Breban, M.; Tap, J.; Leboime, A.; Said-Nahal, R.; Langella, P.; Chiocchia, G.; Furet, J.P.; Sokol, H. Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 1614–1622. [Google Scholar] [CrossRef]
- Taurog, J.D.; Richardson, J.A.; Croft, J.T.; Simmons, W.A.; Zhou, M.; Fernandez-Sueiro, J.L.; Balish, E.; Hammer, R.E. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J. Exp. Med. 1994, 180, 2359–2364. [Google Scholar] [CrossRef]
- Costello, M.E.; Ciccia, F.; Willner, D.; Warrington, N.; Robinson, P.C.; Gardiner, B.; Marshall, M.; Kenna, T.J.; Triolo, G.; Brown, M.A. Brief Report: Intestinal Dysbiosis in Ankylosing Spondylitis. Arthritis Rheumatol. 2015, 67, 686–691. [Google Scholar] [CrossRef]
- Chen, Z.; Qi, J.; Wei, Q.; Zheng, X.; Wu, X.; Li, X.; Liao, Z.; Lin, Z.; Gu, J. Variations in gut microbial profiles in ankylosing spondylitis: Disease phenotyperelated dysbiosis. Ann. Transl. Med. 2019, 7, 571. [Google Scholar] [CrossRef]
- Zhang, L.; Han, R.; Zhang, X.; Fang, G.; Chen, J.; Li, J.; Xu, S.; Qian, L.; Chen, W.; Pan, F. Fecal microbiota in patients with ankylosing spondylitis: Correlation with dietary factors and disease activity. Clin. Chim. Acta 2019, 497, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Tito, R.Y.; Cypers, H.; Joossens, M.; Varkas, G.; Van Praet, L.; Glorieus, E.; Van den Bosch, F.; De Vos, M.; Raes, J.; Elewaut, D. Brief report: Dialister as a microbial marker of disease activity in spondyloarthritis. Arthritis Rheumatol. 2017, 69, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Cardoneanu, A.; Cozma, S.; Rezus, C.; Petrariu, F.; Burlui, A.M.; Rezus, E. Characteristics of the intestinal microbiome in ankylosing spondylitis. Exp. Ther. Med. 2021, 22, 676. [Google Scholar] [CrossRef]
- Cotillard, A.; Kennedy, S.; Kong, L.C.; Prifti, E.; Pons, N.; Le Chatelier, E.; Almeida, M.; Quinquis, B.; Levenez, F.; Galleron, N.; et al. Dietary intervention impact on gut microbial gene richness. Nature 2013, 500, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Ebringer, R.W.; Cawdell, D.R.; Cowling, P.; Ebringer, A. Sequential studies in ankylosing spondylitis. Association of Klebsiella pneumoniae with active disease. Ann. Rheum. Dis. 1978, 37, 146–151. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.J.; Chen, J.; Huang, X.L.; Fang, G.S.; Yang, L.J.; Duan, Y.; Want, J. The association of HLA-B27 and Klebsiella pneumoniae in ankylosing spondylitis: A systematic review. Microb. Pathog. 2018, 117, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Conway, P.L.; Brown, I.L.; Evans, A.J. In Vitro Utilization of Amylopectin and High-Amylose Maize (Amylomaize) Starch Granules by Human Colonic Bacteria. Appl. Environ. Microbiol. 1999, 65, 4848–4854. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, A.M.; Richard, C.; Lebeault, J.M. Bacteriology and nutrition of environmental strains of Klebsiella pneumoniae involved in wood and bark decay. Ann. L’institut Pasteur Microbiol. 1983, 134, 189–196. [Google Scholar] [CrossRef]
- Appelboom, T.; Durez, P. Effect of milk product deprivation on spondyloarthropathy. Ann. Rheum. Dis. 1994, 53, 481–482. [Google Scholar] [CrossRef]
- Sundström, B.; Wållberg-Jonsson, S.; Johansson, G. Diet, disease activity, and gastrointestinal symptoms in patients with ankylosing spondylitis. Clin. Rheumatol. 2011, 30, 71–76. [Google Scholar] [CrossRef]
- Haugen, M.; Kjeldsen-Kragh, J.; Nordvåg, B.Y.; Førre, O. Diet and disease symptoms in rheumatic diseases-results of a questionnaire based survey. Clin. Rheumatol. 1991, 10, 401–407. [Google Scholar] [CrossRef]
