Modelling the Impact of Reducing Ultra-Processed Foods Based on the NOVA Classification in Australian Women of Reproductive Age
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Data
2.3. Dietary Scenarios
2.4. Dietary Modelling
3. Results
3.1. Population Baseline Intakes
3.2. Strategy 1
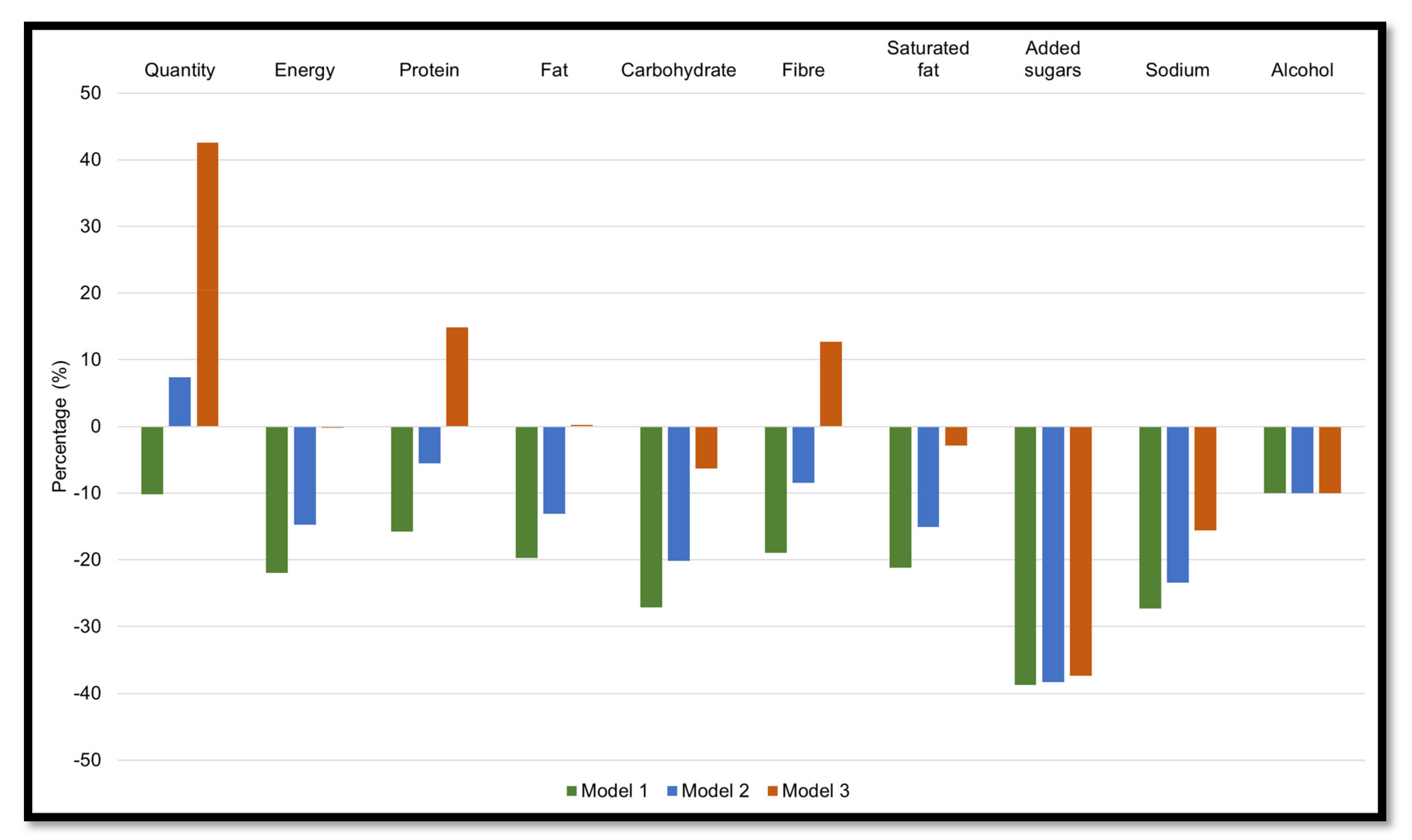
3.2.1. Model 1: Reducing Ultra-Processed Foods by 50%
3.2.2. Models 2: Reducing Ultra-Processed Foods by 50% and Increasing Unprocessed or Minimally Processed Foods by 25%
3.2.3. Model 3: Reducing Ultra-Processed Foods by 50% and Increasing Unprocessed or Minimally Processed Foods by 75%
3.3. Strategy 2
3.3.1. Model 1: Reducing Processed Foods by 50%
3.3.2. Model 2: Reducing Processed Foods by 50% and Increasing Unprocessed or Minimally Processed Foods by 25%
3.3.3. Model 3: Reducing Processed Foods by 50% and Increasing Unprocessed or Minimally Processed Foods by 75%
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organisation (WHO). Reproductive Health Indicators. Reproductive Health and Research Guidelines for Their Generation, Interpretation and Analysis for Global Monitoring. 2006. Available online: http://apps.who.int/iris/bitstream/handle/10665/43185/924156315X_eng.pdf;jsessionid=13280E8CC5E65685D35F6AF1DA27B394?sequence=1 (accessed on 18 November 2021).
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Gomersall, S.R.; Dobson, A.J.; Brown, W.J. Weight Gain, Overweight, and Obesity: Determinants and Health Outcomes from the Australian Longitudinal Study on Women’s Health. Curr. Obes. Rep. 2014, 3, 46–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieger, J.A.; Hutchesson, M.J.; Cooray, S.D.; Bahri Khomami, M.; Zaman, S.; Segan, L.; Teede, H.; Moran, L.J. A review of maternal overweight and obesity and its impact on cardiometabolic outcomes during pregnancy and postpartum. Ther. Adv. Reprod. Health 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Patro Golab, B.; Santos, S.; Voerman, E.; Lawlor, D.A.; Jaddoe, V.W.V.; Gaillard, R.; Authors, M.S.G. Influence of maternal obesity on the association between common pregnancy complications and risk of childhood obesity: An individual participant data meta-analysis. Lancet Child Adolesc. Health 2018, 2, 812–821. [Google Scholar] [CrossRef] [Green Version]
- Jacob, C.M.; Baird, J.; Barker, M.; Cooper, C.; Hanson, M.; World Health Organization. The Importance of a Life Course Approach to Health: Chronic Disease Risk from Preconception through Adolescence and Adulthood. 2017. Available online: https://www.who.int/life-course/publications/life-course-approach-to-health.pdf (accessed on 18 November 2021).
- Rooney, B.L.; Schauberger, C.W.; Mathiason, M.A. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet. Gynecol. 2005, 106, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Linné, Y.; Dye, L.; Barkeling, B.; Rössner, S. Long-term weight development in women: A 15-year follow-up of the effects of pregnancy. Obes. Res. 2004, 12, 1166–1178. [Google Scholar] [CrossRef] [PubMed]
- Blumfield, M.L.; Hure, A.J.; Macdonald-Wicks, L.K.; Patterson, A.J.; Smith, R.; Collins, C.E. Disparities exist between National food group recommendations and the dietary intakes of women. BMC Womens Health 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J.C.; Moran, L.J.; Teede, H.J.; Ranasinha, S.; Lombard, C.B.; Harrison, C.L. Exploring Diet Quality between Urban and Rural Dwelling Women of Reproductive Age. Nutrients 2017, 9, 586. [Google Scholar] [CrossRef] [Green Version]
- Mishra, G.D.; Schoenaker, D.A.; Mihrshahi, S.; Dobson, A.J. How do women’s diets compare with the new Australian dietary guidelines? Public Health Nutr. 2015, 18, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, S.; Sayami, J.T.; Thapa, P.; Sayami, M.; Kandel, B.P.; Banjara, M.R. Dietary intake patterns and nutritional status of women of reproductive age in Nepal: Findings from a health survey. Archives of Public Health 2016, 74, 2. [Google Scholar] [CrossRef] [Green Version]
- Devarshi, P.P.; Legette, L.L.; Grant, R.W.; Mitmesser, S.H. Total estimated usual nutrient intake and nutrient status biomarkers in women of childbearing age and women of menopausal age. Am. J. Clin. Nutr. 2021, 113, 1042–1052. [Google Scholar] [CrossRef]
- Padmadas, S.S.; Dias, J.G.; Willekens, F.J. Disentangling women’s responses on complex dietary intake patterns from an Indian cross-sectional survey: A latent class analysis. Public Health Nutr. 2006, 9, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, Z.; Yang, H.; Qiu, P.; Wang, H.; Wang, F.; Zhao, Q.; Fang, J.; Nie, J. Consumption of ultra-processed foods and health outcomes: A systematic review of epidemiological studies. Nutr. J. 2020, 19, 86. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013, 14 (Suppl. S2), 21–28. [Google Scholar] [CrossRef]