- Macfarlane, T.V.; Abbood, H.M.; Pathan, E.; Gordon, K.; Hinz, J.; Macfarlane, G.J. Relationship between diet and ankylosing spondylitis: A systematic review. Eur. J. Rheumatol. 2018, 5, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Feldtkeller, E.; Lind-Albrecht, G.; Rudwaleit, M. Core set of recommendations for patients with ankylosing spondylitis concerning behaviour and environmental adaptations. Rheumatol. Int. 2013, 33, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, D.V.; Gladman, D. Psoriatic arthritis. F1000Research 2019, 8, 1665. [Google Scholar] [CrossRef] [PubMed]
- Ritchlin, C.T.; Colbert, R.A.; Gladman, D.D. Psoriatic Arthritis. N. Engl. J. Med. 2017, 376, 957–970. [Google Scholar] [CrossRef]
- Ogdie, A.; Weiss, P. The Epidemiology of Psoriatic Arthritis. Rheum. Dis. Clin. North Am. 2015, 41, 545–568. [Google Scholar] [CrossRef]
- FitzGerald, O.; Haroon, M.; Giles, J.T.; Winchester, R. Concepts of pathogenesis in psoriatic arthritis: Genotype determines clinical phenotype. Arthritis Res. Ther. 2015, 17, 115. [Google Scholar] [CrossRef]
- Michelsen, B.; Fiane, R.; Diamantopoulos, A.P.; Soldal, D.M.; Hansen, I.J.W.; Sokka, T.; Kavanaugh, A.; Haugeberg, G. A comparison of disease burden in rheumatoid arthritis, psoriatic arthritis and axial spondyloarthritis. PLoS ONE 2015, 10, e0123582. [Google Scholar] [CrossRef] [PubMed]
- Kotsis, K.; Voulgari, P.V.; Tsifetaki, N.; Machado, M.O.; Carvalho, A.F.; Creed, F.; Drosos, A.A.; Hyphantis, T. Anxiety and depressive symptoms and illness perceptions in psoriatic arthritis and associations with physical health-related quality of life. Arthritis Care Res. 2012, 64, 1593–1601. [Google Scholar] [CrossRef]
- Di Minno, M.N.D.; Peluso, R.; Iervolino, S.; Russolillo, A.; Lupoli, R.; Scarpa, R. Weight loss and achievement of minimal disease activity in patients with psoriatic arthritis starting treatment with tumour necrosis factor α blockers. Ann. Rheum. Dis. 2013, 73, 1157–1162. [Google Scholar] [CrossRef]
- Kharaeva, Z.; Gostova, E.; De Luca, C.; Raskovic, D.; Korkina, L. Clinical and biochemical effects of coenzyme Q, vitamin E, and selenium supplementation to psoriasis patients. Nutrition 2009, 25, 295–302. [Google Scholar] [CrossRef]
- Kristensen, S.; Schmidt, E.B.; Schlemmer, A.; Rasmussen, C.; Johansen, M.B.; Christensen, J.H. Beneficial effect of n-3 polyunsaturated fatty acids on inflammation and analgesic use in psoriatic arthritis: A randomized, double blind, placebo-controlled trial. Scand. J. Rheumatol. 2018, 47, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Adawi, M.; Damiani, G.; Bragazzi, N.L.; Bridgewood, C.; Pacifico, A.; Conic, R.R.Z.; Morrone, A.; Malagoli, P.; Pigatto, P.D.M.; Amital, H.; et al. The Impact of Intermittent Fasting (Ramadan Fasting) on Psoriatic Arthritis Disease Activity, Enthesitis, and Dactylitis: A Multicentre Study. Nutrients 2019, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Michaëlsson, G.; Gerdén, B.; Hagforsen, E.; Nilsson, B.; Pihl-Lundin, I.; Kraaz, W.; Hjelmquist, G.; Loof, L. Psoriasis patients with antibodies to gliadin can be improved by a gluten-free diet. Br. J. Dermatol. 2000, 142, 44–51. [Google Scholar] [CrossRef]
- Michaëlsson, G.; Ahs, S.; Hammarström, I.; Lundin, I.P.; Hagforsen, E. Gluten-free diet in psoriasis patients with antibodies to gliadin results in decreased expression of tissue transglutaminase and fewer Ki67+ cells in the dermis. Acta Derm. Venereol. 2003, 83, 425–429. [Google Scholar] [CrossRef] [PubMed]
- De Bastiani, R.; Gabrielli, M.; Lora, L.; Napoli, L.; Tosetti, C.; Pirrotta, E.; Ubaldi, E.; Bertolusso, L.; Zamparella, M.; De Polo, M.; et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology 2015, 230, 156–160. [Google Scholar] [CrossRef]
- Gaál, J.; Lakos, G.; Szodoray, P.; Kiss, J.; Horváth, I.; Horkay, E.; Nagy, G.; Szegedi, A. Immunological and clinical effects of alphacalcidol in patients with psoriatic arthropathy: Results of an open, follow-up pilot study. Acta Derm. Venereol. 2009, 89, 140–144. [Google Scholar] [CrossRef][Green Version]