- Aguirre, A.; Borneo, M.T.; El Khori, S.; Borneo, R. Exploring the understanding of the term “ultra-processed foods” by young consumers. Food Res. Int. 2019, 115, 535–540. [Google Scholar] [CrossRef]
- Ares, G.; Vidal, L.; Allegue, G.; Gimenez, A.; Bandeira, E.; Moratorio, X.; Molina, V.; Curutchet, M.R. Consumers’ conceptualization of ultra-processed foods. Appetite 2016, 105, 611–617. [Google Scholar] [CrossRef]
- Adams, J.; White, M. Characterisation of UK diets according to degree of food processing and associations with socio-demographics and obesity: Cross-sectional analysis of UK National Diet and Nutrition Survey (2008–2012). Int. J. Behav. Nutr. Phys. Act. 2015, 12, 160. [Google Scholar] [CrossRef] [Green Version]
- Cediel, G.; Reyes, M.; da Costa Louzada, M.L.; Martinez Steele, E.; Monteiro, C.A.; Corvalan, C.; Uauy, R. Ultra-processed foods and added sugars in the Chilean diet (2010). Public Health Nutr 2018, 21, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Nardocci, M.; Leclerc, B.S.; Louzada, M.L.; Monteiro, C.A.; Batal, M.; Moubarac, J.C. Consumption of ultra-processed foods and obesity in Canada. Can. J. Public Health 2019, 110, 4–14. [Google Scholar] [CrossRef]
- Machado, P.P.; Steele, E.M.; Levy, R.B.; da Costa Louzada, M.L.; Rangan, A.; Woods, J.; Gill, T.; Scrinis, G.; Monteiro, C.A. Ultra-processed food consumption and obesity in the Australian adult population. Nutr. Diabetes 2020, 10, 39. [Google Scholar] [CrossRef]
- Rauber, F.; Steele, E.M.; Louzada, M.; Millett, C.; Monteiro, C.A.; Levy, R.B. Ultra-processed food consumption and indicators of obesity in the United Kingdom population (2008–2016). PLoS ONE 2020, 15, e0232676. [Google Scholar] [CrossRef] [PubMed]
- Bielemann, R.M.; Motta, J.V.S.; Minten, G.C.; Horta, B.L.; Gigante, D.P. Consumption of ultra-processed foods and their impact on the diet of young adults. Rev. Saude Publica 2015, 49, 28. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Park, J.M.; Oh, S.U.; Ha, K.; Joung, H. Consumption of Ultra-Processed Foods Increases the Likelihood of Having Obesity in Korean Women. Nutrients 2021, 13, 698. [Google Scholar] [CrossRef] [PubMed]
- Srour, B.; Fezeu, L.K.; Kesse-Guyot, E.; Alles, B.; Mejean, C.; Andrianasolo, R.M.; Chazelas, E.; Deschasaux, M.; Hercberg, S.; Galan, P.; et al. Ultra-processed food intake and risk of cardiovascular disease: Prospective cohort study (NutriNet-Sante). BMJ 2019, 365, l1451. [Google Scholar] [CrossRef] [Green Version]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Alles, B.; Mejean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Sante prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef] [Green Version]
- Machado, P.P.; Steele, E.M.; Louzada, M.; Levy, R.B.; Rangan, A.; Woods, J.; Gill, T.; Scrinis, G.; Monteiro, C.A. Ultra-processed food consumption drives excessive free sugar intake among all age groups in Australia. Eur. J. Nutr. 2020, 59, 2783–2792. [Google Scholar] [CrossRef]
- Weinstein, M.C.; O’Brien, B.; Hornberger, J.; Jackson, J.; Johannesson, M.; McCabe, C.; Luce, B.R. Principles of good practice for decision analytic modeling in health-care evaluation: Report of the ISPOR Task Force on Good Research Practices--Modeling Studies. Value Health 2003, 6, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Australian Government. Department of Health and Aging, and the National Health and Medical Research Council. A Modelling System to Inform the Revision of the Australian Guide to Healthy Eating. 2011. Available online: https://www.eatforhealth.gov.au/sites/default/files/files/public_consultation/n55a_dietary_guidelines_food_modelling_111216.pdf (accessed on 16 June 2021).