- Huckins, D.; Felson, D.T.; Holick, M. Treatment of psoriatic arthritis with oral 1,25-dihydroxyvitamin D3: A pilot study. Arthritis Rheum. 1990, 33, 1723–1727. [Google Scholar] [CrossRef]
- Fernando, H.A.; Zibellini, J.; Harris, R.A.; Seimon, R.V.; Sainsbury, A. Effect of Ramadan Fasting on Weight and Body Composition in Healthy Non-Athlete Adults: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 478. [Google Scholar] [CrossRef]
- Chang, J.T. Pathophysiology of Inflammatory Bowel Diseases. N. Engl. J. Med. 2020, 383, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Cosnes, J.; Gower-Rousseau, C.; Seksik, P.; Cortot, A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011, 140, 1785–1794. [Google Scholar] [CrossRef] [PubMed]
- Thia, K.T.; Loftus, E.V., Jr.; Sandborn, W.J.; Yang, S.K. An update on the epidemiology of inflammatory bowel disease in Asia. Am. J. Gastroenterol. 2008, 103, 3167–3182. [Google Scholar] [CrossRef] [PubMed]
- Molodecky, N.A.; Kaplan, G.G. Environmental risk factors for inflammatory bowel disease. Gastroenterol. Hepatol. 2010, 6, 339–346. [Google Scholar]
- Rogler, G.; Zeitz, J.; Biedermann, L. The Search for Causative Environmental Factors in Inflammatory Bowel Disease. Dig. Dis. 2016, 34, 48–55. [Google Scholar] [CrossRef]
- Danese, S.; Fiocchi, C. Etiopathogenesis of inflammatory bowel diseases. World J. Gastroenterol. 2006, 12, 4807–4812. [Google Scholar] [CrossRef]
- Rudwaleit, M.; Baeten, D. Ankylosing spondylitis and bowel disease. Best Pract. Res. Clin. Rheumatol. 2006, 20, 451–471. [Google Scholar] [CrossRef]
- Dekker-Saeys, B.J.; Meuwissen, S.G.; Van Den Berg-Loonen, E.M.; De Haas, W.H.; Agenant, D.; Tytgat, G.N. Ankylosing spondylitis and inflammatory bowel disease. II. Prevalence of peripheral arthritis, sacroiliitis, and ankylosing spondylitis in patients suffering from inflammatory bowel disease. Ann. Rheum. Dis. 1978, 37, 33–35. [Google Scholar] [CrossRef] [PubMed]
- de Vlam, K.; Mielants, H.; Cuvelier, C.; De Keyser, F.; Veys, E.M.; De Vos, M. Spondyloarthropathy is underestimated in inflammatory bowel disease: Prevalence and HLA association. J. Rheumatol. 2000, 27, 2860–2865. [Google Scholar]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Amre, D.K.; D’Souza, S.; Morgan, K.; Seidman, G.; Lambrette, P.; Grimard, G.; Israel, D.; Mack, D.; Ghadirian, P.; Deslandres, C.; et al. Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn’s disease in children. Am. J. Gastroenterol. 2007, 102, 2016–2025. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, N.; Kono, S.; Wakai, K.; Fukuda, Y.; Satomi, M.; Shimoyama, T.; Inaba, Y.; Miyake, Y.; Sasaki, S.; Okamoto, K.; et al. Dietary risk factors for inflammatory bowel disease: A multicenter case-control study in Japan. Inflamm. Bowel Dis. 2005, 11, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, F.; Spisni, E.; Giovanardi, E.; Imbesi, V.; Salice, M.; Alvisi, P.; Valerii, M.C.; Gionchetti, P. Implications of the Westernized Diet in the Onset and Progression of IBD. Nutrients 2019, 11, 1033. [Google Scholar] [CrossRef] [PubMed]
- Martínez Steele, E.; Baraldi, L.G.; Louzada, M.L.; Moubarac, J.C.; Mozaffarian, D.; Monteiro, C.A. Ultra-processed foods and added sugars in the US diet: Evidence from a nationally representative cross-sectional study. BMJ Open 2016, 6, e009892. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.G.; Ahlbom, A.; Hellers, G. Diet and inflammatory bowel disease: A case-control study. Epidemiology 1992, 3, 47–52. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef]
- Chicco, F.; Magrì, S.; Cingolani, A.; Paduano, D.; Pesenti, M.; Zara, F.; Tumbarello, F.; Urru, E.; Melis, A.; Casula, L.; et al. Multidimensional Impact of Mediterranean Diet on IBD Patients. Inflamm. Bowel Dis. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Vrdoljak, J.; Vilović, M.; Živković, P.M.; Tadin Hadjina, I.; Rušić, D.; Bukić, J.; Borovac, J.A.; Božić, J. Mediterranean Diet Adherence and Dietary Attitudes in Patients with Inflammatory Bowel Disease. Nutrients 2020, 12, 3429. [Google Scholar] [CrossRef]
- Khalili, H.; Håkansson, N.; Chan, S.S.; Chen, Y.; Lochhead, P.; Ludvigsson, J.F.; Chan, A.T.; Hart, A.R.; Olén, O.; Wolk, A. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: Results from two large prospective cohort studies. Gut 2020, 69, 1637–1644. [Google Scholar] [CrossRef]
- Bodini, G.; Zanella, C.; Crespi, M.; Lo Pumo, S.; Demarzo, M.G.; Savarino, E.; Savarino, V.; Giannini, E.G. A randomized, 6-wk trial of a low FODMAP diet in patients with inflammatory bowel disease. Nutrition 2019, 67–68, 10542. [Google Scholar] [CrossRef]
- Suskind, D.L.; Wahbeh, G.; Cohen, S.A.; Damman, C.J.; Klein, J.; Braly, K.; Shaffer, M.; Lee, D. Patients Perceive Clinical Benefit with the Specific Carbohydrate Diet for Inflammatory Bowel Disease. Dig. Dis. Sci. 2016, 61, 3255–3260. [Google Scholar] [CrossRef]
- Tosti, V.; Bertozzi, B.; Fontana, L. Health Benefits of the Mediterranean Diet: Metabolic and Molecular Mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Popa, S.L.; Pop, C.; Dumitrascu, D.L. Diet Advice for Crohn’s Disease: FODMAP and Beyond. Nutrients 2020, 12, 3751. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.; Gold, B.D.; Oliva, S.; Jeffery, L.; Angela, S.; Bailey, K.; Laura, E.; David, M. Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Suskind, D.L.; Wahbeh, G.; Gregory, N.; Vendettuoli, H.; Christie, D. Nutritional therapy in pediatric Crohn disease: The specific carbohydrate diet. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Guyatt, G.; Ogdie, A.; Gladman, D.D.; Deal, C.; Deodhar, A.; Dubreuil, M.; Dunham, J.; Husni, M.E.; Kenny, S.; et al. 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol. 2019, 71, 5–32. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. Clin. Nutr. 2020, 39, 632–653. [Google Scholar] [CrossRef]
| Author (Year) | Study Design and Participants’ Characteristics | Type of Diet | Variables Evaluated | Outcome |
|---|---|---|---|---|
| Ebringer et al. (1996) [15] | Longitudinal prospective; 36 patients with AS | Low-starch | IgA ESR | Both IgA and ESR have significantly decreased over the 9-month period of the study, with the majority of patients reporting a reduction in the severity of symptoms |
| Appelboom and Durez (1994) [38] | Longitudinal prospective; 25 patients with AS, reporting morning stiffness of over 30 min, inflammatory lower back pain, multiple joint swelling | Dairy-free | General wellbeing Pain severity Duration of morning stiffness Articular stiffness Changes in NSAID treatment Considering the continuation of the diet | 18 out of 25 patients had a good compliance with the diet, with 17 patients reporting moderate or good efficacy; 8 out of 13 patients could discontinue their NSAID therapy, with 6 patients continuing the diet for over 2 years, without any additional medication |
| Sundström et al. (2011) [39] | Longitudinal prospective; 111 patients (84 males, 27 females) with AS | Various dietary habits | Dietary habits Physical activity Medication Gastrointestinal symptoms Disease activity and functional capacity | 27% of the patients reported gastrointestinal symptoms after consuming dairy, fatty or flour-rich foods. Nevertheless, the study did not yield conclusive evidence of a relationship between diet and disease activity but rather a correlation with gastrointestinal symptoms in patients with AS |
| Haugen et al. (1991) [40] | Questionnaire-based; 41 patients with AS | Fasting | Pain Stiffness Joint-swelling | More than half of the respondents reported less pain, stiffness and reduced joint-swelling |
| Author (Year) | Study Design and Participants’ Characteristics | Type of Diet | Variables Evaluated | Outcome |
|---|---|---|---|---|