- Heart Foundation. Rapid Review of the Evidence. Effectiveness of Food Reformulation as a Strategy to Improve Population Health. 2012. Available online: https://www.heartfoundation.org.au/getmedia/2bcb1637-1a86-48fc-bb6c-b243c4746272/RapidReview_FoodReformulation.pdf (accessed on 8 July 2021).
- Cobiac, L.J.; Tam, K.; Veerman, L.; Blakely, T. Taxes and Subsidies for Improving Diet and Population Health in Australia: A Cost-Effectiveness Modelling Study. PLoS Med. 2017, 14, e1002232. [Google Scholar] [CrossRef]
- Scarborough, P.; Nnoaham, K.E.; Clarke, D.; Capewell, S.; Rayner, M. Modelling the impact of a healthy diet on cardiovascular disease and cancer mortality. J. Epidemiol. Community Health 2012, 66, 420–426. [Google Scholar] [CrossRef]
- Hutchesson, M.J.; de Jonge Mulock Houwer, M.; Brown, H.M.; Lim, S.; Moran, L.J.; Vincze, L.; Rollo, M.E.; Hollis, J.L. Supporting women of childbearing age in the prevention and treatment of overweight and obesity: A scoping review of randomized control trials of behavioral interventions. BMC Womens Health 2020, 20, 14. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. ABS. Australian Health Survey: Users’ Guide 2011–2013 Catalogue No. 4363.0.55.001. 2015. Available online: http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4363.0.55.001Chapter1002011-13 (accessed on 16 June 2021).
- Food Standards Australia New Zealand. FSANZ. AUSNUT 2011–2013. Available online: http://www.foodstandards.gov.au/science/monitoringnutrients/ausnut/Pages/about.aspx (accessed on 8 July 2021).
- O’Halloran, S.A.; Lacy, K.E.; Grimes, C.A.; Woods, J.; Campbell, K.J.; Nowson, C.A. A novel processed food classification system applied to Australian food composition databases. J. Hum. Nutr. Diet. 2017, 30, 534–541. [Google Scholar] [CrossRef]
- Machado, P.P.; Steele, E.M.; Levy, R.B.; Sui, Z.; Rangan, A.; Woods, J.; Gill, T.; Scrinis, G.; Monteiro, C.A. Ultra-processed foods and recommended intake levels of nutrients linked to non-communicable diseases in Australia: Evidence from a nationally representative cross-sectional study. BMJ Open 2019, 9, e029544. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, C.A.; Cannon, G.; Levy, R.B.; Moubarac, J.C.; Louzada, M.L.; Rauber, F.; Khandpur, N.; Cediel, G.; Neri, D.; Martinez-Steele, E.; et al. Ultra-processed foods: What they are and how to identify them. Public Health Nutr. 2019, 22, 936–941. [Google Scholar] [CrossRef]
- Moubarac, J.C.; Parra, D.C.; Cannon, G.; Monteiro, C.A. Food Classification Systems Based on Food Processing: Significance and Implications for Policies and Actions: A Systematic Literature Review and Assessment. Curr. Obes. Rep. 2014, 3, 256–272. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. AIHW. Poor Diet. 2019. Available online: https://www.aihw.gov.au/reports/food-nutrition/poor-diet/contents/poor-diet-in-adults (accessed on 8 July 2021).
- Habibi, N.; Livingstone, K.M.; Edwards, S.; Grieger, J.A. Do Older Women of Reproductive Age Have Better Diet Quality than Younger Women of Reproductive Age? Nutrients 2021, 13, 3830. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Plates, Pyramids, Planet. Developments in National Healthy and Sustainable Dietary Guidelines: A State of Play Assessment. FAO/University of Oxford. Rome. 2016. Available online: https://www.fao.org/documents/card/en/c/d8dfeaf1-f859-4191-954f-e8e1388cd0b7/ (accessed on 24 September 2021).
- Crimarco, A.; Landry, M.J.; Gardner, C.D. Ultra-processed Foods, Weight Gain, and Co-morbidity Risk. Curr. Obes. Rep. 2021. [Google Scholar] [CrossRef]
- Pulker, C.E.; Scott, J.A.; Pollard, C.M. Ultra-processed family foods in Australia: Nutrition claims, health claims and marketing techniques. Public Health Nutr. 2018, 21, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Forde, C.G. From perception to ingestion; the role of sensory properties in energy selection, eating behaviour and food intake. Food Qual. Prefer. 2018, 66, 171–177. [Google Scholar] [CrossRef]
- Australian Government. National Health and Medical Research Council. Eat for Health. Discretionary Food and Drink Choices. 2017. Available online: https://www.eatforhealth.gov.au/food-essentials/discretionary-food-and-drink-choices (accessed on 16 June 2021).
- Ryan, D.; Heaner, M. Guidelines (2013) for managing overweight and obesity in adults. Preface to the full report. Obesity 2014, 22 (Suppl. S2), S1–S3. [Google Scholar] [CrossRef]
- Greenway, F.L. Physiological adaptations to weight loss and factors favouring weight regain. Int. J. Obes. 2015, 39, 1188–1196. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, J.A.; Drapeau, V.; Astrup, A.; Tremblay, A. Relationship between diet-induced changes in body fat and appetite sensations in women. Appetite 2009, 52, 809–812. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 2011, 365, 1597–1604. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.J. The psychology of food craving. Proc. Nutr. Soc. 2007, 66, 277–285. [Google Scholar] [CrossRef]
- Hasan, H.; Nayfeh, T.; Alzuabi, M.; Wang, Z.; Kuchkuntla, A.R.; Prokop, L.J.; Newman, C.B.; Murad, M.H.; Rajjo, T.I. Weight Loss and Serum Lipids in Overweight and Obese Adults: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2020, 105, 3695–3703. [Google Scholar] [CrossRef]
- Xiang, L.; Wu, H.; Pan, A.; Patel, B.; Xiang, G.; Qi, L.; Kaplan, R.C.; Hu, F.; Wylie-Rosett, J.; Qi, Q. FTO genotype and weight loss in diet and lifestyle interventions: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 1162–1170. [Google Scholar] [CrossRef]
- Melby, C.L.; Paris, H.L.; Foright, R.M.; Peth, J. Attenuating the Biologic Drive for Weight Regain Following Weight Loss: Must What Goes Down Always Go Back Up? Nutrients 2017, 9, 468. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, N.A.; Rigby, B.R.; La Bounty, P.; Shelmadine, B.; Bowden, R.G. A review of weight control strategies and their effects on the regulation of hormonal balance. J. Nutr. Metab. 2011, 2011, 237932. [Google Scholar] [CrossRef] [Green Version]
- Grieger, J.A.; Johnson, B.J.; Wycherley, T.P.; Golley, R.K. Comparing the Nutritional Impact of Dietary Strategies to Reduce Discretionary Choice Intake in the Australian Adult Population: A Simulation Modelling Study. Nutrients 2017, 9, 442. [Google Scholar] [CrossRef] [Green Version]
- Australian Institute of Health and Welfare. AIHW. Australian Government. The Health of Australia’s Females. 2019. Available online: https://www.aihw.gov.au/reports/men-women/female-health/contents/lifestyle-risk-factors/tobacco-smoking-alcohol-and-illicit-drugs (accessed on 24 September 2021).