| Caso et al. (2020) [19] | Cross-sectional observational study; 211 patients with PsA: 131 females and 80 males | Mediterranean diet | Disease activity Metabolic parameters | Most patients had moderate and high adherence to the diet; low adherence was associated with higher PsA activity |
| Klingberg et al. (2019) [20] | Interventional study; 41 patients with PsA | Low-energy diet Energy-restricted diet | Disease activity Weight loss | Significant reduction of disease activity after 6 months of diet Higher weight loss was associated with improvement in a dose–response manner |
| Di Minno et al. (2013) [49] | Interventional study; 126 overweight patients with PsA | Hypocaloric diet Free-managed diet | Minimal Disease Activity Metabolic parameters | 74 patients managed a ≥5% weight loss, this being a predictor of minimal disease activity Regardless of diet, weight loss was crucial in predicting disease activity |
| Kharaeva et al. (2009) [50] | Case–control; 30 patients with PsA | Selenium aspartate, coenzyme Q10, vitamin E | Disease activity Pro/antioxidant balance in granulocytes, plasma Involved epidermis RBC sedimentation rate | Diet was effective in reducing oxidative stress in patients with PsA, together with improvement of clinical condition |
| Kristensen et al. (2018) [51] | Randomized controlled trial; 133 patients with PsA | Fish oil supplementation | Disease activity Use of analgesics Leukotriene formation from activated granulocytes | Decreased activity score for patients receiving the diet, decreased use of NSAID and paracetamol, reduced formation of leukotriene from activated granulocytes |
| Adawi et al. (2019) [52] | Cohort study; 37 patients with PsA | Ramadan practice | Disease activity Body mass index | Diet had a beneficial impact on disease activity, although weight loss did not vary between groups |
| Author (Year) | Study Design and Participants’ Characteristics | Type of Diet | Variables Evaluated | Outcome |
|---|---|---|---|---|
| Herfarth et al. (2014) [18] | Cross-sectional observational study; 1647 IBD patients enrolled in the CCFA Partners internet-based cohort, 616 diagnosed with UC and 1031 with CD | Gluten-free diet | Symptom improvement Dietary adherence | 65.6% of patients who have followed or are still on a gluten-free diet reported improvement in at least 1 symptom. Adherence was only associated with a reduction in fatigue, not any other symptoms |
| Gearry et al. (2008) [21] | Longitudinal retrospective; 72 IBD patients, 20 diagnosed with UC and 52 with CD | Low-FODMAP diet | Dietary adherence Change in gastrointestinal symptoms | Reduction of FODMAP intake is correlated with a reduction in abdominal pain, diarrhea, wind and bloating |
| Grammatikopoulou et al. (2020) [22] | Meta-analysis of randomized controlled trials; 205 IBD patients | Low-FODMAP diet | Disease activity Quality of life CRP and calprotectin levels | Studies showed conflicting results in terms of disease activity and quality of life. Most trials also found no significant difference in calprotectin or CRP levels after dietary restriction |
| Chicco et al. (2020) [76] | Interventional study; 142 IBD patients, 84 diagnosed with UC and 58 with CD | Mediterranean diet | Body mass index Fat body mass Lean body mass Disease activity Quality of life CRP and calprotectin levels Liver steatosis Serum lipid levels | Adherence to the Mediterranean diet showed an improvement in disease activity and reductions in inflammation markers and obesity-related parameters. Liver steatosis also showed notable improvement |
| Vrdoljak et al. (2020) [77] | Cross-sectional observational study; 94 IBD patients, 44 diagnosed with UC and 50 with CD | Mediterranean diet | Body mass index Waist circumference Disease activity Serum lipid levels hsCRP levels Dietary adherence Attitude regarding disease and eating habits | IBD patients adhering to the Mediterranean diet had higher HDL cholesterol levels. Although only 9 participants fulfilled the criteria for Mediterranean diet adherence, a majority of the patients considered that a more controlled diet would beneficially impact their symptoms |