- Anwar, M.Y.; Marcus, M.; Taylor, K.C. The association between alcohol intake and fecundability during menstrual cycle phases. Hum. Reprod. 2021, 36, 2538–2548. [Google Scholar] [CrossRef]
- Lawrence, M.A.; Dickie, S.; Woods, J.L. Do Nutrient-Based Front-of-Pack Labelling Schemes Support or Undermine Food-Based Dietary Guideline Recommendations? Lessons from the Australian Health Star Rating System. Nutrients 2018, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Dickie, S.; Woods, J.L.; Lawrence, M. Analysing the use of the Australian Health Star Rating system by level of food processing. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 128. [Google Scholar] [CrossRef] [PubMed]
- Page, B.; Sharp, A.; Lockshin, L.; Sorensen, H. Parents and children in supermarkets: Incidence and influence. J. Retail. Consum. Serv. 2018, 40, 31–39. [Google Scholar] [CrossRef]
- Marchese, L.; Livingstone, K.M.; Woods, J.L.; Wingrove, K.; Machado, P. Ultra-processed food consumption, socio-demographics and diet quality in Australian adults. Public Health Nutr. 2022, 25, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Kane, S.; Herron, L.M.; Matsuyama, M.; Lewis, M. A tale of two cities: The cost, price-differential and affordability of current and healthy diets in Sydney and Canberra, Australia. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 80. [Google Scholar] [CrossRef]
- Lee, A.J.; Kane, S.; Ramsey, R.; Good, E.; Dick, M. Testing the price and affordability of healthy and current (unhealthy) diets and the potential impacts of policy change in Australia. BMC Public Health 2016, 16, 315. [Google Scholar] [CrossRef] [Green Version]
- Baraldi, L.G.; Martinez Steele, E.; Canella, D.S.; Monteiro, C.A. Consumption of ultra-processed foods and associated sociodemographic factors in the USA between 2007 and 2012: Evidence from a nationally representative cross-sectional study. BMJ Open 2018, 8, e020574. [Google Scholar] [CrossRef] [Green Version]
- Conde, W.L.; Monteiro, C.A. Nutrition transition and double burden of undernutrition and excess of weight in Brazil. Am. J. Clin. Nutr. 2014, 100, 1617S–1622S. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; de Castro, I.R.; Cannon, G. Increasing consumption of ultra-processed foods and likely impact on human health: Evidence from Brazil. Public Health Nutr. 2011, 14, 5–13. [Google Scholar] [CrossRef]

| Mean Baseline Intake | Intake from Ultra-Processed Foods (%) | Intake from Processed Foods (%) | Intake from Unprocessed or Minimally Processed Foods (%) | Intake from Processed Culinary Ingredients (%) | |
|---|---|---|---|---|---|
| Quantity (g) | 3112.6 | 548.9 (17.6) | 175.3 (5.6) | 2361.8 (75.9) | 26.6 (0.9) |
| Energy (kJ) * | 7388.2 | 3056.2 (41.4) | 1093.0 (14.8) | 2656.1 (36.0) | 582.9 (7.9) |
| Protein (g) | 74.6 | 20.8 (27.9) | 10.9 (14.6) | 42.7 (57.2) | 0.2 (0.3) |
| Fat (g) | 63.9 | 26.5 (41.5) | 8.7 (13.6) | 17.1 (26.8) | 11.6 (18.2) |
| Carbohydrate (g) | 198.7 | 97.1 (48.9) | 20.8 (10.5) | 71.5 (36.0) | 9.3 (4.7) |
| Fibre (g) | 19.8 | 6.3 (31.8) | 2.4 (12.1) | 11.1 (56.1) | 0.0 (0.0) |
| Saturated Fat (g) | 24.3 | 10.6 (43.6) | 4.0 (16.5) | 6.0 (24.7) | 3.7 (15.2) |
| Added sugar (g) | 46.2 | 36.8 (79.7) | 1.4 (3.0) | 0.2 (0.3) | 7.9 (17.1) |