| Khalili et al. (2019) [78] | Prospective cohort 83,147 participants enrolled in the Swedish Mammography Cohort and the Cohort of Swedish Men, respectively | Mediterranean diet | Dietary adherence Risk of CD and UC | Adherence to the Mediterranean diet is correlated with a lower risk of later-onset CD but not UC |
| Bodini et al. (2019) [79] | Randomized controlled trial; 55 IBD patients, 20 patients with UC, 35 patients with CD) | Low-FODMAP diet | Body mass index Disease activity CRP and calprotectin levels Quality of life | Although disease activity showed improvement in CD patients in the low FODMAP group, the UC cohort had similar scores after the 6-week dietary intervention regardless of diet. CRP levels showed no improvement; however, there was a significant reduction in calprotectin levels coupled with a modest increase in quality of life in the low-FODMAP group |
| Suskind et al. (2016) [80] | Cross-sectional observational study; 417 IBD patients, 221 diagnosed with UC and 196 with CD | Specific carbohydrate diet | Change in gastrointestinal symptomatology and association with laboratory values | Patients on the specific carbohydrate diet reported a decrease in abdominal pain, daily activity limitation, diarrhea and blood in the stools. However, less than half reported an improvement in laboratory values associated with the perceived remission |
| Disease | Diet | Recommendations |
|---|---|---|
| Psoriatic arthritis | Low-energy diet Hypocaloric diet Energy-restricted diet | Weight loss is recommended in patients with PsA for potential increase in pharmacological response |
| Grade of Recommendation * | Recommendation | |
|---|---|---|
| IBD | Strong consensus | A diet rich in fruit and vegetables, rich in n-3 fatty acids and low in n-6 fatty acids is associated with a decreased risk of developing Crohn’s disease or ulcerative colitis and is therefore recommended |
| Strong consensus | There is no “IBD diet” that can be generally recommended to promote remission in IBD patients with active disease | |
| Strong consensus | No specific diet needs to be followed during remission phases of IBD | |
| CD | Strong consensus | Exclusion diets cannot be recommended to achieve remission in active CD, even if the patient suffers from individual intolerances |
| Strong consensus | Probiotic therapy should not be used for maintenance of remission in CD | |
| UC | Strong consensus | Probiotic therapy should be considered for the maintenance of remission in ulcerative colitis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, S.L.; Dumitrascu, D.I.; Brata, V.D.; Duse, T.A.; Florea, M.D.; Ismaiel, A.; Muntean, L.M.; Grad, S. Nutrition in Spondyloarthritis and Related Immune-Mediated Disorders. Nutrients 2022, 14, 1278. https://doi.org/10.3390/nu14061278
Popa SL, Dumitrascu DI, Brata VD, Duse TA, Florea MD, Ismaiel A, Muntean LM, Grad S. Nutrition in Spondyloarthritis and Related Immune-Mediated Disorders. Nutrients. 2022; 14(6):1278. https://doi.org/10.3390/nu14061278
Chicago/Turabian StylePopa, Stefan Lucian, Dinu Iuliu Dumitrascu, Vlad Dumitru Brata, Traian Adrian Duse, Maria Delia Florea, Abdulrahman Ismaiel, Laura Mirela Muntean, and Simona Grad. 2022. "Nutrition in Spondyloarthritis and Related Immune-Mediated Disorders" Nutrients 14, no. 6: 1278. https://doi.org/10.3390/nu14061278
APA StylePopa, S. L., Dumitrascu, D. I., Brata, V. D., Duse, T. A., Florea, M. D., Ismaiel, A., Muntean, L. M., & Grad, S. (2022). Nutrition in Spondyloarthritis and Related Immune-Mediated Disorders. Nutrients, 14(6), 1278. https://doi.org/10.3390/nu14061278