| Sodium (mg) | 2142.3 | 1309.5 (61.1) | 463.4 (21.6) | 289.8 (13.5) | 79.6 (3.7) |
| Alcohol (g) | 9.1 | 1.8 (19.8) | 7.3 (80.2) | 0.0 (0.0) | 0 (0.0) |
| NOVA Food Groups | Energy (kJ) | Energy (kcal) | % of Total Energy Intake |
|---|---|---|---|
| Ultra-processed foods | 3056.2 | 730.4 | 41.4 |
| Mass-produced packaged breads | 333.5 | 79.7 | 4.5 |
| Pastries, buns, and cakes | 292.3 | 69.9 | 4.0 |
| Fast foods dishes a | 286.7 | 68.5 | 3.9 |
| Confectionery | 247.9 | 59.2 | 3.4 |
| Frozen and shelf stable ready meals b | 237.4 | 56.7 | 3.2 |
| Fruit drinks and iced teas | 206.2 | 49.3 | 2.8 |
| Breakfast cereals | 190.4 | 45.5 | 2.6 |
| Biscuits | 180.4 | 43.1 | 2.4 |
| Carbonated soft drinks | 171.7 | 41.0 | 2.3 |
| Milk-based drinks | 168.5 | 40.3 | 2.3 |
| Sausage and other reconstituted meat products | 163.5 | 39.1 | 2.2 |
| Sauces, dressing, and gravies | 157.7 | 37.7 | 2.1 |
| Salty snacks | 118.5 | 28.3 | 1.6 |
| Ice cream, ice pops, and frozen yoghurts | 101.7 | 24.3 | 1.4 |
| Margarine and other spreads | 91.3 | 21.8 | 1.2 |
| Alcoholic distilled drinks | 53.7 | 12.8 | 0.7 |
| Other c | 54.8 | 13.1 | 0.7 |
| Processed foods | 1093.0 | 261.2 | 14.8 |
| Processed breads | 427.0 | 102.1 | 5.8 |
| Beer and wine | 233.5 | 55.8 | 3.2 |
| Cheese | 220.2 | 52.6 | 3.0 |
| Bacon and other salted, smoked, or canned meat or fish | 84.0 | 20.1 | 1.1 |
| Vegetables and other plant foods preserved in brine | 36.2 | 8.7 | 0.5 |
| Other d | 92.1 | 22.0 | 1.2 |
| Unprocessed or minimally processed foods | 2656.1 | 634.8 | 36.0 |
| Red meat and poultry | 582.5 | 139.2 | 7.9 |
| Cereal grains and flours | 485.8 | 116.1 | 6.6 |
| Milk and plain yoghurt | 452.7 | 108.2 | 6.1 |
| Fruits e | 323.2 | 77.2 | 4.4 |
| Vegetables | 239.2 | 57.2 | 3.2 |
| Pasta | 204.8 | 48.9 | 2.8 |
| Nuts and seeds | 96.3 | 23.0 | 1.3 |
| Potatoes and other tubers and roots | 80.5 | 19.2 | 1.1 |
| Eggs | 71.7 | 17.1 | 1.0 |
| Fish | 62.1 | 14.8 | 0.8 |
| Legumes | 31.7 | 7.6 | 0.4 |
| Other f | 25.8 | 6.2 | 0.3 |
| Processed culinary ingredients | 582.9 | 139.3 | 7.9 |
| Plant oils | 269.6 | 64.4 | 3.6 |
| Animal fats | 164.3 | 39.3 | 2.2 |
| Table sugar | 125.6 | 30.0 | 1.7 |
| Other g | 23.4 | 5.6 | 0.3 |
| Total | 7388.2 | 1765.8 | 100.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habibi, N.; Leemaqz, S.Y.-L.; Grieger, J.A. Modelling the Impact of Reducing Ultra-Processed Foods Based on the NOVA Classification in Australian Women of Reproductive Age. Nutrients 2022, 14, 1518. https://doi.org/10.3390/nu14071518
Habibi N, Leemaqz SY-L, Grieger JA. Modelling the Impact of Reducing Ultra-Processed Foods Based on the NOVA Classification in Australian Women of Reproductive Age. Nutrients. 2022; 14(7):1518. https://doi.org/10.3390/nu14071518
Chicago/Turabian StyleHabibi, Nahal, Shalem Yiner-Lee Leemaqz, and Jessica Anne Grieger. 2022. "Modelling the Impact of Reducing Ultra-Processed Foods Based on the NOVA Classification in Australian Women of Reproductive Age" Nutrients 14, no. 7: 1518. https://doi.org/10.3390/nu14071518
APA StyleHabibi, N., Leemaqz, S. Y.-L., & Grieger, J. A. (2022). Modelling the Impact of Reducing Ultra-Processed Foods Based on the NOVA Classification in Australian Women of Reproductive Age. Nutrients, 14(7), 1518. https://doi.org/10.3390/nu14071518